Abstract
Every year 40 per 100.000 people suffer from peripheral facial palsy (FP). So, it is one of the most common nerve diseases. The paralysis leads to a wide range of daily impairments and results in a loss of ADLs, like reduced facial expression, problems with eating and drinking, or incomplete eyelid closure. In many cases, a purely pharmaceutical-based therapy does not lead to any improvement. In the last few years, in addition to the most common existing conservative therapy options, the focus was placed on scientific research of extended options, including functional electrical stimulation (FES). Research results and practical experience manifest positive aspects of FES as additional therapeutical approach. Indications and contraindications of FES in the treatment of FP are shown. Furthermore, an application example gives an insight in the practical FP therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Facial paralysis
- Facial palsy and functional electric stimulation
- Facial rehabilitation
- Facial reanimation
10.1 N. facialis
Peripheral facial palsy (FP) can be a form of facial paralysis (complete denervation) or facial paresis (incomplete denervation or weakness) on mostly only one side of the face that results from a lesion to the facial nerve. Every year, 7–40 per 100,000 people suffer from idiopathic facial palsy, which is one of the most common cranial nerve lesions leading to facial muscle dysfunction [1]. While the diagnosis includes numerous medical consequences, one must also consider the social consequences it entails. FP is caused by different etiological factors and can lead to functional deficits in eating, speaking, nasal breathing, eye closure as well as verbal and nonverbal communication, which will be further described in Chap. 10.6. These functional tasks necessary for daily self-care can also be defined as “activities of daily living (ADLs)”. An inability to perform these fundamental activities may lead to poor quality of life. The complex interaction of facial muscles enables us to express emotions, such as happiness, sadness, anger, fear, and disgust. But what happens when this is no longer possible? When eating in public becomes an unpleasant experience, physical appearance has changed, and muscle weakness reduces intelligibility of speech? Thus far, people affected by FP have often been left to deal with the unavoidable impact of the disease on mental health by themselves. In many cases, this led to social withdrawal and, in a few cases, secondary depressions [2]. The German Society of Neurology [1] outlined recommendations for pharmaceutical treatment in the acute phase. However, there do not exist recommendations for physical or speech and language therapy intervention in the acute, acute-chronic, and chronic phase. Even though interest in this topic has grown in recent years and treatment options are becoming more diverse, the treatment of FP still lacks in many areas a strong evidence-based research base. Due to the complexity of the topic and numerous treatment options, it is essential that the patient is taken care by an interdisciplinary team where treatment options are carefully presented to the patient, while severity and duration of the disease are being considered. This will allow the patient to make an informed choice about treatment options and the team to optimally collaborate with the patient to work together on the rehabilitation process. From a clinical perspective, the process of healing significantly depends on the cause, the location, and severity of the lesion.
Therefore, anatomy, physiology, and pathology as well as everyday impairments will be discussed in the following chapters.
10.2 Anatomy
The n. facialis is the seventh cranial nerve (CN VII). The motor facial nucleus emerges from the pons, lateral to the cerebellopontine angle [3]. In this area, the cranial nerve forms an inner angle, surrounding the abducens nerve. After building the inner angle, fibers of the facial nerve merge with fibers of the vestibulocochlear nerve and extend into the bony structure of the skull base. The fibers then pass through the porus acusticus internus of the temporal bone, into the immediate vicinity of the inner and middle ear. These fibers carry afferent and efferent signals from the cranial nerves. At the foramen stylomastoideum, the n. facialis exists the bony structure of the petrosal bone and extends to the glandula parotidea, where it divides into further branches, supplying the occipital, neck, and face muscles.
The main trunks are:
-
Posterior auricular nerve: supplies sensory muscles of the inner and outer ear and the occipital muscle.
-
Ramus stylohyoideus: leads to the m. stylohyoideus.
-
Ramus digastricus: supplies the posterior digastric muscle.
-
Plexus parotideus: is the generic term for the nerve plexus between the inner and outer parotic leaf, which supplies the facial muscles and is usually distinguished in five branches.
-
Ramus temporalis,
-
Ramus zygomatici,
-
Ramus buccales,
-
Ramus marginalis mandibule: along the margin of the lower jaw,
-
Ramus colli: supplies the platysma.
-
In addition, the n. intermedius proceeds with the n. vestibulocochlearis and the n. facialis. This so-called intermediate nerve carries afferent and efferent fibers to supply the nasal and lacrimal secretions, salivary glands and the sense of taste. Damage in the early course of the n. facialis can therefore be associated with a tear secretion disorder, a taste disorder, or a salivary gland secretion disorder.
10.3 Causes for FP
A variety of causes can lead to the development of FP. In general, differentiation is made between cryptogenic and symptomatic causes [4]. In case of cryptogenic FP, also known as idiopathic peripheral FP or Bell’s palsy, the cause is by definition unknown.
If there is evidence of damage (e.g., iatrogenic, tumor-related, virus-associated) it can be considered symptomatic FP. While the cause of acute paresis continues to remain unclear in more than half of the cases, idiopathic FP has a higher chance of spontaneous recovery.
The most common causes that lead to FP are [5]
-
Birth trauma or genetic.
-
Neurological diseases.
-
Infections.
-
Iatrogenic.
-
Metabolic.
-
Toxic.
-
Neoplastic.
-
Idiopathic.
10.4 Pathology
The disease is characterized by limited mobility of the facial muscles of one side, reduced facial expressions and gestures, difficulty eating and/or drinking, and reduced articulatory movements. A common complaint of those affected is an incomplete closure of the eyelid, which often results in eye dryness and redness and may also lead to permanent corneal damage.
While unilateral cranial nerve lesions are more commonly observed, a bilateral facial nerve paresis may also occur (e.g., with an onset of meningeal neuroborreliosis). Depending on the location of nerve damage, the following deficits may occur (Table 10.1) [4]:
-
A nerve lesion distal to the stylomastoid foramen leads to a purely motor paresis of the facial muscles. If the lesion is severe, all facial muscles may be affected, which may be noted by incomplete eyelid closure and the inability to wrinkle skin, located on the forehead.
-
A lesion to the nerve in the bony structure of the skull base may cause a paralysis of the whole mimic musculature. Depending on the location of the lesion, a lacrimal and/or salivary gland disorder, a taste disorder, and hyperacusis may be observed.
-
A lesion to the facial nucleus or the fascicle in the brainstem predominantly causes motor deficits. Deficits may include an incomplete closure of the affected eyelid and inability to wrinkle skin located on the forehead. Lacrimal and salivary secretory functions as well as the sensation of taste typically remain preserved as responsible nerve fibers extend in the petrous bone, together with fibers of the n. facialis.
-
A lesion above the facial nucleus (so-called “central facial nerve palsy”) may result in a perioral palsy. Due to bilateral supply of the upper facial muscles, eye closure and forehead elevation typically remain intact.
10.5 Incomplete vs. Complete Facial Palsy
The terms complete and/or incomplete FP are not synonymous of central and peripheral FP, rather they refer the degree of facial expression loss. There exist various definitions of complete and incomplete FP. When all branches of the n. facialis are reduced in the range of motion (i.e., forehead, eye, cheek, mouth, and neck), some people may refer to it as a complete FP. However, a more stringent definition of complete FP requires a complete failure of all facial muscle movement. The better-defined neurological pair of terms of paresis and paralysis means something similar: In a paresis, there still should be a residual function of the nerve, while all axons are interrupted by a paralysis. This state of paralysis can be diagnosed by conducting a needle electromyogram of all innervated muscles in question. A further differentiated method for diagnosing a FP is the frequently used House-Brackmann scale (HBS) (Table 10.2).
Complete vs. Incomplete FP
Complete peripheral facial nerve palsy: The patient presents with the most severe of FP and would be rated as grade 6 on HBS.
Incomplete peripheral FP: FP can be classified after a spontaneous remission and with moderately severe symptoms f.e. as grade 3 on the HBS.
10.6 Daily Impairment
An overview about daily impairment and loss of ADLs [6]:
-
Impaired facial expression: restricted nonverbal communication.
-
Eyes: impaired eyelid closure or reduced blinking leads to drying out of the cornea, inflamed cornea, visual disturbances, pain and lacrimal secretion disorder.
-
Ear: possible impairment of the m. stapedius may lead to hyperacusis.
-
Eating and drinking: impaired bolus control; difficulty with chewing and bolus formation; food residues in the cheek and associated difficulties removing residues from the cheek, as a consequence of sensory deficits of the mucous membrane of the cheek (cheek biting).
-
Teeth: protective function of saliva against caries and other diseases in the oral cavity; diminished saliva production, dry oral mucosa, disturbed movement-patters of the cheek-muscles, so that self-cleaning, but also brushing teeth becomes more difficult.
-
Facial pain: even though the n. facilis is purely motoric, it should not be associated with facial pain, it is often reported by patients. An initial ear pain, which often occurs days before the palsy appears, is often related to the local inflammation of the facial canal. Despite the pain, it is important to distinguish between the initial and muscle pain that occurs months after reinnervation, which is likely associated with the sudden overstraining due to the defective reinnervation and as a response to massage and botulinum toxin injections.
-
Speech: Reduced orofacial musculature difficulties, such as slurred speech or an altered vocal quality, may arise.
-
“Spasms”: misguided reinnervation may cause unilateral cramps or uncoordinated muscle twitching. Although the underlying pathology differs from spasticity, this term is often erroneously used.
-
Synkinesias/dyskinesias: In the course of the disease synkinesias or dyskinesias may develop. These terms also refer to involuntary muscle movement and can be explained by errors in reinnervation. For example, nerve fibers that were initially responsible for eye closure may now innervate the m. orbicularis oris, and therefore trigger lid-beat synchronous twitching in the corner of the mouth.
10.7 Consequences in the Tissue
Even a mild and short-term form of incomplete facial paresis will have an impact on the soft tissue. This minimal form of FP will course an immediate restriction of movement, a rapid decrease of muscle tone, and thereby several indirect effects like a reduced lymph circulation. If in more severe cases of FP axons and their motor end plates are fully destroyed, it is called a paralysis that leads to denervation atrophy and a complete loss of voluntary movement and muscle tone. The denervation of the VII cranial nerve may subsequently lead to a loss of muscle mass, although there are also indications that muscle breakdown in the face is slower than on the great muscles of the extremities. The lack of neural input may lead to a decrease in muscle fiber thickness and a pathological increase in collagen fibers and connective tissue cells, as a long-term consequence. Therefore, muscle fibers are often remodeled, and affected muscles gradually decrease in tone. This loss of muscle mass may be observed as hypotonic tissue and asymmetry of the facial muscles at rest and in motion [7]. However, facial muscles may also behave similar to striated muscle fibers of the extremities that continue to persist despite denervation of the muscle tissue [8]. When these fibers are stimulated using FES, also a severely damaged muscle may be rebuilt. When stimulation is applied early, the muscle ideally retains its size and function, and the remodeling processes in the muscle tissue may be slowed down or even be stopped entirely.
10.8 Treatment Options of FES
The FES is currently being established as a treatment for facial paralysis. For a long time, the treatment’s potential was not recognized, as side effects were assumed to cause difficulty with facial application. Interestingly, two contradicting side effects of electrical stimulation have been reported: On the one hand, it is repeatedly argued that a FES would prevent or reduce reinnervation. On the other hand, there exists the assertion that FES increases aberrant reinnervation as dys- or/and synkinesis may be noted during FES. Although both claims are repeatedly made, they are not supported by scientific evidence. A recent study investigated this question while treating patients with completely denervated facial muscles. Results show that surface electrical stimulation neither delayed reinnervation nor decreased functional outcomes [9]. A further review of literature on the current state of studies of electrical stimulation in facial paralysis concludes that there is no evidence for a positive effect of FES on acute paresis and only weak evidence in chronic paresis. One explanation for this may be the poor quality of the studies [10].
To overcome this lack of high-quality evidence, a prospective (but not controlled) study is running in Jena. First, results of the stimulation parameters of the first five paralysis patients were published. By surface electrical stimulation a selective m. zygomaticus response in absence of discomfort and unselective contraction of other facial muscle was reproducibly obtained for all the assessed patients. The most effective results with single pulses were observed with pulse width greater or equal to 50 ms. The required amplitude was remarkably lower (≤5 mA vs. up to 15 mA) in freshly diagnosed (≤3 months) than in long-term facial paralysis patients (>5 years). A triangular waveform was more effective than a rectangular waveform, mostly because of the lower discomfort threshold of the latter. Delivery of trains of stimulation showed similar results to the single pulse setting, though lower amplitudes were necessary to achieve the selective m. zygomaticus response [11]. While this shows well, that FES is tolerated in the face, positive effects like increase of muscle tone self-reported by the patients but also objectively visible and measurable are not yet published (Table 10.3).
However, various studies about FES and peripheral nerve damage in other anatomical illustrate regions and on animals, regarding that the FES in case of damage to muscle groups demonstrate a positive effect on muscles. It is believed that after denervation of the muscle a race between breaking down and reinnervation begins. The output then depends on the speed of nerve growth and the rate of atrophy. From animal experiments as well as clinical observations, we know that the facial nerve growth rate is about 1 mm per day. Very close to reality, no data on the rate of muscle atrophy has been published. Successful reinnervation after over a year, but also the sonographic evidence of paralytic facial muscles after more than a year of denervation let hypothesize that the muscular atrophy turns out to be a disintegration process without a defined end. There exist first confirmations that the low-frequency FES affects positive axonal sprouting and functional improvement. A possible reason for this may be that that sensory-mechanical stimulation of the muscles through targeted stimulation of the trigeminal afferents and during the de- and reinnervation period could have positive effects on functional reinnervation. Positive effects of mechanical stimulation, even after surgical nerve reconstruction (i.e., neuromuscular replacement surgery) have already been proven successful for rats [12]. The results of this study can be underpinned through a randomized study of human subjects. This study investigated the effectiveness of low-frequency FES in 20 patients whose Bell palsy had not recovered after 5 months. After randomization, 10 patients received low-frequency FES for 4 weeks, five times a week for 30 minutes, and completed movement exercises under supervision. The other 10 patients merely completed the movement exercises. The evaluation by experts through the Sunnybrook Rating Scale as well as the automatic movement measurement both demonstrated significant improvements of voluntary movements, especially the m. zygomaticus [13]. These results suggest that targeting FP using FES may be a benefit for those affected. In the future FES should present an additional treatment to the occupational, the conservative, and surgical treatment of FP. Before treatment options will be discussed, fields of applications of the FES, indications and contraindications will be discussed in further detail.
10.9 Indications and Contraindications Plus Advantages of FES
The use of FES as a treatment option for patients with FP should be adapted to the individual needs and the situation of the patient. A requirement for the application of FES in speech and language therapy or physical therapy is a qualified therapist as well as an in-depth education of the patient when dealing with the electrical stimulation device. Because of the possibility to apply FES during home practice, the person affected receives FES more frequently than those who only receive FES during therapy sessions. From a therapeutic and medical standpoint, the following contraindications discouraged patients using FES [14]:
-
Type and severity of pain.
-
Lack of motivation, mental state, cognitive limitations.
Acute infections (e.g., herpes zoster) and/or wound healing disorders
-
Acute inflammatory processes.
-
Tumor diseases in the to be stimulated area (except after R0-Resection).
-
Radiotherapy.
-
Internal diseases (internal clarification and statement necessary).
-
Surgical sutures in the immediate area proximity to electrical stimulation.
-
Limited motor skills (device handling), if no assistance by another person (therapist/relative) is possible.
-
Titanium plates in the facial area after severe facial injuries.
-
Pacemaker/defibrillator (internal clarification and statement by the internist necessary).
Indications for FES
-
Good compliance and good cognitive skills to understand the device and the training plan.
-
High motivation of the patient.
Advantages of FES
-
FES as a motivational measure.
-
Independent application of the FES during home practice.
-
The patient receives immediate feedback and can take corrective measures to improve self-perception.
-
Controlling the target muscles at least partially possible.
-
Delaying or reducing muscle atrophy.
-
Shortening the rehabilitation phase.
10.10 Further Recommendations for the Application of FES
-
In case of poor reinnervation after occurrence of idiopathic FP.
-
With poor reinnervation after occurrence and subsidence of all symptoms after herpes zoster induced FP.
-
Immediate use after iatrogenic damage (e.g., acoustic neuroma removal, parotidectomy) after consultation with the attending surgeon to preserve denervated muscle fibers.
-
FES with initially long biphasic triangular pulses until the nerve fibers sprout out again, so that the muscle cannot degenerate in this phase and convert into fat or connective tissue.
-
If there is a surgical restitution of the VII cranial nerve planned, the optimal setting should be discussed in consultation with the surgeons. It is recommended that the patient carries out FES until the surgery, so that the muscle tissue does not convert in connective tissue and the skin trophic stays preserved. It is expected that a cross-face nerve transplant (CFNTPL) or a hypoglossal facial jump resultanastomosis results in reinnervation of the denervated musculature. FES should be offered for at least 6 months or until the first action potentials are observed in needle electromyography.
-
With a two-stage CFNTPL, in combination with a free gracilis graft, FES should be offered 7 days postoperatively for at least 3 months to minimize the atrophy of muscle fibers of the gracilis graft.
10.11 Electrodes to Use
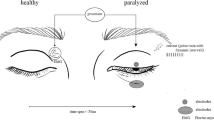
The muscles of the face are laid out in several layers, and the muscles to be stimulated are very small and often extend directly into the area above. Therefore, targeted stimulation of individual muscles may be difficult. The size of the electrodes is crucial so that the affected muscles are stimulated in a targeted manner. Additionally, it must be noted that activity of mimic muscles often shifts the facial skin. Thus, adequate adhesion of the electrodes is a prerequisite for successful stimulation. When stimulating facial muscles, it is further recommended that electrodes with a diameter of 2.5 cm (Figs. 10.1, 10.2, 10.3 and 10.4) be utilized [15]. The advantage of small self-adhesive electrodes is that insulated potentials of small muscle groups can be derived. The disadvantage is poor adhesion of the electrodes to the skin, most likely due to the special texture of the facial skin. As previously mentioned, the skin of the face shifts and stretches due to the movement of the underlying muscles. To improve the adhesiveness of the electrodes it is recommended to fix them with a kinesio-tape, band-aid, or elastic bands with Velcro. The latter is often used by patients, however mostly perceived as an unpleasant restriction. Using a elastic-tape may also reduce the range of motion of the desired movement. In patients with completely denervated FP or after surgically reanimated FP, so-called “sponge electrodes” (Fig. 10.5) may be suitable, which enable a wider area of muscle stimulation. Lastly, there is the option to use oval self-adhesive electrodes (Fig. 10.6) (6 × 4 cm), which are more convenient to use due to sponge electrodes.
10.12 EMG Biofeedback Incomplete Peripheral FP
As stated in the introduction of this chapter (Sects. 10.8, 10.9, 10.10, and 10.11), there is a variety of options for the treatment of FP [14]. If there is an incomplete or aberrant reinnervation, for example, in peripheral facial palsy, EMG biofeedback programs can be applied. If the patient demonstrates measured action potentials in the muscles when using needle EMG, these programs can be used to build strength and endurance of the muscles. Regular and active practice as possible in home-training is an integral part of the disorder’s healing rehabilitation process. The goal is for the patient to independently trigger muscle activity and subsequently be rewarded with electrical stimulation. Biofeedback programs can be used in the following areas:
-
Forehead branch area.
-
Lateral circular muscles of the eye.
-
Cheek area.
-
Lateral circular muscles of the mouth.
Using extended options can be used to achieve maximal muscle strength, endurance, and relaxation. In addition, biofeedback aims to improve intermuscular coordination. Therefore, the use of a FES device can have a positive effect on a patient’s motivation. The application improves perception of the patient to control facial muscles. Consequently, this leads to an improvement of arbitrary movement activation and control (Fig. 10.7, 10.8, and 10.9). To derive the potential of the target muscles, the round adhesive electrodes with a diameter of <2.5 cm should be used. In the forehead branch area, the oval self-adhesive electrodes with a diameter of 6 × 4 cm can be used. The size of the electrodes should always be tailored to the structural features of the patient.
10.13 FES at Completely Denervated FP
A completely denervated FP can occur, for example, in patients during a vestibular schwannoma surgery or a parotidectomy. In some cases, an injury of the facial nerve or complete severance can occur intraoperatively. In this case, the goal of postoperative FES would be to counteract atrophy of the facial muscles [14]. Regardless of the surgeon being able to preserve the nerve intraoperatively or surgical nerve reconstruction being performed, re-innervation of the muscles may take several months. Usually, a nerve regrows approximately 1 mm per day and every nerve suture requires approximately an additional 4 weeks for the axons to grow through. Without FES, atrophy of the mimic musculature would occur due to muscle inactivity. Therefore, FES is recommended to bridge the time till reinnervation to minimize denervation atrophy. So-called exponential or triangular currents with long pulse widths are used in FES as they immediately stimulate muscles rather than nerves. While a nerve can be stimulated with as little as 50 μs, a denervated muscle may need at least 10 ms (10,000 μs) pulse width to demonstrate a motor response. When selecting a suitable electrical stimulation device, ensure that the device offers the possibility of choosing between different current forms as well as long pulse widths to generate at least 250 ms [11]. Furthermore, a course FES may be deemed necessary before surgical intervention. This is because when target muscles stay preserved, a more complicated and invasive replacement surgery could be avoided and instead a reinnervation of the preserved original facial muscles could be achieved (Sect. 10.10). Thus, the significantly more complex free muscle transfer could be handed in. If the FP is completely denervated, long pulse times of up to 250 ms phase duration may be needed and sponge electrodes or large self-adhesive electrodes are typically used during stimulation. However, it should be noted that the electrical stimulation device must approve the use of such electrodes to do so (Table 10.4).
10.14 FES at Central FP
Personal experiences show that the application of FES must become viewed in a differentiated manner when treating central FP [14]. Treatment differentiates from peripheral FP due to the degree of impaired facial expression as well as the occurrence and duration of the disease. Of note, often a kind of pain may occur during early stages of stimulation. If a patient reports an uncomfortable stabbing sensation or pain during FES, stimulation should be discontinued. However, it is important to discriminate between pain experienced during stimulation and facial palsy trigeminal pain, which may occur simultaneously to FP. In some cases, FES may also have an analgesic effect on the patient. During the remission phase and when there are visible reinnervation potentials, biofeedback programs can be used in combination with emotionally supported facial expression movements.
10.15 FES After Operative Reanimated/Supplied FP
The term “surgically reanimated FP” describes reconstructive ways to improve symmetry and motor function of the paralyzed facial musculature. A distinction is made between three levels of function, which varies from the surgical options:
-
The static plane.
-
The dynamic-arbitrary level.
-
The emotional level.
Azizzadeh [16] points out that after severe facial palsy with destruction of the nerve fibers and despite successful operation the original condition of the muscles cannot be fully restored. Regardless of spontaneous remission or surgical reconstruction, facial muscles will no longer be controlled as differentiated as before the onset of FP. In individual cases of nerve fiber loss, certain motor units and muscle fibers must be supplied by other axons. Incorrect or new innervations may be observed during the brain’s motor learning process, for example, after cross-face nerve transplant (CFNTPL) or other neuromuscular surgeries. Therefore, FES may be a valuable contribution to motor learning, motor control, and ultimately improve the symmetry of the face (Figs. 10.5 and 10.6).
Muscle replacement surgeries, coupled with nerve surgeries, may also improve overall emotional muscle function. These include dynamic, arbitrary muscle substitute operations and nerve replacement operations. Examples of nerve replacement surgeries are hypoglossal facial nerve jump anastomosis, masseteric facial anastomosis, and accessory facial nerve anastomosis. The goal of a nerve transposition is for nerve fibers to sprout out to the facial muscles, discontinue muscular atrophy, and/or lower the increased muscle tone. As a result of surgery, patients should be able to activate movement potentials and trigger movement in the target muscles. One of the most frequently performed muscle replacement surgeries is the transplantation of the m. temporalis, the m. masseter, and the free gracilis graft. Especially after muscle and nerve replacement surgeries, post-operative therapy, including FES and biofeedback training, is recommended [14]. FES should be introduced 14 days post-surgery, as described in Sect. 10.10. During primary stimulation, it is recommended to use sponge pockets with rubber electrodes. Once complete sprouting of the nerve fibers in the target muscles has occurred and first action potentials in the target muscles appear, the use of biofeedback programs is recommended. At this time, the patient will learn to control individual muscles and increase perception of motor movements. From a speech and language therapy point of view, this approach is highly recommended as the patient increases awareness for the new structures and precision of motor movement. To generate emotionally coupled facial movements, a one- or two-stage surgery must be carried out. At the first stage, a so-called cross-face nerve graft is required. Therefore, the sural nerve, a sensitive nerve of the lower leg, is removed with the intend to relocate. The nerve graft is then connected to a nerve suture and attached to a strong branch of the n. facialis located at healthy side of the face. It is then connected to the paralyzed side through a subcutaneous tunnel extending underneath the upper lip. With the aim of reinnervation, the nerve graft is attached to the denervated mimic muscle, there would be a low likelihood for successful reinnervation. In these cases, a free muscle graft may be connected during a follow-up surgery (approx. 9–12 months later). The m. gracilis from the thigh is often used for this procedure. FES can take on several functions in this complex two-stage surgery process [14]. A rarely used function is to use FES to locate denervated muscles.
If the treatment with exponential current demonstrates a contraction of the m. zygomaticus before the initial surgery, the patient should be informed, that if the original mimic musculature still will be reinnervated by FES and a free muscle transfer may be avoided. If gradual training over several weeks does not result in muscle contraction, it must be assumed that the musculature is too damaged to be used for reinnervation. In this case, e.g., a free muscle transfer is indicated. Until re-innervation occurs, exponential or triangular current treatment may minimize atrophy of the remaining facial muscles and the free muscle transplant. After successful reinnervation the EMG biofeedback programs may be used. This way, patients learn how to control the once immobile facial muscles and restore facial functions, such as the range of motion of mouth and cheek muscles and achieve a normal resting tone.
10.16 Patient Example
A 45-year-old patient presents in the specialized facial clinic 3 months after a surgical removal of a vestibular schwannoma. According to the surgical report, the n. facialis was stimulated throughout the surgical procedure. Postoperatively, the patient developed a complete unilateral FP and asks the SLP about his prognosis and therapy options. A needle electromyography was conducted but revealed no preserved arbitrary activity. However, excess pathological spontaneous activity could be observed, which may indicate severe axonal damage denervation of the facial muscles. A sonography of the paralyzed facial muscles revealed significantly smaller muscle mass as compared to muscles on the healthy side and no indications of voluntary movement. As a result of the absence of a spontaneous reinnervation, the option of surgical restoration using the hypoglossal nerve or the r. massetericus of the trigeminal nerve is discussed with the patient. To counteract atrophy of the denervated mimic muscles up to the decision about the operation and the time after nerve reconstruction, electrical stimulation using long triangular pulses is recommended. A trial stimulation using a FES device is carried out during consultation. The stimulation using biphasic triangular pulses (150 ms, 5-7 mA) above the cheek and below the corner of the mouth (6x4 cm self-adhesive electrodes) triggers contraction of the m. orbicularis oculi and m. zygomaticus. Of note, proper electrode placement is adapted to the patient’s individual needs and is photographically documented to serve as an instruction for the patient during home practice. Further, the patient is provided with detailed written instructions on how to properly place the electrodes and self-conduct electrical stimulation. Electrical stimulation should be carried out twice a day for 5 min each for the first week and at least 6 h apart (e.g., morning and evening). Further, stimulation should be conducted in front of a mirror to obtain immediate visual feedback. Triggering clearly visible contractions of the exercised muscles is crucial for minimizing the denervation atrophy of the affected facial muscles. Stretching of the trained muscles after each therapy unit as well as frequent breaks are recommended to prevent fatigue. From the second week onward, practice should increase to 2 × 5 min in the morning and 2 × 5 min in the evening with a 5-min break between sets. Finally, from the third week on, the patient should practice 3 × 5 min in the morning and evening with 5-min breaks between sets. A medical prescription is issued to the patient and a check-up appointment is scheduled for 3 months in the future. The patient is provided a telephone number in case any questions or problems with electrical stimulation occur during home practice. Further, the patient is educated that electrical stimulation may only minimize muscle atrophy, however, the chances of reinnervation do not increase through treatment. Surgical restoration is indicated even if the patient perceives the symptoms of FP as less severe following electrical stimulation.
Summary
Both, the literature and clinical practice recommend the use of FES in facial palsy therapy.
References
Heckmann, Prof. Dr. Josef G. Therapie der idiopathischen Fazialisparese (Bell’s Palsy). 3 31, 2017. Leitlinien für Diagnostik und Therapie in der Neurologie. https://dgn.org/wp-content/uploads/2013/01/030013_Therapie_der_idiopathischen_Fazialisparese.pdf.
Dobel C, et al. Emotionale Auswirkungen einer Fazialisparese (Emotional Impact of Facial Palsy). Laryngo-Rhino-Otol. 2013;92(01):9–23.
Ferner P. Anatomie des Nervensystems und der Sinnesorgane des Menschen. Ernst Reinhardt Verlag; 1967.
Mumenthaler M, Mattle H. Kurzlehrbuch Neurologie. 1. Auflage. Stuttgard: Georg Thieme Verlag; 2006.
Cheney M, Hadlock T. Facial surgery - plastic and reconstructive. 2014.
Facial Palsy UK. Physical Issues. Facial palsy UK; 2021. https://www.facialpalsy.org.uk/inform/what-is-facial-palsy/physical-issues/.
Willand MP, et al. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair. 2016;30(5):490–6.
Cararro U, et al. Persistent muscle fibre regeneraltion in long term denervation. Past, present, future. Muscle fibre regenration in longterm denervation. 2015, pp. 77–92.
Puls W, et al. Surface electrical stimulation for facial paralysis is not harmful. Muscle Nerve. 2020;61(3):347–53.
Fargher KA, Coulson S. Effectiveness of electrical stimulation for rehabilitation of facial nerve paralysis. Phys Therapy Rev. 2017;22(3-4):169–76.
Arnold D, et al. Selective surface electrostimulation of the denervated zygomaticus muscle. Diagnostics (Basel). 2021;11(2):188. https://doi.org/10.3390/diagnostics11020188.
Angelov DN, et al. Mechanical Stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery od whisking. Neurobiol Dis. 2007;26(1):229–42.
Marotta N, et al. Neuromuscular electrical stimulation and shortwave diathermy in unrecovered Bell palsy - A randomized controlles study. Medicine. 2020;99(8)
Repitsch CA, Volk GF. Funktionelle Elektrostimulation bei Fazialisparesen. In: Schick T, editor. Funktionelle Elektrostimulation in der Neurorehabilitation. 2. Auflage. Deutschland: Springer Verlag GmbH; 2020. https://springerlink.bibliotecabuap.elogim.com/chapter/10.1007%2F978-3-662-61705-2_10#DOI.
Volk GF, Finkensieper M, Guntinas-Lichius O. EMG Biofeedback Training zuhause zur Therapie der Defektheilung bei chronischer Fazialisparese. Laryngo-Rhino-Otol. 2014;93:15–24.
Azizzadeh B. Latest treatment options for facial paralysis. 062014. Latest treatment options for facial paralysis with Dr. Babak Azizzadeh; 2014. https://facialparalysisfound.wistia.com/medias/352hsepmqg
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Repitsch, C.A., Volk, G.F. (2022). Functional Electrical Stimulation in Facial Rehabilitation. In: Schick, T. (eds) Functional Electrical Stimulation in Neurorehabilitation. Springer, Cham. https://doi.org/10.1007/978-3-030-90123-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-90123-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90122-6
Online ISBN: 978-3-030-90123-3
eBook Packages: MedicineMedicine (R0)