Abstract
Renal hyperparathyroidism (rHPT) is a complex and challenging disorder. It develops early in renal failure, mainly as a consequence of reduced levels of vitamin D, hypocalcemia, diminished excretion of phosphate, and inability to activate vitamin D. RHPT is associated with increased risk of fractures, cardiovascular disease and death. The diagnosis of rHPT rests on demonstrating elevated levels of parathyroid hormone, PTH. Treatment consists of supplying vitamin D, reducing phosphate intake, and treatment with active vitamin D analogs. In later stages, calcimimetics might be added. However, in rHPT, parathyroid glands grow and can become refractory to medical treatment. Patients with rHPT refractory to medical treatment should be considered for parathyroidectomy, PTX. A close collaboration between nephrologists, endocrinologists, and endocrine surgeons is required to achieve optimal outcomes. Risks of surgery are small but not negligible. Surgery should likely not be too radical, especially if the patient is a candidate for future renal transplantation. Subtotal or total parathyroidectomy with autotransplantation are recognized surgical options. Hence, a classical, systematic, standard bilateral exploration to identify all parathyroid glands, including supranumerary glands, should be carried out. The endocrine surgeon performing this operation needs to be familiar with both the pathophysiology of rHPT but also needs a detailed knowledge of parathyroid embryology and anatomy. Surgery cannot cure rHPT, but will lower PTH levels and reduce morbidity and mortality. Intraoperative measurement of PTH can be helpful; the value of preoperative imaging studies to localize parathyroid glands has not been established for PTX in rHPT.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Case
A 57-year-old man who had been undergoing chronic hemodialysis for 6 years was listed for kidney transplantation. He presented with bone pain, symptoms of weakness, tiredness, and nausea. He had a history of cardiac bypass operation 3 years ago.
He was treated for arterial hypertension. Further medication included sevelamer and calcium carbonate as phosphate binders and cinacalcet as calcimimetic. Paricalcitol was stopped because of elevated calcium phosphate product.
His laboratory findings were the following:
-
Ca 2.3 mmol/l (normal range 2.1–2.6)
-
iPTH 1250 pg/ml (normal range 15–65)
-
P 4.5 mmol/l (normal range 0.84–1.45)
-
Vitamin D 25-OH 10 ng/ml (normal range >37.5).
Questions
-
1.
Which therapy would you suggest for this patient?
-
1.
Increase dose of cinacalcet
-
2.
Add cholecalciferol
-
3.
Waiting for kidney transplantation and no change of the medical treatment
-
4.
Parathyroid operation
-
5.
Switch from cinacalcet to etelcalcetide (iv)
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
2.
What are the indications for operation in renal hyperparathyroidism?
-
1.
Resistance to medical treatment
-
2.
Hypocalcemia
-
3.
PTH >800 pg/ml
-
4.
Hypercalcemia
-
5.
Calciphylaxis
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(1) and (3) and (4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
3.
Which factors play a role in the pathophysiology of renal hyperparathyroidism?
-
1.
Phosphate
-
2.
Vitamin D
-
3.
PTH
-
4.
Fibroblast growth factor 23
-
5.
Alpha-Klotho
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(2) and (3) and (4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
4.
Complications of renal hyperparathyroidism are:
-
1.
Kidney stones
-
2.
Vascular calcification
-
3.
Bone disease
-
4.
Pruritus
-
5.
Tissue calcification
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(2) and (3) and (4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
5.
What are medical treatment options for renal hyperparathyroidism
-
1.
Phosphate binders
-
2.
Supplementation with calciferol
-
3.
Calcimimetics
-
4.
Diuretics
-
5.
Calcium supplementation
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(1) and (2) and (3) and (4) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
6.
Phosphate binders in the treatment of renal hyperparathyroidism are:
-
1.
Paricalcitol
-
2.
Lanthanum carbonate
-
3.
Ferric citrate
-
4.
Calcium carbonate
-
5.
Sevelamer
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(2) and (3) and (4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
7.
Which statements regarding cinacalcet are correct?
-
1.
Cinacalcet reduces the PTH level.
-
2.
Cinacalcet reduces the risk of death.
-
3.
Cinacalcet reduces the risk of cardiovascular disease.
-
4.
Cinacalcet reduces the risk of fractures.
-
5.
Cinacalcet reduces gastrointestinal symptoms like nausea.
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(2) and (3) and (4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
8.
Which statements regarding renal hyperparathyroidism are correct?
-
1.
Adynamic bone disease is an indication for operation.
-
2.
The drop of intraoperative PTH is slower in renal hyperparathyroidism than in primary hyperparathyroidism.
-
3.
Supernumerary glands play a role in renal hyperparathyroidism.
-
4.
Recurrent disease is common in renal hyperparathyroidism due to the natural course of the disease.
-
5.
Kidney transplantation is the best therapy.
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(2) and (3) and (4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
9.
What statement(s) regarding preoperative management in renal hyperparathyroidism is(are) correct?
-
1.
Preoperative laryngoscopy should be performed.
-
2.
Preoperative ultrasound of the thyroid should be performed.
-
3.
A preoperative MIBI scan should be performed.
-
4.
Localization procedures are not mandatory in primary operation.
-
5.
Localization procedures should be performed in recurrent disease.
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) and (5) are correct.
-
(d)
(1) and (2) and (3) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
10.
Which operative procedures are advisable in this patient (more than 1 choice)?
-
1.
Subtotal parathyroidectomy
-
2.
Subtotal parathyroidectomy with transcervical thymectomy
-
3.
Total parathyroidectomy with autotransplantation and with transcervical thymectomy
-
4.
Total parathyroidectomy without autotransplantation
-
5.
Removal of enlarged parathyroid glands
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(1) and (2) and (3) and (4) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
11.
Which statement(s) regarding the operative strategy is(are) correct?
-
1.
Leaving too much parathyroid tissue increases the risk of recurrence.
-
2.
Leaving too little parathyroid tissue increases the risk of permanent hypocalcemia.
-
3.
The optimal level of postoperative PTH is unknown.
-
4.
A postoperative PTH level between 100 and 600 pg/ml seems to be associated with the lowest risk of mortality.
-
5.
Intraoperative PTH should be measured no earlier than 20 minutes post-resection.
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(2) and (3) and (4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
12.
If less than four parathyroid glands are found intraoperatively, what would you suggest to do:
-
1.
Autotransplantation of parathyroid tissue and termination of the operation
-
2.
Transcervical thymectomy
-
3.
Intraoperative PTH
-
4.
Venous sampling for ioPTH from the right and the left jugular vein
-
5.
Sternotomy
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (3) and (5) are correct.
-
(c)
Only (1) and (2) and (4) are correct.
-
(d)
(2) and (3) and (4) are correct.
-
(e)
All are correct.
-
(a)
-
1.
1 Introduction
Hyperparathyroidism (HPT) secondary to chronic kidney disease (CKD) is common in patients with chronic renal failure [1]. In this chapter, this condition is referred to throughout as renal hyperparathyroidism (rHPT) . Renal hyperparathyroidism is associated with decreased quality of life, increased risk of skeletal and cardiovascular complications, and mortality. Most patients with rHPT can be successfully managed medically, but some patients require surgery to control their hyperparathyroidism. Surgery with parathyroidectomy (PTX) cannot cure rHPT but will usually lead to markedly decreased levels of parathyroid hormone (PTH) and improved outcomes for patients with rHPT [1]. PTX should, in the vast majority of patients with rHPT, be performed as a bilateral, four-gland exploration. The endocrine surgeon performing parathyroid surgery in patients with rHPT thus must be familiar with the pathophysiology and the aspects of surgical and medical treatment, including their complications, of rHPT, and parathyroid embryology and anatomy, including variations. In this chapter, the conditions that cause parathyroid hyperplasia and autonomous production of parathyroid hormone (PTH) are outlined; indications and surgical technique are discussed, together with preoperative investigations and postoperative management.
2 Clinical Presentation
In most developed countries, patients with end-stage CKD are managed by nephrologists, i.e., internal medicine physicians specialized in kidney care. Almost all patients with CKD have rHPT to some extent, and the endocrine surgeon will usually only become involved once medical treatment can no longer control rHPT [1]. Hence, the common clinical presentation for the endocrine surgeon is that of a referral from the nephrologist for parathyroidectomy (PTX). In some units, patients are presented by the nephrologist to a multidisciplinary group, where endocrine surgeons, nephrologists, and kidney transplant surgeons discuss patients together.
Commonly, patients with rHPT referred for PTX suffer from the effects of long-standing renal disease; they also can have other complications to the underlying condition causing renal failure, such as hypertension or diabetes mellitus. Thus, patients with rHPT often have multiple comorbidities that have to be addressed before accepting and scheduling the patient for surgery. More detailed workup to establish whether the patient is fit for surgery, especially regarding the cardiopulmonary system, might be indicated.
Patients with rHPT referred for PTX usually have high levels of PTH, together with normal or high levels of calcium and phosphate. Apart from these laboratory manifestations, patients can also exhibit symptoms such as pruritus and thirst. Further symptoms are listed in ► Box 14.1. As rHPT becomes more pronounced, muscle weakness and fatigue are common. Vascular calcification and osteodystrophy can occur already during the early stages of CKD and progress as glomerular filtration rate (GFR) declines [2]. With advanced rHPT, patients can experience mood swings, conjunctivitis, as well as bone and joint pain. Late manifestations include soft tissue calcifications (◘ Fig. 14.1), brown tumors in the skeleton and calciphylaxis, and a severe, painful deposition of calcium salts in soft tissues [3].
3 Natural History
3.1 Chronic Kidney Disease (CKD)
Chronic kidney disease, CKD, is defined as abnormalities of kidney structure or function, present for more than 3 months, with implications for health [4]. CKD ranges from a mild asymptomatic decrease in renal function that remains stable for decades to a rapidly decreasing renal function with multiple complications and finally renal failure. Renal failure affects almost all organs in the human body, and patients with ESRD have 10–20 times increased mortality compared to the general population [5]. The main cause of this increased mortality is cardiovascular disease, but patients with renal failure also develop bone disease. The complex relation between vascular calcifications, bone, and kidney has led the international group Kidney Disease Improving Global Outcomes (KDIGO) to formulate clinical guidelines for the management of Chronic Kidney Disease – Mineral and Bone Disorder (CKD-MBD) [2].
Renal hyperparathyroidism, rHPT, with increasing levels of parathyroid hormone (PTH) and parathyroid gland hyperplasia, is a major part of CKD-MBD and develops in all patients with CKD as renal function deteriorates [2]. CKD is divided into five stages based on glomerular filtration rate (GFR) [4], ranging from stage 1 where the GFR is >90 ml/min/1.73m2 to stage 5 where GFR is <15 ml/min/1.73m2 (see ◘ Table 14.1). In a recent review, the global prevalence of CKD stages 3–5 was about 10% [6]. Thus, CKD is common, and with an aging population, it is a growing global problem [7]. Both death and disability-adjusted life years lost due to CKD are increasing [8]. The medical costs attributable to CKD are substantial and increase as disease severity worsens, particularly if renal replacement therapy (RRT) has to be initiated [9]. Renal hyperparathyroidism worsens outcomes in patients with CKD, especially regarding vascular and bone-related outcomes, but it also leads to shorter life expectancy [10].
3.2 FGF23, Klotho
A central hormone in rHPT is fibroblast growth factor 23 (FGF23). This hormone is produced by osteoblasts and osteocytes and is the major phosphate regulatory hormone [11]. FGF23 is induced by high levels of phosphate, active vitamin D, and PTH [12, 13] and increases early in CKD [11]. FGF23 binds to its receptor, FGF Receptor 1 (FGFR1), which requires a co-receptor, α-klotho, to function [14]. α-Klotho is expressed in the kidney and parathyroid tissue [15]. FGF23 decreases the phosphate reuptake in the distal tubule and thus lowers blood levels of phosphate [16]. FGF23 also downregulates 1- α-hydroxylase and upregulates D-24-hydroxylase in the kidney with the net effect of lower levels of active vitamin D and lower uptake of phosphate in the intestines [17].
FGF23 has two key effects on calcium-phosphate-vitamin D homeostasis: it suppresses the activation of vitamin D in the proximal tubules, functioning as a counterregulatory hormone for 1,25 dihydroxivitamin D3 (calcitriol) and it suppresses the reabsorption of phosphate, thus inducing phosphaturia. In the course of progressive renal failure FGF23 increases first, followed by a decrease in 1, 25 dihydroxivitamin D3 after which levels of parathyroid hormone increase. Finally, plasma phosphate further stimulates hyperparathyroidism [11, 18].
3.3 Phosphate Retention
A positive phosphate balance is another central factor in the development of rHPT. With declining renal function, the ability to maintain mineral homeostasis is impaired, both by a reduced capacity to filter phosphate due to loss of renal function and by the disturbed function of the bone. In CKD, various types of bone disease occur, all characterized by excessive bone resorption compared to formation [19]. This occurs early in CKD and reduces the capacity for the skeleton to buffer phosphate load. Instead, the skeleton contributes to hyperphosphatemia [20]. The positive phosphate balance leads to elevated levels of FGF23. Phosphate also stimulates the release of PTH. The phosphaturic actions of PTH together with FGF23 keep phosphate levels regulated in early CKD [16]. Phosphate levels in the blood remain normal until CKD stages 4–5 when hyperphosphatemia is common [21]. This is due to the loss of functioning nephrons and because tubular reabsorption is already maximally inhibited by FGF23 and PTH. In CKD, due to bone disease, the phosphate reservoir is shifted to soft tissue (e.g., vasculature), a process that is driven by multiple bone-specific signaling pathways, many of them directly activated by phosphate itself [20]. Long-lasting hyperphosphatemia thus leads to vascular calcifications [22] and is a central element of the development of CKD-MBD and rHPT (see also ◘ Fig. 14.2).
3.4 Vitamin D and Calcium
Vitamin D plays an important role in mineral homeostasis. Native vitamin D (25-hydroxyvitamin-D) is activated in the kidney via 1- α-hydroxylase [23] to the active form 1,25-dihydroxyvitamin-D. Activated vitamin D acts via the vitamin D receptor (VDR) in the intestines to stimulate calcium and phosphate uptake [24]. In the parathyroid gland, activation of vitamin D-receptors leads to reduced production and release of PTH and suppression of parathyroid gland proliferation [25]. The elevated levels of FGF23 in early CKD contribute to low levels of activated vitamin D, and later on, loss of nephrons also contributes to a deficiency of active vitamin D [21]. Patients with CKD also have low levels of native vitamin D due to albuminuria, low exposure to sunlight, and poor dietary intake [26]. The result of vitamin D deficiency is hypocalcemia. In late CKD, both high phosphate and vitamin D deficiency leads to hypocalcemia which is the most potent stimulator of PTH release via the calcium-sensing receptor in the parathyroid gland (CaSR) [27]. Apart from other effects of PTH described earlier, the most potent effect is to increase serum calcium levels by enhancing renal tubular calcium reabsorption, stimulating net bone resorption, and increasing the production of activated vitamin D (1,25(OH)2D3) [28]. Low levels of active vitamin D also directly result in PTH release and parathyroid cell proliferation.
3.5 Parathyroid Gland Hyperplasia
The leading factors for parathyroid gland hyperplasia are active vitamin D, calcium, and phosphate. Transforming growth factor-alpha (TGF-α) is a potent proliferative agent for parathyroid cells via the activation of the epidermal growth factor receptor (EGFR). Activation of EGFR both leads to the proliferation of parathyroid cells and lesser expression of VDR [29]. The anti-proliferation pathway is mediated via cyclin-dependent kinase inhibitor p21 and also reduced expression of TGF-α. Both active vitamin D and high levels of calcium inhibit parathyroid cell proliferation through this pathway [30, 31]. Thus, low levels of active vitamin D in CKD contribute to parathyroid cell proliferation [32]. In uremic rats, high dietary intake of phosphate increases TGF-α, and low dietary intake of phosphate enhances the expression of p21 independent of vitamin D, which is why phosphate also contributes to parathyroid cell proliferation [33]. In early CKD, the parathyroid gland often shows polyclonal proliferation, and in late CKD, monoclonal/nodular proliferation is more common. However, different pathological changes often coexist in the same parathyroid gland [34]. With more severe rHPT, the expression of VDR CaSR and α-klotho is reduced [35,36,37].
3.6 Tertiary Hyperparathyroidism
Long-standing CKD with rHPT leads to polyclonal and eventually monoclonal proliferation of parathyroid tissue with a loss of regulatory receptors [38]. This condition of autonomous parathyroid gland function is sometimes referred to as tertiary hyperparathyroidism and is characterized by high levels of PTH in the presence of persistent hypercalcemia [39]. A histological finding of severe hyperplasia together with high levels of parathyroid hormone and persistent hypercalcemia and hyperphosphatemia in patients with rHPT is associated with failure to respond to medical treatment [40]. Tertiary (autonomous) HPT is a complication of long-term CKD and can persist after successful renal transplantation. Two years after renal transplantation, an incidence of about 30% has been reported [41]. However, the term tertiary HPT has also been defined as persistent HPT after renal transplantation [42]. In reality, there is a gradual increase of autonomy in the parathyroid glands with increasing time of CKD, and even if renal transplantation ameliorates rHPT, it never corrects it completely.
4 Diagnosis
The diagnosis of rHPT is a process. Initially plasma calcium (low to normal), phosphate (elevated), PTH (elevated), and vitamin D (low) is sufficient for the diagnosis of rHPTH. Moreover, in patients with evidence of CKD-MBD or osteoporosis, monitoring of bone mineral density with dual-energy X-ray absorptiometry (DEXA) is recommended (KDIGO). As long as rHPT can be controlled with medical therapy, further investigations are generally not required. However, when PTH, plasma calcium, and phosphate no longer can be controlled, further investigation is necessary. Firstly, patient symptoms should be explored to guide the extent of the investigation. If the nephrologist is convinced that the patient has rHPT resistant to medical treatment, contact with an endocrine surgeon should be established.
5 Non-surgical Treatment
5.1 Medical
Most patients with rHPT are treated medically; only a minority require surgery. Medical treatment options are summarized in ◘ Table 14.2. The surgeon needs to have a general understanding of the medical treatment options, to be able to make balanced decisions on when and whom to operate.
5.1.1 Vitamin D
The first line of treatment is supplementation with vitamin D (calciferol), which is recommended in non-dialysis patients with CKD stages 3a to 5 [2, 43]. In a recent randomized controlled trial in patients with CKD stages 3 and 4, 12 weeks of supplementation with cholecalciferol resulted in a decrease in PTH with stable levels of plasma calcium [44].
5.1.2 Control of Calcium and Phosphate
5.1.2.1 Restriction of Dietary Intake
As plasma phosphate begins to rise, phosphate intake restriction is recommended. This can be difficult. Dietary protein restriction leads to lower phosphate intake and can be used for patients not yet on dialysis. Patients on dialysis need extra protein, which makes phosphate restriction more complicated. Thus, most patients will require treatment with phosphate binders.
5.1.2.2 Treatment with Phosphate Binders
Phosphate binders are central in the treatment of hyperphosphatemia. Phosphate levels are positively associated with mortality [45, 46]. Modern phosphate binders, such as sevelamer hydrochloride and lanthanum carbonate are effective and safe [47]. Phosphate control is an important priority in patients with CKD and levels should be maintained within the normal range [2].
5.1.2.3 Active Vitamin D Analogs
The active vitamin D analogs bind to vitamin D receptors in many tissues such as the parathyroid gland and the intestine. They decrease levels of PTH but can cause hypercalcemia [48].
5.1.2.4 Calcimimetics
In 2002, cinacalcet was shown to successfully lower PTH in patients on hemodialysis [49]. A randomized controlled trial in 2004 showed that cinacalcet decreased PTH, calcium, and phosphate levels in patients on hemodialysis [50]. Similar positive results were also reported in patients with CKD stages 3–4 [51]. The introduction of cinacalcet carried high hopes and was thought of as a “medical parathyroidectomy.” Disappointingly, cinacalcet has not been as effective as initially expected. In an observational study using data from a French registry, cinacalcet did not lower PTH values compared with patients without the treatment [52]. Further, cinacalcet treatment did not reduce the risk of death or major cardiovascular events in the EVOLVE trial, a large, double-blind, multi-center randomized trial (RCT) [53]. However, cinacalcet decreased rates of bone formation, and some biochemical markers of high-turnover bone disease as PTH was reduced, with 26% of the patients achieving normal bone histology after 12 months of treatment [54]. Cinacalet has some unwanted side effects. All studies report that hypocalcemia, nausea, and vomiting are frequent and difficult side effects in patients treated with cinacalcet [50, 52, 53], and these symptoms often cause the patient to stop treatment.
The latest calcimimetic, etelcalcetide, is administered intravenously. In an RCT in hemodialysis patients, the effects of etelcalcetide on PTH were found to be non-inferior to cinacalcet [55]. The frequency of nausea and vomiting was similar in both treatment groups, but the etelcalcetide group was more likely to experience hypocalcemia compared with the cinacalcet group [55].
5.2 Renal Transplantation
Renal transplantation offers the best outcomes for patients with CKD needing renal replacement therapy [56]. Mortality and morbidity are much lower, and quality of life higher, than with dialysis [56].
RHPT also improves after renal transplantation [57]. After successful renal transplantation, the mineral homeostasis changes completely. The remaining high FGF23 and PTH increase the secretion of phosphate in the urine, resulting in hypophosphatemia [58]. Levels of FGF23 decrease, and the expression of α-klotho increases after transplantation [59]. Levels of vitamin D and calcium increase [60]. Hypercalcemia in the first one to six months is common and is associated with high levels of PTH [61]. Levels of PTH accumulate during ESRD, and a rapid decrease in PTH is seen immediately after transplantation. Thereafter, levels of PTH keep decreasing slowly and stabilize after the first 6 months [57]. However, the majority of patients still have PTH levels above the reference range 1 year after transplantation [62]. Risk factors for post-transplant rHPT are pre-transplant levels of PTH and calcium, time spent on dialysis before transplantation, and nodular hyperplasia of the parathyroid glands [63]. Cardiovascular disease is the leading cause of death in renal transplant recipients [64], and some data support an association with rHPT [65, 66]. Bone disease after renal transplantation can be both due to rHPT but also to factors specific to transplantation such as corticosteroids and immunosuppressive agents [67, 68].
6 Surgical Treatment: Parathyroidectomy
6.1 Indications
As stated above, rHPT is initially a physiologic adaptation to the decreasing renal function. However, with time, hyperparathyroidism becomes deleterious, increasing the risk for cardiovascular and skeletal disease, and can lead to shortened survival in patients with CKD [1]. Most patients are successfully managed medically, as outlined above. However, in a small but important subset of patients, medical treatment cannot control rHPT. In these patients, surgical treatment with parathyroidectomy (PTX) is an option. KDIGO CKD-MBD guidelines state that PTX is indicated in “patients with ESRD and severe HPT who fail to respond to pharmacological treatment” [2]. The European Society of Endocrine Surgeons in 2015 stated that “PTX is an option in any patient with rHPT, but that in most patients, the condition can be managed medically.” Specifically, PTX would be indicated when “medical treatment fails to correct metabolic parameters – PTH>85 pmol/l, hypercalcemia and hyperphosphatemia” [69].
No RCTs compare PTX to medical treatment. Hence, guideline recommendations rely on data from observational studies. Given the heterogeneity of patients with rHPT, the differences in types of dialysis, whether patients had or had not previously received a renal transplant, differences in medication, etc., it has been hard to define specific indications for surgery in a given patient. Indications likely differ according to sex, age, and type of underlying renal disease, whether the patient has a functioning transplant or the patient’s chance of receiving a transplant.
Epidemiologic studies indicate that parathyroidectomy rates decreased in the first years after the introduction of calcimimetics but have since risen again [70]. They also point to regional differences within and between countries, probably due to different access to nephrologists and/or endocrine surgeons, and to different therapy strategies between institutions. Multiple regression models suggest that women, younger patients, and non-diabetic patients have a greater probability of undergoing PTX [70].
There is also evidence that PTX is associated with reduced risk of fractures [71], cardiovascular disease [72], and mortality [73]. Further, studies show improved quality of life after PTX [74]. PTX is also more cost-efficient than calcimimetics in most patients with ESRD [75]. However, morbidity and even mortality after PTX are not insignificant [76, 77]; hence, in all patients, surgical risk must be weighed against potential long-term improvement in outcomes.
Even if most of these studies tried to adjust for confounders, a selection bias cannot be completely ruled out. Patients that are referred for parathyroidectomy are healthier and have a better prognosis than patients who do not get referred for surgery. Unfortunately, it is unlikely that an RCT comparing medical treatment to PTX will ever be performed, given the large number of centers that would be needed to perform such a study.
6.1.1 Is There an Absolute Threshold of PTH When PTX Is Indicated?
In patients on dialysis, according to KDIGO, PTH levels should be maintained between 2 and 9 times the upper normal limit, corresponding to approximately between 15 and 55 pmol/L [2]. Although not explicitly stated in the guidelines, if medical treatment fails to keep PTH in this range, this value, 55 pmol/L, could be used as an indication for PTX. Other authors recommend PTX only at higher levels, 80–100 pmol/L [69, 78]. Published series report mean preoperative PTH levels ranging from 87 pmol/L [79] to 233 pmol/L [80]. Thus, there is no clear, absolute threshold of PTH levels where surgery is indicated.
6.1.2 Are There Other Specific, Absolute Indications for PTX, Apart from PTH Levels?
Calciphylaxis has by many been reported as an absolute indication for PTX [3], although this has also been disputed [81].
6.1.3 Should PTX Be Performed Before or After Renal Transplantation?
Whether to perform PTX or not is also influenced by potential future or previous renal transplantation. As discussed above, renal transplantation can be expected to ameliorate some but not all renal hyperparathyroidism. Some studies showed no difference in outcome [82], whereas others found better outcomes if PTX was performed before renal transplantation [83].
6.1.4 What, Exactly, Constitutes “Medical Failure”?
This is not exactly defined [2]. Patients with CKD are complex; they can have many comorbidities; the number of pills needed to compensate for the failing kidney and treat any underlying disease can be staggering [1]. Non-compliance is a common problem, often due to side effects [2]. Costs of treatment also need to be taken into consideration [75]. Thus, whether rHPT can be controlled medically or not has to be evaluated in each patient. In most settings, a specialized nephrologist is responsible for the patient and makes this evaluation. Accepted and pragmatic indications for PTX in rHPT are summarized in ► Box 14.2.
6.2 Surgical Technique
In almost all patients, PTX for rHPT should be conducted as a classical, bilateral, four-gland exploration [69] (◘ Figs. 14.3 and 14.4). Details of how to perform this operation are covered in other chapters. It has to be emphasized, that to perform parathyroid surgery successfully, the surgeon needs a detailed knowledge of parathyroid embryology and anatomy and its variations [84].
PTX is performed as either subtotal PTX, where the aim is to keep parathyroid tissue corresponding to one normal gland, or total parathyroidectomy, aiming at removing all parathyroid tissue (◘ Fig. 14.5). PTX is usually performed with open surgery through a Kocher cervical incision in general anesthesia [69], although there have been reports on minimally invasive PTX [85,86,87]. Subtotal and total PTX can both be combined with transcervical thymus resection and/or parathyroid autotransplantation (AT). However, subtotal PTX is normally not combined with AT, and total parathyroidectomy without AT is often performed without thymus resection. The lower parathyroids are often found in or close to the thymus, and nests of parathyroid tissue are also often found in normal thymic tissue. Hence, many authors recommend performing transcervical thymectomy together with PTX [69, 88].
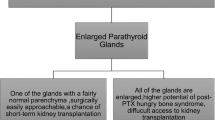
There has been a debate among endocrine surgeons as to whether less (subtotal/focused) or more (total) radical surgery is optimal in rHPT. Large population-based studies [89] and a meta-analysis [90] could not find any difference in long-term outcomes such as the risk of fracture, cardiovascular disease, and mortality between the two procedures. Furthermore, there has been a misunderstanding in that some authors believe that rHPT can be cured [91], analogous to primary HPT (pHPT), which has very high cure rates with the resection of one or more parathyroid glands [92]. However, pHPT and rHPT are different entities. It is evident from the discussion above that rHPT also persists even in mild renal dysfunction, even if the patient receives a renal transplant [93]. Hence, PTX cannot cure rHPT. Instead, PTX aims to reduce the amount of parathyroid tissue to such an extent that an optimal level of PTH post-PTX is achieved. This is similar to the situation in hereditary pHPT, e.g., multiple endocrine neoplasia type 1 (MEN1), which also cannot be cured, and where surgery aims to give the patient as many years with normocalcemia as possible [94].
The optimal level of PTH after PTX for rHPT is unknown. Probably, profound hypoparathyroidism is just as detrimental as severe hyperparathyroidism [78]– in patients with rHPT, as we have seen above, the initial adaptation of the parathyroids is physiologic, helping the body get rid of excess phosphate not cleared by the kidneys. Hence, leaving too little viable parathyroid tissue is suboptimal. On the other hand, leaving too much increases the risk of reoperation, due to persistent/recurrent disease. Thus, the question for the endocrine surgeon is not how much to remove, but how much to leave behind. Support for this concept comes from studies examining the correlation between PTH levels and long-term outcomes in patients with ESRD. Thus, a report from the DOPPS study in 2008 showed that PTH levels between 10 pmol/L and 60 pmol/L were associated with the lowest risk of mortality [45]. The same authors re-examined this issue and in 2015 reported similar findings [95]. In their multivariate analysis, patients in the reference group with levels of PTH between 15 and 30 pmol/L had the lowest mortality risk [95]. Data also show that PTH levels vary significantly after both subtotal and total PTX [89, 91].
6.2.1 Intraoperative Measurement of Parathyroid Hormone (ioPTH)
In primary HPT, intraoperative measurement of PTH (ioPTH) helps the surgeon to determine if there is more hyperfunctioning tissue left after resection or whether the operation can be terminated. There have been numerous studies investigating whether ioPTH also assists the surgeon performing PTX for rHPT [80, 96,97,98,99,100,101,102,103,104]. Most, but not all, of these studies indicate that there is a correlation between levels of ioPTH and postoperative PTH and that ioPTH helps determine the extent of PTX. Since PTH is cleared by the kidneys, the half-life of PTH, and hence the time needed to wait for a drop in intraoperative PTH, is longer after PTX for renal HPT. Probably, PTH should be measured no earlier than 15–20 minutes post-resection. Different criteria on the optimal level of ioPTH post-resection have been proposed, but there is no consensus on what level of ioPTH yields the best outcomes.
6.2.2 Preoperative Localization
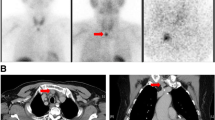
The outcome of PTX is highly dependent on the skills and experience of the surgeon. In experienced hands, the main cause of persistent or recurrent rHPT after PTX is the inability to localize ectopic parathyroid glands [105]. From a surgical point of view, a distinction exists between minor ectopy (such as in the thyrothymic horn and upper anterior mediastinum, or beneath thyroid capsule) and major ectopy (such as low mediastinal, retro esophageal, above the level of the hyoid, in the carotid sheath, or within the thyroid parenchyma – truly intrathyroidal) [106]. Ectopic and/or supernumerary glands are common in rHPT [69] and the surgeon must identify all parathyroid glands. The experienced surgeon will usually find all non-ectopic glands; preoperative localization should therefore positively and accurately localize all ectopic parathyroid glands. Similar to primary HPT, preoperative imaging, with modalities such as ultrasonography, 99-Technetium sestamibi scintigraphy, and four-dimensional computed tomography (4D-CT), has been evaluated but has not been shown to have greater accuracy in finding all parathyroid glands than traditional surgical exploration. A meta-analysis [107] reported that the sensitivity of the 99mTc-sestamibi scan in secondary HPT was only 58%. It was concluded that 99mTc-sestamibi is not a first-line diagnostic imaging method before PTX for rHPT. The sensitivity of ultrasound for the detection of enlarged parathyroid glands has been reported to be 46–81% in patients with secondary HPT [108,109,110]. The combination of ultrasound with 99mTc-sestamibi SPECT/CT had a higher sensitivity than US or 99mTc-sestamibi SPECT/CT alone [110]. Most authors thus conclude that ultrasound and sestamibi scintigraphy offer little benefit in localizing ectopic glands and rarely change the conduct of a standard four-gland exploration [38, 111, 112], although ESES recommended ultrasound pre-PTX, also to rule out co-existing thyroid disease [69]. However, some authors have found that SPECT-CT offers useful information [106]. On the contrary, in the setting of re-PTX, i.e. surgery for persistent or recurrent HPT after previous PTX, imaging studies are mandatory [69] (◘ Figs. 14.6 and 14.7).
6.2.3 Intraoperative Angiography
A further issue complicating PTX is that it is difficult to be certain that the parathyroid tissue left in the neck at surgery is viable – unintentional devascularization of parathyroid glands is common, both during parathyroid and thyroid surgery. Recently, intraoperative angiography of the parathyroids using indocyanine green has shown great promise in aiding the surgeon to determine whether parathyroid glands are functioning or not [113]. Combined with ioPTH and possibly with cross-sectional imaging, these tools might enable the surgeon to deliver a more precise PTX, yielding an optimal postoperative level of PTH [114].
6.2.4 Surgical Complications
Risks of PTX include damage to the recurrent laryngeal nerve, bleeding, and infection. These risks are small in the hands of experienced surgeons, and nationwide studies have shown these complications to be rare [115]. However, complications related to abnormal mineral metabolism are common and expected.
6.2.5 Postoperative Management
Patients undergoing PTX for renal hyperparathyroidism are best managed by nephrologists perioperatively, with input from the endocrine surgeon if needed. Profound postoperative hypocalcemia is not uncommon and perhaps ameliorated with preoperative calcitriol loading [116]. Admissions to intensive care units for hypocalcemia and re-admissions due to mineral metabolism imbalances are common; protocols for postoperative care after PTX might reduce these [117].
7 Outcomes and Prognosis
Overall, patient outcome after PTX is mainly determined by whether the patient will receive renal transplantation or not. Chronic dialysis is associated with a markedly reduced life-span; patients with renal transplants have an expected survival that is close to that of the normal population. Both patient and other factors are related to the chance of receiving a transplant; this is outside the scope of this chapter.
Regarding outcome after PTX, studies indicate that PTX diminishes the risk of fractures and is associated with better survival. As noted above, there is a significant risk of re-PTX after subtotal PTX; this risk is much lower after total PTX. However, studies also indicate that persistently low levels of PTH are associated with an increased risk of cardiovascular disease. No difference in survival has been established between total and subtotal PTX.
More research is needed to establish the exact indications for PTX, especially concerning its timing concerning renal transplantation. Knowledge of the optimal level of PTH after PTX for favorable long-term outcomes would also be useful, and application of modern tools (fluorescence, angiography) together with ioPTH to arrive at this level of PTH might be ways to improve outcomes in the future.
In conclusion, renal hyperparathyroidism develops early in renal failure, mainly as a consequence of reduced levels of vitamin D, hypocalcemia, diminished excretion of phosphate, and inability to activate vitamin D. RHPT is associated with increased morbidity and mortality. RHPT is a continuum and diagnosis depends on demonstrating elevated levels of parathyroid hormone, PTH. Treatment consists of supplying vitamin D, reducing phosphate intake, and treatment with active vitamin D analogs. In later stages, calcimimetics might be added. In rHPT, parathyroid glands grow and can become refractory to medical treatment. Patients with rHPT refractory to medical treatment should be considered for parathyroidectomy, PTX. A close collaboration between nephrologists, endocrinologists, and endocrine surgeons is required to achieve optimal outcomes. Risks of surgery are small but not negligible. Surgery should likely not be too radical, especially if the patient is a candidate for future renal transplantation. Subtotal or total parathyroidectomy with autotransplantation is recognized surgical options. Intraoperative measurement of PTH can be helpful; the value of preoperative imaging studies to localize parathyroid glands has not been established for PTX in rHPT.
Answers to Questions
1. (d); 2. (d); 3. (e); 4. (d); 5. (a); 6. (d); 7. (a); 8. (d); 9. (c); 10. (a); 11. (e); 12. (d)
References
Almquist M, Isaksson E, Clyne N. The treatment of renal hyperparathyroidism. Endocr Relat Cancer. 2020;27(1):R21–34.
Kidney Disease: Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1–130.
McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91(10):1384–94.
Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013;3(1):19–62.
Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–9.
Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765.
Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47.
Jager KJ, Fraser SDS. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant. 2017;32(suppl_2):ii121–8.
Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9):1478–83.
Young EW, Akiba T, Albert JM, et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(5 Suppl 2):34–8.
Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–8.
Bon N, Frangi G, Sourice S, Guicheux J, Beck-Cormier S, Beck L. Phosphate-dependent FGF23 secretion is modulated by PiT2/Slc20a2. Mol Metab. 2018;11:197–204.
Meir T, Durlacher K, Pan Z, et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 2014;86(6):1106–15.
Kurosu H, Kuro OM. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol. 2009;299(1):72–8.
Lim K, Groen A, Molostvov G, et al. Alpha-klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100(10):E1308–18.
Sneddon WB, Ruiz GW, Gallo LI, et al. Convergent signaling pathways regulate parathyroid hormone and fibroblast growth Factor-23 action on NPT2A-mediated phosphate transport. J Biol Chem. 2016;291(36):18632–42.
Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–35.
Kuro OM. Klotho and endocrine fibroblast growth factors: markers of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant. 2019;34(1):15–21.
Drueke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89(2):289–302.
Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74(2):148–57.
Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8.
Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19(6):1092–105.
Brunette MG, Chan M, Ferriere C, Roberts KD. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978;276(5685):287–9.
Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136(4):1317–27, e1311–1312.
Silver J, Naveh-Many T, Mayer H, Schmelzer HJ, Popovtzer MM. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986;78(5):1296–301.
Obi Y, Hamano T, Isaka Y. Prevalence and prognostic implications of vitamin D deficiency in chronic kidney disease. Dis Markers. 2015;2015:868961.
Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–80.
Pocotte SL, Ehrenstein G, Fitzpatrick LA. Regulation of parathyroid hormone secretion. Endocr Rev. 1991;12(3):291–301.
Arcidiacono MV, Sato T, Alvarez-Hernandez D, et al. EGFR activation increases parathyroid hyperplasia and calcitriol resistance in kidney disease. J Am Soc Nephrol. 2008;19(2):310–20.
Cordero JB, Cozzolino M, Lu Y, et al. 1,25-Dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. J Biol Chem. 2002;277(41):38965–71.
Cozzolino M, Lu Y, Finch J, Slatopolsky E, Dusso AS. p21WAF1 and TGF-alpha mediate parathyroid growth arrest by vitamin D and high calcium. Kidney Int. 2001;60(6):2109–17.
Tokumoto M, Tsuruya K, Fukuda K, Kanai H, Kuroki S, Hirakata H. Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002;62(4):1196–207.
Dusso AS, Pavlopoulos T, Naumovich L, et al. p21(WAF1) and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth. Kidney Int. 2001;59(3):855–65.
Basile C, Lomonte C, Vernaglione L, et al. A high body mass index and female gender are associated with an increased risk of nodular hyperplasia of parathyroid glands in chronic uraemia. Nephrol Dial Transplant. 2006;21(4):968–74.
Fukuda N, Tanaka H, Tominaga Y, Fukagawa M, Kurokawa K, Seino Y. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993;92(3):1436–43.
Lee HJ, Seo UH, Kim WY, Woo SU, Lee JB. Calcium-sensing receptor and apoptosis in parathyroid hyperplasia of patients with secondary hyperparathyroidism. J Int Med Res. 2013;41(1):97–105.
Komaba H, Goto S, Fujii H, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77(3):232–8.
van der Plas WY, Noltes ME, van Ginhoven TM, Kruijff S. Secondary and tertiary hyperparathyroidism: a narrative review. Scand J Surg. 2019:1457496919866015.
Pitt SC, Sippel RS, Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am. 2009;89(5):1227–39.
Tominaga Y, Matsuoka S, Sato T, et al. Clinical features and hyperplastic patterns of parathyroid glands in hemodialysis patients with advanced secondary hyperparathyroidism refractory to maxacalcitol treatment and required parathyroidectomy. Ther Apher Dial. 2007;11(4):266–73.
Shindo M, Lee JA, Lubitz CC, et al. The changing landscape of primary, secondary, and tertiary hyperparathyroidism: highlights from the American College of Surgeons Panel, “What’s New for the Surgeon Caring for Patients with Hyperparathyroidism”. J Am Coll Surg. 2016;222(6):1240–50.
Dulfer RR, Franssen GJH, Hesselink DA, Hoorn EJ, van Eijck CHJ, van Ginhoven TM. Systematic review of surgical and medical treatment for tertiary hyperparathyroidism. Br J Surg. 2017;104(7):804–13.
Goldsmith DJ. Pro: Should we correct vitamin D deficiency/insufficiency in chronic kidney disease patients with inactive forms of vitamin D or just treat them with active vitamin D forms? Nephrol Dial Transplant. 2016;31(5):698–705.
Westerberg PA, Sterner G, Ljunggren O, et al. High doses of cholecalciferol alleviate the progression of hyperparathyroidism in patients with CKD Stages 3–4: results of a 12-week double-blind, randomized, controlled study. Nephrol Dial Transplant. 2018;33(3):466–71.
Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008;52(3):519–30.
Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10(1):98–109.
Patel L, Bernard LM, Elder GJ. Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2016;11(2):232–44.
Zand L, Kumar R. The use of vitamin D metabolites and analogues in the treatment of chronic kidney disease. Endocrinol Metab Clin N Am. 2017;46(4):983–1007.
Goodman WG, Hladik GA, Turner SA, et al. The Calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol. 2002;13(4):1017–24.
Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–25.
Charytan C, Coburn JW, Chonchol M, et al. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis. 2005;46(1):58–67.
Brunaud L, Ngueyon Sime W, Filipozzi P, et al. Minimal impact of calcimimetics on the management of hyperparathyroidism in chronic dialysis. Surgery. 2016;159(1):183–91.
Investigators ET, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–94.
Behets GJ, Spasovski G, Sterling LR, et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;87(4):846–56.
Block GA, Bushinsky DA, Cheng S, et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317(2):156–64.
Ortiz A, Covic A, Fliser D, et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383(9931):1831–43.
Isaksson E, Sterner G. Early development of secondary hyperparathyroidism following renal transplantation. Nephron Clin Pract. 2012;121(1–2):c68–72.
Bhan I, Shah A, Holmes J, et al. Post-transplant hypophosphatemia: tertiary ‘hyper-phosphatoninism’? Kidney Int. 2006;70(8):1486–94.
Kimura T, Akimoto T, Watanabe Y, et al. Impact of renal transplantation and nephrectomy on urinary soluble klotho protein. Transplant Proc. 2015;47(6):1697–9.
Reinhardt W, Bartelworth H, Jockenhovel F, et al. Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol Dial Transplant. 1998;13(2):436–42.
Amin T, Coates PT, Barbara J, Hakendorf P, Karim N. Prevalence of hypercalcaemia in a renal transplant population: a single centre study. Int J Nephrol. 2016;2016:7126290.
Sprague SM, Belozeroff V, Danese MD, Martin LP, Olgaard K. Abnormal bone and mineral metabolism in kidney transplant patients–a review. Am J Nephrol. 2008;28(2):246–53.
Taweesedt PT, Disthabanchong S. Mineral and bone disorder after kidney transplantation. World J Transplant. 2015;5(4):231–42.
Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. J Am Soc Nephrol. 1996;7(1):158–65.
Pihlstrom H, Dahle DO, Mjoen G, et al. Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation. 2015;99(2):351–9.
Bleskestad IH, Bergrem H, Leivestad T, Hartmann A, Goransson LG. Parathyroid hormone and clinical outcome in kidney transplant patients with optimal transplant function. Clin Transpl. 2014;28(4):479–86.
Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325(8):544–50.
Weisinger JR, Carlini RG, Rojas E, Bellorin-Font E. Bone disease after renal transplantation. Clin J Am Soc Nephrol. 2006;1(6):1300–13.
Lorenz K, Bartsch DK, Sancho JJ, Guigard S, Triponez F. Surgical management of secondary hyperparathyroidism in chronic kidney disease--a consensus report of the European Society of Endocrine Surgeons. Langenbeck's Arch Surg. 2015;400(8):907–27.
Akaberi S, Clyne N, Sterner G, et al. Temporal trends and risk factors for parathyroidectomy in the Swedish dialysis and transplant population – a nationwide, population-based study 1991–2009. BMC Nephrol. 2014;15:75.
Isaksson E, Ivarsson K, Akaberi S, et al. The effect of parathyroidectomy on risk of hip fracture in secondary hyperparathyroidism. World J Surg. 2017;41(9):2304–11.
Ivarsson KM, Akaberi S, Isaksson E, et al. Cardiovascular and cerebrovascular events after parathyroidectomy in patients on renal replacement therapy. World J Surg. 2019;
Ivarsson KM, Akaberi S, Isaksson E, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015;30(12):2027–33.
van der Plas WY, Dulfer RR, Engelsman AF, et al. Effect of parathyroidectomy and cinacalcet on quality of life in patients with end-stage renal disease-related hyperparathyroidism: a systematic review. Nephrol Dial Transplant. 2017;32(11):1902–8.
Narayan R, Perkins RM, Berbano EP, et al. Parathyroidectomy versus cinacalcet hydrochloride-based medical therapy in the management of hyperparathyroidism in ESRD: a cost utility analysis. Am J Kidney Dis. 2007;49(6):801–13.
Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004;66(5):2010–6.
Ishani A, Liu J, Wetmore JB, et al. Clinical outcomes after parathyroidectomy in a nationwide cohort of patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10(1):90–7.
El-Husseini A, Wang K, Edon AA, Sawaya BP. Parathyroidectomy-A last resort for hyperparathyroidism in dialysis patients. Semin Dial. 2017;30(5):385–9.
Matsuoka S, Tominaga Y, Sato T, et al. QuiCk-IntraOperative Bio-Intact PTH assay at parathyroidectomy for secondary hyperparathyroidism. World J Surg. 2007;31(4):824–31.
Zhang L, Xing C, Shen C, et al. Diagnostic accuracy study of intraoperative and perioperative serum intact PTH level for successful parathyroidectomy in 501 secondary hyperparathyroidism patients. Sci Rep. 2016;6:26841.
Nigwekar SU, Sprague SM. We do too many parathyroidectomies for calciphylaxis. Semin Dial. 2016;29(4):312–4.
van der Plas WY, El Moumni M, von Forstner PJ, et al. Timing of parathyroidectomy does not influence renal function after kidney transplantation. World J Surg. 2019;43(8):1972–80.
Schwarz A, Rustien G, Merkel S, Radermacher J, Haller H. Decreased renal transplant function after parathyroidectomy. Nephrol Dial Transplant. 2007;22(2):584–91.
Akerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of human parathyroid glands. Surgery. 1984;95(1):14–21.
Alesina PF, Hinrichs J, Kribben A, Walz MK. Minimally invasive video-assisted parathyroidectomy (MIVAP) for secondary hyperparathyroidism: report of initial experience. Am J Surg. 2010;199(6):851–5.
Sun Y, Cai H, Bai J, Zhao H, Miao Y. Endoscopic total parathyroidectomy and partial parathyroid tissue autotransplantation for patients with secondary hyperparathyroidism: a new surgical approach. World J Surg. 2009;33(8):1674–9.
Barbaros U, Erbil Y, Yildirim A, Saricam G, Yazici H, Ozarmagan S. Minimally invasive video-assisted subtotal parathyroidectomy with thymectomy for secondary hyperparathyroidism. Langenbeck's Arch Surg. 2009;394(3):451–5.
Schneider R, Bartsch DK, Schlosser K. Relevance of bilateral cervical thymectomy in patients with renal hyperparathyroidism: analysis of 161 patients undergoing reoperative parathyroidectomy. World J Surg. 2013;37(9):2155–61.
Isaksson E, Ivarsson K, Akaberi S, et al. Total versus subtotal parathyroidectomy for secondary hyperparathyroidism. Surgery. 2019;165(1):142–50.
Chen J, Zhou QY, Wang JD. Comparison between subtotal parathyroidectomy and total parathyroidectomy with autotransplantation for secondary hyperparathyroidism in patients with chronic renal failure: a meta-analysis. Horm Metab Res. 2015;47(9):643–51.
Burgstaller T, Selberherr A, Brammen L, Scheuba C, Kaczirek K, Riss P. How radical is total parathyroidectomy in patients with renal hyperparathyroidism? Langenbeck's Arch Surg. 2018;403(8):1007–13.
Bergenfelz AO, Jansson SK, Wallin GK, et al. Impact of modern techniques on short-term outcome after surgery for primary hyperparathyroidism: a multicenter study comprising 2,708 patients. Langenbeck's Arch Surg. 2009;394(5):851–60.
Lou I, Foley D, Odorico SK, et al. How well does renal transplantation cure hyperparathyroidism? Ann Surg. 2015;262(4):653–9.
Schreinemakers JM, Pieterman CR, Scholten A, Vriens MR, Valk GD, Rinkes IH. The optimal surgical treatment for primary hyperparathyroidism in MEN1 patients: a systematic review. World J Surg. 2011;35(9):1993–2005.
Tentori F, Zepel L, Fuller DS, et al. The DOPPS practice monitor for US dialysis care: PTH levels and management of mineral and bone disorder in US hemodialysis patients. Am J Kidney Dis. 2015;66(3):536–9.
Marcadis AR, Teo R, Ouyang W, Farra JC, Lew JI. Successful parathyroidectomy guided by intraoperative parathyroid hormone monitoring for primary hyperparathyroidism is preserved in mild and moderate renal insufficiency. Surgery. 2018;163(3):633–7.
El-Husseini A, Wang K, Edon A, et al. Value of intraoperative parathyroid hormone assay during parathyroidectomy in dialysis and renal transplant patients with secondary and tertiary hyperparathyroidism. Nephron. 2018;138(2):119–28.
Sohn JA, Oltmann SC, Schneider DF, Sippel RS, Chen H, Elfenbein DM. Is intraoperative parathyroid hormone testing in patients with renal insufficiency undergoing parathyroidectomy for primary hyperparathyroidism accurate? Am J Surg. 2015;209(3):483–7.
Freriks K, Hermus AR, de Sevaux RG, et al. Usefulness of intraoperative parathyroid hormone measurements in patients with renal hyperparathyroidism. Head Neck. 2010;32(10):1328–35.
Meyer SK, Zorn M, Frank-Raue K, Buchler MW, Nawroth P, Weber T. Clinical impact of two different intraoperative parathyroid hormone assays in primary and renal hyperparathyroidism. Eur J Endocrinol. 2009;160(2):275–81.
Haustein SV, Mack E, Starling JR, Chen H. The role of intraoperative parathyroid hormone testing in patients with tertiary hyperparathyroidism after renal transplantation. Surgery. 2005;138(6):1066–71; discussion 1071.
Weber T, Zeier M, Hinz U, Schilling T, Buchler MW. Impact of intraoperative parathyroid hormone levels on surgical results in patients with renal hyperparathyroidism. World J Surg. 2005;29(9):1176–9.
Seehofer D, Rayes N, Ulrich F, et al. Intraoperative measurement of intact parathyroid hormone in renal hyperparathyroidism by an inexpensive routine assay. Langenbeck's Arch Surg. 2001;386(6):440–3.
Lokey J, Pattou F, Mondragon-Sanchez A, et al. Intraoperative decay profile of intact (1–84) parathyroid hormone in surgery for renal hyperparathyroidism–a consecutive series of 80 patients. Surgery. 2000;128(6):1029–34.
Dotzenrath C, Cupisti K, Goretzki E, et al. Operative treatment of renal autonomous hyperparathyroidism: cause of persistent or recurrent disease in 304 patients. Langenbeck's Arch Surg. 2003;387(9–10):348–54.
Taieb D, Urena-Torres P, Zanotti-Fregonara P, et al. Parathyroid scintigraphy in renal hyperparathyroidism: the added diagnostic value of SPECT and SPECT/CT. Clin Nucl Med. 2013;38(8):630–5.
Caldarella C, Treglia G, Pontecorvi A, Giordano A. Diagnostic performance of planar scintigraphy using (9)(9)mTc-MIBI in patients with secondary hyperparathyroidism: a meta-analysis. Ann Nucl Med. 2012;26(10):794–803.
Sukan A, Reyhan M, Aydin M, et al. Preoperative evaluation of hyperparathyroidism: the role of dual-phase parathyroid scintigraphy and ultrasound imaging. Ann Nucl Med. 2008;22(2):123–31.
Li P, Liu Q, Tang D, et al. Lesion based diagnostic performance of dual phase (99m)Tc-MIBI SPECT/CT imaging and ultrasonography in patients with secondary hyperparathyroidism. BMC Med Imaging. 2017;17(1):60.
Yuan LL, Kan Y, Ma DQ, Yang JG. Combined application of ultrasound and SPECT/CT has incremental value in detecting parathyroid tissue in SHPT patients. Diagn Interv Imaging. 2016;97(2):219–25.
Alkhalili E, Tasci Y, Aksoy E, et al. The utility of neck ultrasound and sestamibi scans in patients with secondary and tertiary hyperparathyroidism. World J Surg. 2015;39(3):701–5.
Karipineni F, Sahli Z, Somervell H, et al. Are preoperative sestamibi scans useful for identifying ectopic parathyroid glands in patients with expected multigland parathyroid disease? Surgery. 2018;163(1):35–41.
Cui L, Gao Y, Yu H, et al. Intraoperative parathyroid localization with near-infrared fluorescence imaging using indocyanine green during total parathyroidectomy for secondary hyperparathyroidism. Sci Rep. 2017;7(1):8193.
Vidal Fortuny J, Guigard S, Diaper J, Karenovics W, Triponez F. Subtotal parathyroidectomy under indocyanine green angiography. Video Endocrinol. 2016;3(1)
van der Plas WY, Dulfer RR, Koh EY, et al. Safety and efficacy of subtotal or total parathyroidectomy for patients with secondary or tertiary hyperparathyroidism in four academic centers in the Netherlands. Langenbeck's Arch Surg. 2018;403(8):999–1005.
Alsafran S, Sherman SK, Dahdaleh FS, et al. Preoperative calcitriol reduces postoperative intravenous calcium requirements and length of stay in parathyroidectomy for renal-origin hyperparathyroidism. Surgery. 2019;165(1):151–7.
Walsh NJ, Caten AJ, White JJ, Terris DJ. Protocol driven outcomes in renal parathyroid surgery. Head Neck. 2019;41(4):880–4.
Pasieka JL, Parsons LL. A prospective surgical outcome study assessing the impact of parathyroidectomy on symptoms in patients with secondary and tertiary hyperparathyroidism. Surgery. 2000;128(4):531–9.
Acknowledgments
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. There were no sources of funding other than the authors’ salaries. This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector. Both authors contributed to the literature search, drafting of the manuscript, and critical review. There are no specific acknowledgments.
Sources of Support
There were no other sources of support than the authors' salaries.
Conflict of Interest
There are no financial or other relationships that might lead to any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Almquist, M., Dotzenrath, C. (2021). Renal Hyperparathyroidism. In: Shifrin, A.L., Raffaelli, M., Randolph, G.W., Gimm, O. (eds) Endocrine Surgery Comprehensive Board Exam Guide. Springer, Cham. https://doi.org/10.1007/978-3-030-84737-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-84737-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84736-4
Online ISBN: 978-3-030-84737-1
eBook Packages: MedicineMedicine (R0)