Abstract
A microcosm study has been done to explain the disparities of nutrient variability vis-à-vis the partial pressure of carbon dioxide (CO2) in water [pCO2(water)] of brackish water pond of Frazergaunge, Namkhana block, Indian Sundarbans. The present study showed that there are a relationship between the decrease in pCO2(water) and nutrient removal from the brackish water pond system. Although there are exceptions. The present study revealed that the brackish water pond of Indian Sundarbans acted as an important source of CO2 during monsoon and pre-monsoon season although having a substantial amount of chlorophyll-a in the pond water to make the pond an autotrophic ecosystem. The present research showed that if the optimum photosynthesis can be continued in a shallow aquaculture pond, it can act as a sink of CO2. During the post-monsoon season (water column pH is high) the ecosystem acted as a CO2 sink. The present experiment suggests that the controlled lime treatment could be useful to reverse the character of this shallow aquaculture pond.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aquaculture pond
- Nutrient variability
- Photosynthetic potential
- Optimum photosysnthesis
- Indian sundarbans
13.1 Introduction
Wetlands are sink in nature of carbon dioxide in the tropical areas of the World. Globally, Mitsch et al. (2013) described that tropical wetlands acted as a net carbon sink (~830 Tg C/yr) as well occupied ~5–8% of the land area. Several tropical marshlands or wetlands contained numerous inland water bodies like aquaculture ponds, swamps, riverine wetlands, etc. In India, most of the wetlands are the source of CO2 (Panneer Selvam et al. 2014). The age and the functioning of the aquaculture pond are the main criteria to be shown the source/sink character in terms of carbon dioxide (Mitsch et al. 2013). St. Louis et al. (2000) described that anthropogenic conversion of wetlands can alter the carbon dioxide flux.
The sink/source environment of the wetlands like aquaculture ponds depends upon the evenness between the community respiration and the primary production (Natchimuthu et al. 2014). In the aquaculture ponds, available resources (nutrients, etc.) and CO2/pH coordinations play an important character in determining the trophic status (Huszar et al. 1998). Because aquaculture effluents contain high concentrations of organic matter and different nutrients. These effluents have a role to degrade the water quality as well as source/sink nature in terms of carbon dioxide. The biogeochemical processes of tropical lentic ecosystems (e.g. aquaculture ponds) are dependent on two abiotic constituents: dissolved carbon dioxide and nutrients. Dissolved carbon dioxide coexists with numerous dissociation goods like HCO3−, H2CO3, and CO32−. Therefore, primary producers can use them during the process of photosynthesis.
However, CO32− and HCO3− and are the key suppliers of the total alkalinity and the involvement of dissolved inorganic carbon (DIC) is reliant on the solubilization of particulate matter and pH of the water column (Dickson 1992). In general, tropical inland waters are supersaturated with carbon dioxide (Aufdenkampe et al. 2011). Holgerson and Raymond (2016) portrayed that several factors such as autotrophic primary production, benthopelagic respiration, and remineralization of organic materials to carbon dioxide by biological decay are responsible for variable the net biological pump of the aquaculture pond in the tropical areas. On the other hand, Song (2011) described that the dissolved nutrient pool could alter the carbon sequestration potential and phytoplankton community of aquaculture. According to Downing et al. (1993), the optimal nutrient pool is responsible to increase the utilization rate of carbon as well the increased primary production.
The present research has shown that the waters acquired from aquaculture (brackish water pond) positioned in the Frazergaunge, Namkhana Block of Indian Sundarbans. The objectives of the present research were: Change in nutrient concentration and water pCO2 with time and the seasonal changeability of the nutrient deduction concerning pCO2(water) dynamics.
To perform the present study, a microcosm experiment applied the water column’s nutrient concentration was not changed by external nutrient addition as the chief determination of the present research was to scrutinize the rate at which pre-existent nutrients are getting drained, hence, the change in pCO2(water) of the corresponding samples.
13.2 Materials and Method
13.2.1 Study Location and Sampling Strategy
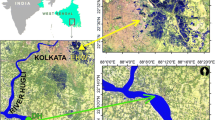
The present study was carried out in pre-monsoon, monsoon, and post-monsoon seasons of the tropical weather. Surface (water) samples were stored from the aquaculture pond (S) of Frazergaunge, Namkhana Block, Indian Sundarbans (Fig. 13.1) around 8:00 a.m., on the 20th January 2018 (post-monsoon), 12th May 2018 (pre-monsoon), and 25th August 2018 (monsoon). Two reservoirs or tanks (made: polyvinyl chloride) was positioned on the roof of the SOS’s laboratory, Jadavpur University, Kolkata. The reservoir's height was one m (the depth of the aquaculture pond is also near one meter). Both tanks were filled up with collected sample (aquaculture pond) waters. According to Biswas et al. (2015), eight microcosm bottles (5 L transparent polycarbonate sealed bottle) filled with sample (aquaculture pond surface) waters were nurtured and kept floating in the respective tanks. 01 bottle was treated as control by poisoning the microcosm bottles with HgCl2 (Kattner 1999). Each bottle was mixed moderately to avoid the bottom settlement. The microcosm experiment was carried out for eight consecutive days. Samples were collected each day from one microcosm bottle (floating) respectively. The experiment was accompanied for 15 days, however, in the present research, we have conveyed the experimental data of eight days only, because most of the nutrients were used by the end of the 8th day.
13.2.2 Analytical Methods
Water temperature and pH were observed with a micro-pH meter (Thermo Scientific, U.S.A.) with a precision of 0.1 °C and 0.001, respectively. The pH meter was calibrated daily (before sampling). During calibration, technical buffers of pH 4.01, pH 7.00, and pH 9.00 were used (at 25 °C). Conductivity was examined using a Multikit (Merck, Germany). A Digital DO meter (Mettler Toledo, USA) was used to monitor the dissolved oxygen (DO) of the samples. The precision of the DO meter was 0.01 mg/L. A standard sensor with a data logger (UWQ 8247, Li-Cor, NE, USA, precision 0.1 μ.mol/m2/s) was used to observe the underwater photosynthetically active radiation (PAR) of the samples.
Sample turbidity was checked with a digital meter (Eutech Instruments, Singapore) with a precision of 0.1 NTU. A Lux digital meter (Lutron, Czech Republic) was used to monitor the incoming solar radiation on the surface water of samples. According to Parsons et al. (1992), standard spectrophotometric procedures were used to estimate the concentration of nitrite-nitrogen (NO2–N), dissolved nitrate-nitrogen (NO3–N), ammonium–nitrogen (NH4–N), silicate, phosphate, iron with a precision of 0.01 μM. Chlorophyll-a was also measured following standard spectrophotometric procedures (Parsons et al. 1992) with a precision of 0.01 mg/m3. Total alkalinity (TAlk) was monitored using an auto-titrator (Titrando, Switzerland). Dissolved inorganic carbon (DIC) and the pCO2(water) were calculated through the CO2SYS.EXE software. Water temperature, pH, TAlk, silicate, and phosphate were used as primary data to calculate DIC and pCO2 (Lewis and Wallace 1998). According to Parsons et al. (1992), the gross primary production (GPP), net primary production (NPP), and community respiration (CR) data were recorded for 08 successive days in pre-monsoon, monsoon, and post-monsoon season.
13.3 Results
13.3.1 Seasonal Changeability (In-Situ Aquatic State) of the Aquaculture Pond
The surface water temperature of the aquaculture pond ranged from ~23 to ~33 °C. Conductivity values ranging between ~389 and ~620 μS/cm (Table 13.1). D.O. was highest in pre-monsoon months (8.8 mg/L) and lowest in the monsoon (7.3 mg/L). Turbidity was comparatively high in the monsoon compared to pre-monsoon and post-monsoon. The in-situ pH was observed as the order post-monsoon > pre-monsoon > monsoon season. The in-situ pCO2(water) surveyed the opposite order. The higher TAlk value was detected in the pre-monsoon season (5434 μmol/kg) and the lower in the post-monsoon season (2844 μmol/kg). DIC was also found to change similarly to TAlk. Minimum in-situ Chl-a concentration was detected during pre-monsoon months (34.2 mg/m3) followed by monsoon (61.2 mg/m3) and post-monsoon months (69.4 mg/m3). NO3−–N, NH4+–N, PO43−–P and SiO32− were maximum concentrations during pre-monsoon i.e. 7.8, 21.2, 19.3, and 80.5 μM. All the above-mentioned parameters were showed the following order as pre-monsoon > post-monsoon > monsoon except ammonium. Iron was observed maximum in the post-monsoon months and minimum in the monsoon.
13.3.2 Physicochemical Factors—Temporal Trend During the Experiments days (Eight days of Microcosm)
The microcosm test was carried out for 08 consecutive days in pre-monsoon, monsoon, and post-monsoon season. In the post-monsoon and pre-monsoon, pH revealed an early increase followed by a decrease from 2nd to 4th days and started increasing again from 5 to 8th days (Fig. 13.2). During monsoon season, a steady rise of pH was found till the 8th day. The highest increase of pH happened during the pre-monsoon season (Fig. 13.2). DO exhibite an initial boost during the 1st day of the test followed by a firm decrease between day one and day four which is followed by a fringe rise from 5th day to 8th day (Fig. 13.2). Net reduction of DO was considerable in the pre-monsoon season from the initial value (maximum). The DIC and TAlk trends were almost same throughout the all seasons (Fig. 13.2). DIC and Talk gradually declined in monsoon and post-monsoon months. In the pre-monsoon, both DIC and TAlk exhibited an elevating tendency from 3rd to 5th days followed by a clear decrease during pre-monsoon season (Fig. 13.2).
Positive values indicates reduction and negative values indicates increase.
13.3.3 Temporal Trend of pCO2(Water) Along with Chlorophyll and Nutrient During the 08 days (a Microcosm Study)
We have tabulated the % reduction of pCO2(water), Chl-a, and nutrients during the eight (08) days microcosm study in Table 13.2. A preliminary reduction in pCO2(water) till 2nd day was detected in all the three (03) seasons. During monsoon, pCO2(water) were more or less constant from 2nd to 6th days followed by a reduction in 7th day, whereas in pre-monsoon a sharp increase in pCO2(water) was detected between 2nd and 4th days followed by a strong reduction. An alike rise in pCO2(water) during the middle of the research was detected in post-monsoon. But it was low compared to pre-monsoon (Table 13.2). Chlorophyll-a in pre-monsoon reduced from 0th day to 4th day. During post-monsoon season, chl-a declined from 1st day to 3rd day followed by a fringe rise till 5th day and it again reduced till 8th day. Chl-a in monsoon season reduced gradually from the start till the end.
Nitrate (aquaculture pond water) reduced gradually in the pre-monsoon and post-monsoon. During monsoon, an increase was noticed between the 3rd and 4th days. The net decrease in nitrate was ~88% in both post-monsoon and pre-monsoon. In monsoon, the net reduction was only 59% (8th day) (Table 13.2). A net reduction in ammonium was noticed only in the post-monsoon (~81%), whereas in pre-monsoon and monsoon, the net reduction was less (Table 13.2). The maximum net reduction of phosphate was found in the monsoon (~86.5% by the end of the 8th day) though it showed a rise during the initial days. In the pre-monsoon and post-monsoon, phosphate concentration decreased. However, a lower reduction was noticed in the pre-monsoon (Table 13.2). A net decrease in Fe value was almost the same in all three seasons (70–75% reduction by 8th day). In contrast to other nutrients, the use of SiO32− was noticed less (pre-monsoon ~4% and post-monsoon ~50%).
13.3.4 Link Between pCO2(Water), chl-a and Nutrients
All the nutrients of this microcosm experiment revealed a positive relationship with chl-a in pre-monsoon, monsoon, and post-monsoon season. Most of all were statistically significant (exceptions are there). Nitrate showed a positive (strong) correlation (R2 = 0.96) during monsoon. There was no significant relationship in the post-monsoon. On the contrary, ammonium displayed a strong correlation in the pre-monsoon. It was not significant during monsoon and post-monsoon. SiO32− value also showed a similar relationship like ammonium concentration with chl-a value. Iron concentration revealed a significant (positive) relationship with chl-a in all the three seasons. Alike, ammonium and silicate, phosphate also showed a significant (positive) correlation with chl-a only during pre-monsoon. Comparison to chl-a, pCO2(water) also showed a positive correlation with the respective nutrients. But the relationship was not statistically important all the time. The R2 values were much less than the detected chl-a value.
13.4 Discussion
There are several studies on the coastal water or estuarine water of Indian Sundarbans regarding CO2 emission or uptake. But there is no study on the carbon dynamics of the aquaculture pond. The present study is the first approach to reveal that the brackish pond water acted as a source of CO2 in the months of pre-monsoon and monsoon in respect to pCO2(water).
During the post-monsoon, the surface waters showed lower pCO2(water) values (under-saturated concerning atmospheric CO2 level). Bhattacharyya et al. (2018) also observed the same trend while working the aquaculture ponds of East Kolkata Wetland. During pre-monsoon and monsoon season, the pCO2(water) level was reduced (drastically) during the eight-day experiments (even below the atmospheric CO2 level). The present study demonstrated that the photosynthetic potential of the aquaculture pond of Indian Sundarbans is high. That means the pond system must be in a net autotrophic state. But in reality, the system is net heterotrophic. Moriarty (1997) stated that there is a source of organic matter to make the pond water system net heterotrophic in this type of situation. While pCO2(water) showed decreasing trend throughout the microcosm, chl-a increased in corresponding. But the chl-a was decreased steadily in post-monsoon and monsoon season. Lindsey et al. (2010) also observed the same trend and explained the lower life cycles of phytoplankton standing stock in the aquaculture pond system. During pre-monsoon months, the chl-a reduced until the fourth day, it rose significantly at the final stage of the microcosm study. Higher surface temperature and lesser light penetration in the water column are the common features during pre-monsoon months, thus lower concentration of chl-a present in the surface water. Roy and Pal (2015) also revealed a similar trend during pre-monsoon months. They have found a lesser amount of phytoplankton in the same season.
During monsoon season, surface water of brackish water pond act as a source of CO2 under in-situ and microcosm experiment till the 7th day. The higher amount of organic load and heterotrophic decomposition are the principal reason behind the above-mentioned observation. Boyd and Tucker (2012) described that a higher amount of DIC produced during monsoon months in the surrounding areas of aquaculture ponds and makes this pond net heterotrophic. They also identified diatoms as a controller of photosynthetic activity during monsoon. But DIC makes the aquaculture pond a source of CO2 in-situ conditions. According to Giordano et al. (2005), the present research experienced sufficient CO2 consumption and nutrient removal during the microcosm experiment.
During post-monsoon, because of the higher amount of sunlight and lower turbidity of pond water, the photosynthesis occurred at its higher level and reduced the pCO2(water). In most of the case, liming activities are performed in the aquaculture ponds of Indian Sundarbans during post-monsoon months. Due to these activities, the pH values of the pond water are high in this season. According to Wurts and Durborow (1992) nutrients are more available at higher pH. All the above-mentioned statements explained the higher photosynthesis rate of the aquaculture pond water during post-monsoon in Indian Sundarbans. Thus, pond water act as a sink of carbon-di-oxide.
The key finding of the present research is the higher pH value of the aquaculture pond water during post-monsoon. This is a traditional practice of local fishermen to perform lime treatment in their pond to lower the turbidity in Indian Sundarbans. This traditional practice is the key reason behind the low pCO2(water) values in this season. Finally, it is understood that nutrient removal make the aquaculture pond as a sink of carbon di-oxide in all three seasons from the microcosm experiment.
In conclusion, it was found that the pCO2(water) variability of the aquaculture pond was generally dependent on the photosynthetic potential of pond water and the environments which hamper or enhance the frequency of photosynthesis in Indian Sundarbans. For the management purpose, it can be suggested that the lime treatment in the fixed interval could increase the nutrient deduction as well as minimize the CO2 as a source from the aquaculture pond of the Indian Sundarbans.
References
Aufdenkampe AK, Mayorga E, Raymond PA, Melack JM, Doney SC, Alin SR (2011) Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front Ecol Environ 9(1):53–60. https://doi.org/10.1890/100014
Bhattacharyya S, Chanda A, Das S, Akhand A, Pattanaik S, Choudhury SB (2018) Nutrient removal Vis-a-Vis change in partial pressure of CO2 during post-monsoon season in a tropical lentic and lotic aquatic body: a comparative study. Earth Syst Environ 2:233–245
Biswas H, Jie J, Li Y, Zhang G, Zhu ZY, Wu Y (2015) Response of a natural Phytoplankton community from the Qingdao coast (Yellow Sea, China) to variable CO2 levels over a short-term incubation experiment. Curr Sci 108:1901–1909
Boyd CE, Tucker CS (2012) Pond aquaculture water quality management. Springer, Boston. https://doi.org/10.1007/978-1-4615-5407-3
Dickson AG (1992) The determination of total dissolved inorganic carbon in sea water using extraction/coulometry: the first stage of a collaborative study (No. DOE/RL-01830T-H14). Scripps Institution of Oceanography, Marine Physical Lab, San Diego, U.S
Downing JP, Meybeck M, Orr JC, Twilley RR, Scharpenseel HW (1993) Land and water interface zones. Water Air Soil Pollut 70(1–4):123–137
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Ann Rev Plant Bio 56:99–131
Holgerson MA, Raymond PA (2016) Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat Geosci 9(3):222–226
Huszar VL, Silva LHS, Domingos P, Marinho M, Melo S (1998) Phytoplankton species composition is more sensitive than OECD criteria to the trophic status of three Brazilian tropical lakes. In: Alvarez-Cobelas M, Reynolds CS, Sanchez-Castillo P, Kristiansen J (eds) Phytoplankton and trophic gradients. Springer, Dordrecht, pp 59–71. https://doi.org/10.1023/A:1017047221384
Kattner G (1999) Storage of dissolved inorganic nutrients in seawater: poisoning with mercuric chloride. Mar Chem 67(1–2):61–66
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations, ORNL/CDIAC-105. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy
Lindsey R, Scott M, Simmon R (2010) What are Phytoplankton. NASA’s Earth Observatory (online). http://earthobservatory.nasa.gov/Library/phytoplankton. Accessed 22 October 2020
Mitsch WJ, Bernal B, Nahlik AM, Mander U, Zhang L, Anderson CJ (2013) Wetlands, carbon, and climate change. Landsc Ecol 28(4):583–597
Moriarty DJ (1997) The role of microorganisms in aquaculture ponds. Aquaculture 151(1–4):333–349
Natchimuthu S, Selvam BP, Bastviken D (2014) Influence of weather variables on methane and carbon dioxide flux from a shallow pond. Biogeochemistry 119(1–3):403–413
Panneer Selvam B, Natchimuthu S, Arunachalam L, Bastviken D (2014) Methane and carbon dioxide emissions from inland waters in India–implications for large scale greenhouse gas balances. Glob Change Biol 20(11):3397–3407
Parsons TR, Maita Y, Lalli CM (1992) A manual of chemical and biological methods for sea water analysis. Pergamon, New York
Roy AS, Pal R (2015) Planktonic Cyanoprokaryota and Bacillariophyta of East Kolkata Wetlands Ecosystem, a Ramsar Site of India with reference to diversity and taxonomic study. J Algal Biomass Util 6(3):47–59
Song C (2011) Carbon dynamics in aquatic ecosystems in response to elevated atmospheric CO2 and altered nutrients availability. Doctoral dissertation, University of Kansas, U.S
St. Louis VL, Kelly CA, Duchemin E, Rudd JW, Rosenberg DM, (2000) Reservoir surfaces as sources of greenhouse gases to the atmosphere: a global estimate: reservoirs are sources of greenhouse gases to the atmosphere, and their surface areas have increased to the point where they should be included in global inventories of anthropogenic emissions of greenhouse gases. AIBS Bull 50(9):766–775
Wurts WA, Durborow RM (1992) Interactions of pH, carbon dioxide, alkalinity and hardness in fishponds. Southern Regional Aquaculture Center publication no. 464. Liming Fishponds 3. Auburn University
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Das, S. (2022). Portraying the Nutrient Variability with Relation to Variation in Partial Pressure of Carbon Dioxide in an Aquaculture Pond of Indian Sundarbans. In: Das, S., Chanda, A., Ghosh, T. (eds) Pond Ecosystems of the Indian Sundarbans. Water Science and Technology Library, vol 112. Springer, Cham. https://doi.org/10.1007/978-3-030-86786-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-86786-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86785-0
Online ISBN: 978-3-030-86786-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)