Abstract
The hypothalamus orchestrates various essential physiological and behavioral processes via neuropeptide secretion. The adenohypophysis (anterior pituitary) is a major center for peripheral endocrine organs, which secretes systemic hormones responding to hypothalamic neuropeptides as releasing factors. This functional connection between the hypothalamus and adenohypophysis is indispensable for the endocrine system and homeostasis. Pluripotent stem cells are promising tools for studying the process of human organ development, disease modeling, and regenerative medicine. Differentiation methods derived from pluripotent stem cells have been studied over the last quarter of a century. Recent studies have succeeded in the differentiation into hypothalamus and adenohypophysis from mouse and human pluripotent stem cells by a three-dimensional floating culture method of embryonic bodies. The induced hypothalamic-like progenitors generate hypothalamic neurons such as vasopressin neurons. In the induction of adenohypophysis, Rathke’s pouch-like structures are self-organized as seen in embryogenesis in vivo, and functional anterior pituitary hormone-producing cells are subsequently differentiated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The hypothalamus and pituitary gland are essential for fundamental physiological processes such as stress responses, growth, sexual development, reproduction, regulation of food intake and energy expenditure and circadian rhythms. In the central part of the brain, hypothalamic neurons integrate afferent information from the periphery and respond by releasing neuropeptides and neurotransmitters. The pituitary gland consists of the adenohypophysis (anterior pituitary gland) and neurohypophysis (posterior pituitary gland). Some of the hypothalamic neuropeptides reach the adenohypophysis through the pituitary portal vein and regulate anterior pituitary hormones, including adrenocorticotropic hormone (ACTH), growth hormone (GH), prolactin (PRL), thyroid-stimulating hormone (TSH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). Through the regulation of these anterior pituitary hormones, the adenohypophysis, in turn, stimulates or inhibits hormone secretion from peripheral endocrine glands such as the adrenal cortex, thyroid, and gonads. The neurohypophysis contains axon terminals of hypothalamic neurons producing arginine vasopressin (AVP) and oxytocin, the somas of which are located in the supraoptic and paraventricular nuclei in the hypothalamus.
Dysfunction of the hypothalamus and pituitary gland causes various systemic symptoms (Schneider et al. 2007). Currently, the only available treatment for hypothalamic and pituitary disorders is hormone replacement therapy, which is not able to fully and precisely meet the demands of hormone requirements, which may change from moment to moment under various physiological conditions. Indeed, adrenal crisis (a life-threatening condition due to glucocorticoid deficiency) occurs in a substantial proportion of patients with hypopituitarism (deficiency of pituitary hormones), and also adrenal crisis-associated death could occur even in educated patients treated with glucocorticoid replacement therapy (Hahner et al. 2015). Moreover, patients with ACTH-dependent adrenal insufficiency (glucocorticoid deficiency) have higher risks of diabetes mellitus, hypertension, hyperlipidemia, depression, and anxiety, presumably due to glucocorticoid overreplacement (Stewart et al. 2016). Furthermore, adipsic patients with diabetes insipidus (polyuria due to antidiuretic hormone AVP) exhibit severe fluctuations in serum sodium levels, resulting in poor prognosis (Arima et al. 2014). These still unsatisfactory results of conventional hormone replacement therapies are attributed to the lack of a hormonal feedback mechanism, which is an essential feature of the endocrine system.
To overcome the difficulties in the current treatment for hypothalamic and pituitary disorders, high expectations have been growing for regenerative medicine using hypothalamic and pituitary hormone-producing cells differentiated from human embryonic stem (ES) and induced pluripotent stem (iPS) cells. Theoretically, ES/iPS cells are able to differentiate into all types of cells in our body. In addition, these pluripotent stem cells provide an unlimited cell source because of their self-renewal properties. Since recent studies have succeeded in the differentiation into hypothalamic neurons and adenohypophysis cells from mouse and human ES/iPS cells (Wataya et al. 2008; Suga et al. 2011; Dincer et al. 2013; Wang et al. 2015; Merkle et al. 2015; Ozone et al. 2016; Zimmer et al. 2016; Lund et al. 2016; Yamada-Goto et al. 2017; Ogawa et al. 2018; Kano et al. 2019; Mitsumoto et al. 2019; Kasai et al. 2020), regenerative medicine is coming closer to reality in the neuroendocrine field. Furthermore, these differentiated hypothalamic neurons and adenohypophysis cells can also be used for developmental basic research and disease modeling. In this chapter, we introduce achievements to date of differentiation into hypothalamic neurons and adenohypophysis cells from mouse and human ES/iPS cells.
2 Three-Dimensional Culture Method for Embryoid Body
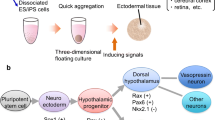
Organ formation during embryogenesis consists of complicated and sophisticated processes with various local interactions among distinct cells and tissues. To imitate these developmental processes during embryogenesis, a three-dimensional culture is an ideal approach. An efficient three-dimensional culture method has been established for selective neural differentiation from ES cells, which is called “serum-free culture of embryoid body-like aggregates with quick re-aggregation (SFEBq)” (Watanabe et al. 2005; Eiraku et al. 2008). Dissociated ES/iPS cells are autonomically and quickly aggregated in low-cell adhesion well plates in the differentiation medium (Fig. 3.1).
Schema of SFEBq method. Dissociated ES/iPS cells are seeded into the low-cell-adhesion 96-well plate and quickly aggregated. Using inducing signals in the culture medium, the aggregates differentiate into aimed ectodermal tissues. Reproduced from Suga (2019), with permission
The SFEBq method is appropriate for differentiation into various ectodermal derivatives from ES/iPS cells. In the SFEBq method, the ES/iPS cell aggregates exhibit self-organization (Sasai et al. 2012) and spontaneous formation of a highly ordered structure and patterning. This floating culture has revealed intrinsic programs driving locally autonomous modes of organogenesis and homeostasis. Based on the SFEBq method, cortex neurons (Eiraku et al. 2008; Danjo et al. 2011; Kadoshima et al. 2013), the optic cup (Ikeda et al. 2005; Osakada et al. 2008; Eiraku et al. 2011; Nakano et al. 2012), cerebellar neurons (Muguruma et al. 2010) and hippocampal neurons (Sakaguchi et al. 2015) have been generated from ES cells.
3 Mouse ES Cells as a Pioneer of a Human Model
Fundamental processes in differentiation studied in mouse ES cells can apply to human ES cells. For example, the retinal differentiation process in human ES cells (Nakano et al. 2012) has been established based on a previous report using mouse ES cells (Eiraku et al. 2011). Furthermore, the duration of mouse fetal development is approximately 20 days, which is considerably shorter than the 300 days of human fetal development. Since numerous trials and errors are required to establish a novel differentiation method, we should take advantage of mouse ES cells as a first step.
3.1 Hypothalamic Neuron Differentiation from Mouse ES Cells
SFEBq-cultured ES cells spontaneously differentiate into neural progenitors of the rostral forebrain and efficiently generate telencephalic progenitors (Watanabe et al. 2005). In the SFEBq method, ES cell aggregates are cultured in serum-free medium containing knockout serum replacement (KSR) without major exogenous inductive factors such as fibroblast growth factor (FGF), bone morphogenetic protein (BMP) or Wnt. However, the serum-free medium used in the original SFEBq culture still includes some exogenous signals that might affect the differentiation pathway. In particular, KSR, widely used for the maintenance and differentiation of ES cells, contains bioactive growth factors, including a high concentration of insulin and lipid-rich albumin purified from bovine serum.
Wataya et al. have established the differentiation method for hypothalamic neurons from mouse ES cells using a growth factor-free, chemically defined medium (gfCDM). Strict removal of exogenous patterning factors during early differentiation steps induces efficient generation of rostral hypothalamic progenitors (Rax+/Six3+/Vax1+) in mouse ES cell aggregates. The addition of insulin to the SFEBq/gfCDM culture strongly inhibits differentiation into Rax+ hypothalamic progenitors from mouse ES cells via the PI3K/Akt pathway. The ES cell-derived Rax+ progenitors generate rostral-dorsal hypothalamic precursors (Pax6+/Nks2.1−) and subsequently AVP neurons that efficiently release the hormone upon stimulation. Besides, rostral-ventral hypothalamic precursors (Pax6−/Nks2.1+) and neurons are differentiated from ES cell-derived Rax+ progenitors by treatment with the Sonic hedgehog (SHH) (Wataya et al. 2008).
ES cell differentiation into hypothalamic progenitors in SFEBq/gfCDM culture is summarized in Fig. 3.2. When cultured in the chemically defined medium without additional growth factors, including insulin, FGF, BMP, Wnt, and retinoic acid, ES cells frequently differentiate into Sox1+ naïve neuroectodermal cells and subsequent rostral hypothalamic progenitors (Rax+/nestin+/Six3+/Vax1+/Irx3+). Without SHH treatment, these rostral hypothalamic progenitors have the characteristics of dorsal hypothalamic progenitors (Pax6+/Nks2.1−), while SHH treatment promotes differentiation into ventral hypothalamic progenitors (Pax6−/Nks2.1+). ES cell-derived dorsal hypothalamic progenitors generate AVP neurons, presumably via Otp+/Brn2+ intermediate precursors. SHH-treated ES cell-derived ventral hypothalamic progenitors give rise to neurons characteristic of the ventral hypothalamus (e.g., SF1+ glutamatergic neurons in the ventromedial hypothalamic nucleus, A12 dopaminergic neurons, and neuropeptide Y/agouti-related peptide neurons in the arcuate nucleus).
Schema of hypothalamic differentiation. When cultured in the chemically defined medium without additional growth factors, pluripotent stem cells frequently differentiate into Sox1+ naïve neuroectodermal cells and subsequent rostral hypothalamic progenitors (Rax+/nestin+/Six3+/Vax1+/Irx3+). Without SHH treatment, these rostral hypothalamic progenitors have the characteristic of dorsal hypothalamic progenitors (Pax6+/Nks2.1−), while SHH treatment promotes differentiation into ventral hypothalamic progenitors (Pax6−/Nks2.1+). Dorsal hypothalamic progenitors generate AVP neurons. SHH-treated ventral hypothalamic progenitors give rise to neurons characteristic of the ventral hypothalamus. Modified from Suga (2019), with permission
3.2 Adenohypophysis Differentiation from Mouse ES Cells
The principal aspect of the SFEBq method is replicating the embryonic differentiation environment and imitating complicated and sophisticated processes during embryogenesis. Therefore, a detailed understanding of developmental biology is essential for the establishment of differentiation methods from ES cells.
Box 3.1 Adenohypophysis Development In Vivo
The adenohypophysis anlage originates as a placode in the non-neural ectoderm adjacent to the hypothalamic anlage situated in the top of the anterior neural plate (Fig. 3.3a). The adenohypophysis placode and hypothalamic anlage interact with each other during early development. In particular, the thickened placode invaginates and subsequently detaches from the oral ectoderm, leading to the formation of a hollowed vesicle termed Rathke’s pouch (Fig. 3.3b) (Zhu et al. 2007). The molecular nature of local inductive interaction underlying the initial phase of adenohypophysis formation has been intensively investigated, revealing that FGF, BMP, and SHH signals are involved as important factors (Takuma et al. 1998; Brinkmeier et al. 2007). Also, epithelial cells of Rathke’s pouch express Lhx3 (also called Lim3). Lhx3+ adenohypophysis progenitors generate several lineages of anterior pituitary hormone-producing cells (Fig. 3.3c). Among them, the ACTH-producing corticotroph lineage requires a transcription factor, Tbx19. GH-, PRL-, and TSH-producing cell lineages are differentiated from Pit1+ intermediate precursors. The third lineage differentiates into LH- and FSH-producing cells. Lhx3 knockout mice reveal that Lhx3 is essential for these anterior pituitary hormone-producing cell lineages (Sheng et al. 1996).
Diagram of adenohypophysis differentiation. (a) Dorsal view of neural plate and placodes. The adenohypophysis anlage (pituitary placode) is located in the non-neural ectoderm adjacent to the hypothalamus anlage (rostral hypothalamus). (b) Sagittal view of adenohypophysis embryogenesis. The thickened placode invaginates and subsequently detaches from the oral ectoderm, leading to the formation of a hollowed vesicle termed Rathke’s pouch. The epithelial cells of Rathke’s pouch express Lim3 (also called Lhx3). (c) Schema of adenohypophysis differentiation and subsequent generation of anterior pituitary hormone-producing cell lineages. Lhx3+ Rathke’s pouch progenitors (adenohypophysis progenitors) are derived from the oral ectoderm (Pitx1/2+) and subsequently generate several lineages of anterior pituitary hormone-producing cells. Among them, the ACTH-producing cell lineage requires a transcription factor Tbx19. GH-, PRL-, and TSH-producing cell lineages are differentiated from Pit1+ intermediate precursors. The third lineage differentiates into LH- and FSH-producing cells. Modified from Suga (2019), with permission
3.2.1 Two-Layer Formation In Vitro as the First Step of Adenohypophysis Differentiation
As discussed above, the formation of Rathke’s pouch is attributed to interactions between the hypothalamus and neighboring oral ectoderm. By inducing the hypothalamus and oral ectoderm simultaneously within the same ES cell aggregate in vitro to recapitulate these developmental processes in embryos, Suga et al. have established the differentiation method for adenohypophysis from mouse ES cells (Suga et al. 2011).
In the ES cells cultured by the SFEBq method for hypothalamic differentiation (Wataya et al. 2008), the expression of an oral ectoderm marker Pitx2 is low. Since oral ectoderm and hypothalamic progenitors are adjacent, a slight shift in the positional information is expected to promote the simultaneous generation of both tissues within the same aggregate in SFEBq culture. A key positioning factor for the oral ectoderm is BMP4. BMP4 treatment increased Pitx2 expression in SFEBq-cultured ES cells, whereas the BMP4 antagonist dorsomorphin suppresses the generation of the oral ectoderm. However, treatment with exogeneous BMP4 in contrast inhibits hypothalamic differentiation even at a low dose. An optional condition is a large-cell aggregation (LCA) culture (Fig. 3.4a; starting differentiation culture at 10,000 cells per aggregate, instead of 3000 in the original SFEBq culture). In the LCA-SFEBq culture, both the oral ectoderm (Pitx1/2+) and hypothalamic progenitors (Rax+) are differentiated simultaneously within the same aggregate (Fig. 3.4b, b′) as a result of the moderate elevation of endogenous BMP4 (Suga et al. 2011).
In vivo differentiation into adenohypophysis from mouse ES cells. (a) Schema of LCA-SFEBq method. (b, b′) Two-layer formation of the oral ectoderm and hypothalamic progenitors in the LCA-SFEBq-cultured aggregates (b). Immunostaining of the aggregates (b′, scale bar 100 μm). (c–c′) Self-formation of Rathke’s pouches (c). Bright field images (c′, scale bar 100 μm) and immunostaining of the aggregates with Rathke’s pouches (c″, scale bar 100 μm). (d–d″) Generation of ACTH+ cells in the differentiated adenohypophysis (d). Low-power field view (d′, scale bar 50 μm) and high-power field view (d″, scale bar 20 μm) of ACTH+ area. Modified from Suga (2019), with permission
3.2.2 Self-Formation of Rathke’s Pouch In Vitro
SHH signals are known to provide positional information to adjust towards the midline in vivo (Zhu et al. 2007). During embryogenesis, Rathke’s pouch receives SHH signals from neighboring tissues and develops in the middle of the rostral head ectoderm. Also in vitro, treatment of smoothened agonist (SAG), a hedgehog agonist induces multiple oval epithelial clusters in the LCA-SFEBq-cultured mouse EB cell aggregates (Fig. 3.4c, c′). These oval structures are located between the oral ectoderm and hypothalamic neurons (Fig. 3.4c″). Rathke’s pouch marker Lim3 (also called Lhx3) is expressed in epithelial cells comprising these oval structures. The long axis of the Lim3+ (Lhx3+) pouches reaches a diameter of 150–200 μm, comparable to that of early Rathke’s pouch in vivo. Interactions between the oral ectoderm and hypothalamic neurons are critical for in vitro induction of Rathke’s pouch. Neither isolated surface ectoderm nor hypothalamus alone generates Lim3+ (Lhx3+) pouches (Suga et al. 2011).
3.2.3 Generation of Multiple Endocrine Lineages
During early adenohypophysis development, Lhx3+ adenohypophysis progenitors commit to several hormone-type-specific lineages (Davis et al. 2011). Among them, the ACTH-producing corticotroph lineage requires the transcription factor Tbx19 (Lamolet et al. 2001), the expression of which is inhibited by Notch signaling (Zhu et al. 2006; Kita et al. 2007). Treatment with the Notch inhibitor DAPT increases Txb19 expression in SAG-treated LCA-SFEBq-cultured ES cell aggregates. Substantial numbers of ACTH+ cells accumulate in the Tbx19+ domains of DAPT-treated pouch tissues (Fig. 3.4d–d″) (Suga et al. 2011).
Previous reports have shown that canonical Wnt signaling promotes Pit1 expression (DiMattia et al. 1997; Olson et al. 2006). Consistent with these findings, treatment with the Wnt agonist BIO increases Pit1 expression, leading to subsequent GH+ and PRL+ cell differentiation in the SAG-treated LCA-SFEBq-cultured ES cell aggregates. Head mesenchyme has been suggested to promote adenohypophysis development in vivo (Gleiberman et al. 1999). In the SAG-treated LCA-SFEBq-cultured ES cell aggregates with conditioned medium of PA6 stromal cells, LH+, FSH+, and TSH+ cells are successfully differentiated (Suga et al. 2011).
Positive and negative feedback systems are characteristic of endocrine cells. The complete recapitulation of these properties is the ultimate goal for endocrine regenerative medicine. To investigate in vitro functionality, a corticotropin-releasing hormone (CRH) loading test has been performed on induced ACTH+ cells generated in SAG+DAPT-treated LCA-SFEBq-cultured aggregates of mouse ES cells. From the SAG+DAPT-treated LCA-SFEBq-cultured aggregates, substantial amounts of ACTH are secreted, comparable to peripheral ACTH levels in mice. ACTH secretion from the adenohypophysis is negatively regulated by the downstream glucocorticoid hormone. Consistent with this hormonal regulation in vivo, ACTH release in vitro after CRH stimulation is suppressed by glucocorticoid pre-treatment. These findings demonstrate that the ES-cell-derived endocrine cells in the adenohypophysis actively secrete ACTH and respond to both positive and negative regulators that work for endocrine homeostasis in vivo (Suga et al. 2011).
3.2.4 Functional Rescue in Hypophysectomized Model Animals
In hypophysectomized mice with the SAG+DAPT-treated LCA-SFEBq-cultured aggregates transplanted into the kidney capsule, CRH loading induces a substantial elevation of blood ACTH levels and downstream glucocorticoid hormone corticosterone, indicating that ACTH from the graft sufficiently induced the downstream hormone. Even without CRH loading, the basal levels of ACTH and corticosterone are also increased after transplantation, suggesting that partial recovery of blood ACTH has a moderate but biologically significant effect. With hormonal recovery, the transplanted hypophysectomized mice exhibit higher spontaneous locomotor activities and survive longer. Although CRH, secreted from the hypothalamus, should be diluted at the peripheral site, mouse ES cell-derived adenohypophysis tissues improve survival and spontaneous activities even when transplanted into the kidney capsule (Suga et al. 2011).
3.3 Application to Human ES/iPS Cell Culture
Although human ES cells are vulnerable to apoptosis upon cellular detachment and dissociation, it has been demonstrated by Watanabe et al. that Y-27632, a selective inhibitor of Rho-associated kinase (Rock), markedly diminishes dissociation-induced apoptosis of human ES cells, which enables human ES cells to aggregate in the SFEBq method (Watanabe et al. 2007). Based on differentiation methods into hypothalamic neurons (Wataya et al. 2008) and adenohypophysis (Suga et al. 2011) from mouse ES cells using the SFEBq culture, differentiation methods have been established for human hypothalamic neurons (Merkle et al. 2015; Ogawa et al. 2018) and adenohypophysis cells (Ozone et al. 2016) from human ES cells. Very recently, a functional hypothalamic-adenohypophysis unit has been generated using human iPS cells (Kasai et al. 2020).
3.3.1 Hypothalamic Neuron Differentiation from Human ES Cells
Merkle et al. have reported hypothalamic neuronal differentiation from human pluripotent stem cells using the SFEBq method, although hormonal secretion from differentiated hypothalamic neurons has not been demonstrated (Merkle et al. 2015). Recently, Ogawa et al. established a differentiation method for functional hypothalamic neurons from human ES cells (Ogawa et al. 2018).
In the development of hypothalamic neurons from mouse ES cells, strict removal of exogenous patterning factors is essential in the SFEBq/gfCDM culture (Wataya et al. 2008); however, human ES cells fail to aggregate and die within several days in this method. A small amount of KSR as well as Rock inhibitor Y-27632 enables human ES cells to aggregate in the gfCDM, although the cells differentiate towards the telencephalon under these conditions. Therefore, by the slight modification of positional information with BMP4, SAG, and Akt inhibitor, SFEBq/gfCDM-cultured human ES cell aggregates differentiate into rostral hypothalamic progenitors (Rax+). Furthermore, the tuning of SHH signals induces dorsal (Pax6+/Nks2.1−) or ventral (Pax6−/Nks2.1+) hypothalamic progenitors. Via Otp+/Brn2+ intermediate precursors, dorsal hypothalamic progenitors generate AVP neurons that efficiently release the hormone upon stimulation (Ogawa et al. 2018). A schematic summary of human ES cell differentiation into hypothalamic neurons is shown in Fig. 3.5.
Human ES cell differentiation into hypothalamus. Schematic summary of development from human ES cells to AVP neurons. Hypothalamic progenitors expressing Rax (a). Turning of SHH signals induces dorsal hypothalamus (Pax6+/Nks2.1−, b), the subsequent generation of AVP precursor cells (Otp, b′) and AVP neurons (b″), or ventral hypothalamus (Pax6−/Nks2.1+, c). Scale bars 50 μm. Modified from Suga (2019), with permission
3.3.2 Adenohypophysis Differentiation from Human ES/iPS Cells
As demonstrated in the adenohypophysis differentiation method from mouse ES cells (Suga et al. 2011), the stages of differentiating pluripotent stem cells into adenohypophysis are as follows; (1) simultaneous induction of neighboring hypothalamic neuroectoderm and oral ectoderm, (2) self-formation of adenohypophysis anlage (Rathke’s pouch) as a result of interaction between two layers of the hypothalamic neuroectoderm and oral ectoderm, (3) generation of multiple endocrine lineages from Lhx3+ adenohypophysis progenitors, and (4) differentiation into functional anterior pituitary hormone-producing cells. By following the above steps and mimicking the differentiation method in mice (Suga et al. 2011), Ozone et al. have established the adenohypophysis differentiation method from human ES cells (Ozone et al. 2016).
In SFEBq-cultured human ES cell aggregates with gfCDM/KSR/Y-27632 medium, the addition of SAG, BMP4, and FGF2 to the differentiation medium induces hypothalamic neuroectoderm and oral ectoderm, following self-formation of Pitx1+/Lhx3+ Rathke’s pouch-like oval structures (Fig. 3.6a). These structures subsequently differentiate into all lineages of anterior pituitary hormone-producing cells (Fig. 3.6b, c–c″, d–d″, e–e′). Among them, human ES cell-derived ACTH-producing corticotrophs and GH-producing somatotrophs have been demonstrated feedback systems. Moreover, electron microscopy reveals secretory granules characteristic of endocrine cells stored in the cytoplasm of these cells (Fig. 3.6f). Furthermore, transplantation with human ES cell-derived adenohypophysis tissues into the kidney capsule improved survival and spontaneous activities in hypophysectomized mice (Ozone et al. 2016).
Applied adenohypophysis differentiation in human ES cells. (a) Self-formation of Rathke’s pouch-like structures. Scale bars 50 μm. (b) Schema of differentiation into multiple lineages of anterior pituitary hormone-producing cells. (c–c″) Corticotroph. Scale bars 50 μm. (d–d″) Somatotroph (d), lactotroph (d′), and thyrotroph (d″). Scale bars 50 μm. (e, e′) Gonadotrophs. Scale bars 50 μm. (f) Secretory granules characteristic of endocrine cells in electron microscopy. Scale bars 2 μm. Modified from Suga (2019), with permission
By following the above differentiation method with slight modifications, Kasai et al. have succeeded in generating a functional hypothalamic-adenohypophysis unit from human iPS cells (Kasai et al. 2020). This hybrid organoid exhibits simultaneous differentiation and maturation of the hypothalamic neurons and anterior pituitary hormone-producing cells within the same aggregates. Therefore, ACTH secretion capacity is comparable to that in vivo since CRH from the hypothalamic area regulates ACTH-producing cells in analogy with the hypothalamic-pituitary axis (Kasai et al. 2020).
4 Perspectives: Applications of Human ES/iPS Cell-Derived Hypothalamic Neurons and Adenohypophysis
4.1 In Vitro Human Model of Development and Disease
Human iPS cells are promising tools for studying the process of human organ development and its disorders. Recently, Matsumoto et al. have established a disease model of congenital pituitary hypoplasia (CPH) using iPS cells derived from patients with a heterozygous mutation in the orthodenticle homeobox 2 (OTX2) gene. The patient-derived iPS cells retain the potential to differentiate into the oral ectoderm but exhibit a severely impaired adenohypophysis differentiation. OTX2 in the hypothalamus is essential for progenitor cell maintenance by regulating Lhx3 expression in the ectoderm via FGF10 expression in the hypothalamus (Matsumoto et al. 2020).
iPS cell lines from patients with various hereditary diseases have been generated so far. Regarding hypothalamic and pituitary diseases, besides CPH described above, iPS cell lines from patients afflicted with familial neurohypophysial diabetes insipidus (Yoshida et al. 2020b) and multiple endocrine neoplasia type 1 (Yoshida et al. 2020a) have recently been generated.
4.2 Transplantation of Human ES/iPS Cell-Derived Hypothalamic Neurons and Adenohypophysis
ES cell-derived ACTH-producing cells function with hormonal regulation and improve survival and spontaneous activities in hypophysectomized mice even ectopically transplanted in the kidney capsule (Suga et al. 2011; Ozone et al. 2016). These findings raise the possibility of simple grafting in a peripheral site; however, physiological CRH release from the hypothalamus does not directly affect these peripheral grafts. Therefore, orthotopic transplantation into the sella or hypothalamus is one of the future candidates.
There are several challenges for regenerative medicine using human ES/iPS cell-derived hypothalamic neurons and adenohypophysis. First, the differentiation method still needs to be optimized: the maturity of ACTH- and GH-producing cells and the differentiation of other lineages are not enough. Also, xeno-free culture systems are required for clinical application. Second, ensuring safety is essential for clinical use. Contamination of immature cells increases the risk of tumorigenesis. Therefore, purification methods for target cells need to be developed. Third, ethical issues should be considered appropriately, even though human iPS cells have fewer issues than ES cells in general.
Key References
-
Watanabe et al. (2005) The first paper to introduce a method of serum-free suspension culture (SFEBq; serum-free floating culture of embryoid body-like aggregates with quick re-aggregation).
-
Wataya et al. (2008) This paper demonstrates that strict removal of exogeneous patterning factors during early differentiation step induces hypothalamic progenitors in mouse ES cells.
-
Suga et al. (2011) Innovative work on self-formation of Rathke’s pouch-like adenohypophysis progenitors and subsequent differentiation into functional adenohypophysis from mouse ES cells.
-
Ozone et al. (2016) This paper illustrates the differentiation method into adenohypophysis from human ES cells.
-
Kasai et al. (2020) Seminal work generating hypothalamic-adenohypophysis functional units from human iPS cells.
Abbreviations
- ACTH:
-
adrenocorticotropic hormone
- AVP:
-
arginine vasopressin
- BIO:
-
6-bromo-3-[(3E)-1,3-dihydro-3-(hydroxyimino)-2H-indol-2-ylidene]-1,3-dihydro-(3Z)-2H-indol-2-one
- BMP:
-
bone morphogenetic protein
- CPH:
-
congenital pituitary hypoplasia
- CRH:
-
corticotropin-releasing hormone
- DAPT:
-
(2S)-N-[N-(3,5-Difluorophenacetyl)-l-alanyl]-2-phenylglycine tert-butyl ester
- ES:
-
embryonic stem
- FGF:
-
fibroblast growth factor
- FSH:
-
follicle-stimulating hormone
- GATA2:
-
GATA-binding factor 2
- gfCGM:
-
growth factor-free chemically defined medium
- GH:
-
growth hormone
- iPS:
-
induced pluripotent stem
- IRX3:
-
Iroquois homeobox gene 3
- KSR:
-
knockout serum replacement
- LCA:
-
large-cell aggregate
- LH:
-
luteinizing hormone
- LHX3:
-
LIM-homeobox 3
- NKX2.1:
-
NK2 homeobox 1
- OTP:
-
orthopedia homeobox
- OTX2:
-
orthodenticle homeobox 2
- PAX6:
-
paired box 6
- PI3K:
-
phosphoinositide 3-kinase
- PIT1:
-
pituitary transcription factor 1
- PRL:
-
prolactin
- Rock:
-
Rho-associated kinase
- SAG:
-
smoothened agonist
- SF1:
-
steroidogenic factor 1
- SFEBq:
-
serum-free culture of embryoid body-like aggregates with quick re-aggregation
- SHH:
-
sonic hedgehog
- SIX3:
-
SIX homeobox 3
- SOX1:
-
SRY-box transcription factor 1
- TBX19:
-
T-box transcription factor
- TSH:
-
thyroid-stimulating hormone
- VAX1:
-
ventral anterior homeobox 1
References
Arima H, Wakabayashi T, Nagatani T, Fujii M, Hirakawa A, Murase T, Yambe Y, Yamada T, Yamakawa F, Yamamori I, Yamauchi M, Oiso Y (2014) Adipsia increases risk of death in patients with central diabetes insipidus. Endocr J 61:143–148
Brinkmeier ML, Potok MA, Davis SW, Camper SA (2007) TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol 311:396–407
Danjo T, Eiraku M, Muguruma K, Watanabe K, Kawada M, Yanagawa Y, Rubenstein JL, Sasai Y (2011) Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci 31:1919–1933
Davis SW, Mortensen AH, Camper SA (2011) Birthdating studies reshape models for pituitary gland cell specification. Dev Biol 352:215–227
DiMattia GE, Rhodes SJ, Krones A, Carrière C, O’Connell S, Kalla K, Arias C, Sawchenko P, Rosenfeld MG (1997) The Pit-1 gene is regulated by distinct early and late pituitary-specific enhancers. Dev Biol 182:180–190
Dincer Z, Piao J, Niu L, Ganat Y, Kriks S, Zimmer B, Shi SH, Tabar V, Studer L (2013) Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Rep 5:1387–1402
Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3:519–532
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472:51–56
Gleiberman AS, Fedtsova NG, Rosenfeld MG (1999) Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol 213:340–353
Hahner S, Spinnler C, Fassnacht M, Burger-Stritt S, Lang K, Milovanovic D, Beuschlein F, Willenberg HS, Quinkler M, Allolio B (2015) High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab 100:407–416
Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N, Takahashi M, Sasai Y (2005) Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci USA 102:11331–11336
Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y (2013) Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci USA 110:20284–20289
Kano M, Suga H, Ishihara T, Sakakibara M, Soen M, Yamada T, Ozaki H, Mitsumoto K, Kasai T, Sugiyama M, Onoue T, Tsunekawa T, Takagi H, Hagiwara D, Ito Y, Iwama S, Goto M, Banno R, Arima H (2019) Tanycyte-like cells derived from mouse embryonic stem culture show hypothalamic neural stem/progenitor cell functions. Endocrinology 160:1701–1718
Kasai T, Suga H, Sakakibara M, Ozone C, Matsumoto R, Kano M, Mitsumoto K, Ogawa K, Kodani Y, Nagasaki H, Inoshita N, Sugiyama M, Onoue T, Tsunekawa T, Ito Y, Takagi H, Hagiwara D, Iwama S, Goto M, Banno R, Takahashi J, Arima H (2020) Hypothalamic contribution to pituitary functions is recapitulated in vitro using 3D-cultured human iPS cells. Cell Rep 30:18–24.e15
Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N (2007) Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol Endocrinol 21:1458–1466
Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J (2001) A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104:849–859
Lund C, Pulli K, Yellapragada V, Giacobini P, Lundin K, Vuoristo S, Tuuri T, Noisa P, Raivio T (2016) Development of gonadotropin-releasing hormone-secreting neurons from human pluripotent stem cells. Stem Cell Reports 7:149–157
Matsumoto R, Suga H, Aoi T, Bando H, Fukuoka H, Iguchi G, Narumi S, Hasegawa T, Muguruma K, Ogawa W, Takahashi Y (2020) Congenital pituitary hypoplasia model demonstrates hypothalamic OTX2 regulation of pituitary progenitor cells. J Clin Invest 130:641–654
Merkle FT, Maroof A, Wataya T, Sasai Y, Studer L, Eggan K, Schier AF (2015) Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development 142:633–643
Mitsumoto K, Suga H, Sakakibara M, Soen M, Yamada T, Ozaki H, Nagai T, Kano M, Kasai T, Ozone C, Ogawa K, Sugiyama M, Onoue T, Tsunekawa T, Takagi H, Hagiwara D, Ito Y, Iwama S, Goto M, Banno R, Arima H (2019) Improved methods for the differentiation of hypothalamic vasopressin neurons using mouse induced pluripotent stem cells. Stem Cell Res 40:101572
Muguruma K, Nishiyama A, Ono Y, Miyawaki H, Mizuhara E, Hori S, Kakizuka A, Obata K, Yanagawa Y, Hirano T, Sasai Y (2010) Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat Neurosci 13:1171–1180
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785
Ogawa K, Suga H, Ozone C, Sakakibara M, Yamada T, Kano M, Mitsumoto K, Kasai T, Kodani Y, Nagasaki H, Yamamoto N, Hagiwara D, Goto M, Banno R, Sugimura Y, Arima H (2018) Vasopressin-secreting neurons derived from human embryonic stem cells through specific induction of dorsal hypothalamic progenitors. Sci Rep 8:3615
Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG (2006) Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell 125:593–605
Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M (2008) Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 26:215–224
Ozone C, Suga H, Eiraku M, Kadoshima T, Yonemura S, Takata N, Oiso Y, Tsuji T, Sasai Y (2016) Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun 7:10351
Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, Sasai Y (2015) Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 6:8896
Sasai Y, Eiraku M, Suga H (2012) In vitro organogenesis in three dimensions: self-organising stem cells. Development 139:4111–4121
Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E (2007) Hypopituitarism. Lancet 369:1461–1470
Sheng HZ, Zhadanov AB, Mosinger B, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H (1996) Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272:1004–1007
Stewart PM, Biller BM, Marelli C, Gunnarsson C, Ryan MP, Johannsson G (2016) Exploring inpatient hospitalizations and morbidity in patients with adrenal insufficiency. J Clin Endocrinol Metab 101:4843–4850
Suga H (2019) Application of pluripotent stem cells for treatment of human neuroendocrine disorders. Cell Tissue Res 375:267–278
Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y (2011) Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480:57–62
Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA (1998) Formation of Rathke’s pouch requires dual induction from the diencephalon. Development 125:4835–4840
Wang L, Meece K, Williams DJ, Lo KA, Zimmer M, Heinrich G, Martin Carli J, Leduc CA, Sun L, Zeltser LM, Freeby M, Goland R, Tsang SH, Wardlaw SL, Egli D, Leibel RL (2015) Differentiation of hypothalamic-like neurons from human pluripotent stem cells. J Clin Invest 125:796–808
Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y (2005) Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 8:288–296
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25:681–686
Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N, Sasai Y (2008) Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc Natl Acad Sci USA 105:11796–11801
Yamada-Goto N, Ochi Y, Katsuura G, Yamashita Y, Ebihara K, Noguchi M, Fujikura J, Taura D, Sone M, Hosoda K, Gottschall PE, Nakao K (2017) Neuronal cells derived from human induced pluripotent stem cells as a functional tool of melanocortin system. Neuropeptides 65:10–20
Yoshida S, Okura H, Suga H, Nishitomi T, Sakurai A, Arima H, Matsuyama A (2020a) Generation of three induced pluripotent stem cell (iPSC) lines from a multiple endocrine neoplasia type 1 (MEN1) patient and three iPSC lines from an unaffected relative of the patient. Stem Cell Res 46:101846
Yoshida S, Okura H, Suga H, Soen M, Kawaguchi Y, Kurimoto J, Miyata T, Takagi H, Arima H, Fujikawa T, Otsuka F, Matsuyama A (2020b) Generation of four induced pluripotent stem cell lines (FHUi003-A, FHUi003-B, FHUi004-A and FHUi004-B) from two affected individuals of a familial neurohypophyseal diabetes insipidus family. Stem Cell Res 48:101960
Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG (2006) Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev 20:2739–2753
Zhu X, Gleiberman AS, Rosenfeld MG (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963
Zimmer B, Piao J, Ramnarine K, Tomishima MJ, Tabar V, Studer L (2016) Derivation of diverse hormone-releasing pituitary cells from human pluripotent stem cells. Stem Cell Reports 6:858–872
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hagiwara, D., Suga, H., Arima, H. (2021). Generation of Hypothalamus and Adenohypophysis from Human Pluripotent Stem Cells. In: Grinevich, V., Dobolyi, Á. (eds) Neuroanatomy of Neuroendocrine Systems. Masterclass in Neuroendocrinology, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-030-86630-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-86630-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86629-7
Online ISBN: 978-3-030-86630-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)