Abstract
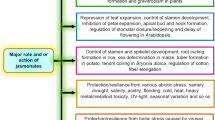
Jasmonates (JAs) are well known new class of lipid based phytohormones which are produced endogenously in plants growing under stress. They play significant role in regulating plant adaptation to several biotic and abiotic stresses like wounding, predator attack, salt stress and UV radiation etc. Studies have shown that besides playing role of stress hormone JAs are also involved in many growth and development activities in vegetative as well as reproductive parts of plants including roots. Since the time of its discovery many detailed studies have been done in understanding its biosynthetic and signalling pathway, its crosstalk with other phytohormones. Many genes and transcription factors have been identified which are involved in positive and negative regulation of these pathways and other root growth related activities such as inhibition of primary root growth, growth of lateral and adventitious roots, gravitotropic response, root—microbe interactions. In this chapter we have given an overview on mechanism of JA action and its effects on various aspects of root growth and development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Jasmonates (JAs) are phospholipids derived cyclopentanones that included Jasmonic acid (JA) and its derivatives methyl Jasmonates (MetJA). In year 1962 (Met JA) was first isolated from essential oil of Jasminium grandilorum flower (Demole et al. 1962) and the free acid was isolated later from the culture filtrate of fungus Botryodiplodia theohormae (Aldrige et al. 1971), Cucurbita pepo (Fukui et al. 1977), Vicia faba (Dathe et al. 1981). In 1980, JA and its derivatives were synthesised chemically and their biological activity was tested on growing rice seedling (Yamane et al. 1980). JA are basically stress hormone as they slow down normal growth and development processes in plants which are sensitive to environmental stress and promotes several stress related responses in plants. Since the MeJA is a volatile compound it can easily escape from a plant under stress and raises an alarm in neighbouring plants to the prevailing biotic and abiotic stresses. Therefore this hormone has several ecological and physiological implications. In last few years almost all genes, proteins and transcription factors involved in biosynthetic and signalling pathways of JA have been identified, isolated and characterized. In this chapter we have outlined the mechanism of JAs biosynthesis, signal transduction, crosstalk with other phytohormones and molecular basis of the effects shown by them in regulating various growth and development related activities in roots.
2 Jasmonates
Jasmonates include Jasmonic acid (JA) its methyl ester MeJA and isoleucine conjugates of JA. This is a class of phytohormones which are involved in plant defence against biotic and abiotic stress (Du et al. 2013). Along with plant defence these are also involved in plant growth and development, reproduction (Wasternack 2007; Browse 2009), floral development, trichome formation, vegetative storage protein (VSP) formation, fruit ripening, tendril formation, mycorrhizal association, male fertility and development of roots in plant. Chemically JA is 3-oxo-2’-2’-cis pentenyl-cyclopentane 1-actic acid.
3 Biosynthesis of Jasmonates
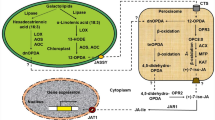
α-linolenic acid (α-LeA) serves as a precursor for biosynthesis of JA (Browse 2005; Wasternack and Hause, 2013) (Fig. 1). Biosynthesis process begins in plastids, where α-linolenic acid is produced by joint action of two enzymes fatty acid desaturase (FAD) and phospholipase A1 (PLA). It is then converted to (13S)-hydroperoxyoctadecatrienoic acid (13-HPOT), 12,13(S)-epoxyoctadecatrienoic acid (12,13-EOT), and (9S,13S)-12-oxo-phytodienoic acid (OPDA) in a stepwise manner through the action of enzymes 13-lipoxygenase (LOX), allene oxide synthase (AOS), and allene oxide cyclase (AOC), respectively (Fig. 1). OPDA thus formed is transported to peroxisomes, where it is reduced to 3-oxo-2-(cis-2ʹ-pentenyl)-cyclopentane-1-octanoic acid by OPDA reductase (OPR). OPC is subsequently shortened to jasmonic acid by three rounds of β-oxidation catalyzed by three different enzymes: acyl-CoA oxidase (ACX), multifunctional protein (MFP), and 3-ketoacyl-CoA thiolase (KAT). Jasmonic acid is finally exported to the cytoplasm, where it is conjugated with isoleucine to form bioactive (+)-7-iso-JA-Ile (Wasternack and Strnad 2016).
4 Biosignalling of Jasmonates
A JA response mutant coronatine insensitive 1 (coi1) in Arabidopsis thaliana enlighten our understanding in JA signalling pathway (Feys et al. 1994). COI1 encodes for a F-box protein which is a putative JA receptor and it function in E3—ubiquitin ligase mediated degradation of target proteins (Thines et al. 2007; Chini et al. 2007; Xu et al. 2002) JASMONATE ZIM DOMAIN (JAZ) protein (Fig. 1). Further, identification of JA responsive MYC transcription factors, revealed pathways for JA perception and JA dependent gene regulation. At low level of JA, expression of JA response and JA responsive genes are not active and MYC2 transcription factors are also inactive by interacting with JAZ protein, the JA signalling repressor. JAZ protein contain two domains, ZIM and JAS. The ZIM domain regulate its dimerization and interaction with NINJA further connecting it to another protein TOPLESS, a transcription suppressor in JA signalling pathway (Huang et al. 2017). The JAS domain regulate interaction of JAZ with COI1. In response to endogenous or environmental signals JA biosynthesis pathway gets activated by binding of JA to COI1 receptor. This interaction results in degradation of JAZ protein followed by release of MYC2 from JAZ (Fig. 1). On its release MYC2 activates transcription of JA responsive genes. Both JAZ and MYC2 play significant role in plant growth and development as positive and negative regulator by regulating JA dependent inhibition of growth under various biotic and abiotic stresses.
5 Role of Jasmonates in Root Growth and Development
5.1 Gravitotropism Response
According to Cholodny and Went, shift in auxin transport from basipetal to lateral results in development of lateral auxin gradient and hence asymmetric growth thereby showing gravitotropism. In a study done on rice coleoptiles (Gutjahr et al. 2005) it is clearly evident that gravitropism is not caused only by auxin gradient but it also involve role of JA. The total JA content rises significantly in the gravitropically stimulated rice coleoptiles during the time course of stimulation and also JA is distributed in a gradient reciprocally oriented to the IAA-gradient. In Arabidopsis (Moseyko et al. 2002) wheat seedling (Kramer et al. 2003) expression of enzyme lipoxygenase the first enzyme of JA biosynthesis pathway is upregulated during gravitropic stimulation (León and Sánchez-Serrano 1999) further in rice coleoptile split in two halves, the transcript level of the JA-responsive gene GER1 increases in both halves. Since JA concentration generally increases in both flanks during initial stages of gravitropic stimulation but later a gradient is developed due to more synthesis in the upper flank. In order to find out whether the JA-gradient has any significant role in gravitropism two test were done in rice coleoptile (1) stimulated coleoptiles were flooded the with exogenous methyl-jasmonate (Me-JA) (2) JA-deficient rice mutant hebiba was compared with the wild type for time course of bending (Gutjahr et al. 2005). Results obtained showed that flooding with jasmonate delays the onset of gravitropic bending moreover a jasmonate-deficient rice mutant bends more slowly and much late in comparison to the wild type. This clearly indicates that that JA is not absolutely necessary for gravitropic bending but mainly seems to accelerate the bending process.
Investigations were also carried using 5 uM concentration of NPA to determine whether the JA-gradient is induced independently or it’s a downstream effect of the IAA-gradient. Results obtained showed that 5uM NPA efficiently suppressed the establishment of an IAA-gradient but has no effect on the JA-gradient (Gutjahr et al. 2005).
In conclusion JA is not absolutely necessary for gravitropic bending but at the same time it accelerates the gravitropic response.
5.2 Inhibition of Primary Root Growth
Exogenous application of JA inhibits growth of primary root. In Arabidopsis thaliana COI1 together with JAZ and inositol pentakisposphate (InsP5) form a coreceptor for JA–Ile (Sheard et al. 2010; Huang et al. 2017) to inhibit root growth. Mutation in COI1 makes the coreceptor complex insensitive to this inhibitory response. Several JAZ proteins have been identified in Arabidopsis of which few can directly recruit the corepressor TPL and related proteins to suppress JA response whereas many perform this function by interacting with NINJA and uses its EAR domain to recruit these co-repressors (Chini et al. 2007, 2016; Pauwels et al. 2010; Shyu et al. 2012; Thines et al. 2007; Thireault et al. 2015; Yan et al. 2007). The inhibitory effect of JAs on primary root growth can be suppressed by overexpression of some NINJA or JAZ protein mutants (e.g. JAZ1Δ3A, JAZ3ΔC, JAZ10.3/JAS1, JAZ10.4, JAZ8, and JAZ13). In response to JA-Ile the E3-ligase SCFCOI1 targets JAZ for degradation via 26S proteasome pathway. Several TFs in Arbidopsis including MYC2, 3 and 4 which are present in primary root apex function to promote inhibitory action of JA on growth of primary root (Fig. 2). MYC 2 interact with a mediator complex (MED 25) and repress expression of two genes PLT1 and PLT2 (PLETHORA genes), which results in restricted activity of root meristem and hence inhibit growth of primary root. MYC3 also interact with MED 25 and regulate the effect. Ubiquitination and phosphorylation of MYC2 by PLANT U-Box protein (PUB-10) and MAPK decreases inhibitory effect of JA on primary root growth (Fig. 2). Basic loop helix (bHLH) like TF also interact with JAZ. They compete with transcription activators MYC2 for common promoter sequences of target genes, inactivate them subsequently by binding to them and hence negatively regulate inhibition of primary root growth by JA.
An ethylene signalling TF EIN3-LIKE1 (EIL1) also interact with JAZ protein and positively regulate JA induced primary root growth inhibition and JA dependent root hair formation (Zhu et al. 2011) (Fig. 2). Effect of high salt condition on JA mediated inhibition of root growth was analysed in some rice mutants and it was observed that the inhibitory effect of JA on root growth decreases in JA biosynthesis mutants whereas in loss of function mutants the root growth was severely affected under high salt conditions (Hazman et al. 2015).
Lateral root formation is promoted by JA in Arabidopsis. This response is mediated by overexpression of ERF 109 which binds and activate promotor of an auxin biosynthetic gene ANTHRANICATE SYSNTHASE A1 (ASA1) and YUCCA (Cai et al. 2014b; Sun et al. 2009). At the same time JA negatively regulate adventitious root formation in Arabidopsis. This effect is controlled by auxin induced overexpression of GH3 enzymes. These enzymes inactivate JA by conjugating it to amino acids aspartic acid, methionine and tryptophan and hence promotes adventitious root formation (Gutierrez et al. 2012). In Petunia plant however JA enhances adventitious root formation (Lischweski et al. 2015) emphasizing differential effect of JA on adventitious root formation in different plant species.
5.3 Effect on Nodulation
In order to maintain balance in symbiotic relation, leguminous plants have a systemic regulation system called autoregulation of nodulation (AUT). Mechanism of AUT is similar to systemic acquired resistance (SAR). Exogenous application of methyl jasmonates (MeJA) in Lotus japonicas wild type and one of its mutant har-1 resulted in suppression of nodulation in wild type and also suppression of hypernodulation in the har1-4 (Nakagawa and Kawaguchi 2006). Higher concentration of MeJA showed similar response in both wild type as well as mutant whereas at lower concentration suppression effect is more pronounced in mutant. Since JA are known to inhibit plant growth and degradation of photosynthetic pigment therefore suppression of nodule formation may be a secondary effect of growth inhibition. When MeJA applied at conc 10–4 M it result in significant reduction of root hair curling, infection threads and nodule primordia formation moreover higher concentration of the rate 10–3 completely block root hair deformation and curling. These results indicate that shoot applied with MeJA inhibits early stages of bacterial infection and nodule initiation. A gene NIN which is already nodulin gene induced in response to nod factor, coded for a putative transcription regulator which is required for formation of infection thread and inception of nodule primordia. Expression of NIN gene is significantly reduced in legumes shoot treated with MeJA. These finding suggests that inhibitory effect of MeJA on infection and nodulation in legumes occur upstream of the induction of NIN transcript.
5.4 Jasmonate Mediated Root Curling
A gene identified as Oryza sativa root meander curling (OSRMC) is expressed largely in roots of rice plant. It results in production of a putative receptor protein OSRLK, AAL87185. Expression of this gene is induced by application of JA. RNAi based knockdown of this gene in transgenic rice plants results in altered root development and coiling pattern. The primary root in RNAi transgenic rice plant meanded and curled more efficiently than wild type plant roots when treated with JA. In these transgenic plants primary roots were shorter, number of lateral was low whereas adventitious roots increased in number. Transgenic rice also showed increased expression of one of the JA signalling pathway gene RSOsPR10. It is very evident from the results obtained that OSRMC a DUF26 subfamily gene is directly involved in JA signalling mediated root development process and negatively regulates root curling in rice (Jiang et al. 2007).
5.5 Disruption of Root Mitochondria
Application of MeJA results in reduction in accumulation of protein related to energy metabolism. Treating the hairy roots (HR) with MeJA increases in accumulation of H2O2 in the initial 48 h and gradually the concentration decreases thereafter due to disruption of root tissues and also the mitochondrial membrane in the roots. The disintegration of mitochondrial membrane, reduction in ATP synthesis and increased accumulation of H2O2 suggest that mitochondria in hairy root (HR) might be the target organelle for MeJA signalling. Activity of enzymes like POX and CAT al decreases in HR treated with MeJA which are responsible for accumulation of H2O2. In overall H2O2 outburst due to MeJA could be a initiating response for disruption of root mitochondria (Loyola-Vargas et al. 2012).
5.6 Regulation of Beneficial Microbe—Root Interaction
JA promotes interaction between plant roots and beneficial bacteria or fungi. Generally JA signalling at moderate rate promotes symbiotic association while at the same time high rate of JA signalling inhibits this response. Besides JA signalling rate the mutualism is also dependent on compatibility between microbe—host and environmental factors. A recent study has shown that when arbascular mycorrhiza colonizes barley roots it results in elevation of endogenous JA level, expression of JA responsive genes and JA biosynthetic genes in cells containing arbascular (Hause et al. 2002). Further, studies have also shown that treatment with JA stimulates mycorrhizal development in endo and ectomycorrhizal associations (Regvar et al. 1996, 1997) and expression of symbiotic nod genes in Rhizobium (Rosas et al. 1998).
6 Crosstalk of Jasmonates with Other Phytohormones During Root Development
Auxin: Wild plants of Arabidopsis when treated with JA showed shorter roots due to decreased apical growth of roots while JA signalling mutant showed normal size roots even on treatment with JA (Jang et al. 2017). In contrast auxin deficient or auxin signalling mutants like (trp2-12) and (arx 3-1) from very short roots compared to wild type plants (Ursache et al. 2014; Zhang et al. 2019). This clearly indicates that JA induced inhibition of root growth might be regulated by its interaction with auxin (Chen et al. 2011). Inhibition of root growth is actually a result of reduced meristem activity. Application of JA on plants suppress expression of auxin responsive transcription factor PLETHORAs (PLTs) which maintain stem cells and their proliferation in meristem (Mähönen et al. 2014). However in JA signalling mutant like coi I and myc2, PLTs expression is not suppressed indicating thereby that COI1—dependent JA signalling mediates JA induced root phenotype and transcription factor MYC2 suppresses expression of PLTs. Therefore JA and auxin acts antagonistically for regulating apical growth of roots. Formation of lateral and adventitious roots in plants is also due to interplay between JA and auxin biosynthetic genes.
Cytokinin: Water and minerals transporting xylem elements develop form procambium cells in their roots. Cytokinin transcription mutant of type B, ARRSs and transgenics overexpressing AHP6—a negative regulation of cytokinin signalling form extra xylem (Yokoyama et al. 2007; Jang et al. 2017). JA deficient OPDA reductase 3 (opr3) when treated with JA showed an extra xylem phenotype, whereas JA signalling mutant coiI, jasmonate resistant I (jar I), failed to do so (Jang et al. 2017). From these studies conclusion can be drawn that stress hormone JA antagonistically interact with cytokinin in xylem development in plant roots. Molecular studies done further validates that JA reduces expression of cytokinin responsive gene PINFORMED (PIN 7) which controls xylem development. Further myc2 mutant fail to form extra xylem on exogenous application of JA and it also lacks expression of AHP6 a cytokinin signalling inhibitor.
Ethylene: JA and ethylene coordinate together to regulate many plant stress responses via JAZs—MYC2 and EIN3/EIL1. In plants EIL 1 an essential TF in ethylene signalling, interact with JAZ protein and positively regulates both JA—dependent primary root growth inhibition and JA induced root hair formation (Zhu et al. 2011).
ABA: JAZ—MYCs are involved in crosstalk between JA and ABA signalling pathways, affecting various aspects of plant growth (Chen et al. 2011). A ABA receptor PYRABACTIN RESISTANCE 1 Like protein (PYLS) forms a complex with JA2, which interacts with MYC2 and activates its transcriptional activity. The activated MYC2 inhibits the expression of PLT 1 and PLT2 (PLETHORA 1 and 2) and hence the primary root growth.
7 Conclusions
Jasmonic acid and its derivatives are involved in regulating various developmental and growth related processes in plants. It is produced in response to various biotic and abiotic stresses. JA controls various aspect of root growth and development like, inhibition of primary growth of roots, promotes lateral roots, inhibits formation of adventitious roots, colonization of roots with beneficial microbes, gravitropism, root curling behaviour, nodulation etc. Most of the effect on roots are negatively controlled by JA signalling in plants. JA is involved in crosstalk with several other hormone like auxin, cytokinin, ethylene and ABA for mediating root growth and development in plants. All effects regulated by JA and its interaction with other phytohormone in genetically controlled and well elucidated by several studies carried out in recent past.
Abbreviations
- α-LeA:
-
α-Linolenic acid
- LOX:
-
Lipoxygenase
- COI 1:
-
Coronatine insensitive 1
- MeJA:
-
Methyl jasmonates
- AOS:
-
Allene oxide synthase
- AOC:
-
Allene oxide cyclase
- OPDA:
-
(9S,13S)-12-oxo-phytodienoic acid
- JAZ:
-
JASMONATE ZIM DOMAIN
- AUT:
-
Autoregulation of nodulation
- PLT:
-
Plethora
- JA-Ile:
-
Jasmonate isoleucine
- bHLH:
-
Basic loop helix
- HR:
-
Hairy root
- ASA1:
-
Anthranicate sysnthase A1
- TF:
-
Transcription factor
References
Aldrige DC, Galt S, Giles D, Turner WB (1971) Metabolites of Lasiodiploidia theobromae. J Chem Soc C Organic 1623–1627
Browse J (2005) Jasmonate: an oxylipin signal with many roles in plants. Vitam Horm 72:431–456
Browse J (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60:183–205
Cai ZY, Liu JJ, Wang HJ, Yang CJ, Chen YX, Li YC, Pan SJ, Dong R, Tang GL, Barajas-Lopez JD (2014b) GSK3-like kinases positively modulate abscisic acid signalling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc Natl Acad Sci USA 111:9651–9656
Chen Q, Sun J, Zhai Q, Zhou W, Qi L, Xu L, Wang B, Chen R, Jiang H, Qi J (2011) The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 23:3335–3352
Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, LorenzoO, Garcia-Casado, G, Lopez-Vidriero I, Lozano FM, Ponce MR et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448:666–671
Chini A, Gimenez-Ibanez S, Goossens A, Solano R (2016) Redundancy and specificity in jasmonate signalling. Curr Opin Plant Biol 33:147–156
Dathe W, Rönsch H, Preiss A, Schade W, Sembdner G, Schreiber K (1981) Endogenous plant hormones of the broad bean, Vicia faba L. (-)-jasmonic acid, a plant growth inhibitor in pericarp. Planta 153:530–535
Demole E, Lederer E, Mercier D (1962) Isolement et détermination de la structure du jasmonate de méthyle, constituant odorant caractéristique de l’essence de jasmine. Helvitica 45(2):675–685
Du H, Liu H, Xiong L (2013) Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci 4:397
Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6(5):751–759
Fukui H, Koshimizu K, Yamazaki Y, Usuda S (1977) Structure of plant growth inhibitors in seeds of Cucurbita pepo L. Agricult Biol Chem 41:189–194
Gutjahr C, Riemann M, Müller A, Düchting P, Weiler EW, Nick P (2005) Cholodny-Went revisited: a role for jasmonate in gravitropism of rice coleoptiles. Planta 222:575–585
Gutierrez L, Mongelard G, Flokova K et al (2012) Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 24:2515–2527
Hause B, Maier W, Miersch O, Kramell R, Strack D (2002) Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol 130:1213–1220
Hazman M, Hause B, Eiche E, Nick P, Riemann M (2015) Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J Exp Bot 66:3339–3352
Huang H, Liu B, Liu L, Song S (2017) Jasmonate action in plant growth and development. J Exp Bot 68(6):1349–1359
Jang G, Chang SH, Um TY, Lee S, Kim J-K, Do CY (2017) Antagonistic interaction between jasmonic acid and cytokinin in xylem development. Sci Rep 7:10212
Jiang J, Li J, Xu Y, Han Y, Bai Y, Zhou G, Lou Y, Xu Z, Chong K (2007) RNAi knockdown of Oryza sativa root meander curling gene led to altered root development and coiling which were mediated by jasmonic acid signalling in rice. Plant Cell Environ 30:690–699
Kramer S, Piotrowski M, Kühnemann F, Edelmann HG (2003) Physiological and biochemical characterization of ethylenegenerated gravicompetence in primary shoots of coleoptile-less gravi-incompetent rye seedlings. J Exp Bot 54:2723–2732
León J, Sánchez-Serrano JJ (1999) Molecular biology of jasmonate biosynthesis in plants. Plant Physiol Biochem 37:373–380
Lischweski S, Muchow A, Guthörl D, Hause B (2015) Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol 15:229
Loyola-Vargas V, Ruíz-May E, Galaz-Ávalos R, De-la-Peña C (2012) The role of jasmonic acid in root mitochondria disruption. Plant Signal Behav 7(6):611–614
Mähönen AP, Tusscher KT, Scheres B (2014) PLETHORA gradient formation mechanism separates auxin responses. Nature 515:125–129
Moseyko N, Zhu T, Chang HS, Wang Z, Feldman LJ (2002) Transcription profiling of the early gravitropic response in Arabidopsis using high-density oligonucleotide probe microarrays. Plant Physiol 130:720–728
Nakagawa T, Kawaguchi M (2006) Shoot-applied MeJA suppresses root nodulation in Lotus japonicas. Plant Cell Physiol 47(1):176–180
Pauwels L, Barbero GF, Geerinck J et al (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464:788–791
Regvar M, Gogala N, Zalar P (1996) Effects of jasmonic acid on mycorrhizal Allium sativum. New Phytol 134:703–707
Regvar M, Gogala N, Znidarsic N (1997) Jasmonic acid effects mycorrhization of spruce seedlings with Laccaria laccata. Trees-Struct Funct 11:511–514
Rosas S, Soria R, Correa N, Abdala G (1998) Jasmonic acid stimulates the expression of nod genes in Rhizobium. Plant Mol Biol 38:1161–1168
Sheard LB, Tan X, Mao H et al (2010) Jasmonate perception by inositolphosphate-potentiated COI1-JAZ co-receptor. Nature 468:400–405
Shyu C, Figueroa P, Depew CL et al (2012) JAZ8 lacks a canonical degron and has an EAR motif that mediates transcriptional repression of jasmonate responses in Arabidopsis. Plant Cell 24:536–550
Sun J, Xu Y, Ye S et al (2009) Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21:1495–1511
Thireault C, Shyu C, Yoshida Y, St Aubin B, Campos ML, Howe GA (2015) Repression of jasmonate signaling by a non-TIFY JAZ protein in Arabidopsis. Plant J 82:669–679
Thines B, Katsir L, Melotto M et al (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448:661–665
Ursache R, Miyashima S, Chen Q, Vatén A, Nakajima K, Carlsbecker A, Zhao Y, Helariutta Y, Dettmer J (2014) Tryptophan-dependent auxin biosynthesis is required for HD-ZIP III-mediated xylem patterning. Development 141:1250–1259
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response growth and development. Ann Bot 100(4):681–697
Wasternack C, Strnad M (2016) Jasmonate signaling in plant stress responses and development—active and inactive compounds. New Biotechnol 33:604–613
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in annals of botany. Ann Bot 111:1021–1058
Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang D, Xie D (2002) The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14:1919–1935
Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19:2470–2483
Yamane H, Sugawara S, Suzuki Y, Shimamura E, Takahashi N (1980) Syntheses of jasmonic acid related compounds and their structure-activity relationships on the growth of rice seedlings. Agr Biol Chem 44:2857–2864
Yokoyama A, Yamashino T, Amano Y-I, Tajima Y, Imamura A, Sakakibara H, Mizuno T (2007) Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol 48:84–96
Zhu Z, An F, Feng Y et al (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci USA 108:12539–12544
Zhang Y, He P, Ma X, Yang Z, Pang C, Yu J, Wang G, Friml J, Xiao G (2019) Auxin-mediated statolith production for root gravitropism. New Phytol 224:761–774
Acknowledgements
The authors gratefully acknowledge contribution of Ms. Bhavini Sharma in preparation of figures included in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sharma, S., Banerjee, M. (2021). Crosstalk of Jasmonates with Phytohormones Accompanying Root Growth, Development and Microbe-Interaction. In: Mukherjee, S., Baluška, F. (eds) Rhizobiology: Molecular Physiology of Plant Roots. Signaling and Communication in Plants. Springer, Cham. https://doi.org/10.1007/978-3-030-84985-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-84985-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84984-9
Online ISBN: 978-3-030-84985-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)