Abstract
Lentic ecosystems though encompass a much smaller area compared to the lotic water bodies of the world, are found to emit substantial quantities of greenhouse gases like CO2, towards the atmosphere. The Sundarbans Biosphere Reserve (SBR) of India, besides being the abode of the world’s largest mangrove forest, shelters almost 4.4 million people with a substantially high population density. The CO2 dynamics from several compartments of this biosphere reserve is studied in the recent past; however, the ponds are yet to receive any attention as such. The present chapter reports the variability of the partial pressure of CO2 in water [pCO2(water)] and the air–water CO2 flux from four different types of ponds situated within the SBR. One of these selected ponds is abandoned, and not used for any human purpose and another pond is well-maintained and not at all used for any human purpose. The rest of the two ponds are typical homestead ponds with varying degrees of anthropogenic disturbances. The results indicated that all four ponds acted as a source of CO2 towards the atmosphere; however, the rate of emission varied across the ponds. The most well-maintained least anthropogenically disturbed pond emitted CO2 at the lowest rate, whereas the dilapidated and abandoned pond, which exist in a hypereutrophic state emitted the most. The other two ponds showed an intermediate range of fluxes. Biological processes played a dominant role over physical processes in governing the CO2 fluxes. Water temperature showed a strong, positive, and statistically significant relationship with pCO2(water). Thus, given the ongoing climate-change-induced rise in temperature can effectively enhance the emission rate of these ponds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pond
- pCO2(water)
- Air–Water CO2 flux
- Water temperature
- Primary productivity
- Greenhouse gas emission
- Lentic system
- Indian Sundarban
6.1 Introduction
Aquatic ecosystems are perhaps one of the largest repositories of the world, which store various elements and compounds that are in dynamic equilibrium with the lithosphere and the atmosphere. The aquatic ecosystems continually exchange several gases with the ambient atmosphere. Some of these gases are extremely crucial to maintain life activities within the aquatic systems like oxygen and carbon dioxide gases. However, many of these gases when found in the atmosphere at higher concentrations leads to climatic changes that are not desirable. There are several gaseous compounds in the atmosphere, which impart a greenhouse effect on the planet Earth. In other words, the prevalence of these gases in the atmosphere in higher concentrations implies that the lesser would be the energy emission from the earth back to space, which mostly takes place in the form of longer-wavelength infrared radiation (Elrod 1999). The gas molecules, which can impart a greenhouse effect to the earth and enhance the radiative forcing within the Earth’s atmosphere, are collectively referred to as ‘greenhouse gases’. The different greenhouse gases present in the atmosphere have the varying capability to absorb the outgoing longer wavelength radiation from the Earth and this capability is characterized by quantifying their global warming potential (GWP) (Lashof and Ahuja 1990). These greenhouse gases also happen to vary in the atmosphere in terms of their concentration. Many of these greenhouse gases occur naturally in the atmosphere and participates in several biogeochemical processes of nature. Water vapor, carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) are some such greenhouse gases that can naturally occur in the atmosphere. Whereas, the emission of some of the greenhouse gases such as perfluorocarbons, hydro-chlorofluorocarbons, hydrofluorocarbons, and sulfur hexafluoride to the atmosphere is exclusively associated with certain anthropogenic activities like refrigeration, air-conditioning, metal manufacturing plant, etc. (Khalil 1999). Though the fluorinated greenhouse gases have much higher GWP (Purohit and Höglund Isaksson 2017), it is the conventional greenhouse gases like CO2, CH4, and N2O, that remain to be the point of major concern, from the perspective of the global climate (Köhler et al. 2017).
Ever since the scientific community realized the potential of greenhouse gases in altering the global climate, research with a thrust and emphasis on understanding the role of several natural ecosystems has increased manifold. After having carried out extensive research, the global scientific community can confidently and unanimously state that the terrestrial biosphere and the open oceans act as a sink for carbon (mainly by absorbing CO2 through autotrophic activities) with considerable spatiotemporal variability (DeVries et al. 2019; Pugh et al. 2019). Though several pieces of research indicated that the inland aquatic bodies like rivers, lakes, ponds, reservoirs, dams, play a very crucial role in regulating the global carbon cycle, the knowledge, and understanding about the behavior of the different types of inland aquatic bodies concerning being a source or a sink of greenhouse gases is still poorly constrained (Harmon 2020). According to the estimates of Drake et al. (2018) terrestrial compartments export ~5.1 Pg C to the inland aquatic bodies, out of which only 33% reaches the global oceanic waters through the rivers and estuaries. However, during the transit of carbon from the terrestrial compartments to the oceans, around 25 to 44% of the carbon is respired back to the atmosphere (Harmon 2020). Researchers usually derive such estimates from the meta-analyses of the existing data. However, among the different types of inland aquatic bodies, lotic aquatic systems have received comparatively more attention (Aufdenkampe et al. 2011; Benstead and Leigh 2012; Crosswell et al. 2017) than the lentic aquatic bodies. Again, among the various lentic water bodies, large lakes and reservoirs have received a substantially higher share of attention compared to the small ponds (Bastviken et al. 2004, 2008; Åberg et al. 2010; DelSontro et al. 2018).
The ponds owing to their small size are often neglected while drawing estimates of hydrological carbon budgets but their contribution in acting as a link between the terrestrial environment and water cycle has been recognized long ago (Torgersen and Branco 2008). As far as the processing of terrestrial carbon in the pond ecosystems is concerned, several schools of thought exist at present. Overall, the small ponds usually act as a consistent source of CH4 round the year, and both source and sink for CO2 and N2O; depending upon several, other biogeochemical factors that regulate these gas exchanges (Fig. 6.1) (Macrae et al. 2004; Ferrón et al. 2012). Though the air–water greenhouse gas exchange from the ponds mostly recorded a source character, Smith et al. (2002) along with many others argued that pond ecosystems are most likely a net sink of carbon due to having a very high rate of sedimentation (Chen et al. 2018). Downing et al. (2006) estimated that small ponds having a surface area less than 0.1 km2 occupy an area of about 6,90,000 km2 throughout the world with an additional 12,000 km2 area covered by man-made impoundments. These figures indicate that there is no reason to neglect such a crucial aquatic ecosystem while estimating the carbon budgets and these aquatic bodies can play a significant role in combating or promoting climate change.

(modified after Glaz et al. 2016)
A schematic diagram showing the pathways that lead to exchanges of several important gases between the pond system and the immediate atmosphere
6.2 Pond Ecosystem Biogeochemistry
Compared to medium-sized and large lakes and ponds, the small ponds are present in much more abundance than the former classes throughout the world. These small ponds usually have a shallow water column and exhibit a wide range of biogeochemical variability over space and time (Martinsen et al. 2019). Owing to their small size and shallow depths, the terrestrial setting surrounding these ponds exert a substantial influence on these water bodies (Christensen et al. 2013), which in turn results in some unique biogeochemical conditions in such ponds. Water temperature happens to be one of the key physicochemical parameters of any aquatic system that regulates several biogeochemical processes and the biodiversity of the water column (Yvon-Durocher et al. 2012). All the atmospheric parameters that regulate the ambient temperature near the earth's surface play a crucial role in regulating the water temperature of the ponds near the air–water interface. This includes the ambient temperature itself, the degree of insolation, wind speed near the air–water interface of the pond, relative humidity, and the cloud cover (Woolway et al. 2015, 2016). The shallow ponds due to land effect typically experience rapid heating and subsequent cooling during a diurnal cycle and thus exhibits a wide range of diel variability in water temperature, which in turn regulates the stratification and mixing in the water column (Boehrer and Schultze 2008; Woolway et al. 2016).
Several researchers used to believe that shallow lakes and ponds, under their small water volume, remain homogeneously mixed, however, recent estimates indicate that such perception is wrong (Andersen et al. 2017). Contrary to such common perception, shallow ponds often undergo clear stratification during the daytime, especially if these ponds have submerged aquatic vegetation, and during the nighttime, the mixing of the water column takes place. This not only leads to high diel variability of the water temperature as mentioned above, but also in pH levels, dissolved oxygen (DO), and CO2 concentrations (Andersen et al. 2016). Usually, the shallow ponds experience vertical stratification during the daytime, influenced by the heating of the air–water interface depending on the attenuation of light and morphometry of the lake (Fee et al. 1996). During the nighttime, the cooling of the water mass coupled with wind shear usually destabilizes the stratified water column and consequences to vertical mixing (Spigel et al. 1986). When the water column remains stratified, the ponds can be conceptually demarcated into three prominent zones from the air–water interface to the pond bottom: (i) the turbulent and disturbed epilimnion (upper layer), (ii) the metalimnion where the density sharply changes with depth (middle layer), and lastly (iii) the cold hypolimnion with almost no turbulence (bottom layer) (Kalff 2002) (Fig. 6.2). However, the degree of vertical stratification during a diel cycle might vary across the ponds of a certain region depending on multiple micrometeorological factors like shades offered by the tree canopy surrounding the ponds, hindrances to wind flow, presence of submerged aquatic vegetation like macrophytes, and so forth (Branco and Torgersen 2009; Markfort et al. 2010).
A schematic diagram showing the vertical stratification and mixing of shallow pond waters during the day and night time. The upward and downward blue arrows indicate an increase and decrease respectively. The black arrows indicate the movement of water masses. The red arrows indicate heat exchange between land and pond water
The shallow tropical ponds usually experience high turbidity, especially where land runoffs are very prominent (Sarnelle et al. 1998). Such high turbidity enables the water column to capture and sustain heat (Condie and Webster 2002) and at the same time promotes net heterotrophy as it obstructs the penetration of the photosynthetically active radiation to the deeper layers. Most of the freshwater ponds in tropical regions have zero salinity with few ponds having measurable electrical conductivity, which is situated, close to marine regions. Salt-water intrusion from shallow aquifers often enhances the salinity of some natural ponds in the deltaic and coastal regions and sometimes cyclonic events associated with storm surges and coastal flooding lead to salinization of freshwater ponds (Chand et al. 2012). In some cases like that of brackish aquaculture ponds, saltwater is deliberately introduced to some of the manmade ponds to facilitate the aquaculture of selected species, mainly shrimps (Biswas et al. 2019). Besides this, the local people to meet the water demand for several household activities use many of the small ponds, which are situated in less developed regions like that of Sundarbans. This usage of pond water for activities like bathing, washing utensils, and clothes coupled with runoffs from adjacent agricultural fields treated with fertilizers, in turn, significantly alters the biogeochemistry of such ponds (Mukhopadhyay et al. 2004).
6.3 Nutrient Dynamics in the Pond Ecosystem
There is a group of elements and compounds, which plays a crucial role in the primary autotrophic process, despite being present in minute quantities. We collectively refer to these substances as essential micronutrients. These micronutrients remain in the soil substratum and the terrestrial plants can absorb these through root absorption. Similarly, micronutrients are essential for the primary autotrophs of an aquatic ecosystem as well. Nitrogen, phosphorus, and silica are some of the most vital micronutrients that play a central role in regulating the functioning and the species composition of this base-level floristic community. Not only the absolute quantity of these dissolved nutrients (mostly in the inorganic form) but also their proportion to each other, can significantly govern the phytoplankton dynamics in any aquatic body (Elser et al. 2005; Leflaive et al. 2008). The entire microbial diversity constituting both the microscopic and macroscopic species are, in turn, dependent on the concentrations of these nutrients, especially nitrogen and phosphorus (Torsvik et al. 2002; Groszkopf and Soyer 2016). Pond ecosystems are also no exception, in this regard.
Like many other aquatic ecosystems, ponds also host a suite of microbial species that are dependent on these nutrients to carry out all the essential activities required for thriving. The different kinds of taxonomic groups or individual species have varying capabilities to utilize the respective components of these nutrients (Nelson and Carlson 2011; Corman et al. 2016). Such differences arise out of varying metabolic activities and ecological strategies of these microbes to survive in a particular type of environment (Carbonero et al. 2014). Some microbes can survive in a given state of nutrients, whereas others cannot. Changes in nutrient levels and ratios due to natural or anthropogenic reasons can often lead to only minute changes. However, pieces of evidence show that it can also bring about a paradigm shift in the species composition, coupled with major changes in species richness and evenness (Hewson et al. 2003; Van Horn et al. 2011; Soininen and Meier 2014). The capability of nutrient alteration to modify the biodiversity of a particular system, in turn, plays a major influence on the productivity of the same system. Particularly, the ponds that are small and shallow are more susceptible to such changes, as evaporation takes place at a rapid rate in such ponds and with the fast change in the volume of water, the nutrient concentration also changes (Lee et al. 2017).
Essential nutrients like nitrogen and phosphorus end up accumulating in the ponds through several pathways. Land runoff plays one of the most pivotal roles in regulating the concentration of these substances in the ponds. The ponds in the urbanized sector such as retention ponds and storm-water ponds have received substantial attention in this regard (Yang and Lusk 2018; Taguchi et al. 2020); however, the understanding of rural ponds like that of Sundarbans are still limited. However, in the Indian setup, where many of the ponds are ill-managed and indiscriminately used for various domestic purposes, aquatic pollution takes place due to activities like washing utensils and clothes, bathing, and waste disposal (Kant et al. 2019). These activities, in turn, lead to enhanced nutrient input in these waters and deteriorates the water quality. Excessive presence of nutrients leads to harmful impacts like eutrophication, which in turn, consequences to undesirable manifestations like bad odor, harmful algal blooms, and fish mortality (Howarth and Paerl 2008; Conley et al. 2009). The occurrence of such situations demands intervention in the form of pond management, however, the absence of such initiative compels the local people to abandon such ponds, and in this way, we lose the vital ecosystem services of such aquatic ecosystems.
Once introduced to the ponds, nitrogen, and phosphorus goes through several biogeochemical reactions by which these elements change their chemical speciation and find their way out of the system. Nitrogen gas is abundant in the atmosphere; however, most of the autotrophs and other life forms cannot make use of nitrogen in the gaseous form. There are certain microbes, which specialize in fixing atmospheric nitrogen to the soluble and bioavailable form of dissolved inorganic nitrogen like nitrate, nitrite, and ammonia. This process, known as nitrogen fixation, is one of the most important natural processes, which regulates the nitrogen content of any lentic aquatic body, like that of ponds (Howarth et al. 1988; Scott et al. 2008; Newell et al. 2016). Similarly, a microbial-mediated process that transforms nitrogen-bearing inorganic nutrient compounds to molecular nitrogen, commonly referred to as the denitrification process effectively removes nitrogen from the system (Seitzinger et al. 2006; Groffman et al. 2009; Collins et al. 2010; Bettez and Groffman 2012).
Like nitrogen, phosphorus also exists in different chemical forms. All these varying chemical forms have different mobilizing patterns. Some are easily accessible for aquatic plants, whereas some forms are biochemically inert. The ironbound and labile organic phosphorus is one of the most abundant forms that the aquatic biotas can readily utilize (Hansen et al. 2003; Søndergaard et al. 2003; James 2011). The physicochemical properties of the pond sediments, like the aluminum, sulfur, calcium, and iron content and dissolution potential play a crucial role in regulating the proportion of the different forms of phosphorus (Hupfer and Lewandowski 2008). Compared to nitrogen, the concentration of phosphorus prevails at much less magnitudes in natural ponds; however, a slight alteration in phosphorus concentration can lead to eutrophication (Frost et al. 2019). Like denitrification, phosphorus removal from any closed aquatic body also has to rely on biotic assimilation of phosphorus carried out by a suite of microorganisms, mainly algae (Sañudo-Wilhelmy et al. 2004; Barat et al. 2011; Zhimiao et al. 2016). A delicate balance maintains the equilibrium of these nutrients’ concentration in the pond ecosystem, disturbing which can lead to far-reaching impacts, including the destruction of this ecosystem and eventual conversion to a terrestrial landform.
6.4 Eutrophication and Hypoxia
Eutrophication is the excessive growth of a suite of algal organisms and aquatic plants that take place under favorable conditions accompanied by an abundance of limiting factors essential for photosynthesis, like sunlight, CO2, and primary nutrients (Schindler 2006; Chislock et al. 2013). Lentic systems accumulate sediments in the bottom and gradually become shallow. The lakes and ponds, in this way, undergo eutrophication over the natural course of the cycle, which takes more than a hundred years (Carpenter 1981). However, various anthropogenic activities have enhanced the rate of eutrophication. Human beings are responsible for enhancing the rate of nitrogen and phosphorus input to these shallow lentic bodies, which directly reflects an anomalous growth of macroalgae and other unwanted aquatic vegetation (Carpenter et al. 1998). We refer to this phenomenon as cultural eutrophication. During the late twentieth century, many scholars throughout the world associated cultural eutrophication with a range of industrial, domestic, and fishery-based activities (Schindler 1974). The eutrophication leads to the formation of an algal mat in the pond-air interface, which restricts the exchange of essential gases like oxygen with the ambient atmosphere. The lowering of dissolved oxygen levels in the aquatic bodies beyond a certain threshold (mostly <2 mg l−1) leads to hypoxia. Several aquatic organisms find it challenging to survive under such low oxygen levels, and eventually dies. Thus, eutrophication can lead to fish mortality and enhances heterotrophy. Microbial degradation of dead remains of various flora and fauna supersedes the autotrophic CO2 fixation. The degradation of overall water quality and foul odor are some of the common consequences of eutrophication, which leaves the aquatic bodies in an unusable state. The ill effects of this phenomenon incur substantial financial loss, as we cease to reap the benefits of several ecosystem services (like drinking water supply and recreational use) from these shallow aquatic bodies (Dodds et al. 2009).
Characterizing the trophic state of an inland lentic body is essential to understand the structure and function of such ecosystems and to project future inclination under incessantly altering environmental and climatic conditions (Zhang et al. 2018). We can quantify the trophic state of a pond based on the concentrations of several parameters like total nitrogen, total phosphorus, chlorophyll-a, and the degree of transparency. The combined concentrations of these indicators enable us to determine whether a pond is oligotrophic, mesotrophic, eutrophic, or hypertrophic. Minimal concentrations of nutrients accompanied by modest levels of chlorophyll-a denote the oligotrophic state, whereas the excessive presence of total N and P with very high chlorophyll-a levels indicates a hypereutrophic state (Carlson 1977; Kratzer and Brezonik 1981; Wetzel 2001). Besides the nutrient levels, this trophic state depends substantially on the degree of photosynthesis. Photosynthesis and aerobic respiration leading to the production of oxygen and carbon dioxide, respectively, are the principal metabolic pathways through which the production as well as the destruction of organic matter takes place in nature (Cole et al. 2000), and maintains the gross metabolic balance in any ecosystem (Howarth et al. 1996). Removal of organic matter from any aquatic system exclusively depends on the microbial community strength and supply of enough oxygen to decompose the organic matter (Sobek et al. 2009). However, inland aquatic bodies like lakes and ponds often receive a substantially high load of organics (Tranvik et al. 2009) and lead to an enhanced rate of respiration in degrading this organic matter (Vaquer-Sunyer and Duarte 2008). Such respiratory processes consequences of overutilization of oxygen leaving the adjacent water column deprived of the necessary oxygen levels required for many of the life forms to thrive and promoting anaerobic conditions (Conrad et al. 2011). Hypoxia can cause naturally over time with prolonged aging of any pond or lake; however, anthropogenic inputs to such water bodies accelerate the rate and intensifies the degree of hypoxic conditions (Marotta et al. 2012). In the tropical climate, warmer temperatures favor the deterioration of hypoxic conditions to total anoxia, which in turn leads to deleterious consequences to the biological life (Marotta et al. 2012).
6.5 Pond Ecosystem Productivity
The primary productivity of any ecosystem is integral to the well-being and overall health of that system. The lentic ecosystems like lakes and ponds are no exception. To restore and conserve the ponds of any region, one must properly evaluate the intrinsic algal dynamics and the nature of primary production (Mayer 2020). A suite of biotic and abiotic factors govern the ecological food chain of the ponds, and hence the net ecosystem productivity (Kitchell and Carpenter 1993). The algal species assemblage and count often follows a top-down control, driven by biotic factors like zooplankton grazing and ingestion by fish. Similarly, abiotic factors like light availability and nutrient concentrations regulate the community structure through bottom-up control (Menezes et al. 2010). Besides light penetrability, the residence time of water, the depth of the ponds, the water level, the mixing rate, and the flushing frequency also play a critical role in regulating productivity. The tropical shallow ponds are particularly susceptible to all these parameters. The ponds of the tropics are usually small and experience warmer temperatures and a high rate of evaporation, which in turn fluctuates the water level of the ponds throughout the year. The ratio of surface area to volume is substantially high with ample scope of interaction between pond bottom and the above-lying water mass. All these characteristics play a significant role in governing the primary productivity of these ponds (Zohary et al. 2010; Jeppesen et al. 2015).
The pond substratum and the nature and quality of sediments can indirectly regulate the rate of primary production and shape the algal community structure that thrives therein. The vertical movement of the loose sediments controls the degree of transparency of the water column as these particulate matters impart turbidity. The higher the concentration of these turbid materials the lower would be the light penetration, and hence, it can potentially compromise the rate of photosynthesis. The bottom-churning induced sediment resuspension takes place in particular in the shallow ponds, as wind-driven shear can disturb the pond bottom (Talling 2001). Rapid changes in water level in the seasonal time scale can exacerbate the situation, as, under the circumstances, sediments tend to leave the bottom and remains suspended in the water column (Rodrigues et al. 2016). However, there could be some positive feedbacks of sediment suspension towards enhancing primary productivity, as they often release dissolved nutrient matter to the water, thereby promoting photosynthesis (Dantas et al. 2019).
Among the biotic factors, zooplankton and fish dynamics cast a significant impact on the phytoplankton community structure and the associated gross primary production. Zooplankton grazing can directly control the phytoplankton counts. However, the grazing rate depends on the size and ingestion capability of the zooplankton (Iglesias et al. 2011). Fish population, especially in the tropics, can both promote as well as negate the primary productivity by controlling the phytoplankton community composition (Attayde et al. 2010). The consumption of phytoplankton by the fishes reduces the productivity potential of the ponds. However, by preferential consumption of zooplankton, fishes occasionally relive the phytoplankton from grazing pressure (Torres et al. 2016). Besides, some of the bottom feeders disturb the pond bottom in search of food and release nutrients, which in turn helps in enhancing productivity (Starling et al. 2002). Thus, we can infer that several biotic options can alter and control the primary productivity of the ponds.
6.6 Ponds and Greenhouse Gases
Downing (2009) strongly emphasized that inland aquatic bodies like lakes and ponds, though neglected due to their small size and discrete distribution throughout the land surface, play a crucial role in regulating the greenhouse gases in the atmosphere. Downing et al. (2006) established that these shallow aquatic bodies encompass substantially large areas compared to previous estimates. Contrary to the pre-existing belief, many scholars proved that the smaller ponds and lakes emitted higher magnitudes of greenhouse gases towards the atmosphere (Kortelainen et al. 2006; Juutinen et al. 2009). The production of greenhouse gases, its removal, transformation, and emission towards the atmosphere depends on several factors like temperature, size and depth, hydrology, maintenance, and usage types, and many others (van Hulzen et al. 1999; Rantakari and Kortelainen 2005; Battin et al. 2009; Duc et al. 2010; Kosten et al. 2010). Atmospheric variables like ambient temperature, relative humidity, solar insolation, wind speed change from minutes to years to decades, and play a crucial role in regulating the greenhouse gas fluxes. However, it is difficult to ascertain the role of climate change in changing the flux patterns from shallow ponds, due to the absence of long-term data and repeated measurements in the same spots (Natchimuthu et al. 2014).
Besides the phytoplankton, all the other life forms breathe out CO2 in the aquatic column of the ponds. If the rate of CO2 production by the autotrophs supersedes the rate of CO2 respiration by the rest of the biotic community in a diurnal cycle, the system altogether becomes net autotrophic, and usually acts as a sink of CO2. However, in most cases, the reverse scenario prevails, which makes the system net heterotrophic and hence, a source of CO2. As ponds often receive substantial quantities of organic load and suffer from hypoxia due to human-induced eutrophication, the ponds experience anaerobic conditions, especially near the bottom. This type of condition activates the methanogens, and instead of CO2, leads to the production of a more potent greenhouse gas, CH4. The input of nitrogenous materials leads to the generation of N2O under hypoxic conditions during denitrification (Codispoti 2010). The global warming potential of N2O is much higher than CO2 as well as CH4, and research shows that small water bodies can emit substantial quantities of N2O (Gorsky et al. 2019). The enhanced use of nitrogenous fertilizers and domestic waste through land runoff ends in the lentic ecosystems, whereby, these substances undergo denitrification and emit N2O towards the atmosphere (Blaszczak et al. 2018). In this chapter, we have dealt with only CO2 emissions from the homestead ponds of the Sundarbans region.
6.7 Household Ponds of Sundarbans and Their Characteristic Features
Not only in the Sundarbans but also in many rural parts of this country, ponds are an integral part of many domestic activities. In the Sundarbans, a small pond within the household of a kaccha or even pukka houses is a very common sight. Mandal et al. (2015) reported that in the rural sectors of Sundarban, the majority of the households possess a pond in its adjacency, having an average surface of 400 to 500 m2. The landowners own most of these ponds legally as these ponds are essentially a part of the entire land area purchased by an individual or passed on through a family heirloom. Many households with a larger area under their share have more than one pond of varying size. These ponds vary in size and shape. However, household ponds larger than 1000 m2 are scarce. Most of these ponds exist within a distance of 10–15 m from the dwelling. It is a common practice in this part of the world to dig ponds and utilize the unearthed soils to increase the basement of the dwelling or even construct the house itself (the kaccha houses). Besides these small ponds, which are in the true sense personal property of the landowner, there are some large ponds as well. These large ponds are less in number; however, exist in one or two numbers across a village. This type of ponds are public properties and local villagers can access these ponds to meet several daily life activities.
These ponds are mostly perennial. During the non-monsoon dry months, the water level in many of these ponds goes down leaving a limited amount of water. The rain during the monsoon season recharges these ponds up to the brim. The depth of these ponds varies from a meter to three meters. Most of these are manually dug ponds, and these have a typical shape of steadily inclining edges with the highest depth around the center. Mandal et al. (2015) observed that the average age of the ponds in the Sundarbans is around 60 years. Some of the ponds are as old as hundred years, whereas, very few ponds came into operation in the last decade. The older the ponds the higher is the rate of sedimentation, which affects the primary productivity of these ponds (Boyd 2012). These ponds are in a way, lifelines for the rural population in the Sundarbans. They rely on these ponds for several services. Fish rearing is a very common practice in these ponds. Local people grow a wide variety of freshwater fishes in these homestead ponds, which mainly includes Labeo rohita, Labeo catla, Catla catla, Cirrhinus cirrhosis, Puntius sophore, Hypophthalmichthys molitrix, Mystus tengara, Lates calcarifer, Oreochromis niloticus, Amblypharyngodon mola, Probarbus jullieni, Clarias batrachus, and many more (Mandal et al. 2015). The local population does not rear fish for commercial purposes. They harvest these fishes regularly from the ponds for their daily meals. They can rear fish for almost eight to nine months, excluding the dry months when the water column becomes extremely shallow, and hence, unfavorable for the fishes to survive. They also do not practice any scientific management for the well-being of these ponds, mostly because they cannot afford to do so. Hence, most of these ponds usually do not undergo conventional pond management practices like periodic dredging, lime treatment, and de-silting. The fish rearing practiced in these ponds also does not follow any stringent aquaculture protocols. The traditional practice just includes the introduction of seedlings at different times of the year. However, the local people do not care to take into account the conventional aquaculture parameters like stocking density, and fish composition. They also do not provide specific feeds for the fishes. The fishes mostly grow on their own depending on the natural foods that end up in these ponds, mostly out of primary autotrophic processes. Mandal et al. (2015) observed that the local people could enhance the aquaculture potential of these ponds; however, the financial constraints bar them from doing so.
Besides being a source of fish, the pond water finds its utility in growing fruits and vegetables in the homestead gardens for household consumption. Mandal et al. (2015) mentioned in their study that local households of Sundarbans deploy about 120–240 m2 of their land plot to grow a wide range of vegetables throughout the year. The adjacent ponds serve as the sole source of water to sustain this miniature form of agriculture. Besides growing vegetables and rearing fishes, the local people also use this water for bathing and washing purposes. Many people prefer to take a dip and swim across the ponds as a daily routine. Washing utensils along the edge of the ponds is also a very common sight in this region.
6.8 Materials and Methods
Though we understand the importance of these small lentic bodies in regulating the greenhouse gas concentration, there are almost no studies that focused on the air–water exchange of the conventional greenhouse gases in these ponds of Sundarbans. The ongoing climate change is significantly altering the ambient temperature and the rainfall pattern. Sundarbans experience the wrath of climate change mainly in the form of tropical cyclones, which have exhibited an alarming increase both in terms of frequency as well intensity (Dubey et al. 2017). Storm surges and relative sea-level rise have posed a severe threat to the functioning of several aquaculture ponds situated near the coastline due to a change in the overall salinity regime of the aquatic column. All these factors can potentially alter the CO2 dynamics of these small ecosystems as well. To characterize the air–water CO2 exchange across the pond interface, we have sampled in few of the representative ponds within the Indian Sundarbans Biosphere Reserve. In the present date, there are several established protocols for measuring the air–water CO2 fluxes. Eddy covariance technique has become extremely popular among meteorologists, and it has replaced all the measurement protocols because of its robustness and long-term flux monitoring capability. However, this method requires a sophisticated set of instruments and proper stationing, which was beyond the ambit of the author’s reach. We implemented the bulk density method for spot measurements and estimated the partial pressure of CO2 in water [pCO2(water)]. The concentration gradient across the air–water interface enabled us to compute the CO2 fluxes. We have also measured a suite of other biogeochemical parameters and observed the relationship of those with the CO2 fluxes. We have detailed the methodology adopted for the present study in the following sub-sections.
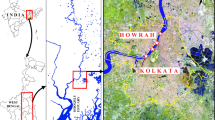
6.8.1 Location of Ponds Selected for This Study
We carried out the sampling at four selected ponds distantly located throughout the South 24 Paraganas district of the Sundarbans Biosphere Reserve. Two of these ponds are in the Namkhana Block, one in the Patharpratima Block, and the other in the Basanti Block. Figure 6.3 shows the location of the respective ponds in the study area. We tried to encompass the different varieties of ponds based on their state of health, water usage, size, and shape. Some of these are typical household ponds used for multifarious domestic activities. Some ponds receive comparatively less domestic attention, as the local people are not exclusively dependent on the services provided by these ponds. Besides, there are some water bodies, which are usually larger than the other two types of ponds mentioned in the preceding sentence, and these undergo proper maintenance, mainly for their aesthetic value and beautification purposes. Lastly, there exists a class of ponds, which are most neglected, not used for any domestic activity, and suffer from intense eutrophication. Somehow, these ponds can manage a water column for a substantially long time, as these ponds get replenishment of water from monsoonal rain.
The pond in the Basanti Block exists near a popular tourist destination named Jharkhali (hereafter referred to as P1). This pond roughly covers an area of 250 m2. This pond is not a typical household pond as it lies within the premises of a government office. However, stationed officials make use of the pond water. Occasionally very few people bathe and wash utensils in this pond. The pond has fishes but the people surrounding this pond are not very dependent on it, and they harvest fishes rarely. Overall, this pond has limited use for domestic purposes.
The second pond selected for this study is in Ramganga (hereafter referred to as P2), which is one of the busiest places in the Patharpratima Block, as many people commute through this critical junction to go towards Kolkata and the suburban localities. The pond merely covers an area of about 400 m2. Situated in a typical locality, this pond is almost of no use to the local people. A thin algal mat prevailed over the water surface almost for the entire duration of an annual cycle. In the monsoon season, heavy rain sometimes broke off the intact mat cover. The visual scenario of the very pond can tell us that it is abandoned and suffering from eutrophication. We selected this pond as one of the representative classes, as these types of ponds are prevalent all around the Sundarban.
The third pond sampled in this study represents the most typical type of household ponds. This pond is in the Namkhana Block (hereafter referred to as P3), situated in a typical semi-rural locality. We have noticed a substantial number of people bathing in this pond all-round the year. Women from nearby houses occasionally use the pond water to wash the utensils or even clothes, sitting near the periphery of the pond. This pond occupies an area of about 380 m2.
Lastly, we selected a pond, situated in the same Namkhana Block, but it is located within the premises of a Guest House (hereafter referred to as P4) that mainly cater to tourists from Kolkata who ventures into the nearby Bakkhali and Henry Island sea beaches. Out of the four ponds we sampled, this is the largest having an area of about 2700 m2. The pond is full of fishes and occasionally the caretakers harvest fishes out of the pond but they essentially do not rely on these fishes for consumption. There is a strict prohibition for bathing and washing clothes or utensils in this pond. The guesthouse authority maintains this pond mainly for beautification purposes. All the four ponds had one thing in common. There are no concrete embankments in any of these ponds. Almost all the ponds have some naturally growing bushes and shrubs in their periphery.
6.8.2 Sampling Strategy
We sampled the above-mentioned four ponds during the years 2016 to 2018. The broad demarcation of seasons in this part of the world includes the pre-monsoon season (February to May), the monsoon season (June to September), and the post-monsoon season (October to January). We sampled in each of the three seasons during the three years (2016–2018). However, we carried out the sampling in different months of a particular season in the three respective years to characterize the short-term temporal variability in the physicochemical parameters as well as the CO2 fluxes. In this way, we sampled in all the twelve months but of different calendar years. We presented the seasonal mean data taking into account the measurements carried out in all the months of that particular season. We assumed that there was no significant inter-annual variability in the measured parameters.
We sampled once every month during the daytime (between 10:00 a.m. and 12:00 p.m.) and the nighttime (between 06:00 p.m. and 08:00 p.m.) from all four ponds. We collected both the day and night samples to analyze the difference in the CO2 flux dynamics in the presence and absence of photosynthesis. In the year 2016, we sampled in March, May, September, and November. Similarly, in the year 2017, we sampled in January, April, August, and October. Finally, in the year 2018, we sampled in February, June, July, and December. We collected three replicate samples from each of the ponds with the help of a pre-cleaned polypropylene glass bottle from the surface water. We collected the water samples from at least a meter distance from the edge of the ponds.
6.8.3 Analytical Protocol
We measured most of the parameters in situ by deploying standard probes. However, for those parameters, which require laboratory analysis, we collected the samples in specific containers, preserved the samples with the addition of necessary preservatives for each parameter, and transferred the bottles in freezing conditions to the laboratory. We took adequate care to calibrate and standardize the sensors and probes used in this study. We ensured that all the reagents used for the different laboratory analyses were of analytic grade.
6.8.3.1 Spot Measurements of Atmospheric and Biogeochemical Parameters
Most of these ponds sampled in the present study were essential of freshwater type. Thus, the total dissolved solid content was much low to have any reportable salinity. Thus, we measured the in-situ electrical conductivity of the surface water of these ponds with the help of a Multikit (Multi 340i set, WTW, Germany) fitted with a standard probe (Tetracon 325, WTW, Germany). We also monitored the in-situ water temperature with the same instrument. The sensors had a measurement resolution of 1 μS cm−1 and 0.1 °C, for electrical conductivity and water temperature, respectively. We estimated the analytical precision of the sensors by measuring a few of the same samples repeatedly for ten times. The precision of measurements for electrical conductivity was ±2 μS cm−1 and for water temperature, it was 0.1 °C. We measured the dissolved oxygen (DO) concentration by deploying a standard probe (FiveGo portable F4 Dissolved Oxygen meter, Mettler Toledo, Switzerland) having a resolution of 0.01 mg l−1. We measured the DO once during each sampling endeavor following the modified Winkler’s titrimetric method. We checked the offset and calibrated the sensor’s DO measurement readings with the help of the results obtained from Winkler’s method. We also regularly calibrated the DO sensor with the help of boiled water (having zero DO) and fully oxygenated water (having a hundred percent oxygen saturation). The DO sensor exhibited an accuracy of ±1 percentage. We also measured the saturation state of DO using the empirical formula of Weiss (1970).
We measured the in-situ pH with the help of a micro-pH meter equipped with standard glass electrodes (Orion PerpHecT ROSS Combination pH Microelectrode) fitted to a data logger [Thermo Scientific, U.S.A.]. The analytical resolution of the pH meter was 0.001. We calibrated the glass electrodes for pH measurements (once before the sampling endeavor begun for each month and once after the completion) using the NBS scale technical buffers (Merck, Germany) at a controlled temperature of 25 °C. We deployed a nephelometer (Eutech TN-100, Singapore) to measure the turbidity of the water samples. With the help of a standard underwater light sensor (LI-192SA, Li-Cor, USA; analytical resolution 0.1 μ mol m−2 s−1) fitted to a data logger (Li-250A, Li-Cor, USA), we measured the underwater photosynthetically active radiation (UWPAR). We carried a portable weather station (WS-2350, La Crosse Technology, Wisconsin, USA) to the sampling spots to measure the ambient temperature, atmospheric pressure, and wind velocity. We implemented the standard light–dark bottle method by incubating the samples for 12 h from dawn to dusk and measured the gross primary productivity (GPP) and community respiration (CR) of the surface water by measuring the DO concentrations with standard probes. We deployed three replicate sets of bottles for GPP and CR measurements.
6.8.3.2 Estimation of pCO2(Water) and Other Laboratory Measurements
We filtered 100 ml water samples through GF/C filter papers and preserved the samples for total alkalinity (TAlk) analysis (Frankignoulle et al. 1996) by poisoning each sample with 20 μl saturated HgCl2 solution (7.2 g HgCl2 in 100 ml distilled water) (Kattner 1999). We used an automated titrator (905 Titrando, Metrohm, Switzerland) to measure the TAlk following a closed chamber titration. We estimated pCO2(water), dissolved inorganic carbon (DIC), and hydroxyl ion (OH−) concentration using the water temperature, atmospheric pressure, TAlk, and pH data with the help of the software CO2SYS.EXE (Lewis and Wallace 1998). Oceanographers and marine scientists mostly use this software to estimate pCO2 in seawater. However, the software has a provision to estimate pCO2(water) in absolutely fresh water. Recent studies like Chanda et al. (2020) have successfully used this software to estimate the pCO2(water) in the urban tidal river of Hooghly, which flows by the twin cities of Kolkata and Howrah, situated in the north of the Sundarbans Biosphere Reserve. The Hooghly river water in this part had negligible salinity. Following Chanda et al. (2020) we used the dissociation constants, K1 and K2, of Millero (1979) for zero salinity water on the NBS scale. We used the corrections of Khoo et al. (1977) for sulfate concentrations. We used a nondispersive infrared (NDIR) sensor (Li-840A; Li-COR, USA) to measure the CO2 concentration in the ambient air. We calibrated the instrument before each sampling campaign with certified reference standard gases of known concentrations of CO2 (0, 300, and 600 CO2 concentration) in N2 as base gas (Chemtron Science Laboratories, India). We converted the mole fraction of CO2 in ambient air to the partial pressure of air [pCO2(air)] by using the ambient temperature and atmospheric pressure, and the virial equation of state (Weiss 1974).
We collected 1 l of a water sample from the pond surface, stored the same in an amber-colored container, preserved under ice-cold condition, and sent the samples back to the laboratory within 24 h for chlorophyll-a (chl-a) analysis. We kept the samples in the dark in a freezer until further analysis. We implemented standard spectrophotometric protocols (Parsons et al. 1992) to measure chl-a. The precision of the chl-a measurement was ±0.02 mg m−3. We used standard lyophilized chlorophyll-a (Sigma-Aldrich, Merck, Germany) for the preparation of standard stock solutions and calibration of the spectrophotometer.
6.8.3.3 Computation of Air–Water CO2 Fluxes
For the sake of the CO2 flux computation across the air–water interface, we converted the pCO2(water) and the pCO2(air) to concentrations of carbon dioxide in water (CO2wc) and air (CO2ac), respectively, following the equations given below (Weiss 1974; Anderson 2002).
where KH stands for the gas partition constant of CO2 in freshwater at the sampling temperature. We computed KH (in mole l−1 atm−1) following the equation below.
where TK denotes the water temperature in the unit of Kelvin (Weiss 1974).
We computed the air–water CO2 exchange rate (flux) according to the formula furnished in MacIntyre et al. (1995) as shown below.
where kx stands for the gas transfer coefficient (cm h−1) that we estimated according to the formula given by Wanninkhof (1992).
where Sc denotes the Schmidt number (for CO2). The Sc depends on the water temperature (T, in the unit of Kelvin) according to the formula given below.
We calculated k600 using the wind speed at a height of 10 m above the pond interface (U10), according to Cole and Caraco (1998), where, the magnitude ‘x’ is equal to 0.5 and 0.66 for wind speeds >3 m s−1 and ≤3 m s−1, respectively. We measured the wind speed with the help of a weather station at 1 m height from the pond interface and converted the speed magnitudes for 10 m height, using the empirical wind profile equations given by Kondo (2000).
6.8.4 Statistical Analyses
We used the Statistical Product and Service Solutions software (SPSS version 16.0, Inc., USA) and Microsoft Excel for Windows 2010 to carry out all the statistical analyses and prepare the graphical illustrations for this chapter. We examined whether the arithmetic means of pCO2(water), CO2 fluxes, and other biogeochemical parameters were significantly different or not between all the four ponds and across all the seasons by carrying out a one-way analysis of variance test (ANOVA; F-test). We conducted a posthoc Tukey's honest significant difference (HSD) test to specify the difference in mean of the biogeochemical parameters among the selected ponds if any. To analyze the inter-relationship between the pCO2(water) and the other regulating factors, we calculated the Pearson correlation coefficient. We considered the outcomes of these tests statistically significant at p ≤ 0.05.
6.9 Results and Discussion
6.9.1 Variability of Physicochemical Parameters
The surface water temperature in the ponds varied between 20.5 and 35.4 °C (Table 6.1). The annual mean temperature was close to 30 °C in all four ponds. However, P2 which is an abandoned pond recorded slightly higher temperature than the other three ponds consistently throughout the year though the difference was not statistically significant (p > 0.05). Due to a comparatively lesser volume of water in P2, the influence of land could have been slightly higher in this pond, which in turn, led to this marginal difference. Seasonally, monsoon recorded the highest temperature followed by pre-monsoon and post-monsoon in all four ponds. The surface water temperature is mainly governed by the ambient temperature. In this region, the temperature remains consistently high throughout the monsoon. Though the summer months (April and May) fall under the pre-monsoon season, the other two months of this season (February and March) experiences much lower temperatures. All four ponds were essentially freshwater types. The electrical conductivity ranged between 856 and 1563 µS cm−1. P2, the abandoned pond showed significantly higher electrical conductivity than the three other ponds (p > 0.05). One of the main reasons could be the lesser volume of water coupled with the dilapidated condition of the pond, which enabled the dissociation of various litter in the pond water that enhanced the total dissolved solids. P2 not being used for any purpose was left neglected and such ponds often receive a higher degree of anthropogenic litters than the ones which are consistently in use for some purpose. Dissolved oxygen exhibited significant differences among the four ponds (p < 0.05). Again, the most notable difference was observed between P2 and all the other ponds. P2 almost recorded half the annual mean dissolved oxygen observed in the other three ponds. We observed an algal mat over P2 almost every month, except few monsoon months when heavy torrential downpour cleared off some portion of the algal cover. Thus, it was expected that net heterotrophy is quite persistent in this pond compared to the others. This aspect could have led to the lowered dissolved oxygen in P2. It is also worth mentioning that P1 and P4 showed a significant difference, with P4 showing the highest annual mean dissolved oxygen among the four ponds. Similar to dissolved oxygen, turbidity also showed a significant difference among the ponds, and in this case, also P2 exhibited significantly high turbidity compared to the other three ponds. The shallow depth in P2 might have enabled bottom churning and the public lettering might have added more suspended materials, which led to the enhanced turbidity. The presence of a thick algal cover might have also contributed to the enhanced turbidity. Besides P2, the other ponds also showed a subtle but significant difference in turbidity among them. P4, the one that is maintained properly, showed the least annual mean turbidity. The variation of the physicochemical parameters is illustrated in Fig. 6.4.
6.9.2 Variability of Primary Productivity Parameters
The UWPAR gives us an idea about how conducive the aquatic environment is, in terms of carrying out primary autotrophic processes. It depends on the solar insolation as well as the light penetrating ability of the water column. In this study, a significant difference in UWPAR was observed among the ponds (p < 0.05). P2 recorded the lowest annual mean UWPAR, whereas P4 recorded the highest annual mean UWPAR. The ponds with minimal anthropogenic disturbance (P1 and P4) exhibited comparatively higher UWPAR, which showed that proper maintenance can enhance the UWPAR in the water column and can increase the autotrophic potential of these ponds. The high turbidity in P2 could be one of the major reasons behind the low UWPAR observed in this pond. Chl-a concentration gives us an idea about the standing stock of phytoplankton biomass in an aquatic system. The results obtained from this study showed that P1, P3, and P4 did not exhibit any significant difference in chl-a concentration amongst each other. However, P2 showed significantly (p < 0.01) higher chl-a concentration all through the sampling period. The annual mean chl-a concentration indicated that P2 exhibited 4–5 times the concentration observed in the other three ponds. This shows that the neglected pond, P2 is prone to eutrophication and most likely exists in a hypereutrophic state. It is interesting to note that despite having the highest mean concentration of chl-a, P2 did not record the highest rate of GPP. P4 exhibited the highest GPP, followed by P2, P1, and P3. The GPP of P1 and P3 had no statistically significant difference. Similarly, P2 and P4 did not show any statistically significant difference. However, it is worth mentioning that the GPP in P1 and P3 was nearly half of what was observed in the case of P2 and P4. The reason behind high GPP in P2 could be the higher concentration of chl-a, whereas in the case of P4 despite having a modest chl-a concentration, favorable conditions of photosynthesis could have led to the higher GPP, compared to P1 and P3. CR magnitudes indicated that all four ponds acted as net heterotrophic systems. However, in P4 there was hardly any difference between the GPP and CR, which indicated that this pond was neither net autotrophic, not net heterotrophic, in the strict sense. CR followed the trend like that of chl-a. P2 exhibited significantly high CR compared to the other three ponds and P1, P3, and P4 did not exhibit any significant difference in CR amongst each other. This showed that excessively high chl-a led to hyper-eutrophic conditions, which in turn, promoted community respiration. The NPP followed the order P4 (−0.2 ± 3.0 gO2 m−2 d−1) > P1 (–6.2 ± 2.0 gO2 m−2 d−1) > P3 (−7.5 ± 2.1 gO2 m−2 d−1) > P2 (−18.2 ± 5.5 gO2 m−2 d−1). The variation of productivity parameters in the four ponds is illustrated in Fig. 6.5.
6.9.3 Variability in Carbonate Chemistry Parameters
All four ponds were alkaline throughout the year. However, P2 exhibited the lowest mean pH compared to the other three ponds. At the same time, all four ponds showed significantly different pH amongst each other. P4 recorded the highest pH (8.368 ± 0.109) among the four ponds. Overall, pH remained the lowest in the monsoon season compared to the other two seasons, except in P2. In P2, pH increased during the monsoon. Monsoonal rain usually brings with low pH water, as CO2 remains dissolved in rainwater. However, in P2, the already low pH water was probably diluted by the rain which could have increased the pH. Total alkalinity in P1, P2, and P4 did not show any significant difference. However, the annual mean TAlk in P3 was almost double the other three ponds. This could be due to both geogenic or anthropogenic reasons (like liming); however, it could not be delineated from the present study. The TAlk concentration of the respective ponds remained more or less constant throughout the year and did not any significant seasonal variability. Estimated DIC also mirrored the variability of TAlk in all the ponds. Hydroxyl ion concentration followed the same trend as that of pH. The variability of the carbonate chemistry parameters is illustrated in Fig. 6.6.
6.9.4 Variability of pCO2(Water) and Air–Water CO2 Flux
The present study indicated that P1, P2, and P3 were always supersaturated with CO2 in terms of the atmospheric CO2 concentration, as the minimum pCO2(water) observed in these ponds was higher than the atmospheric CO2 concentration. The atmospheric CO2 concentration during the sampling period did not vary significantly among the ponds. Altogether, the atmospheric CO2 concentration ranged between 402 and 413 µatm. Thus, it is clear that pCO2(water) was mainly responsible for governing the ΔpCO2, and hence the air–water CO2 flux. All four ponds exhibited significant differences in pCO2(water) amongst each other. However, P2 showed the highest mean pCO2(water) among all four ponds (2798 ± 472 µatm). This indicated that the least favorable photosynthetic conditions coupled with a hyper-eutrophic state enhanced the pCO2(water) to a great extent in P2. P1 and P3 also showed significantly high pCO2(water). The only exception in this regard was P4. The pCO2(water) in P4 was undersaturated in some months during the late half of the post-monsoon season and the early half of the pre-monsoon season. Apart from these months, the other months had pCO2(water) higher than the atmospheric CO2 concentration. Thus, this pond, P4, exhibited a dual character in terms of source and sink of CO2. The air–water CO2 flux mirrored the variability of pCO2(water), as ΔpCO2 was mainly governed by this parameter, and pCO2(air) exhibited marginal variation. The annual mean air–water CO2 flux varied in the order: P2 (4707 ± 1099 µmol m−2 h−1) > P3 (1915 ± 729 µmol m−2 h−1) > P1 (1666 ± 771 µmol m−2 h−1) > P4 (69 ± 281 µmol m−2 h−1) (Fig. 6.7). Thus it is evident that the abandoned pond, which is in the most dilapidated state emits CO2 at the highest rate and the well-maintained pond with the least anthropogenic disturbance emits CO2 at the lowest rate. Sometimes this well-maintained pond acted as a CO2 sink also.
6.9.5 Relationship Between pCO2(Water) and Other Biogeochemical Variables
Water temperature, electrical conductivity, dissolved oxygen, oxygen saturation, turbidity, UWPAR, chl-a, GPP, CR, and NPP were correlated with pCO2(water) to identify the potential regulators of the air–water CO2 fluxes (Table 6.2). Three out of four ponds (except P2) showed a statistically significant positive relationship between water temperature and pCO2(water). This shows that temperature plays a crucial role in aiding and enhancing the net heterotrophy, which led to this positive relationship. Given the ongoing climate change and the increase in ambient temperature, these ponds are expected to emit more CO2 in the near future.
Electrical conductivity, which did not vary to a great extent amongst the ponds (as all the ponds are of freshwater type) did not show any significant relationship in any of the ponds. Usually, high pCO2(water) is accompanied by low dissolved oxygen and vice-versa, when both of these parameters are governed exclusively by biological mechanisms. However, such a negative relationship between the two parameters was only observed in the case of P4, which is a well-maintained pond with the least anthropogenic disturbance. Oxygen saturation, however, showed a negative relationship with pCO2(water) in P1, P3, and P4. This indicated that biological mechanisms played a crucial role in the O2-CO2 equilibrium. Though chl-a and GPP did not show any significant relationship with pCO2(water), CR and NPP showed a significant relationship in P1, P3, and P4. This showed that the pCO2(water) dynamics in these three ponds were mainly regulated by the biological net heterotrophy. This is why temperature and pCO2(water) also could have shown a positive relationship because increased temperature facilitates the degradation of organic matter, and enhances net heterotrophy. It is also worth mentioning and quite intriguing that P2 did not show any significant relationship with any of the parameters. This led us to conclude that under hyper-eutrophic and abandoned state, the dynamics of pCO2(water) depends on a combination of factors like net heterotrophy, photosynthesis inhibition in the water column, a higher rate of degradation, and so forth, and it is often not observed through a simple linear correlation between the respective parameters.
6.10 Conclusion
Overall, the ponds of Indian Sundarbans are found to be net heterotrophic. The ponds where daily household activities are carried out or are anthropogenically disturbed, emitted more CO2 than the ones that experience lesser anthropogenic intervention. Well-maintained ponds without any human intervention like bathing, washing utensils, and clothes, can even act as sinks for CO2 during some time of the year when conducive conditions for net autotrophy prevails. The results indicate that biological mechanisms mainly govern the CO2 exchange and physical forcing plays a lesser role. Water temperature, dissolved oxygen saturation, community respiration, and net primary productivity showed a significant relationship with pCO2(water), and hence, air–water CO2 flux. These observations indicate that biological organisms responsible for photosynthesis mainly regulates the pCO2(water). Thus, maintaining conditions favorable for photosynthesis in these ponds can provide negative feedback to climate change and vice-versa. This study also inferred that abandoned hypereutrophic ponds are the worst as these ponds emit significantly higher CO2 than the ordinary homestead and multipurpose ponds, where the regular human intervention takes place.
References
Åberg J, Jansson M, Jonsson A (2010) Importance of water temperature and thermal stratification dynamics for temporal variation of surface water CO2 in a boreal lake. J Geophys Res 115:G02024. https://doi.org/10.1029/2009JG001085
Andersen MR, Kragh T, Sand-Jensen K (2017) Extreme diel dissolved oxygen and carbon cycles in shallow vegetated lakes. Proc R Soc B Biol Sci 284(1862):20171427. https://doi.org/10.1098/rspb.2017.1427
Andersen MR, Sand-Jensen K, Iestyn Woolway R, Jones ID (2016) Profound daily vertical stratification and mixing in a small, shallow, wind-exposed lake with submerged macrophytes. Aquat Sci 79:395–406. https://doi.org/10.1007/s00027-016-0505-0
Anderson CB (2002) Understanding carbonate equilibria by measuring alkalinity in experimental and natural systems. J Geosci Educ 50:389–403. https://doi.org/10.5408/1089-9995-50.4.389
Attayde JL, van Nes EH, Araujo AI, Corso G, Scheffer M (2010) Omnivory by planktivores stabilizes plankton dynamics, but may either promote or reduce algal biomass. Ecosystems 13(3):410–420. https://doi.org/10.1007/s10021-010-9327-4
Aufdenkampe AK, Mayorga E, Raymond PA, Melack J, Doney SC, Alin SR, Aalto RE, Yoo K (2011) Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front Ecol Environ 9:53–60. https://doi.org/10.1890/100014
Barat R, Montoya T, Seco A, Ferrer J (2011) Modelling biological and chemically induced precipitation of calcium phosphate in enhanced biological phosphorus removal systems. Water Res 45(12):3744–3752. https://doi.org/10.1016/j.watres.2011.04.028
Bastviken D, Cole J, Pace M, Tranvik L (2004) Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Glob Biogeochem Cycl 18(4). https://doi.org/10.1029/2004GB002238
Bastviken D, Cole JJ, Pace ML, VandeBogert MC (2008) Fates of methane from different lake habitats: connecting whole-lake budgets and CH4 emissions. J Geophys Res Biogeosci 113:G02024. https://doi.org/10.1029/2007JG000608
Battin TJ, Luyssaert S, Kaplan LA, Aufdenkampe AK, Richter A, Tranvik LJ (2009) The boundless carbon cycle. Nat Geosci 2(9):598–600. https://doi.org/10.1038/ngeo618
Benstead JP, Leigh DS (2012) An expanded role for river networks. Nat Geosci 5:678–679. https://doi.org/10.1038/ngeo1593
Bettez ND, Groffman PM (2012) Denitrification potential in stormwater control structures and natural riparian zones in an urban landscape. Environ Sci Technol 46(20):10909–10917. https://doi.org/10.1021/es301409z
Biswas G, Kumar P, Kailasam M, Ghoshal TK, Bera A, Vijayan KK (2019) Application of Integrated Multi Trophic Aquaculture (IMTA) concept in brackishwater ecosystem: the first exploratory trial in the Sundarban, India. J Coast Res 86(SI):49–55. https://doi.org/10.2112/SI86-007.1
Blaszczak JR, Steele MK, Badgley BD et al (2018) Sediment chemistry of urban stormwater ponds and controls on denitrification. Ecosphere 9(6):e02318. https://doi.org/10.1002/ecs2.2318
Boehrer B, Schultze M (2008) Stratification of lakes. Rev Geophys 46:RG2005. https://doi.org/10.1029/2006RG000210
Boyd CE (ed) (2012) Bottom soils, sediment, and pond aquaculture. Springer Science & Business Media, UK
Branco BF, Torgersen T (2009) Predicting the onset of thermal stratification in shallow inland waterbodies. Aquat Sci 71:65–79. https://doi.org/10.1007/s00027-009-8063-3
Carbonero F, Oakley BB, Purdy KJ (2014) Metabolic flexibility as a major predictor of spatial distribution in microbial communities. PLoS ONE 9:e85105. https://doi.org/10.1371/journal.pone.0085105
Carlson RE (1977) A trophic state index for lakes. Limnol Oceanogr 22(2):361–369. https://doi.org/10.4319/lo.1977.22.2.0361
Carpenter SR (1981) Submersed vegetation: an internal factor in lake ecosystem succession. Am Nat 118(3):372–383
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8(3):559–568. https://doi.org/10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2
Chand BK, Trivedi RK, Biswas A, Dubey SK, Beg MM (2012) Study on impact of saline water inundation on freshwater aquaculture in Sundarban using risk analysis tools. Explor Anim Med Res 2:170–178
Chanda A, Das S, Bhattacharyya S et al (2020) CO2 effluxes from an urban tidal river flowing through two of the most populated and polluted cities of India. Environ Sci Pollut Res 27:30093–30107. https://doi.org/10.1007/s11356-020-09254-6
Chen Y, Dong S, Bai Y, Xu S, Yang X, Pan Z (2018) Carbon budgets from mariculture ponds without a food supply. Aquac Environ Interact 10:465–472. https://doi.org/10.3354/aei00279
Chislock MF, Doster E, Zitomer RA, Wilson AE (2013) Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nat Educ Knowl 4(4):10. https://www.nature.com/scitable/knowledge/library/eutrophication-causes-consequences-and-controls-in-aquatic-102364466/
Christensen J, Sand-Jensen K, Staehr PA (2013) Fluctuating water levels control water chemistry and metabolism of a charophyte-dominated pond. Freshw Biol 58:1353–1365. https://doi.org/10.1111/fwb.12132
Codispoti LA (2010) Interesting times for marine N2O. Science 327(5971):1339–1340. https://doi.org/10.1126/science.1184945
Cole JJ, Caraco NF (1998) Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6. Limnol Oceanogr 43(4):647–656. https://doi.org/10.4319/lo.1998.43.4.0647
Cole JJ, Pace ML, Carpenter SR, Kitchell JF (2000) Persistence of net heterotrophy in lakes during nutrient addition and food web manipulations. Limnol Oceanogr 45:1718–1730. https://doi.org/10.4319/lo.2000.45.8.1718
Collins KA, Lawrence TJ, Stander EK et al (2010) Opportunities and challenges for managing nitrogen in urban stormwater: a review and synthesis. Ecol Eng 36(11):1507–1519. https://doi.org/10.1016/j.ecoleng.2010.03.015
Condie SA, Webster IT (2002) Stratification and circulation in a shallow turbid waterbody. Environ Fluid Mech 2:177–196. https://doi.org/10.1023/A:1019898931829
Conley DJ, Paerl HW, Howarth RW et al (2009) Controlling eutrophication: nitrogen and phosphorus. Science 323:1014–1015. https://doi.org/10.1126/science.1167755
Conrad R, Noll M, Claus P, Klose M, Bastos WR, Enrich-Prast A (2011) Stable carbon isotope discrimination and microbiology of methane formation in tropical anoxic lake sediments. Biogeosciences 8:795–814. https://doi.org/10.5194/bg-8-795-2011
Corman JR, Poret-Peterson AT, Uchitel A, Elser JJ (2016) Interaction between lithification and resource availability in the microbialites of Rio Mesquites, Cuatro Ciénegas, México. Geobiology 17:176–189. https://doi.org/10.1111/gbi.12168
Crosswell JR, Anderson IC, Stanhope JW, Van Dam B, Brush MJ, Ensign S, Piehler MF, McKee B, Bost M, Paerl HW (2017) Carbon budget of a shallow, lagoonal estuary: transformations and source-sink dynamics along the river-estuary ocean continuum. Limnol Oceanogr 62:S29–S45. https://doi.org/10.1002/lno.10631
Dantas DDF, Rubim PL, de Oliveira FA, da Costa MRA, Moura CGB, Teixeira LH, Attayde JL (2019) Effects of benthivorous and planktivorous fish on phosphorus cycling, phytoplankton biomass, and water transparency of a tropical shallow lake. Hydrobiologia 829:31–41. https://doi.org/10.1007/s10750-018-3613-0
DelSontro T, Beaulieu JJ, Downing JA (2018) Greenhouse gas emissions from lakes and impoundments: upscaling in the face of global change. Limnol Oceanogr Lett 3:64–75. https://doi.org/10.1002/lol2.10073
DeVries T, Le Quéré C, Andrews O et al (2019) Decadal trends in the ocean carbon sink. Proc Natl Acad Sci 116(24):11646–11651. https://doi.org/10.1073/pnas.1900371116
Dodds WK, Bouska WW, Eitzmann JL et al (2009) Eutrophication of US freshwaters: analysis of potential economic damages. Environ Sci Technol 43(1):12–19. https://doi.org/10.1021/es801217q
Downing JA (2009) Global limnology: up-scaling aquatic services and processes to planet Earth. Verh Internat Verein Limnol 30(8):1149–1166. https://doi.org/10.1080/03680770.2009.11923903
Downing JA, Prairie YT, Cole JJ et al (2006) The global abundance and size distribution of lakes, ponds, and impoundments. Limnol Oceanogr 51:2388–2397. https://doi.org/10.4319/lo.2006.51.5.2388
Drake TW, Raymond PA, Spencer RGM (2018) Terrestrial carbon inputs to inland waters: a current synthesis of estimates and uncertainty. Limnol Oceanogr Lett 3:132–142. https://doi.org/10.1002/lol2.10055
Dubey SK, Trivedi RK, Chand BK, Mandal B, Rout SK (2017) Farmers’ perceptions of climate change, impacts on freshwater aquaculture and adaptation strategies in climatic change hotspots: a case of the Indian Sundarban delta. Environ Dev 21:38–51. https://doi.org/10.1016/j.envdev.2016.12.002
Duc N, Crill P, Bastviken D (2010) Implications of temperature and sediment characteristics on methane formation and oxidation in lake sediments. Biogeochemistry 100(1):185–196. https://doi.org/10.1007/s10533-010-9415-8
Elrod MJ (1999) Greenhouse warming potentials from the infrared spectroscopy of atmospheric gases. J Chem Educ 76(12):1702–1705. https://doi.org/10.1021/ed076p1702
Elser JJ, Schampel JH, Kyle M et al (2005) Response of grazing snails to phosphorus enrichment of modern stromatolitic microbial communities. Freshw Biol 50:1826–1835. https://doi.org/10.1111/j.1365-2427.2005.01453.x
Fee E, Hecky R, Kasian S, Cruikshank D (1996) Effects of lake size, water clarity, and climatic variability on mixing depths in Canadian Shield lakes. Limnol Oceanogr 41:912–920. https://doi.org/10.4319/lo.1996.41.5.0912
Ferrón S, Ho DT, Johnson ZI, Huntley ME (2012) Air-water fluxes of N2O and CH4 during microalgae (Staurosira sp.) cultivation in an open raceway pond. Environ Sci Technol 46(19):10842–10848. https://doi.org/10.1021/es302396j
Frankignoulle M, Bourge I, Wollast R (1996) Atmospheric CO2 fluxes in a highly polluted estuary (the Scheldt). Limnol Oceanogr 41(2):365–369. https://doi.org/10.4319/lo.1996.41.2.0365
Frost PC, Prater C, Scott AB, Song K, Xenopoulos MA (2019) Mobility and bioavailability of sediment phosphorus in urban stormwater ponds. Water Resour Res 55:3680–3688. https://doi.org/10.1029/2018WR023419
Glaz P, Bartosiewicz M, Laurion I, Reichwaldt ES, Maranger R, Ghadouani A (2016) Greenhouse gas emissions from waste stabilization ponds in Western Australia and Quebec (Canada). Water Res 101:64–74. https://doi.org/10.1016/j.watres.2016.05.060
Gorsky AL, Racanelli GA, Belvin AC, Chambers RM (2019) Greenhouse gas flux from stormwater ponds in southeastern Virginia (USA). Anthropocene 28:100218. https://doi.org/10.1016/j.ancene.2019.100218
Groffman PM, Butterbach-Bahl K, Fulweiler RW et al (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93(1–2):49–77. https://doi.org/10.1007/s10533-008-9277-5
Groszkopf T, Soyer OS (2016) Microbial diversity arising from thermodynamic constraints. ISME J 10:2725–2733. https://doi.org/10.1038/ismej.2016.49
Hansen J, Reitzel K, Jensen HS, Andersen FØ (2003) Effects of aluminum, iron, oxygen, and nitrate additions on phosphorus release from the sediment of a Danish softwater lake. Hydrobiologia 492:139–149. https://doi.org/10.1023/A:1024826131327
Harmon TC (2020) Carbon gas flux to and from inland waters: support for a global observation network. Limnology 21:429–442. https://doi.org/10.1007/s10201-020-00623-1
Hewson I, Vargo GA, Fuhrman JA (2003) Bacterial diversity in shallow oligotrophic marine benthos and overlying waters: effects of virus infection, containment, and nutrient enrichment. Microb Ecol 46:322–336. https://doi.org/10.1007/s00248-002-1067-3
Howarth R, Paerl HW (2008) Coastal marine eutrophication: control of both nitrogen and phosphorus is necessary. Proc Natl Acad Sci USA 105:E103. https://doi.org/10.1073/pnas.0807266106
Howarth RW, Marino R, Lane J, Cole JJ (1988) Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1. Rates and importance. Limnol Oceanogr 33(4 part 2):669–687
Howarth RW, Schneider R, Swaney D (1996) Metabolism and organic carbon fluxes in the tidal freshwater Hudson River. Estuaries 19:848–865. https://doi.org/10.2307/1352302
Hupfer M, Lewandowski J (2008) Oxygen controls the phosphorus release from lake sediments—a long-lasting paradigm in limnology. Int Rev Hydrobiol 93:415–432. https://doi.org/10.1002/iroh.200711054
Iglesias C, Mazzeo N, Meerhoff M et al (2011) High predation is of key importance for dominance of small-bodied zooplankton in warm shallow lakes: evidence from lakes, fish exclosures, and surface sediments. Hydrobiologia 667:133–147. https://doi.org/10.1007/s10750-011-0645-0
James WF (2011) Variations in the aluminum: Phosphorus binding ratio and alum dosage considerations for Half Moon Lake, Wisconsin. Lake Reser Manage 27:128–137. https://doi.org/10.1080/07438141.2011.572232
Jeppesen E, Brucet S, Naselli-Flores L et al (2015) Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 750:201–227. https://doi.org/10.1007/s10750-014-2169-x
Juutinen S, Rantakari M, Kortelainen P et al (2009) Methane dynamics in different boreal lake types. Biogeosciences 6(2):209–223. https://doi.org/10.5194/bg-6-209-2009
Kalff J (2002) Limnology: inland water ecosystems. Prentice-Hall, New Jersey
Kant S, Mandal NK, Dubey MK (2019) Impact of anthropogenic activities on physicochemical properties of water and sediment soil of a perennial pond of Godda District (Santal Pargana), Jharkhand. Indian J Environ Sci 23(2):69–74
Kattner G (1999) Storage of dissolved inorganic nutrients in seawater: poisoning with mercuric chloride. Mar Chem 67(1–2):61–66. https://doi.org/10.1016/S0304-4203(99)00049-3
Khalil MAK (1999) Non-CO2 greenhouse gases in the atmosphere. Ann Rev Energy Environ 24(1):645–661
Khoo KH, Ramette RW, Culberson CH, Bates RG (1977) Determination of hydrogen ion concentrations in seawater from 5 to 40 °C: standard potentials at salinities from 20 to 45‰. Anal Chem 49(1):29–34
Kitchell JF, Carpenter SR (1993) Cascading trophic interactions. In: Carpenter SR, Kitchell JF (eds) The trophic cascade in Lakes. Cambridge University Press, New York, pp 1–14
Köhler P, Nehrbass-Ahles C, Schmitt J, Stocker TF, Fischer H (2017) A 156 kyr smoothed history of the atmospheric greenhouse gases CO2, CH4, and N2O and their radiative forcing. Earth Syst Sci Data 9(1):363–387. https://doi.org/10.5194/essd-9-363-2017
Kondo J (2000) Atmospheric science near the ground surface. University of Tokyo, Japan
Kortelainen P, Rantakari M, Huttunen JT, Mattsson T, Alm J, Juutinen S, Larmola T, Silvola J, Martikainen PJ (2006) Sediment respiration and lake trophic state are important predictors of large CO2 evasion from small boreal lakes. Glob Change Biol 12(8):1554–1567. https://doi.org/10.1111/j.1365-2486.2006.01167.x
Kosten S, Roland F, Da MM, Van Nes EH, Mazzeo N, Sternberg LdSL, Scheffer M, Cole JJ (2010) Climate-dependent CO2 emissions from lakes. Glob Biogeochem Cycle 24(2):GB2007. https://doi.org/10.1029/2009GB003618
Kratzer CR, Brezonik PL (1981) A Carlson-type trophic state index for nitrogen in Florida lakes. J Am Water Resour Assoc 17(4):713–715. https://doi.org/10.1111/j.1752-1688.1981.tb01282.x
Lashof DA, Ahuja DR (1990) Relative contributions of greenhouse gas emissions to global warming. Nature 344(6266):529–531. https://doi.org/10.1038/344529a0
Lee ZMP, Poret-Peterson AT, Siefert JL et al (2017) Nutrient stoichiometry shapes microbial community structure in an evaporitic shallow pond. Front Microbiol 8:949. https://doi.org/10.3389/fmicb.2017.00949
Leflaive J, Danger M, Lacroix G, Lyautey E, Oumarou C, Ten-Hage L (2008) Nutrient effects on the genetic and functional diversity of aquatic bacterial communities. FEMS Microbiol Ecol 66:379–390. https://doi.org/10.1111/j.1574-6941.2008.00593.x
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. ORNL/CDIAC–105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee
MacIntyre S, Wanninkhof R, Chanton JP (1995) Trace gas exchange across the air-water interface in freshwater and coastal marine environments. In: Matson PA, Harriss RC (eds) Biogenic trace gases: measuring emissions from soil and water. Blackwell Science, Oxford, pp 52–97
Macrae ML, Bello RL, Molot LA (2004) Long-term carbon storage and hydrological control of CO2 exchange in tundra ponds in the Hudson Bay Lowland. Hydrol Processes 18(11):2051–2069. https://doi.org/10.1002/hyp.1461
Mandal S, Burman D, Sarangi SK, Bandyopadhyay BK, Maji B (2015) Homestead production systems in Sundarbans region of West Bengal, India–Current status and opportunities. CGIAR-CPWF
Markfort CD, Perez AL, Thill JW, Jaster DA, Porte-Agel F, Stefan HG (2010) Wind sheltering of a lake by a tree canopy or bluff topography. Water Resour Res. https://doi.org/10.1029/2009wr007759
Marotta H, Fontes MLS, Petrucio MM (2012) Natural events of anoxia and low respiration index in oligotrophic lakes of the Atlantic Tropical Forest. Biogeosciences 9:2879–2887. https://doi.org/10.5194/bg-9-2879-2012
Martinsen KT, Andersen MR, Sand-Jensen K (2019) Water temperature dynamics and the prevalence of daytime stratification in small temperate shallow lakes. Hydrobiologia 826(1):247–262. https://doi.org/10.1007/s10750-018-3737-2
Mayer T (2020) Interactions of fish, algae, and abiotic factors in a shallow, tropical pond. Hydrobiologia 847(20):4145–4160. https://doi.org/10.1007/s10750-020-04375-y
Menezes RF, Attayde JL, Vasconcelos FR (2010) Effects of omnivorous filter-feeding fish and nutrient enrichment on the plankton community and water transparency of a tropical reservoir. Freshw Biol 55:767–779. https://doi.org/10.1111/j.1365-2427.2009.02319.x
Millero FJ (1979) Thermodynamics of the carbon dioxide system in the oceans. Geochim Cosmochim Acta 59(4):661–677. https://doi.org/10.1016/0016-7037(94)00354-O
Mukhopadhyay G, Mondal D, Biswas P, Dewanji A (2004) Water quality monitoring of tropical ponds: location and depth effect in two case studies. Acta Hydrochim Hydrobiol 32(2):138–148. https://doi.org/10.1002/aheh.200300524
Natchimuthu S, Selvam BP, Bastviken D (2014) Influence of weather variables on methane and carbon dioxide flux from a shallow pond. Biogeochemistry 119(1–3):403–413. https://doi.org/10.1007/s10533-014-9976-z
Nelson CE, Carlson CA (2011) Differential response of high-elevation planktonic bacterial community structure and metabolism to experimental nutrient enrichment. PLoS ONE 6:e18320. https://doi.org/10.1371/journal.pone.0018320
Newell SE, McCarthy MJ, Gardner WS, Fulweiler RW (2016) Sediment nitrogen fixation: a call for re-evaluating coastal N budgets. Estuar Coast 39(6):1626–1638. https://doi.org/10.1007/s12237-016-0116-y
Parsons TR, Maita Y, Lalli CM (1992) A manual of chemical and biological methods for sea water analysis. Pergamon, New York, pp 101–103
Pugh TA, Lindeskog M, Smith B, Poulter B, Arneth A, Haverd V, Calle L (2019) Role of forest regrowth in global carbon sink dynamics. Proc Natl Acad Sci 116(10):4382–4387. https://doi.org/10.1073/pnas.1810512116
Purohit P, Höglund Isaksson L (2017) Global emissions of fluorinated greenhouse gases 2005–2050 with abatement potentials and costs. Atmos Chemi Phys 17:2795–2816. https://doi.org/10.5194/acp-17-2795-2017
Rantakari M, Kortelainen P (2005) Interannual variation and climatic regulation of the CO2 emission from large boreal lakes. Glob Change Biol 11(8):1368–1380. https://doi.org/10.1111/j.1365-2486.2005.00982.x
Rodrigues M, da Costa A, Attayde JL, Becker V (2016) Effects of water level reduction on the dynamics of phytoplankton functional groups in tropical semi-arid shallow lakes. Hydrobiologia 778:75–89. https://doi.org/10.1007/s10750-015-2593-6
Sañudo-Wilhelmy SA, Tovar-Sanchez A, Fu FX, Capone DG, Carpenter EJ, Hutchins DA (2004) The impact of surface-adsorbed phosphorus on phytoplankton Redfield stoichiometry. Nature 432(7019):897–901. https://doi.org/10.1038/nature03125
Sarnelle O, Cooper SD, Wiseman S, Mavuti KM (1998) The relationship between nutrients and trophic-level biomass in turbid tropical ponds. Freshw Biol 40(1):65–75. https://doi.org/10.1046/j.1365-2427.1998.00332.x
Schindler DW (1974) Eutrophication and recovery in experimental lakes:implications for lake management. Science 184(4139):897–899. https://doi.org/10.1126/science.184.4139.897
Schindler DW (2006) Recent advances in the understanding and management of eutrophication. Limnol Oceanogr 51:356–363. https://doi.org/10.4319/lo.2006.51.1_part_2.0356
Scott JT, McCarthy MJ, Gardner WS, Doyle RD (2008) Denitrification, dissimilatory nitrate reduction to ammonium, and nitrogen fixation along a nitrate concentration gradient in a created freshwater wetland. Biogeochemistry 87(1):99–111. https://doi.org/10.1007/s10533-007-9171-6
Seitzinger S, Harrison JA, Böhlke JK et al (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16(6):2064–2090. https://doi.org/10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2
Smith SV, Renwick WH, Bartley JD, Buddemeier RW (2002) Distribution and significance of small, artificial water bodies across the United States landscape. Sci Total Environ 299:21–36. https://doi.org/10.1016/S0048-9697(02)00222-X
Sobek S, Durisch-Kaiser E, Zurbrugg R, Wongfun N, Wessels M, Pasche N, Wehrli B (2009) Organic carbon burial efficiency in lake sediments controlled by oxygen exposure time and sediment source. Limnol Oceanogr 54:2243–2254. https://doi.org/10.4319/lo.2009.54.6.2243
Soininen J, Meier S (2014) Phytoplankton richness is related to nutrient availability, not to pool size, in a subarctic rock pool system. Hydrobiologia 740:137–145. https://doi.org/10.1007/s10750-014-1949-7
Søndergaard M, Peder Jensen J, Jeppesen E (2003) Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506:135–145. https://doi.org/10.1023/B:HYDR.0000008611.12704.dd
Spigel RH, Imberger J, Rayner KN (1986) Modeling the diurnal mixed layer. Limnol Oceanogr 31(3):533–556. https://doi.org/10.4319/lo.1986.31.3.0533
Starling F, Lazzaro X, Cavalcanti C, Moreira R (2002) Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: evidence from a fish kill. Freshw Biol 47:2443–2452. https://doi.org/10.1046/j.1365-2427.2002.01013.x
Taguchi VJ, Olsen TA, Natarajan P, Janke BD, Gulliver JS, Finlay JC, Stefan HG (2020) Internal loading in stormwater ponds as a phosphorus source to downstream waters. Limnol Oceanogr Lett 5(4):322–330. https://doi.org/10.1002/lol2.10155
Talling JF (2001) Environmental controls on the functioning of shallow tropical lakes. Hydrobiologia 458:1–8. https://doi.org/10.1023/A:1013121522321
Torgersen T, Branco B (2008) Carbon and oxygen fluxes from a small pond to the atmosphere: temporal variability and the CO2/O2 imbalance. Water Res Res 44(2):W02417. https://doi.org/10.1029/2006WR005634
Torres SG, Silva LHS, Rangel LM, Attayde JL, Huszar VLM (2016) Cyanobacteria are controlled by omnivorous filter-feeding fish (Nile tilapia) in a tropical eutrophic reservoir. Hydrobiologia 765:115–129. https://doi.org/10.1007/s10750-015-2406-y
Torsvik V, Øvreås L, Thingstad TF (2002) Prokaryotic diversity–magnitude, dynamics, and controlling factors. Science 296:1064–1066. https://doi.org/10.1126/science.1071698
Tranvik LJ, Downing JA, Cotner JB et al (2009) Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr 54:2298–2314. https://doi.org/10.4319/lo.2009.54.6_part_2.2298
Van Horn D, Sinsabaugh R, Takacs-Vesbach C, Mitchell K, Dahm C (2011) Response of heterotrophic stream biofilm communities to a gradient of resources. Aquat Microb Ecol 64:149–161. https://doi.org/10.3354/ame01515
van Hulzen JB, Segers R, van Bodegom PM, Leffelaar PA (1999) Temperature effects on soil methane production: an explanation for observed variability. Soil Biol Biochem 31(14):1919–1929. https://doi.org/10.1016/S0038-0717(99)00109-1
Vaquer-Sunyer R, Duarte CM (2008) Thresholds of hypoxia for marine biodiversity. Proc Nat Acad Sci 105:15452–15457. https://doi.org/10.1073/pnas.0803833105
Wanninkhof R (1992) Relationship between wind speed and gas exchange over the ocean. J Geophys Res 97(CS5):7373–7382. https://doi.org/10.1029/92JC00188
Weiss RF (1970) The solubility of nitrogen, oxygen, and argon in water and seawater. Deep-Sea Res Oceanogr Abstr 17(4):721–735. https://doi.org/10.1016/0011-7471(70)90037-9
Weiss RF (1974) Carbon dioxide in water and seawater: the solubility of non-ideal gas. Mar Chem 2(3):203–215. https://doi.org/10.1016/0304-4203(74)90015-2
Wetzel RG (2001) Limnology: lake and river ecosystems. Gulf Professional Publishing
Woolway RI, Jones ID, Hamilton DP et al (2015) Automated calculation of surface energy fluxes with high frequency lake buoy data. Environ Model Softw 70:191–198. https://doi.org/10.1016/j.envsoft.2015.04.013
Woolway RI, Jones ID, Maberly SC et al (2016) Diel surface temperature range scales with lake size. PLoS ONE 11:e0152466. https://doi.org/10.1371/journal.pone.0152466
Yang YY, Lusk MG (2018) Nutrients in urban stormwater runoff: current state of the science and potential mitigation options. Curr Pollut Rep 4(2):112–127. https://doi.org/10.1007/s40726-018-0087-7
Yvon-Durocher G, Caffrey JM, Cescatti A et al (2012) Reconciling the temperature dependence of respiration across timescales and ecosystem types. Nature 487:472–476. https://doi.org/10.1038/nature11205
Zhang Y, Zhou Y, Shi K, Qin B, Yao X, Zhang Y (2018) Optical properties and composition changes in chromophoric dissolved organic matter along trophic gradients: implications for monitoring and assessing lake eutrophication. Water Res 131:255–263. https://doi.org/10.1016/j.watres.2017.12.051
Zhimiao Z, Xinshan S, Yanping X et al (2016) Influences of seasons, N/P ratios, and chemical compounds on phosphorus removal performance in algal pond combined with constructed wetlands. Sci Total Environ 573:906–914. https://doi.org/10.1016/j.scitotenv.2016.08.148
Zohary T, Padisak J, Naselli-Flores L (2010) Phytoplankton in the physical environment: beyond nutrients, at the end, there is some light. Hydrobiologia 639:261–269. https://doi.org/10.1007/s10750-009-0032-2
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chanda, A., Das, S. (2022). CO2 Exchange Dynamics in the Household and Abandoned Ponds of the Indian Sundarbans from the Perspective of Climate Change. In: Das, S., Chanda, A., Ghosh, T. (eds) Pond Ecosystems of the Indian Sundarbans. Water Science and Technology Library, vol 112. Springer, Cham. https://doi.org/10.1007/978-3-030-86786-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-86786-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86785-0
Online ISBN: 978-3-030-86786-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)