Abstract
Corticotropin-releasing hormone (CRH) is the master regulator of the hypothalamic–pituitary–adrenal (HPA) axis. CRH is highly expressed in parvocellular neurons of the paraventricular nucleus of the hypothalamus (PVN). PVNCRH neurons are primarily recognized for their role in launching the endocrine stress response. These neurons receive multiple inhibitory and excitatory afferents monitoring external environmental threats and internal physiological states. The integrated information is translated into hormonal, autonomic and behavioural responses aiming at maintaining homeostasis and improving chances of survival. The regulation of the HPA axis is closely associated with glucocorticoid-mediated feedback mechanisms but, in recent years, it has become evident that CRH and its high-affinity CRH receptor type 1 are constituents of a microcircuit within the PVN directly involved in HPA axis regulation. Furthermore, our perception of CRHPVN neurons is currently changing as we have witnessed several exciting studies demonstrating that PVNCRH neurons directly engage in rapid behavioural responses in reaction to stressful stimuli beyond their classical role attributed to neuroendocrine regulation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Corticotropin-releasing hormone
- Corticotropin-releasing factor
- Hypothalamic–pituitary–adrenal axis
- Paraventricular nucleus
- Parvocellular neuron
- Stress
- Hypothalamus
1 Introduction
The discovery of corticotropin-releasing hormone (CRH; also designated corticotropin-releasing factor, CRF) as the principal regulator of the hypothalamic–pituitary–adrenal (HPA) axis was a major breakthrough in neuroendocrinology (Vale et al. 1981). CRH is part of a neuropeptide family comprising urocortin 1 (UCN1), UCN2 and UCN3. CRH is synthesized as a precursor and matures into its 41-amino acid biologically active form via proteolytic processing and C-terminal amidation en route to its storage and release sites at axon terminals. The physiological activity of CRH and related peptides is conveyed by two heptahelical receptors from the family B1 of secretin-like G protein-coupled receptors (GPCRs)—CRH receptor type 1 (CRHR1) and CRHR2 (Deussing and Chen 2018).
Intracerebroventricular application of CRH in rodents promotes behavioural and autonomic reactions reminiscent of a response to natural threats. CRH treatment induces general arousal and anxiogenic behaviour in various behavioural paradigms (Dunn and Berridge 1990). Simultaneously, CRH activates physiological reactions, such as increased heart rate, blood pressure, plasma glucose and oxygen consumption, which are indicative of augmented sympathoadrenal outflow (Brown et al. 1982; Fisher and Brown 1991).
Soon after their discovery, clinical observations supported an involvement of CRH and CRHR1 in stress-related diseases, including mood and anxiety disorders. Patients suffering from major depression present with HPA axis disturbances such as elevated plasma cortisol and adrenocorticotropic hormone (ACTH) levels as well as impaired negative feedback regulation (Holsboer 2000). Post mortem studies showed increased levels of CRH in the cerebrospinal fluid, an upregulation of CRH in the PVN and a compensatory reduction of CRHR1 binding sites in the prefrontal cortex of suicide victims (Arato et al. 1989; Nemeroff et al. 1988; Raadsheer et al. 1994). Remarkably, successful antidepressant treatment can restore HPA axis function and CSF levels of CRH (Ising et al. 2007). A wealth of preclinical and clinical findings has implicated CRHR1 as a promising target for the next generation of antidepressants and anxiolytics (Holsboer 1999; Sanders and Nemeroff 2016). However, after an initial successful study, all subsequent clinical trials failed to demonstrate sufficient efficacy and this has stalled any further development of CRHR1 antagonists (Griebel and Holsboer 2012; Zobel et al. 2000). Nevertheless, the CRHR1 remains an interesting target and the implementation of personalized approaches might help to revisit potential therapeutic strategies based on the CRH/CRHR1 system (Spierling and Zorrilla 2017).
Genetic mouse models underscore the implication of the CRH/CRHR1 system in anxiety-related behaviour (Timpl et al. 1998; Smith et al. 1998), which is independent of the HPA axis disturbances present in CRHR1 knockout mice (Muller et al. 2003). Importantly, mouse models have also revealed that the system is more complex than originally anticipated. For example, CRHR1 is capable of modulating anxiety-related behaviour bidirectionally depending on its cellular localization in glutamatergic or dopaminergic neurons (Refojo et al. 2011; Henckens et al. 2016). Moreover, the effects of CRH are influenced by the individual’s previous experience. Severe stress exposure can, for example, switch the response to CRH from appetitive to aversive (Lemos et al. 2012).
The current progress in basic neuroscience research provides refined tools for in-depth analysis and manipulation of complex ligand/receptor systems from the molecular to the neurocircuit level. CRH effects are traditionally segregated functionally and spatially: in the context of the HPA axis, CRH is regarded as a classical hypothalamic releasing hormone while CRH is considered as a neuromodulator when engaged in neurotransmission and interneuronal communication. Along these lines, it has been a long-standing perception, virtually a dogma, that hypothalamic CRH primarily regulates the activity of the neuroendocrine stress system whereas the modulation of stress-related behaviours is attributed to extrahypothalamic CRH sources.
In this chapter we will focus on the hypothalamic CRH/CRHR1 system, its distribution, physiology and regulation. We will particularly highlight recent findings which provide ample evidence for the convergence of neuroendocrine, autonomic and behavioural responses to stress onto CRH-related neurocircuits within the paraventricular hypothalamic nucleus.
2 Hypothalamic Expression of CRH
The antibody-based detection of neuropeptides is frequently hindered by their comparably low baseline expression accompanied by rapid clearance from the neuronal soma via axonal transport in large dense-core vesicles. Experimentally, this can be overcome using a colchicine pretreatment, which blocks vesicular transport and allows visualization of peptide accumulation in the soma (Merchenthaler et al. 1982; Cummings et al. 1983). Application of colchicine, however, is itself a stressor and might also affect the expression of stress-responsive neuropeptides such as CRH (Alonso et al. 1986). Therefore, mRNA in situ hybridization (ISH) has proven to be a valuable and sensitive complementary approach to address the spatial CRH expression pattern in the brain at baseline and following stress (Keegan et al. 1994).
In Vivo Access to CRH Neurons
In the past decade, we have witnessed the establishment of rodent genetic tools, i.e., CRH reporter mice and rats, which provide a previously unmet level of sensitivity to understand peptide expression and distribution of CRH+ neurons in the rodent brain. Direct reporters have been developed, for example, by integrating a fluorescent protein into the CRH gene. Thus, reporter gene expression reflects the current state of CRH production. However, the relatively low expression level usually requires amplification by antibody staining (Kono et al. 2017; Alon et al. 2009). Indirect reporter mice are based on the expression of Cre recombinase under the control of the CRH promoter. CRH+ neurons can be visualized by breeding general Cre reporter mice or by local application of viral vectors expressing Cre-dependent reporters (Taniguchi et al. 2011; Krashes et al. 2014; Pomrenze et al. 2015; Itoi et al. 2014). Indirect reporters provide the highest sensitivity, as the reporter is usually driven by a strong promoter. However, this approach cannot discriminate between current and legacy expression, which is caused by any transient activation of the reporter, e.g., during developmental stages resulting in permanent reporter gene expression. In addition, a significant time lag between induction of CRH expression and detection of the indirect reporter has to be considered in experiments addressing induction of de novo expression of CRH. It is of note that the regulatory elements of the CRH gene are not yet fully understood. Thus, knock-in strategies have proven their superiority compared to transgenic strategies involving short promoter fragments or even bacterial artificial chromosome (BAC)-based constructs when carefully comparing the exogenous with the endogenous expression pattern (Chen et al. 2015; Dedic et al. 2018a).
Within the hypothalamus, CRH expression is dominated by the PVN but cells expressing CRH are also found in the lateral (LPOA) and medial preoptic area (MPOA), the lateral (LH) and dorsomedial hypothalamus (DMH), the perifornical area (PFA) and in scattered neurons of the posterior periventricular zone and the suprachiasmatic nucleus (SCN) (Keegan et al. 1994; Merchenthaler et al. 1982; Cummings et al. 1983). Reporter mice confirmed previously identified hypothalamic expression and identified additional CRH+ neurons in the anterior (AHA) and posterior hypothalamic area (PHA), the ventromedial hypothalamus (VMH) as well as the arcuate nucleus (Arc) (Walker et al. 2019; Peng et al. 2017) (Fig. 9.1).
CRH-expressing neurons in the murine hypothalamus. Representative coronal brain sections covering the murine hypothalamus. CRH-expressing somata are illustrated as filled orange circles. CRH neurons in extrahypothalamic areas are not depicted. Abbreviations: AHA anterior hypothalamic area, DMH dorsomedial hypothalamus, LA lateral hypothalamus, LPOA lateral preoptic area, MPOA median preoptic area, PFA perifornical area, PHA posterior hypothalamic area, PVN paraventricular nucleus of the hypothalamus, SCN suprachiasmatic nucleus
Hypothalamic CRH neurons display rather small somatic volumes with simple dendritic branches and present only limited numbers of spines (Wang et al. 2021). A molecularly more comprehensive characterization has been obtained by single-cell RNA sequencing, demonstrating that hypothalamic CRH is present in different inhibitory and excitatory neuronal populations (Romanov et al. 2017b; Kim et al. 2020). CRH was primarily found in GABAergic neurons either positive for LIM homeobox 6 or G-protein coupled receptor 15-like. In another study, CRH expression defined a subcluster of neurotensin-positive GABAergic neurons in the hypothalamus (Mickelsen et al. 2019). In addition, CRH was identified in two populations of glutamatergic neurons, confirming previous results that had shown that PVNCRH neurons co-express the vesicular glutamate transporter 2, similar to CRH neurons in the piriform cortex (Dabrowska et al. 2013; Dedic et al. 2018b). Further evidence for the PVN-restricted presence of CRH in glutamatergic neurons originates from conditional CRH knockout mice using the Dlx5/6-Cre driver, which directs Cre-mediated recombination to forebrain GABAergic neurons. These knockout mice lack CRH in the entire hypothalamus but spare CRH expression in the PVN and thus exhibit normal HPA axis function (Dedic et al. 2018b). Reporter mice in combination with immunohistochemistry revealed that PVNCRH neurons are unique with regard to their co-expression of other peptides. About 30% of CRH neurons contain neurotensin and 20% enkephalin, while only a small fraction of parvocellular PVNCRH neurons is also positive for cholecystokinin, galanin or vasoactive intestinal polypeptide (Ceccatelli et al. 1989). There is no overlap with thyrotropin-releasing hormone or somatostatin and only limited co-expression with oxytocin and arginine vasopressin (AVP) (Wamsteeker Cusulin et al. 2013). Under conditions of low circulating corticosterone, however, the overlap with AVP increases significantly, which is in line with the potentiation of ACTH secretion by AVP co-release (Gillies et al. 1982; Rivier and Vale 1983; Muller et al. 2000).
The widespread distribution of CRH in different populations of hypothalamic neurons is in accordance with observations in the hippocampus and cortex suggesting that the production of CRH reflects a functional modality that is acquired by different types of neurons, rather than a classifier defining neuronal identity (Gunn et al. 2019; Kubota et al. 2011; Romanov et al. 2017a).
3 Connectivity of Hypothalamic CRH Neurons
The afferent and efferent connections of the vast majority of CRH+ neurons in the hypothalamus have not been explored yet using modern anterograde and retrograde tracing tools. Only parvocellular PVNCRH neurons have been studied in greater detail in this regard. PVNCRH neurons project to the external zone of the median eminence to release their peptide cargo to the portal vasculature (Lennard et al. 1993). Whole-brain mapping of afferents of PVNCRH neurons by rabies virus-mediated trans-synaptic retrograde tracing using CRH-ires-Cre mice revealed that PVNCRH neurons integrate information from a plethora of different stress- and reward-related brain areas (Fig. 9.2). PVNCRH neurons receive excitatory inputs from several stress-related brain areas, such as the prefrontal cortex (PFC), paraventricular thalamus (PVT), ventral hippocampus (vHPC) and parabrachial nucleus (PBN), to rapidly activate PVNCRH neurons. At the same time, several nuclei, such as the lateral septum (LS), raphe magnus nucleus (RMg) and bed nucleus of the stria terminalis (BNST), send direct long-range GABAergic inputs onto PVNCRH neurons. Together, these presynaptic stress and reward circuits provide the means to bidirectionally modulate dynamics and plasticity of PVNCRH neurons (Fig. 9.2) (Yuan et al. 2019).
Presynaptic partners of PVNCRH neurons. Rabies virus (RV)-mediated trans-synaptic retrograde tracing. (a) Upper bar graph illustrates brain-wide distribution of neurons labelled by retrograde trans-synaptic tracing. Lower bar graph illustrates the proportion of rabies virus and glutamate decarboxylase 1 (GAD1)-positive neurons in each input nucleus. (b) A whole-brain model of selected monosynaptic afferents onto PVNCRH neurons. Colours of the arrow encode the proportion of PVNCRH-projecting GABAergic neurons in each input nucleus. Blue, 100%, magenta, 0%. (Modified with permission from Yuan et al. 2019)
Efferent projections of PVNCRH neurons have been characterized by injecting AAVs expressing a Cre-dependent anterograde tracer into the PVN of CRH-ires-Cre mice. As expected, this approach revealed massively labelled axon terminals within the median eminence but also moderate to dense projections in multiple sites throughout the brain. Abundant PVNCRH fibres were identified in the cingulate cortex, anterior and medial amygdala, LS, subnuclei of the BNST and nucleus accumbens, as well as in multiple intrahypothalamic sites (Zhang et al. 2017). In contrast, Fuzesi and colleagues detected projections of PVNCRH neurons to the LH only, which target an electrophysiological defined population of LH neurons (Fuzesi et al. 2016). Whether these neurons are identical to a population of hypocretin (HCRT)-expressing neurons, which were identified by retrograde trans-synaptic rabies tracing as monosynaptically innervated by PVNCRH neurons, remains to be investigated (Li et al. 2020).
4 CRH—Master Regulator of the HPA Axis
CRH controls the daily rhythm of ACTH and glucocorticoid secretion and regulates the stress-induced activation of the HPA axis (Herman et al. 2003). CRH is synthesized in parvocellular neurons in the dorsomedial aspect of the PVN, which integrate excitatory and inhibitory afferents to convey a net secretory signal to the anterior pituitary (Herman et al. 2003). CRH is stored in large dense-core vesicles (LDCVs) and transported to nerve terminals located in the external zone of the median eminence (Merchenthaler et al. 1984). Exocytosis and release of LDCV content is regulated by the formation of a SNARE complex, which allows fusion with the cell membrane (Pang and Sudhof 2010). In the median eminence, CRH is co-localized with the calcium-sensing protein secretagogin (SCGN), which has been found in neuroendocrine cells including parvocellular neurons of the PVN (Mulder et al. 2009). SCGN directly interferes with CRH release, thus limiting hormonal responses to stress (Romanov et al. 2015). After its release, CRH reaches the anterior pituitary via the hypothalamic–pituitary portal vasculature, binds to CRHR1 present on corticotropes and triggers the secretion of ACTH into the circulation. In turn, ACTH stimulates the synthesis and release of glucocorticoids from the zona fasciculata of the adrenal gland. Cortisol (in primates) and corticosterone (in rodents) are the key effectors of the stress response and are indispensable for successful recovery and adaptation to internal or external threats to homeostasis (de Kloet et al. 2005). Glucocorticoid effects are mediated via two nuclear receptors: the glucocorticoid and the mineralocorticoid receptor. These play also a fundamental role in negative feedback inhibition of the HPA axis to keep glucocorticoid levels in a tolerable range involving genomic and non-genomic mechanisms (Tasker et al. 2006). In addition, HPA axis activity is controlled on the level of the PVN by changes in neuronal plasticity. Plasticity is shaped by afferents of local stress-responsive GABAergic neurons (Herman et al. 2002) and by long-lasting suppression of N-methyl-D-aspartate (NMDA) receptors, which converts parvocellular neurons into a primed state and thereby increases hormonal responses to a novel stressor (Kuzmiski et al. 2010; Bains et al. 2015). PVNCRH neurons show tonic activity in the absence of external threat stimuli. PVNCRH neurons adapt to homotypic stressors but this adaptation is not mediated by negative feedback of corticosterone. Although negative corticosterone feedback suppresses ACTH secretion, it has only a minor effect on CRH neuron activity. Accordingly, corticosterone inhibits the tonic activity of PVNCRH neurons but not stress-induced activity (Kim et al. 2019b).
5 A CRH-CRHR1 Microcircuit Within the PVN Controls HPA Axis Activity
The establishment of BAC-transgenic CRHR1-GFP reporter mice revealed potentially CRHR1-expressing neurons in the PVN (Justice et al. 2008). These CRHR1-GFP neurons are responsive to CRH applied by bath application but also to local CRH release induced by photo-stimulation, indicating the presence of functional CRHR1. These neurons resemble a unique population of PVN neurons as they do not express any classical markers of magnocellular or parvocellular neurons but rather possess characteristics of preautonomic neurons that project to brainstem nuclei (Ramot et al. 2017). The majority of PVN CRHR1-GFP neurons are inhibitory, making local GABAergic synapses within the PVN. Additionally, glutamatergic CRHR1-GFP neurons exist and make long-range projections to the LS, BNST, periaqueductual grey (PAG), parabrachial nucleus (PB) and the nucleus of the solitary tract (NTS). Interestingly, a significant portion of CRHR1-GFP neurons express GABAergic as well as glutamatergic markers. PVNCRH neurons make only partially synaptic contacts with CRHR1-GFP neurons but signalling seems to be also possible by CRH release involving volume transmission (Ramot et al. 2017). CRHR1-GFP neurons are positively regulated by glucocorticoids, while low glucocorticoids, as present in CRHR1-knockout mice or adrenalectomized mice, downregulate GFP expression. CRHR1 in the PVN is co-expressed with Sim1, allowing conditional PVN-specific inactivation using Sim1-Cre driver mice. Basal corticosterone levels are unaffected in CRHR1CKO-Sim1 mice compared to control mice. However, chronic social defeat stress resulted in decreased basal corticosterone levels after the end of the stressor. These chronically stressed mice also showed reduced anxiety-related behaviour (Ramot et al. 2017). Selective ablation of PVNCRHR1 neurons by selective expression of diphtheria toxin resulted in HPA axis hyperactivity due to reduced feedback inhibition of PVNCRH neurons (Jiang et al. 2018). These results revealed an intra-PVN CRH-CRHR1 microcircuit (Fig. 9.3) introducing a previously unrecognized level of HPA axis activity (Jiang et al. 2019).
The intra-PVN CRH/CRHR1 microcircuit. Parvocellular CRH neurons signal to CRHR1 neurons in a short negative feedback microcircuit. Released CRH activates recurrent inhibition via neighbouring GABAergic CRHR1+ neurons. Abbreviations: AP anterior pituitary, ME median eminence, PVN paraventricular nucleus of the hypothalamus
6 PVNCRH Neurons Are Activated by Aversive Stimuli and Regulate Stress-Induced Behaviours
Parvocellular PVNCRH neurons have classically and almost exclusively been acknowledged for their role in orchestrating the neuroendocrine stress response via the HPA axis. PVNCRH neurons have been demonstrated to control autonomic outflow. For example, PVNCRH neurons project to sites controlling autonomic function and selective stimulation of PVNCRH terminals in the NTS increases blood pressure (Wang et al. 2019). Early experiments involving electrical stimulation suggested that these cells may also regulate complex behaviours but they gained only limited attention (Kruk et al. 1998). Mice show an immediate reaction to acute stressors, e.g., a foot-shock, reflected by the expression of multiple behaviours, which differ in their duration depending on the encountered stressor and the animals’ environmental context (Fuzesi et al. 2016). Interestingly, instantaneous optogenetic inhibition of PVNCRH neurons following an acute stressor switched the pattern of stress-induced behaviours from self-grooming to rearing and walking. Accordingly, photoactivation of PVNCRH neurons had the opposite effect, reflected by increased grooming and decreased rearing behaviour. These behavioural alterations were independent of the corticosterone surge induced by optogenetic stimulation of PVNCRH neurons. Moreover, the clear context-dependence of stress-induced behavioural profiles was blunted by stimulation of PVNCRH neurons. Double retrograde tracing using retrobeads and fluorogold revealed that individual PVNCRH neurons project to the median eminence but at the same time send axon collaterals to other brain structures, particularly the LH. Photo-stimulation of those PVNCRH fibres present in the LH had behavioural consequences similar to those seen with direct stimulation of PVNCRH neurons (Fuzesi et al. 2016).
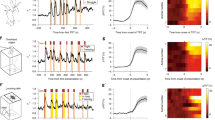
The response of PVNCRH neurons has been addressed in detail by in vivo calcium imaging using fibre photometry. GCaMP6s expressing PVNCRH neurons are immediately activated by a broad array of exteroceptive (e.g. forced swimming, predator odour) and interoceptive (e.g. gastric malaise, food deprivation) stressors/aversive stimuli (Fig. 9.4). On the contrary appetitive or rewarding stimuli such as accessible food or sweet solution rapidly suppressed the activity of PVNCRH neurons (Kim et al. 2019a; Yuan et al. 2019). PVNCRH neurons also responded to social stimuli (Fig. 9.4). Depending on the stimulus, neurons were either suppressed, e.g. when a female mouse was presented with a pup, or activated, e.g. when a mouse was attacked by an aggressive intruder. These bidirectional changes in PVNCRH neuron activity suggest that these neurons convey information with regard to the valence of the encountered stimulus. Accordingly, optogenetic activation of these PVNCRH neurons induces place aversion while optogenetic inhibition of the same neuronal population promotes place preference. Furthermore, photo-stimulation or -inhibition is able to blunt natural preferences (e.g. to food) or aversions (e.g. to LiCl injection), respectively (Kim et al. 2019a).
Activation and Inhibition of PVNCRH neurons. GCaMP6s was selectively expressed in PVNCRH neurons and activity was recorded by fibre photometry. (a) Cartoon illustrating the forced swim test (FST) and a representative trace illustrating an increased GCaMP6 signal recorded from PVNCRH neurons during FST (red bar, above) and decreased activity while back in home cage (white bar). Behavioural epochs, swimming (light blue) and climbing (blue) are annotated in colour-coded shaded bars. The plot shows combined data from all animals tested aligned to the start and end of FST, and the following rest in the home cage. (b) Cartoon illustrating the tail restraint-test (TRT) and a representative trace showing increased GCaMP6 signal from PVNCRH neurons during restraint (red bars, above). Colour-coded shaded bars depict the periods during which mice were chased by a hand (grey) and struggled (beige). The plot shows a peri-event time histogram plot across all tested animals aligned to the start of TRT. (c) Cartoon illustrating presentation of freely accessible chow in a chamber. Representative traces showing GCaMP6 signal from PVNCRH neurons of ad libitum-fed and 22-h fasted animals exposed to a non-food object (grey bar), followed by chow pellet (orange bar). Shaded bars depict the epochs during which mice investigated the non-food object (grey) and consumed the chow pellet (orange). The plot across all animals was aligned to the introduction of chow. (Modified with permission from Kim et al. 2019a)
Moreover, when a rewarding stimulus is presented in conjunction with a stressor, the stress response of PVNCRH neurons is significantly decreased. Similarly, rewarding sucrose-solution is able to diminish signs of a stress response that was artificially induced by direct chemogenetic stimulation of PVNCRH neurons, i.e., reducing the elevated self-grooming, anxiety and corticosterone release. Mechanistically, repeated stress upregulates glutamatergic neurotransmission and induces NMDA receptor-dependent burst firing. In this context, reward consumption is able to rebalance synaptic homeostasis by increasing inhibition and decreasing excitation resulting in abrogation of burst firing (Yuan et al. 2019).
PVNCRH neurons have been further interrogated with respect to their role in innate defensive behaviours using a looming shadow paradigm as threat (Daviu et al. 2020). This advancing threat leads to an activation of PVNCRH neurons and induces escape behaviour. Optogenetic inhibition switches defensive behaviours from escape to freezing, suggesting that PVNCRH neurons control the balance between passive and active response strategies. Interestingly, PVNCRH neurons generate a preparatory signal anticipating escape behaviour, i.e., CRH neurons are activated before the initiation of escape behaviour. Furthermore, this anticipatory signal is sensitive to stressful stimuli that have high or low levels of controllability. Stressors with high outcome control increase PVNCRH anticipatory activity and thus escape behaviour. In contrast, stressors that do not allow control prevent the occurrence of anticipatory activity and subsequent escape behaviour (Daviu et al. 2020).
Another intriguing finding is the capacity of PVNCRH neurons to transmit signals of distress among individuals (Sterley et al. 2018). Exposure to acute stress alters the short-term plasticity of PVNCRH neuron afferents at glutamatergic synapses. Interestingly, similar changes occur at the synaptic level when naïve mice interact with a previously stressed cage-mate. The transmission of synaptic changes does not even require direct interaction between individuals but can be transferred via currently unknown chemosensory signals (Sterley et al. 2018).
7 Hypothalamic CRH Promotes Hyperarousal and Anxiogenic Behaviour
Conditional and constitutive CRHR1-knockout mice consistently exhibit reduced anxiety-related behaviour (Timpl et al. 1998; Muller et al. 2003; Smith et al. 1998). Surprisingly, constitutive CRH knockout mice did not recapitulate the anxiety-related phenotype of CRHR1-mutant mice (Muglia et al. 1995; Muglia et al. 2001). The underlying reasons for the observed discrepancy are unclear but different hypotheses have been put forward: (1) Early inactivation of CRH during embryonic development might induce compensatory mechanisms, including the functional substitution by UCNs or other yet undiscovered family members. (2) The constitutive disruption of CRH might entail pleiotropic effects, which together with the chronic corticosterone deficit mask the consequences on anxiety-related behaviour. (3) CRHR1 possesses to some extent tonic activity independent of ligand-based receptor activation. (4) CRH activity is only relevant under conditions of severe stress. From genomic data, there is no trace of unidentified family members in mammals and it seems unlikely that UCNs can compensate for the CRH loss as their expression is spatially more restricted and no compensatory upregulation has been observed in CRH knockout mice. Moreover, CRHR1 knockout mice are prone to similar pleiotropic effects including a severe corticosterone deficit. Beside the observation that CRHR1 antagonists are still able to block some of the stress-induced behavioural effects in CRH knockout mice (Weninger et al. 1999), there is no experimental evidence for a constitutively active CRHR1.
The generation of a conditional CRH allele amenable to Cre-mediated inactivation allowed contesting some of the postulated explanations for the absence of any anxiety-related phenotype in constitutive CRH knockout mice (Zhang et al. 2017; Dedic et al. 2018b). Combination of the conditional CRH allele with Dlx5/6-Cre driver line results in the deletion of CRH from forebrain GABAergic neurons, including anxiety- and fear-related brain regions such as the central amygdala (CeA) and BNST, while preserving CRH expression in the PVN and thus leaving the HPA axis intact. However, anxiety-related behaviour was unaffected in CRHCKO-Dlx5/6 mice. Interestingly, and in support of a specific role for CRH under conditions of severe or enduring stress, CRH deletion from forebrain GABAergic neurons conferred resilience to chronic social defeat stress (Dedic et al. 2019). Temporally controlled CRH deletion from long-range GABAergic projection neurons of the CeA and BNST using the tamoxifen-inducible Camk2a-CreERT2 driver line resulted in increased anxiety-related behaviour. This is in accordance with results obtained by selective deletion of CRHR1 from dopaminergic neurons in the ventral tegmental area which is the target region of CeA and BNST CRH+ neurons (Refojo et al. 2011). Restricted deletion of CRH from the small population of CRH in glutamatergic neurons mainly in the piriform cortex did not affect anxiety-related behaviour or the response to chronic stress (Dedic et al. 2018b).
The first conditional knockout mice targeting CRH expression in the PVN have been generated by breeding floxed CRH mice to the Sim1-Cre driver line. Sim1-Cre-mediated deletion of CRH in the hypothalamus is not as profound as in constitutive knockout mice. PVN CRH levels are reduced by 70% resulting in decreased basal, diurnal and stress-induced plasma corticosterone levels. Accordingly, the chronic corticosterone deficit results in adrenal atrophy. CRHCKO-Sim1 mice showed markedly reduced anxiety-related behaviour in the open field, hole board, elevated plus maze and dark/light box tests compared to control mice. These behavioural alterations occurred independent of the chronic corticosterone deficit as corticosterone substitution was not able to fully restore normal anxiety related-behaviour in CRHCKO-Sim1 mice (Zhang et al. 2017).
Another line of evidence for a direct involvement of CRH itself in PVN-controlled stress-related behaviours has been demonstrated recently. Restraint stress induces hyperarousal and insomnia, which is accompanied by activation of PVNCRH neurons, as indicated by stress-induced co-expression of the immediate early gene cFos. Restraint stress specifically activates a population of PVNCRH neurons that innervate wake promoting HCRT neurons in the LH. Accordingly, optogenetic stimulation of LH-projecting PVNCRH neurons elicits hyperarousal and wakefulness. In contrast, chemogenetic suppression and ablation of PVNCRH neurons attenuates wakefulness and locomotor activity (Ono et al. 2020). To test the direct impact of CRH on stress-induced arousal, CRH was selectively disrupted in the PVN using CRISPR-Cas9-mediated inactivation (Li et al. 2020). Similar to the ablation of LH HCRT neurons, downregulation of CRH expression in the PVN was sufficient to block the stress-induced hyperarousal. In this context, it is of interest that GABAergic neurons in the SCN—the organism’s central circadian clock—negatively regulate the activity of PVNCRH neurons, which in turn positively regulate wake promoting HCRT neurons (Ono et al. 2020).
Taken together, these results demonstrate that the constitutive deletion of CRH might have been hampered by compensatory and pleiotropic effects due to early deletion throughout the brain, which has been unmasked by conditional strategies of CRH inactivation. In addition, it has become apparent that the function of CRH in parvocellular PVN neurons extends beyond the simple regulation of HPA axis activity but is an integral part of PVN’s capability to orchestrate stress-induced behaviours.
7.1 Perspectives
CRH in parvocellular neurons of the PVN is well known for its role in activating and controlling HPA axis activity. In particular, genomic and non-genomic glucocorticoid-driven mechanisms promote negative feedback inhibition and tightly regulate HPA axis function. Only recently, with the advent of CRHR1 reporter mice, an intra-PVN CRH/CRHR1 system has been identified and characterized. This microcircuit represents an immediate response system providing another level of neuroendocrine control over the HPA axis. To what extent this microcircuit is also involved in stress-induced behaviours remains to be further investigated. Functional interrogation of PVNCRH neurons is complicated by the inseparability of their neuroendocrine and behavioural functions. In this regard, it will be of utmost importance to better understand to what extent PVNCRH neurons projecting to the median eminence simultaneously send axon collaterals to brain regions relevant to behavioural stress responses.
Fostered by the availability of optogenetic and chemogenetic tools, in recent years we have seen an increasing number of studies focussing on the behavioural stress response conveyed by PVNCRH neurons. These studies have demonstrated that PVNCRH neurons are activated immediately and even anticipatorily upon external threats. Conversely, inhibition of PVNCRH neurons, e.g., by appetitive stimuli, is able to attenuate the stress response. PVNCRH neurons encode a broad spectrum of properties allowing for bidirectional control of behaviour, including selection of suitable innate defensive behaviours or social transmission of distress signals. These findings suggest that PVNCRH neurons control the transition to a state that is permissive for motor action enabling the engagement in stress-related behaviours (Daviu and Bains 2021). It is highly likely that PVNCRH neurons are not a homogenous population but might comprise functionally distinct populations, which could be addressed by applying intersectional approaches in the future. Furthermore, it would be highly relevant to better understand the stimuli that trigger neuropeptide release from CRH neurons. With the establishment of G-protein coupled receptor-activation based sensors, in vivo monitoring of CRH release might be within reach in the near future. Finally, it is remarkable that hypothalamic neurons outside of the PVN have largely been neglected in the past with regard to their physiology although there is evidence that they also contribute to the neuroendocrine, autonomic and behavioural stress response.
References
Alon T, Zhou L, Perez CA, Garfield AS, Friedman JM, Heisler LK (2009) Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter. Endocrinology 150:5626–5632
Alonso G, Szafarczyk A, Assenmacher I (1986) Immunoreactivity of hypothalamo-neurohypophysial neurons which secrete corticotropin-releasing hormone (CRH) and vasopressin (Vp): immunocytochemical evidence for a correlation with their functional state in colchicine-treated rats. Exp Brain Res 61:497–505
Arato M, Banki CM, Bissette G, Nemeroff CB (1989) Elevated CSF CRF in suicide victims. Biol Psychiatry 25:355–359
Bains JS, Wamsteeker Cusulin JI, Inoue W (2015) Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci 16:377–388
Brown MR, Fisher LA, Rivier J, Spiess J, Rivier C, Vale W (1982) Corticotropin-releasing factor: effects on the sympathetic nervous system and oxygen consumption. Life Sci 30:207–210
Ceccatelli S, Eriksson M, Hokfelt T (1989) Distribution and coexistence of corticotropin-releasing factor-, neurotensin-, enkephalin-, cholecystokinin-, galanin- and vasoactive intestinal polypeptide/peptide histidine isoleucine-like peptides in the parvocellular part of the paraventricular nucleus. Neuroendocrinology 49:309–323
Chen Y, Molet J, Gunn BG, Ressler K, Baram TZ (2015) Diversity of reporter expression patterns in transgenic mouse lines targeting corticotropin-releasing hormone-expressing neurons. Endocrinology 156:4769–4780
Cummings S, Elde R, Ells J, Lindall A (1983) Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci 3:1355–1368
Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG (2013) Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 7:156
Daviu N, Bains JS (2021) Should i stay or should i go? CRHPVN neurons gate state transitions in stress-related behaviors. Endocrinology 162:bqab061
Daviu N, Fuzesi T, Rosenegger DG, Rasiah NP, Sterley TL, Peringod G, Bains JS (2020) Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat Neurosci 23:398–410
de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475
Dedic N, Chen A, Deussing JM (2018a) The CRF family of neuropeptides and their receptors - mediators of the central stress response. Curr Mol Pharmacol 11:4–31
Dedic N, Kuhne C, Jakovcevski M, Hartmann J, Genewsky AJ, Gomes KS, Anderzhanova E, Pohlmann ML, Chang S, Kolarz A, Vogl AM, Dine J, Metzger MW, Schmid B, Almada RC, Ressler KJ, Wotjak CT, Grinevich V, Chen A, Schmidt MV, Wurst W, Refojo D, Deussing JM (2018b) Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety. Nat Neurosci 21:803–807
Dedic N, Kuhne C, Gomes KS, Hartmann J, Ressler KJ, Schmidt MV, Deussing JM (2019) Deletion of CRH from GABAergic forebrain neurons promotes stress resilience and dampens stress-induced changes in neuronal activity. Front Neurosci 13:986
Deussing JM, Chen A (2018) The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev 98:2225–2286
Dunn AJ, Berridge CW (1990) Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15:71–100
Fisher LA, Brown MR (1991) Central regulation of stress responses: regulation of the autonomic nervous system and visceral function by corticotrophin releasing factor-41. Bailliere Clin Endocrinol Metab 5:35–50
Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS (2016) Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun 7:11937
Gillies GE, Linton EA, Lowry PJ (1982) Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 299:355–357
Griebel G, Holsboer F (2012) Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov 11:462–478
Gunn BG, Sanchez GA, Lynch G, Baram TZ, Chen Y (2019) Hyper-diversity of CRH interneurons in mouse hippocampus. Brain Struct Funct 224:583–598
Henckens MJ, Deussing JM, Chen A (2016) Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat Rev Neurosci 17:636–651
Herman JP, Tasker JG, Ziegler DR, Cullinan WE (2002) Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav 71:457–468
Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180
Holsboer F (1999) The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res 33:181–214
Holsboer F (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501
Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, Kunzel HE, Pfennig A, Uhr M, Holsboer F (2007) Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression - a potential biomarker? Biol Psychiatry 62:47–54
Itoi K, Talukder AH, Fuse T, Kaneko T, Ozawa R, Sato T, Sugaya T, Uchida K, Yamazaki M, Abe M, Natsume R, Sakimura K (2014) Visualization of corticotropin-releasing factor neurons by fluorescent proteins in the mouse brain and characterization of labeled neurons in the paraventricular nucleus of the hypothalamus. Endocrinology 155:4054–4060
Jiang Z, Rajamanickam S, Justice NJ (2018) Local corticotropin-releasing factor signaling in the hypothalamic paraventricular nucleus. J Neurosci 38:1874–1890
Jiang Z, Rajamanickam S, Justice NJ (2019) CRF signaling between neurons in the paraventricular nucleus of the hypothalamus (PVN) coordinates stress responses. Neurobiol Stress 11:100192
Justice NJ, Yuan ZF, Sawchenko PE, Vale W (2008) Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol 511:479–496
Keegan CE, Herman JP, Karolyi IJ, O'Shea KS, Camper SA, Seasholtz AF (1994) Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology 134:2547–2555
Kim J, Lee S, Fang YY, Shin A, Park S, Hashikawa K, Bhat S, Kim D, Sohn JW, Lin D, Suh GSB (2019a) Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat Neurosci 22:576–585
Kim JS, Han SY, Iremonger KJ (2019b) Stress experience and hormone feedback tune distinct components of hypothalamic CRH neuron activity. Nat Commun 10:5696
Kim DW, Washington PW, Wang ZQ, Lin SH, Sun C, Ismail BT, Wang H, Jiang L, Blackshaw S (2020) The cellular and molecular landscape of hypothalamic patterning and differentiation from embryonic to late postnatal development. Nat Commun 11:4360
Kono J, Konno K, Talukder AH, Fuse T, Abe M, Uchida K, Horio S, Sakimura K, Watanabe M, Itoi K (2017) Distribution of corticotropin-releasing factor neurons in the mouse brain: a study using corticotropin-releasing factor-modified yellow fluorescent protein knock-in mouse. Brain Struct Funct 222:1705–1732
Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB (2014) An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507:238–242
Kruk MR, Westphal KG, Van Erp AM, van Asperen J, Cave BJ, Slater E, de Koning J, Haller J (1998) The hypothalamus: cross-roads of endocrine and behavioural regulation in grooming and aggression. Neurosci Biobehav Rev 23:163–177
Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y (2011) Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex 21:1803–1817
Kuzmiski JB, Marty V, Baimoukhametova DV, Bains JS (2010) Stress-induced priming of glutamate synapses unmasks associative short-term plasticity. Nat Neurosci 13:1257–1264
Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE (2012) Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490:402–406
Lennard DE, Eckert WA, Merchenthaler I (1993) Corticotropin-releasing hormone neurons in the paraventricular nucleus project to the external zone of the median eminence: a study combining retrograde labeling with immunocytochemistry. J Neuroendocrinol 5:175–181
Li SB, Borniger JC, Yamaguchi H, Hedou J, Gaudilliere B, de Lecea L (2020) Hypothalamic circuitry underlying stress-induced insomnia and peripheral immunosuppression. Sci Adv 6:eabc2590
Merchenthaler I, Vigh S, Petrusz P, Schally AV (1982) Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat 165:385–396
Merchenthaler I, Hynes MA, Vigh S, Schally AV, Petrusz P (1984) Corticotropin releasing factor (CRF): origin and course of afferent pathways to the median eminence (ME) of the rat hypothalamus. Neuroendocrinology 39:296–306
Mickelsen LE, Bolisetty M, Chimileski BR, Fujita A, Beltrami EJ, Costanzo JT, Naparstek JR, Robson P, Jackson AC (2019) Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat Neurosci 22:642–656
Muglia L, Jacobson L, Dikkes P, Majzoub JA (1995) Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 373:427–432
Muglia LJ, Jacobson L, Weninger SC, Karalis KP, Jeong K, Majzoub JA (2001) The physiology of corticotropin-releasing hormone deficiency in mice. Peptides 22:725–731
Mulder J, Zilberter M, Spence L, Tortoriello G, Uhlen M, Yanagawa Y, Aujard F, Hokfelt T, Harkany T (2009) Secretagogin is a Ca2+-binding protein specifying subpopulations of telencephalic neurons. Proc Natl Acad Sci U S A 106:22492–22497
Muller MB, Landgraf R, Preil J, Sillaber I, Kresse AE, Keck ME, Zimmermann S, Holsboer F, Wurst W (2000) Selective activation of the hypothalamic vasopressinergic system in mice deficient for the corticotropin-releasing hormone receptor 1 is dependent on glucocorticoids. Endocrinology 141:4262–4269
Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W (2003) Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 6:1100–1107
Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M (1988) Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 45:577–579
Ono D, Mukai Y, Hung CJ, Chowdhury S, Sugiyama T, Yamanaka A (2020) The mammalian circadian pacemaker regulates wakefulness via CRF neurons in the paraventricular nucleus of the hypothalamus. Sci Adv 6:eabd0384
Pang ZP, Sudhof TC (2010) Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol 22:496–505
Peng J, Long B, Yuan J, Peng X, Ni H, Li X, Gong H, Luo Q, Li A (2017) A quantitative analysis of the distribution of CRH neurons in whole mouse brain. Front Neuroanat 11:63
Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De GG, Crawford E, Janak PH, George O, Rice KC, Messing RO (2015) A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front Neurosci 9:487
Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF (1994) Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 60:436–444
Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, Chen A (2017) Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat Neurosci 20:385–388
Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM (2011) Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 333:1903–1907
Rivier C, Vale W (1983) Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology 113:939–942
Romanov RA, Alpar A, Zhang MD, Zeisel A, Calas A, Landry M, Fuszard M, Shirran SL, Schnell R, Dobolyi A, Olah M, Spence L, Mulder J, Martens H, Palkovits M, Uhlen M, Sitte HH, Botting CH, Wagner L, Linnarsson S, Hokfelt T, Harkany T (2015) A secretagogin locus of the mammalian hypothalamus controls stress hormone release. EMBO J 34:36–54
Romanov RA, Alpar A, Hokfelt T, Harkany T (2017a) Molecular diversity of corticotropin-releasing hormone mRNA-containing neurons in the hypothalamus. J Endocrinol 232:R161–R172
Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, Alpar A, Mulder J, Clotman F, Keimpema E, Hsueh B, Crow AK, Martens H, Schwindling C, Calvigioni D, Bains JS, Mate Z, Szabo G, Yanagawa Y, Zhang MD, Rendeiro A, Farlik M, Uhlen M, Wulff P, Bock C, Broberger C, Deisseroth K, Hokfelt T, Linnarsson S, Horvath TL, Harkany T (2017b) Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci 20:176–188
Sanders J, Nemeroff C (2016) The CRF system as a therapeutic target for neuropsychiatric disorders. Trends Pharmacol Sci 37:1045–1054
Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF (1998) Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20:1093–1102
Spierling SR, Zorrilla EP (2017) Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology 234:1467–1481
Sterley TL, Baimoukhametova D, Fuzesi T, Zurek AA, Daviu N, Rasiah NP, Rosenegger D, Bains JS (2018) Social transmission and buffering of synaptic changes after stress. Nat Neurosci 21:393–403
Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71:995–1013
Tasker JG, Di S, Malcher-Lopes R (2006) Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology 147:5549–5556
Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W (1998) Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 19:162–166
Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–1397
Walker LC, Cornish LC, Lawrence AJ, Campbell EJ (2019) The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH-IRES-Cre mouse: a validation study. Neuropharmacology 154:96–106
Wamsteeker Cusulin JI, Fuzesi T, Watts AG, Bains JS (2013) Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS One 8:e64943
Wang LA, Nguyen DH, Mifflin SW (2019) Corticotropin-releasing hormone projections from the paraventricular nucleus of the hypothalamus to the nucleus of the solitary tract increase blood pressure. J Neurophysiol 121:602–608
Wang Y, Hu P, Shan Q, Huang C, Huang Z, Chen P, Li A, Gong H, Zhou JN (2021) Single-cell morphological characterization of CRH neurons throughout the whole mouse brain. BMC Biol 19:47
Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA (1999) Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci U S A 96:8283–8288
Yuan Y, Wu W, Chen M, Cai F, Fan C, Shen W, Sun W, Hu J (2019) Reward inhibits paraventricular CRH neurons to relieve stress. Curr Biol 29:1243–1251
Zhang R, Asai M, Mahoney CE, Joachim M, Shen Y, Gunner G, Majzoub JA (2017) Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol Psychiatry 22:733–744
Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F (2000) Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 34:171–181
Acknowledgements and Funding
This work was supported by the German Ministry of Science and Education (IMADAPT, FKZ: 01KU1901) and by the Marie Skłodowska-Curie innovative training network PurinesDX. We thank Elfi Fesl and Jessica Keverne for proofreading and editing of the manuscript.
Key References
Bains JS, Wamsteeker Cusulin JI, Inoue W (2015) Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci 16:377–388. This excellent review provides an overview of mechanisms of plasticity occurring at excitatory and inhibitory synapses of parvocellular neuroendocrine cells
Daviu N, Fuzesi T, Rosenegger DG, Rasiah NP, Sterley TL, Peringod G, Bains JS (2020) Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. The first paper to show the anticipatory activity of PVNCRH neurons and their contribution to selection of different stress response strategies
Deussing JM, Chen A (2018) The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev 98:2225–2286. Comprehensive review of the CRH family of neuropeptides and their cognate receptors
Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS (2016) Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun 7:11937. The first paper to show that PVNCRH neurons regulate a broad array of stress-related behaviours
Henckens MJ, Deussing JM, Chen A (2016) Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat Rev Neurosci 17:636–651. Review of region- and cell type-specific functions of the CRH system
Holsboer F (1999) The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res 33:181–214. Review summarizing clinical findings that supported the development of CRHR1 antagonist for the treatment of mood and anxiety disorders
Jiang Z, Rajamanickam S, Justice NJ (2019) CRF signaling between neurons in the paraventricular nucleus of the hypothalamus (PVN) coordinates stress responses. Neurobiol Stress 11:100192. This review provides a comprehensive overview of the CRH/CRHR1 microcircuit within the PVN
Kim J, Lee S, Fang YY, Shin A, Park S, Hashikawa K, Bhat S, Kim D, Sohn JW, Lin D, Suh GSB (2019) Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat Neurosci 22:576–585. The first paper to demonstrate that PVNCRH neurons assign positive or negative valence to external stimuli
Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM (2011) Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 333:1903–1907. The first paper to demonstrate cell type-specific effects of CRHR1, contesting the previously postulated purely anxiogenic effects of CRHR1
Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, Alpar A, Mulder J, Clotman F, Keimpema E, Hsueh B, Crow AK, Martens H, Schwindling C, Calvigioni D, Bains JS, Mate Z, Szabo G, Yanagawa Y, Zhang MD, Rendeiro A, Farlik M, Uhlen M, Wulff P, Bock C, Broberger C, Deisseroth K, Hokfelt T, Linnarsson S, Horvath TL, Harkany T (2017b) Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci 20:176–188. The first comprehensive single-cell RNA sequencing, unravelling the cellular identity of the murine hypothalamus
Spierling SR, Zorrilla EP (2017) Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology 234:1467–1481. Review exploring potential causes for the failure of CRHR1 antagonists in clinical trials
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chang, S., Deussing, J.M. (2021). Corticotropin-Releasing Hormone in the Paraventricular Nucleus of the Hypothalamus—Beyond Hypothalamic–Pituitary–Adrenal Axis Control. In: Grinevich, V., Dobolyi, Á. (eds) Neuroanatomy of Neuroendocrine Systems. Masterclass in Neuroendocrinology, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-030-86630-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-86630-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86629-7
Online ISBN: 978-3-030-86630-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)