Abstract
Brain delivery of therapeutic antibodies and biologics is restricted due to the presence of the blood-brain barrier (BBB). However, their delivery can be improved with the use of “carrier” antibodies that target receptors on the luminal surface of the BBB which initiate a process termed receptor-mediated transcytosis (RMT). This review describes key steps and transcellular pathways various BBB-crossing antibodies undertake to deliver therapeutic cargos into the brain via RMT. The pathway is initiated with the receptor-mediated endocytosis through clathrin- and/or caveolin-dependent or independent pathways. Once internalized the antibodies are routed to various endosomal compartments where decisions are made regarding their fate during endosomal protein sorting process. During this process antibodies with specific attributes will be either discarded and degraded in lysosomes or rerouted into compartments destined for release on the abluminal surface of the brain endothelial cells. Different RMT receptors may engage different shuttling pathways between the luminal and abluminal sides of the BBB. Based on this knowledge, antibodies can be engineered to add attributes that facilitate preferential routing through pathways that result in enhanced BBB crossing.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Early endosomes
- Multivesicular bodies
- Exosomes
- Clathrin
- Caveolin
- Receptor mediated
- Endocytosis
- Exocytosis
- Transcytosis
- Late endosomes
- Lysosomes
1 Introduction
Therapeutic antibodies have emerged as a novel class of targeted and efficacious biopharmaceuticals, supported by the advancements made in production and downstream processing technologies (Schiel et al. 2014; Ecker et al. 2015). However, the development of antibody therapeutics for diseases of the central nervous system (CNS) remains challenging, because access of therapeutic antibodies to the brain tissue is highly restricted by a tightly sealed layer of endothelial cells in brain microvessels that form the blood-brain barrier (BBB). Improved delivery into the brain can be achieved by using BBB carrier antibodies that bind to receptors expressed on the luminal surface of brain endothelial cells (BEC), shuttle to, and release at the abluminal side in a process termed receptor-mediated transcytosis (RMT). These BBB-crossing antibodies can be engineered into various formats of bi- or multi-specific antibodies where the BBB carrier “arm” enables delivery of the therapeutic antibody “arm” to its target within the brain (Stanimirovic et al. 2014).
Whereas enhanced brain delivery and pharmacological actions on brain targets have been shown for several BBB carriers in experimental animal models, the knowledge of key transcellular pathways they engage while translocating from the luminal to the abluminal side of BECs is still sparse. Further understanding of intracellular compartments and molecular networks BBB-crossing antibodies mobilize during transcytosis is necessary to inform antibody engineering that favor more efficient release pathways.
In this chapter, we describe details of some of the known and emerging pathways involved in the RMT of BBB-crossing antibodies against different BBB receptors.
2 Receptor-Mediated Transcytosis
RMT is a multistep process that involves receptor-mediated endocytosis (RME) of macromolecules at one surface of a polarized cell, followed by their endosomal sorting, and eventual exocytosis at another surface (usually the opposite side) of the cell. Naturally occurring macromolecules utilize the RMT process to bypass various physiological barriers in the body. The informative examples include transferrin and insulin proteins that engage their respective brain endothelial cell receptors, transferrin receptor (TfR), and insulin receptor (IR), to gain access to brain parenchyma via a transcellular transport. As a result, RMT receptors are attractive targets to develop molecular Trojan horses for delivery of macromolecule therapeutics across the BBB. Antibodies and peptides to several RMT receptors (Table 3.1) have been developed including various antibody formats against TfR (Pardridge et al. 1991; Yu et al. 2011, 2014; Niewoehner et al. 2014), humanized IgG against IR (Coloma et al. 2000; Boado et al. 2010), antibodies against the heavy subunit of the large neutral amino-acid transporter CD98 (Lat1) (Zuchero et al. 2016), LRP1-targeting Angiopep2 polypeptide (Xin et al. 2011), species cross-reactive camelid single-domain antibody FC5 that binds a glycosylated epitope of TMEM30A complex (Abulrob et al. 2005; Stanimirovic et al. 2014; Farrington et al. 2014; Webster et al. 2016), and humanized camelid antibodies against IGF1R (Stanimirovic et al. 2017; Ribecco-Lutkiewicz et al. 2018). To better understand how these carriers cross the BBB, we need to dissect various steps involved in the RMT pathway, namely, endocytosis, endosomal sorting, and exocytosis.
2.1 Endocytosis
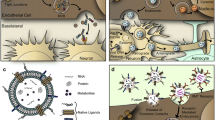
Endocytosis is the uptake of proteins, lipids, extracellular ligands, and soluble molecules, such as nutrients, from the cell surface into the cell interior by endocytic vesicles. While small molecules are absorbed into cells through passive diffusion or transporter-mediated pathways, most macromolecules enter the BBB through endocytosis. The main types of endocytosis include macropinocytosis and micropinocytosis; the latter further distinguishes clathrin-mediated endocytosis (CME), caveolin-mediated endocytosis (CavE), and caveolin- and clathrin-independent endocytosis (CIE). A majority of anti-RMT receptor antibodies have been shown to engage the CME pathway (also traditionally referred to as the RME pathway) to enter the BBB, although other pathways could be engaged through various antibody/ligand displays. The graphical depiction of various endocytosis pathways described in more detail in the subsequent sections is shown in Fig. 3.1.
Endocytosis of macromolecules . Schematic representation of the main pathways that macromolecules (such as antibodies) can undertake to enter cells. (a) Macromolecules present in the extracellular fluids may internalize randomly during the macropinocytosis of larger particles or as bound to exosomes and give rise to endocytic vesicles called macropinosomes. (b) Clathrin-mediated endocytosis involves binding of macromolecules to their receptors followed by formation of clathrin-coated pits that bud into endocytic vesicles called clathrin-coated vesicles (CCV), taking in both the receptor and the bound macromolecule. (c) Caveolin-mediated endocytosis process involves formation of cave-like surface invagination following macromolecule-receptor interaction that internalizes into endocytic vesicles called caveolae. (d) Endocytosis that neither involves clathrin nor caveolin mechanisms usually occurs via the formation of flotillin-regulated lipid rafts resulting in endocytic vesicles called clathrin-independent carriers (CLIC) or GPI-anchored protein-enriched endocytic compartments (GEEC). Once internalized via these endocytic pathways, these vesicles are routed to various endosomes for further sorting
Macropinocytosis
Macropinocytosis is a regulated form of endocytosis that permits non-selective internalization of solute molecules, nutrients, and antigens from extracellular fluids. It is an actin-dependent process initiated from surface membrane ruffles that give rise to large endocytic vesicles of 200–5000 nm in size, known as macropinosomes (Recouvreux and Commisso 2017). The macropinocytosis route is thought to be an effective mechanism for delivery of natural or synthetic particles such as exosomes and nanoparticles, typically ranging in size between 50 and 300 nm and containing plasmid DNA, siRNA, or proteins as payloads (Itakura et al. 2015; Ha et al. 2016; Chen et al. 2016a; Desai et al. 2019). Although smaller macromolecules such as antibodies present in the extracellular fluids may randomly internalize into cells during the macropinocytosis of larger particles, there is a lack of evidence for selective (receptor-mediated) uptake of antibodies via this pathway at the BBB (Itakura et al. 2015; Kähäri et al. 2019). On the other hand, since exosomes may utilize macropinocytosis as one way of entering the BBB (Chen et al. 2016a) and display/contain several RMT receptors (Haqqani et al. 2013), anti-RMT receptor antibodies bound to exosomes may also enter the BBB via the macropinocytosis pathway.
Clathrin-Mediated Endocytosis
CME is the most extensively studied and best understood type of endocytosis. It is also the main pathway for RME because the process is activated when a ligand binds to its receptor on the cell surface. CME itself is a multistep process that starts, following receptor activation, with the formation of clathrin-coated pits (CCPs) on the inner surface of the plasma membrane and involves recruitment of a large endocytic protein machinery, consisting of clathrin and over 50 additional cytosolic proteins. The pit then buds into endocytic vesicle of 85–150 nanometer in diameter called clathrin-coated vesicle, taking in both the receptor and the bound ligand. The vesicle then undergoes un-coating and fuses with early endosomes to release its contents (Conner and Schmid 2003).
Known RMT receptors and BBB-crossing antibodies against these receptors have been shown to internalize primarily through the CME pathway. TfR, the most studied RMT receptor, has been shown to co-localize with clathrin pits/protein by a variety of methods, including immunochemistry, live imaging, subcellular fractionation, and proteomics (Liu et al. 2010; Mayle et al. 2012; Villaseñor et al. 2017; Haqqani et al. 2018a, b). However, the BBB crossing efficiency of TfR antibodies varies depending on their design and affinity; for example, high-affinity bivalent TfR antibodies show poor exocytosis and abluminal release, whereas medium-affinity and monovalent TfR antibodies demonstrate efficient transcytosis and improved brain exposure (Niewoehner et al. 2014; Bien-Ly et al. 2014; Webster et al. 2017; Thom et al. 2018b; Haqqani et al. 2018b). Interestingly, immunofluorescence and live imaging demonstrated that both a weak and a strong BBB-crossing anti-TfR antibodies (bivalent dFab and monovalent sFab, respectively) co-localized with clathrin protein (Sade et al. 2014; Villaseñor et al. 2017). Similarly, bivalent anti-TfR OX26 antibodies of varying affinities and BBB-crossing efficiencies were all shown to co-localize with clathrin fractions using targeted quantitative mass spectrometry after subcellular fractionation of the rat brain endothelial cells (Haqqani et al. 2018b). These studies collectively suggest that the initial step of internalization through CME is common for all TfR antibodies regardless of their transcytosing efficiency, which is likely determined by the subsequent differential sorting through different intracellular routes.
IR has also been shown to co-localize with CME pathway by electron microscopic autoradiography in combination with inhibitors of CCP formation (Fan et al. 1982; Paccaud et al. 1992). However, IR may also internalize via non-CME pathways (McClain and Olefsky 1988; Gustavsson et al. 1999; Fagerholm et al. 2009). Similarly, Angiopep2, a polypeptide shown to cross BBB likely by engaging LRP1, was shown to use both CME and non-CME pathways. An uptake of the fluorescently labeled Angiopep2 into BECs was only moderately reduced in the presence of inhibitors of CCP formation (Xin et al. 2011). FC5, a BBB-crossing single-domain antibody engaging RMT receptor complex containing TMEM30A, was shown to internalize via clathrin-coated vesicles, blocked by inhibitors of CME pathway (Abulrob et al. 2005); in addition, both the receptor and the antibody co-localized with clathrin fractions (Abulrob et al. 2005; Haqqani et al. 2018a) by immunostaining and quantitative mass spectrometry.
Collectively these studies suggest that the CME pathway is the most common route that RMT receptors and their antibodies take to enter cells via endocytosis.
Caveolin-Mediated Endocytosis
Caveolae are usually defined as small cave-like surface invaginations of 50–100 nm in diameter and have been shown to mediate vesicular transport and cell signaling (Sprenger et al. 2006). Caveolae are not present in all cell types but are found abundantly in ECs and aid in regulating numerous endothelial functions such as transcytosis, vascular permeability, and angiogenesis and can serve as docking sites for glycolipids and GPI-linked proteins, as well as for various receptors and signaling molecules (Sprenger et al. 2006). Caveolin-1, the main protein component of these structures, functions as a scaffolding protein and as a potential cholesterol sensor, regulating raft polymerization and lipid trafficking (Pohl et al. 2004; Song et al. 2007). The CavE pathway has been implicated in BBB transcytosis of IR and LRP1 ligands. In a series of experiments using cell fractionation, western blotting, and immunoprecipitation (Fagerholm et al. 2009), IR internalization was shown to occur via CavE pathway and to be insensitive to inhibitors of CCP formation. Similarly, cellular uptake of the fluorescently labeled anti-LRP1 polypeptide Angiopep2 was reduced by >70% in the presence of inhibitors of caveolae (Xin et al. 2011).
Interestingly, while anti-TfR antibodies have been shown to use the CME pathway for internalization/initialization of the RMT process, several studies have demonstrated the role of caveolin in recycling of various receptors, including TfR, on the apical side of polarized epithelial cells (Pol et al. 1999; Gagescu et al. 2000; Hansen et al. 2003; Lapierre et al. 2007; Leyt et al. 2007). The receptor recycling to the apical side is an essential step in maintaining their levels at the luminal membranes in order to allow continuous entry and shuttling of ligands through polarized cells.
CavE pathway has also been implicated in the transcytosis of other macromolecules such as lipids, likely regulated by a protein called major facilitator super family domain containing 2a (Mfsd2a). Ben-Zvi and co-workers identified Mfsd2a in a BBB-specific gene screen and demonstrated that Mfsd2a(−/−) mice have a leaky BBB with a dramatic increase in CNS-endothelial-cell vesicular “bulk” transcytosis from embryonic stages through to adulthood (Ben-Zvi et al. 2014). Furthermore, through unbiased lipidomic analysis in Mfsd2a transgenic mice, they demonstrated that Mfsd2a may act by suppressing lipid transcytosis likely via downregulation of caveola formation in CNS endothelial cells (Andreone et al. 2017).
Caveolin and Clathrin-Independent Endocytosis (CIE)
Macromolecule endocytosis has also been shown to occur through membranes that do not contain either clathrin or caveolin protein. The molecular understanding of the steps involved in the CIE pathway is still in its infancy relative to the vast information known for CME and CavE pathways. The main CIE mechanism that has emerged is the clathrin-independent carrier (CLIC) pathway, also known as the glycosylphosphatidylinositol (GPI)-anchored protein-enriched endocytic compartments (GEEC). The CLIC/GEEC pathway internalizes GPI-anchored proteins, CD44, and some integrins as well as large volumes of fluid and extracellular material that do not have surface receptors (Ferreira and Boucrot 2018). The endocytosis process likely involves a formation of lipid rafts that are regulated by scaffolding protein flotillins, which are believed to stabilize lipid-raft microdomains in phagocytic, caveolin, and non-caveolin-containing membranes (Dermine et al. 2001; Vercauteren et al. 2011). However, there is limited evidence of RMT receptors (or their antibodies) internalizing via the CLIC/GEEC pathway. While both IR and LRP1 have been shown to co-localize with flotillins (Roura et al. 2014; Boothe et al. 2016), the endocytosis is more likely occurring via the CavE pathway as discussed above. A glycoprotein CD98 (SLC3A2) which hetero-dimerizes with SLC7A5 to form large neutral amino acid transporter LAT1 highly enriched in the BBB has been shown recently to shuttle anti-CD98 antibodies into the brain in vivo (Zuchero et al. 2016). This receptor likely utilizes the CLIC/GEEC internalization pathway since it is a GPI-anchored protein. In fact, CD98 has been shown to internalize via CIE pathway with novel downstream sorting mechanisms that may be independent of the widely known sorting at the EE (Eyster et al. 2009).
2.2 Sorting Through the Endosomes
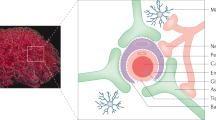
Once receptors and their associated macromolecules are internalized via one of the endocytosis pathways, they are routed to various endosomes where decisions are made regarding their fate during processes known as endosomal protein sorting, graphically shown in Figs. 3.2 and 3.3. The main sorting stations in the cells include the early and late endosomes (Scott et al. 2014).
Endosomal sorting of antibodies during the receptor-mediated transcytosis (RMT). Shown is a schematic depiction of intracellular trafficking pathways triggered by anti-RMT receptor antibodies at the BBB. Once the antibody binds to its receptor, expressed on the luminal membranes, it triggers internalization of the antibody-RMT receptor complex into endocytic vesicles via one of the endocytosis pathways, including extracellular vesicle (EV)- based endocytosis. While most endocytic vesicles fuse to early endosomes (EE), others (such as those containing EVs) may fuse to multivesicular bodies (MVB). In EE, it is decided whether the antibody will recycle back to the luminal side, be degraded, or undergo exocytosis at the abluminal side. Typically recycling vesicles (RV) will recycle the RMT receptor (with or without antibody) back to the luminal side, whereas MVB will receive cargo from EE for degradation or exocytosis. For degradation, the cargo is sent to late endosomes (LE) and lysosomes. Exocytosis may occur through multiple routes from EE: directly from vesicles (e.g., sorting tubules), via trans-Golgi network (TGN), or via a direct fusion of MVBs with abluminal membrane
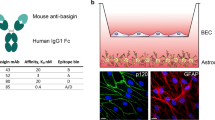
IGF1R VHH co-localization with endocytic vesicles in BEC. (a) Co-localization of the BBB-crossing IGF1R VHH antibody with markers of early endosomes (EE) and late endosomes (LE)/lysosomes in subcellular fractions of SV-ARBEC cells as determined by mass spectrometry. Graph shows relative levels of the antibody, EE markers (e.g., Rab5a, Eea1), and LE/lysosome markers of late endosomes (e.g, Rab7, Lamp1, Lamp2) in each cellular fraction. (b) Co-immunofluorescence detection of IGF1R VHH antibody and Rab5a and Rab7a markers
Early Endosome
All internalized vesicles are first fused to a common early endosome (EE), which functions as the first key sorting station in the cell. Here the cell makes a major decision: Are the cargo and membrane components of the vesicles worth keeping or should they be sent to late endosome (LE)/lysosome for degradation? If the cargo is to be degraded, it goes through the process of early-to-late endosome maturation. This involves the cargo being concentrated in specific regions of the EE membranes that are pinched off to form endosomes that mature into multivesicular bodies (MVBs) and eventually fuse with LEs (Scott et al. 2014; van Weering and Cullen 2014). However, if the cargo does not need to be degraded, it is concentrated in a network of tubular EE subdomains leading to the formation of sorting tubules (Maxfield and McGraw 2004), which are recycled back to the plasma membranes or to the biosynthetic pathway at the level of the trans-Golgi network (TGN). The events in the sorting processes in EEs have been studied in detail at the molecular level and shown to involve an array of protein complexes that direct trafficking events to the appropriate destination (see reviews Scott et al. 2014; van Weering and Cullen 2014; Naslavsky and Caplan 2018). There is strong evidence that RMT receptors, including TfR (Sade et al. 2014; Niewoehner et al. 2014; Bien-Ly et al. 2014; Haqqani et al. 2018b), IR (Hunker et al. 2006), LRP1 (Tian et al. 2015; Haqqani et al. 2018a), and TMEM30A (Haqqani et al. 2018a), predominantly co-localize with EEs, especially when incubated with their respective antibodies that are strong BBB crossers. It is still not well understood how the cell decides whether a specific RMT receptor or its bound ligand should be sent for degradation or rerouted for exocytosis, although some factors that may favor BBB cells to exocytose rather than degrade antibodies have been identified and will be discussed in the next section.
Multivesicular Bodies
MVBs are spherical endosomal organelles containing a number of intraluminal vesicles (ILVs) formed by inward budding of the limiting membrane into the endosomal lumen (Zhang et al. 2019). MVBs have traditionally been considered intermediate endosomes between EE and LE, as they are formed from maturation of EE-released ILV-containing vacuoles that may eventually fuse with LE to deliver the content for degradation (van Weering and Cullen 2014; Naslavsky and Caplan 2018). MVBs are now known to have multiple subpopulations (van Niel et al. 2001; White et al. 2006; Tauro et al. 2013; Chen et al. 2016b; Haqqani et al. 2018a) and to be involved in numerous additional endocytic and trafficking functions including biogenesis and routing of ILVs to and from the plasma membrane to membranes of other organelles (Von Bartheld and Altick 2011; Colombo et al. 2014). The ILVs when released extracellularly are referred to as exosomes, which have recently emerged as natural therapeutic-delivery vehicles; a number of studies have shown that exosomes can cross the BBB and deliver therapeutics into the brain (Zhuang et al. 2011; Alvarez-Erviti et al. 2011; Chen et al. 2016a; Matsumoto et al. 2017). Through proteomic analysis of exosomes derived from BEC, we found that they are enriched with known RMT receptors including TfR, IR, TMEM30A, and others (Haqqani et al. 2013). We have proposed that a subpopulation of MVBs may play a key role as “transcytosing endosomes” trafficking between the apical and basolateral membranes and helping transport exosome-bound ligands from the luminal to the abluminal side of BEC (Haqqani et al. 2013, 2018a).
Late Endosomes and Lysosomes
LE functions as a second trafficking hub in the endosomal system and as a last sorting station in the membrane trafficking cycle to and from lysosomes (Huotari and Helenius 2011; Raposo and Stoorvogel 2013; Bissig and Gruenberg 2014). In fact, live-cell imaging has shown that LEs and lysosomes frequently interact by “kiss-and-run” events and by direct fusion, resulting in the formation of hybrid organelles, in which the degradation of endocytosed macromolecules occurs and from which lysosomes are re-formed. Although LEs and lysosomes can be distinguished by their physical properties and ultrastructure (Scott et al. 2014), two organelles are difficult to differentiate molecularly – both contain highly sialylated membrane proteins LAMP1 and LAMP2 that form a protective glycocalyx lumen against degradative enzymes. Receptors, ligands, and other proteins that need to be downregulated are sorted out of the EE and fused to LE via intraluminal vesicles (Scott et al. 2014). Several studies have shown that higher co-localization of RMT receptors or their ligands with LE markers is associated with their lysosomal degradation at the BBB (Sade et al. 2014; Niewoehner et al. 2014; Haqqani et al. 2018a, b), a mechanism that is considered key for regulating surface expression of RMT receptors. However, not every cargo from LE is sent to lysosomes for degradation, because the LE are empowered to make the last decision; for example, in response to incoming signals via other pathways, LE can divert the cargo to other destinations, including the TGN, MVBs, plasma membrane, or even to cytoplasm via endosomal escape (Huotari and Helenius 2011; Raposo and Stoorvogel 2013; Bissig and Gruenberg 2014; Scott et al. 2014; Tashima 2018). Similar to EE, it is not well understood what regulates LE fusion with lysosomes to enact the final degradation of a specific RMT receptor or its bound ligand.
Antibody Trafficking Through the Endosomes
There is a compelling body of evidence showing that poor BBB-crossing anti-RMT receptor antibodies are targeted for degradation through the LEs and lysosomes, while efficient BBB-crossing antibodies predominantly traffic through the EEs. Comparing intracellular localization of a poor BBB-crossing (high-affinity) anti-TfRA antibody and an efficient BBB-crossing (low-affinity) anti-TfRD antibody using immunofluorescence studies, Watts and co-workers showed that while both antibodies co-localize with EE marker EEA1, poorly crossing anti-TfRA showed more co-localization with lysosomal marker LAMP1 compared with efficient crosser anti-TfRD antibody (Bien-Ly et al. 2014). Similarly, using high-resolution imaging, Freskgård and co-workers demonstrated that a non-BBB-crossing (bivalent) anti-TfR dFab antibody is preferentially co-localized with LAMP1, compared to an efficient BBB-crossing (monovalent) anti-TfR sFab antibody (Niewoehner et al. 2014). We recently evaluated localization of a number of bivalent anti-TfR OX26 affinity variants showing varying BBB-crossing efficiency in subcellular fractions of the rat brain endothelial cells using both targeted quantitative mass spectrometry and immunofluorescence (Haqqani et al. 2018b). While the parental high-affinity OX265 along with TfR co-localized with multiple LE and lysosomal markers, the medium-affinity OX2676 and OX26108 antibodies, along with TfR, routed predominantly into the early/recycling endosomes and demonstrated efficient BBB crossing (Haqqani et al. 2018b).
Additional evidence supporting the relevance of trafficking through the EE for BBB crossing comes from the extensive characterization of species cross-reactive camelid single-domain antibody, FC5 (Tanha et al. 2002; Abulrob et al. 2005; Farrington et al. 2014; Haqqani et al. 2018a). FC5 has been shown to deliver various therapeutic payloads, including peptides and antibodies, to their CNS targets (Farrington et al. 2014; Webster et al. 2016). By examining subcellular distribution of FC5 in rat brain endothelial cells using both targeted quantitative mass spectrometry and immunofluorescence, FC5 enrichment was observed in EEs and a subpopulation of molecularly distinct MVBs with a small proportion being routed to LEs and lysosomes (Haqqani et al. 2018a). Interestingly, FC5 fusion to Fc further enhanced the EE/MVB enrichment, reduced LE/lysosome levels, and increased BBB crossing (Haqqani et al. 2018a). In contrast, a low level of internalized non-BBB-crossing single-domain antibodies, with or without Fc, showed enrichment in LEs/lysosomes and depletion from EEs (Haqqani et al. 2018a).
Similar studies with the BBB-crossing camelid VHH against insulin-like growth factor receptor 1 (IGF1R) (Stanimirovic et al. 2017; Ribecco-Lutkiewicz et al. 2018) revealed a slightly different routing path. IGF1R VHH, after internalizing rat BEC via a CEM pathway, co-localized with the high-density, EE marker-containing subcellular fractions, with further enrichment in the higher density fractions, previously identified as a subset of MVBs (Fig. 3.3a; Haqqani et al. 2018a). No co-localization of internalized IGF1R VHH with the LE marker Rab7a (Fig. 3.3b), and a significant co-localization with Rab5a-containing vesicles (Fig. 3.3c), indicative of EE, was also observed by immunofluorescence detection.
These results collectively strengthen the hypothesis that the lysosomal degradation is a key downstream mechanism by which BECs restrict antibody access to the brain and that BBB-crossing antibodies bypass this pathway and instead follow the EE/MVB route toward exocytosis.
2.3 Exocytosis to the Abluminal Side
The last step of the RMT process involves BBB-crossing antibodies exiting the endosomal pathway and being released on the basolateral side of the barrier. This process is probably the least understood among different RMT steps. Molecules in the EEs that do not need to be degraded are concentrated in a network of tubular EE subdomains leading to the formation of sorting tubules, which are destined for the plasma membrane, MVBs, or TGN, thereby avoiding lysosomal degradation (Maxfield and McGraw 2004; Grant and Donaldson 2009). In fact, using live-cell imaging, it was recently shown that an efficient BBB-crosser anti-TfR sFab localized to sorting tubules, whereas non-BBB-crosser anti-TfR dFab had been size-excluded from these tubules due to receptor cross-linking facilitated by a bivalent receptor binding (Villaseñor et al. 2017). Based on these and our own observations, we postulate that these sorting tubules are either (i) recycled back to the plasma membranes, (ii) evolve to ILV-containing vacuoles and fuse to the MVBs, or (iii) fuse to the TGN. Although recycling of vesicles to plasma membrane is usually believed to be back to apical membranes, similar mechanism may unfold for their movement to the basolateral side for transcytosis. Consistent with this assumption, we have shown that the BBB-crossing FC5 antibody co-localizes with recycling and exocytosing MVBs (Haqqani et al. 2018a), which is different to anti-TfR sFab that was found to undergo transcytosis by avoiding receptor cross-linking and lysosomal degradation (Villaseñor et al. 2017). Mechanisms of exocytosis from both MVBs and TGN have been previously described (Jaiswal et al. 2009; Von Bartheld and Altick 2011; Colombo et al. 2014). MVBs may directly fuse with the basolateral membranes and release the RMT receptor-bound antibodies to the abluminal side of the barrier. On the other hand, the TGN is well known to secrete newly synthesized molecules via exocytotic and secretory vesicles which fuse to the plasma membranes and release their content (Jaiswal et al. 2009). Similar mechanisms may also be involved in exocytosis of antibodies via TGN. A summary of reported pathways for endocytosis, trafficking, and exocytosis for antibodies targeting BBB RMT receptors is shown in Table 3.1.
3 Antibody Attributes That Favor Transcytosis: Designing more Efficient BBB Carriers
Increasing transcytosis efficiency of carrier antibodies developed against BBB RMT receptors could be accomplished by antibody engineering strategies that direct the antibody into endocytic pathways favoring transcytosis instead of lysosomal degradation. Through TfR and FC5 antibody engineering efforts, several antibody attributes that increase the efficiency of BBB crossing have been identified. Many of these are based on specific structure-function relationships that guide antibody docking and binding to its receptor, whereas some others are based on the intracellular milieu that antibody faces while traveling through endocytic pathways. Some of these factors include ligand-receptor affinity, pH sensitivity of ligand-receptor interactions, antibody valency, Fc format, and antibody position in the construct (Niewoehner et al. 2014; Bien-Ly et al. 2014; Villaseñor et al. 2017; Haqqani et al. 2018a, b). Here we describe evidence that these factors have resulted in increased BBB permeability, although it should be noted that the factors might be receptor specific since different receptors undertake different RMT pathways for transporting ligands across the BBB (Table 3.1).
Ligand-Receptor Affinity
A number of studies have demonstrated that manipulating the binding affinity between the carrier antibody and its RMT receptor results in enhanced BBB permeability of the carrier. The strongest evidence exists for anti-TfR antibodies, where several studies have shown that the high-affinity binding to TfR results in receptor cross-linking and lysosomal degradation, whereas a moderate-affinity binding to TfR results in enhanced antibody transcytosis. Watts and co-workers compared BBB crossing of two bispecific antibodies with different binding affinities to TfR, where each antibody had an anti-TfR arm and an anti-BACE1 arm; the low-affinity anti-TfRD antibody showed a significantly enhanced BBB crossing compared to the high-affinity anti-TfRA antibody as demonstrated by labeling experiments both in in vitro and in vivo (Bien-Ly et al. 2014). In addition, live imaging and co-localization experiments demonstrated that high-affinity antibody facilitated degradation of TfR by directing it to lysosomes, resulting in downregulation of TfR in the BBB and reduced brain exposure to a second dose of the BBB-crossing, low-affinity TfR antibody (Bien-Ly et al. 2014). Similarly, in studies with affinity variants of the rat-specific anti-TfR antibody OX26 using a label-free mass spectrometry method that allows simultaneous quantification of antibodies, their receptors, and endosomal markers (Haqqani et al. 2018b), lowering the affinity of OX26 antibody resulted in rerouting of both the TfR and the antibody away from LE and lysosomes and toward the EE/recycling vesicles. OX26 antibodies with affinity range of 70–100 nM displayed a significantly higher BBB transcytosis in a BBB model in vitro (Haqqani et al. 2018b), as well as higher brain penetration in animal studies (Thom et al. 2018a), compared to a parental OX26 having affinity of 5 nM. Other studies have been able to similarly improve the BBB penetration of anti-TfR antibodies in different formats by lowering their affinities (Webster et al. 2017; Johnsen et al. 2018; Karaoglu Hanzatian et al. 2018). It is important to note that the optimal affinity range for maximal transcytosis is different for each TfR antibody, likely because each antibody engages different receptor epitopes. Medium-affinity TfR antibodies also show improved serum pharmacokinetics, resulting in longer brain exposure (Yu et al. 2011; Thom et al. 2018b). However, lowering affinities below the optimal range results in poor receptor engagement and low brain exposure (Yu et al. 2011; Thom et al. 2018b). These studies demonstrate that the optimization of binding affinities between the carrier antibody and its RMT receptor may result in improved efficiency of transcytosis and enhanced brain delivery.
Antibody Valency
Many membrane receptors exist as dimers either at resting state or they dimerize in response to mono- or bivalent ligand binding (De Meyts et al. 1995; Terrillon and Bouvier 2004; Eckenroth et al. 2011). The latter may result in activation of the receptor, leading to signaling cascades and subsequent physiological effects mediated by the receptor. The latter is not a desirable action for BBB carrier antibodies, which aim not to disturb physiological activation/function of the receptor. Among RMT receptors, TfR, IR, and IGF1R are known to dimerize either at resting state or in response to ligand exposure (De Meyts et al. 1995; Eckenroth et al. 2011). To avoid receptor cross-linking and activation by bivalent antibodies, both monovalent and bivalent antibodies have been developed and tested for TfR, FC5, and IGF1R. Freskgård and co-workers engineered a high-affinity anti-TfR antibody at the C-terminus of an anti-amyloid beta antibody in either a bivalent (dFab) or monovalent (sFab) format (Niewoehner et al. 2014). While the bivalent dFab antibody failed to cross the BBB and led to lysosomal degradation, the monovalent sFab antibody exhibited facilitated BBB crossing, localization in sorting tubules, and reduction of amyloid deposits in a mouse model of Alzheimer’s disease (Niewoehner et al. 2014; Villaseñor et al. 2017). Similarly, a monovalent fusion of IGF1R VHH to Fc resulted in improved BBB transcytosis in vitro, compared to the bivalent IGF1R VHH-Fc (unpublished observation).
Influence of antibody valency on BBB transcytosis has also been tested for FC5 (Farrington et al. 2014; Haqqani et al. 2018a). TMEM30A, a putative FC5 receptor, is not known to dimerize but is presented as a heteromeric flippase complex of multiple proteins (Wang et al. 2018). When monomeric FC5 VHH was compared with monovalent FC5Fc or bivalent FC5Fc, the bivalent format showed enhanced BBB permeability in vitro and improved brain exposure and pharmacodynamic effects in vivo (Farrington et al. 2014). Bivalent FC5Fc also displayed stronger partitioning in EE and MVBs in BEC compared to monovalent FC5Fc (Haqqani et al. 2018a). Thus, engineering antibody valency is an important strategy to consider when designing BBB-crossing antibodies, as it could trigger either desired facilitation of receptor traffic or undesired receptor cross-linking, activation, and degradation. These studies also underscore that the nature of receptor-antibody interaction is unique for each antibody-receptor pair and that emerging learnings about factors that facilitate transcytosis cannot be broadly applied to all BBB carriers.
Ligand-Receptor Interaction in Acidic pH
It has been observed that soon after internalization, many receptors that need to be recycled are uncoupled from their ligands at acidic pH in different endosomal compartments (such as EEs and MVBs) during the sorting processes (Goldstein et al. 1985; Scott et al. 2014), while the ligand may continue to sort to other destinations. To test whether such phenomenon may also facilitate antibody transcytosis, an anti-TfR antibody with reduced affinity at pH 5.5 was developed; this antibody demonstrated significant transcytosis, while pH-independent antibodies of comparable affinities at pH 7.4 remained associated with intracellular vesicular compartments (Sade et al. 2014). Therefore, another strategy to improve BBB crossing is to develop antibody variants that have different affinity interactions with the RMT receptor at different pHs.
Fc Format
While an Fc domain of IgG is known to prolong circulatory half-life of antibodies through binding, internalization, and recycling in endothelial cells mediated by the neonatal Fc receptor (FcRn) (Giragossian et al. 2013), the presence of Fc domain has also been shown to enhance BBB permeability of BBB-crossing FC5 VHH. When expressed in fusion with the human Fc in either monovalent or bivalent format, FC5 demonstrated improved BBB transcytosis in vitro, enhanced CSF levels, and improved pharmacodynamic potency in vivo compared to FC5 VHH without the Fc (Farrington et al. 2014; Haqqani et al. 2018a). While the in vivo enhancements were largely due to prolonging of circulatory half-life, the increased BBB transcytosis in vitro might be due partially to FcRn-based rescue from intracellular lysosomal degradation (Lencer and Blumberg 2005). Thus, the addition of Fc to single-domain or single-chain antibodies (or non-antibody ligands) against RMT receptors may not only help extend systemic pharmacokinetics but also improve the efficiency of BBB transcytosis.
Antibody Position in the Construct
A position of the anti-RMT receptor antibody in the bispecific construct may affect the efficiency of its transcytosis. For example, a placement of the FC5 on the C-terminus of the Fc or an antibody cargo resulted in low BBB transcytosis; however, FC5 fused to the N-terminal of Fc (Farrington et al., 2014) or heavy (or light) chain of an antibody (Webster et al., 2016) retained its ability to shuttle cargo across the BBB, suggesting that the N-terminus of FC5 is important for conformational antigen binding that triggers transcytosis.
4 Conclusions
In conclusion, we have described some of the key steps involved in the RMT process and different sorting pathways undertaken by various BBB-crossing antibodies as they “travel” through the BBB. It is apparent that the RMT process is a complex set of cross-communicating pathways comprising of various endocytosing, sorting, and exocytosing sub-pathways. We have assigned individual route(s) to some of the known RMT receptors, which they utilize for transporting ligands across the BBB (Table 3.1). Through better understanding of the RMT of antibodies, several key antibody attributes that facilitate abluminal release have been discovered and engineered to improve their BBB-crossing ability. With discovery of new RMT receptors and development of new carrier antibodies, we believe that these factors may serve as an initial guide for improving brain penetration of bispecific antibody therapeutics.
Abbreviations
- BBB:
-
blood-brain barrier
- BEC:
-
brain endothelial cells
- CavE:
-
caveolin-mediated endocytosis
- CCP:
-
clathrin-coated pit
- CCV:
-
clathrin-coated vesicle
- CIE:
-
caveolin- and clathrin-independent endocytosis
- CLIC:
-
clathrin-independent carriers
- CME:
-
clathrin-mediated endocytosis
- CNS:
-
central nervous system
- EE:
-
early endosome
- EV:
-
extracellular vesicle
- FcRn:
-
neonatal Fc receptor
- GEEC:
-
GPI-anchored protein-enriched endocytic compartments
- GPI:
-
glycosylphosphatidylinositol
- IGF1R:
-
insulin-like growth factor receptor 1
- ILV:
-
intraluminal vesicle
- Lat1:
-
large neutral amino-acid transporter CD98
- LE:
-
late endosome
- MVB:
-
multivesicular bodies
- RME:
-
receptor-mediated endocytosis
- RMT:
-
receptor-mediated transcytosis
- RV:
-
recycling vesicle
- TfR:
-
transferrin receptor
- TGN:
-
trans-Golgi network
References
Abulrob A, Sprong H, Van Bergen en Henegouwen P, Stanimirovic D (2005) The blood-brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem 95:1201–1214. https://doi.org/10.1111/j.1471-4159.2005.03463.x
Alvarez-Erviti L, Seow Y, Yin H et al (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. https://doi.org/10.1038/nbt.1807
Andreone BJ, Chow BW, Tata A et al (2017) Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 94:581–594.e5. https://doi.org/10.1016/j.neuron.2017.03.043
Ben-Zvi A, Lacoste B, Kur E et al (2014) Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 509:507–511. https://doi.org/10.1038/nature13324
Bien-Ly N, Yu YJ, Bumbaca D et al (2014) Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med 211:233–244. https://doi.org/10.1084/jem.20131660
Bissig C, Gruenberg J (2014) ALIX and the multivesicular endosome: ALIX in wonderland. Trends Cell Biol 24:19–25. https://doi.org/10.1016/j.tcb.2013.10.009
Boado RJ, Hui EK-W, Lu JZ et al (2010) Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein. J Biotechnol 146:84–91. https://doi.org/10.1016/j.jbiotec.2010.01.011
Boothe T, Lim GE, Cen H et al (2016) Inter-domain tagging implicates caveolin-1 in insulin receptor trafficking and Erk signaling bias in pancreatic beta-cells. Mol Metab 5:366–378. https://doi.org/10.1016/j.molmet.2016.01.009
Chen CC, Liu L, Ma F et al (2016a) Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 9:509–529. https://doi.org/10.1007/s12195-016-0458-3
Chen Q, Takada R, Noda C et al (2016b) Different populations of Wnt-containing vesicles are individually released from polarized epithelial cells. Sci Rep 6:35562. https://doi.org/10.1038/srep35562
Coloma MJ, Lee HJ, Kurihara A et al (2000) Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res 17:266–274
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422:37–44. https://doi.org/10.1038/nature01451
De Meyts P, Ursø B, Christoffersen CT, Shymko RM (1995) Mechanism of insulin and IGF-I receptor activation and signal transduction specificity. Receptor dimer cross-linking, bell-shaped curves, and sustained versus transient signaling. Ann N Y Acad Sci 766:388–401. https://doi.org/10.1111/j.1749-6632.1995.tb26688.x
Dermine JF, Duclos S, Garin J et al (2001) Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J Biol Chem 276:18507–18512. https://doi.org/10.1074/jbc.M101113200
Desai AS, Hunter MR, Kapustin AN (2019) Using macropinocytosis for intracellular delivery of therapeutic nucleic acids to tumour cells. Philos Trans R Soc Lond Ser B Biol Sci 374:20180156. https://doi.org/10.1098/rstb.2018.0156
Eckenroth BE, Steere AN, Chasteen ND et al (2011) How the binding of human transferrin primes the transferrin receptor potentiating iron release at endosomal pH. Proc Natl Acad Sci 108:13089–13094. https://doi.org/10.1073/pnas.1105786108
Ecker DM, Jones SD, Levine HL (2015) The therapeutic monoclonal antibody market MAbs 7:9–14. https://doi.org/10.4161/19420862.2015.989042
Eyster CA, Higginson JD, Huebner R et al (2009) Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic 10:590–599. https://doi.org/10.1111/j.1600-0854.2009.00894.x
Fagerholm S, Ortegren U, Karlsson M et al (2009) Rapid insulin-dependent endocytosis of the insulin receptor by caveolae in primary adipocytes. PLoS One 4:e5985. https://doi.org/10.1371/journal.pone.0005985
Fan JY, Carpentier JL, Gorden P et al (1982) Receptor-mediated endocytosis of insulin: role of microvilli, coated pits, and coated vesicles. Proc Natl Acad Sci U S A 79:7788–7791. https://doi.org/10.1073/pnas.79.24.7788
Farrington GK, Caram-Salas N, Haqqani AS et al (2014) A novel platform for engineering blood-brain barrier-crossing bispecific biologics. FASEB J 28:4764–4778. https://doi.org/10.1096/fj.14-253369
Ferreira APA, Boucrot E (2018) Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol 28:188–200. https://doi.org/10.1016/j.tcb.2017.11.004
Gagescu R, Demaurex N, Parton RG et al (2000) The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol Biol Cell 11:2775–2791. https://doi.org/10.1091/mbc.11.8.2775
Giragossian C, Clark T, Piché-Nicholas N, Bowman CJ (2013) Neonatal Fc receptor and its role in the absorption, distribution, metabolism and excretion of immunoglobulin G-based biotherapeutics. Curr Drug Metab 14:764–790
Goldstein JL, Brown MS, Anderson RG et al (1985) Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol 1:1–39. https://doi.org/10.1146/annurev.cb.01.110185.000245
Grant BD, Donaldson JG (2009) Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10:597–608. https://doi.org/10.1038/nrm2755
Gustavsson J, Parpal S, Karlsson M et al (1999) Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J 13:1961–1971
Ha KD, Bidlingmaier SM, Liu B (2016) Macropinocytosis exploitation by cancers and cancer therapeutics. Front Physiol 7:381. https://doi.org/10.3389/fphys.2016.00381
Hansen GH, Pedersen J, Niels-Christiansen L-L et al (2003) Deep-apical tubules: dynamic lipid-raft microdomains in the brush-border region of enterocytes. Biochem J 373:125–132. https://doi.org/10.1042/BJ20030235
Haqqani AS, Delaney CE, Tremblay T-L, et al (2013) Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells. Fluids Barriers CNS 10:4. https://doi.org/https://doi.org/10.1186/2045-8118-10-4
Haqqani AS, Delaney CE, Brunette E et al (2018a) Endosomal trafficking regulates receptor-mediated transcytosis of antibodies across the blood brain barrier. J Cereb Blood Flow Metab 38:727–740. https://doi.org/10.1177/0271678X17740031
Haqqani AS, Thom G, Burrell M et al (2018b) Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity. J Neurochem. https://doi.org/10.1111/jnc.14482
Hunker CM, Kruk I, Hall J et al (2006) Role of Rab5 in insulin receptor-mediated endocytosis and signaling. Arch Biochem Biophys 449:130–142. https://doi.org/10.1016/J.ABB.2006.01.020
Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30:3481–3500. https://doi.org/10.1038/emboj.2011.286
Itakura S, Hama S, Ikeda H et al (2015) Effective capture of proteins inside living cells by antibodies indirectly linked to a novel cell-penetrating polymer-modified protein a derivative. FEBS J 282:142–152. https://doi.org/10.1111/febs.13111
Jaiswal JK, Rivera VM, Simon SM (2009) Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell 137:1308–1319. https://doi.org/10.1016/j.cell.2009.04.064
Johnsen KB, Bak M, Kempen PJ et al (2018) Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 8:3416–3436. https://doi.org/10.7150/thno.25228
Kähäri L, Fair-Mäkelä R, Auvinen K et al (2019) Transcytosis route mediates rapid delivery of intact antibodies to draining lymph nodes. J Clin Invest 129:3086–3102. https://doi.org/10.1172/JCI125740
Karaoglu Hanzatian D, Schwartz A, Gizatullin F et al (2018) Brain uptake of multivalent and multi-specific DVD-Ig proteins after systemic administration. MAbs 10:765–777. https://doi.org/10.1080/19420862.2018.1465159
Lapierre LA, Avant KM, Caldwell CM et al (2007) Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol 292:G1249–G1262. https://doi.org/10.1152/ajpgi.00505.2006
Lencer WI, Blumberg RS (2005) A passionate kiss, then run: exocytosis and recycling of IgG by FcRn. Trends Cell Biol 15:5–9. https://doi.org/10.1016/j.tcb.2004.11.004
Leyt J, Melamed-Book N, Vaerman J-P et al (2007) Cholesterol-sensitive modulation of transcytosis. Mol Biol Cell 18:2057–2071. https://doi.org/10.1091/mbc.e06-08-0735
Liu AP, Aguet F, Danuser G, Schmid SL (2010) Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J Cell Biol 191:1381–1393. https://doi.org/10.1083/jcb.201008117
Matsumoto J, Stewart T, Sheng L et al (2017) Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun 5:71. https://doi.org/10.1186/s40478-017-0470-4
Maxfield FR, McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Biol 5:121–132. https://doi.org/10.1038/nrm1315
Mayle KM, Le AM, Kamei DT (2012) The intracellular trafficking pathway of transferrin. Biochim Biophys Acta 1820:264–281. https://doi.org/10.1016/j.bbagen.2011.09.009
McClain DA, Olefsky JM (1988) Evidence for two independent pathways of insulin-receptor internalization in hepatocytes and hepatoma cells. Diabetes 37:806–815. https://doi.org/10.2337/diab.37.6.806
Naslavsky N, Caplan S (2018) The enigmatic endosome – sorting the ins and outs of endocytic trafficking. J Cell Sci 131. https://doi.org/10.1242/jcs.216499
Niewoehner J, Bohrmann B, Collin L et al (2014) Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 81:49–60. https://doi.org/10.1016/j.neuron.2013.10.061
Paccaud JP, Siddle K, Carpentier JL (1992) Internalization of the human insulin receptor. The insulin-independent pathway. J Biol Chem 267:13101–13106
Pardridge WM, Buciak JL, Friden PM (1991) Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther 259:66–70
Pohl J, Ring A, Ehehalt R et al (2004) Long-chain fatty acid uptake into adipocytes depends on lipid raft function. Biochemistry 43:4179–4187. https://doi.org/10.1021/bi035743m
Pol A, Calvo M, Lu A, Enrich C (1999) The “early-sorting”; endocytic compartment of rat hepatocytes is involved in the intracellular pathway of caveolin-1 (VIP-21). Hepatology 29:1848–1857. https://doi.org/10.1002/hep.510290602
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. https://doi.org/10.1083/jcb.201211138
Recouvreux MV, Commisso C (2017) Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front Endocrinol (Lausanne) 8:261. https://doi.org/10.3389/fendo.2017.00261
Ribecco-Lutkiewicz M, Sodja C, Haukenfrers J et al (2018) A novel human induced pluripotent stem cell blood-brain barrier model: applicability to study antibody-triggered receptor-mediated transcytosis. Sci Rep 8. https://doi.org/10.1038/s41598-018-19522-8
Roura S, Cal R, Gálvez-Montón C et al (2014) Inverse relationship between raft LRP1 localization and non-raft ERK1,2/MMP9 activation in idiopathic dilated cardiomyopathy: potential impact in ventricular remodeling. Int J Cardiol 176:805–814. https://doi.org/10.1016/j.ijcard.2014.07.270
Sade H, Baumgartner C, Hugenmatter A et al (2014) A human blood-brain barrier transcytosis assay reveals antibody transcytosis influenced by pH-dependent receptor binding. PLoS One 9:e96340. https://doi.org/10.1371/journal.pone.0096340
Schiel JE, Mire-Sluis A, Davis D (2014) Monoclonal antibody therapeutics: the need for biopharmaceutical reference materials. In: Schiel JE, Davis DL, Borisov OV (eds) State-of-the-art and emerging technologies for therapeutic monoclonal antibody characterization, Monoclonal antibody therapeutics: structure, function, and regulatory space, vol 1. American Chemical Society, Washington, DC, pp 1–34
Scott CC, Vacca F, Gruenberg J (2014) Endosome maturation, transport and functions. Semin Cell Dev Biol 31:2–10. https://doi.org/10.1016/j.semcdb.2014.03.034
Song L, Ge S, Pachter JS (2007) Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood 109:1515–1523. https://doi.org/10.1182/blood-2006-07-034009
Sprenger RR, Fontijn RD, van Marle J et al (2006) Spatial segregation of transport and signalling functions between human endothelial caveolae and lipid raft proteomes. Biochem J 400:401–410. https://doi.org/10.1042/BJ20060355
Stanimirovic D, Kemmerich K, Haqqani AS, Farrington GK (2014) Engineering and pharmacology of blood-brain barrier-permeable bispecific antibodies. Adv Pharmacol 71:301–335. https://doi.org/10.1016/bs.apha.2014.06.005
Stanimirovic D, Kemmerich K, Haqqani AS, et al (2017) Insulin-like growth factor 1 receptor -specific antibodies and uses thereof. Patents US2017015748, US2017015749, US2017022277
Tanha J, Dubuc G, Hirama T et al (2002) Selection by phage display of llama conventional V(H) fragments with heavy chain antibody V(H)H properties. J Immunol Methods 263:97–109
Tashima T (2018) Effective cancer therapy based on selective drug delivery into cells across their membrane using receptor-mediated endocytosis. Bioorg Med Chem Lett 28:3015–3024. https://doi.org/10.1016/j.bmcl.2018.07.012
Tauro BJ, Greening DW, Mathias RA et al (2013) Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics 12:587–598. https://doi.org/10.1074/mcp.M112.021303
Terrillon S, Bouvier M (2004) Roles of G-protein-coupled receptor dimerization. EMBO Rep 5:30–34. https://doi.org/10.1038/sj.embor.7400052
Thom G, Burrell M, Haqqani AS et al (2018a) Enhanced delivery of galanin conjugates to the brain through bioengineering of the anti-transferrin receptor antibody OX26. Mol Pharm 15. https://doi.org/10.1021/acs.molpharmaceut.7b00937
Thom G, Burrell M, Haqqani AS, et al (2018b) Affinity-dependence of the blood-brain barrier crossing and brain disposition of the anti- transferrin receptor antibody OX26. Mol Pharm (in press)
Tian X, Nyberg S, Sharp PS et al (2015) LRP-1-mediated intracellular antibody delivery to the central nervous system. Sci Rep 5:11990. https://doi.org/10.1038/srep11990
van Niel G, Raposo G, Candalh C et al (2001) Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121:337–349. https://doi.org/10.1053/gast.2001.26263
van Weering JRT, Cullen PJ (2014) Membrane-associated cargo recycling by tubule-based endosomal sorting. Semin Cell Dev Biol 31:40–47. https://doi.org/10.1016/j.semcdb.2014.03.015
Vercauteren D, Piest M, van der Aa LJ et al (2011) Flotillin-dependent endocytosis and a phagocytosis-like mechanism for cellular internalization of disulfide-based poly(amido amine)/DNA polyplexes. Biomaterials 32:3072–3084. https://doi.org/10.1016/j.biomaterials.2010.12.045
Villaseñor R, Schilling M, Sundaresan J et al (2017) Sorting tubules regulate blood-brain barrier transcytosis. Cell Rep 21:3256–3270. https://doi.org/10.1016/j.celrep.2017.11.055
Von Bartheld CS, Altick AL (2011) Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol 93:313–340. https://doi.org/10.1016/j.pneurobio.2011.01.003
Wang J, Molday LL, Hii T et al (2018) Proteomic analysis and functional characterization of P4-ATPase phospholipid flippases from murine tissues. Sci Rep 8:10795. https://doi.org/10.1038/s41598-018-29108-z
Webster CI, Caram-Salas N, Haqqani AS et al (2016) Brain penetration, target engagement, and disposition of the blood-brain barrier-crossing bispecific antibody antagonist of metabotropic glutamate receptor type 1. FASEB J 30:1927–1940. https://doi.org/10.1096/fj.201500078
Webster CI, Hatcher J, Burrell M et al (2017) Enhanced delivery of IL-1 receptor antagonist to the central nervous system as a novel anti–transferrin receptor-IL-1RA fusion reverses neuropathic mechanical hypersensitivity. Pain 158:660–668. https://doi.org/10.1097/j.pain.0000000000000810
White IJ, Bailey LM, Aghakhani MR et al (2006) EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J 25:1–12. https://doi.org/10.1038/sj.emboj.7600759
Xin H, Jiang X, Gu J et al (2011) Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 32:4293–4305. https://doi.org/10.1016/j.biomaterials.2011.02.044
Yu YJ, Zhang Y, Kenrick M et al (2011) Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med 3:84ra44. https://doi.org/10.1126/scitranslmed.3002230
Yu YJ, Atwal JK, Zhang Y et al (2014) Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med 6:261ra154. https://doi.org/10.1126/scitranslmed.3009835
Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19. https://doi.org/10.1186/s13578-019-0282-2
Zhuang X, Xiang X, Grizzle W et al (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19:1769–1779. https://doi.org/10.1038/mt.2011.164
Zuchero YJY, Chen X, Bien-Ly N et al (2016) Discovery of novel blood-brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron 89:70–82. https://doi.org/10.1016/j.neuron.2015.11.024
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
Haqqani, A.S., Stanimirovic, D.B. (2022). Brain Delivery of Therapeutics via Transcytosis: Types and Mechanisms of Vesicle-Mediated Transport Across the BBB. In: de Lange, E.C., Hammarlund-Udenaes, M., Thorne, R.G. (eds) Drug Delivery to the Brain. AAPS Advances in the Pharmaceutical Sciences Series, vol 33. Springer, Cham. https://doi.org/10.1007/978-3-030-88773-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-88773-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-88772-8
Online ISBN: 978-3-030-88773-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)