Abstract
Microalgae are one of the most promising raw sources for the production of biofuels (e.g., biodiesel, bioethanol, biohydrogen, and biogas) and high added value compounds (e.g., pigments, antioxidants, proteins, essential fatty acids, polysaccharides, vitamins, and minerals). Microalgae biomass and their cultivation are also explored for various applications such as food, feed animals, bioremediation (wastewater treatment and heavy metals biosorption), biofertilizers, biostimulants, and bioplastics. Nowadays, microalgae study is carried towards biorefinery and circular bioeconomy to have a greater benefit and cost reduction. Still, there are many challenges to developing an efficient process in microalgae cultivation, harvesting and drying of biomass, and recovery of high added value compounds. Membrane technology is one of the most efficient techniques in biomass harvesting, such as the pressure-driven membrane processes (e.g., microfiltration, ultrafiltration) and osmotically driven processes; nonetheless, the membrane fouling caused by the microalgae/debris cake layer and the fast permeate flux decrement is still the greatest issues. This chapter is aimed at summarized the current reports of membrane technology used for biomass harvesting and recovery of high added value compounds; advantages and disadvantages are discussed together with the strategies developed to find the best way of separation, purification, and concentration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Microalgae are photosynthetic microorganisms, they are able to transform inorganic compounds into organic compounds, which are fundamentally used for their cell structure and metabolism. They are inhabitants in fresh, brackish, and marine water. Their growth is mainly in autotrophic medium, where they use CO2 as carbon source, light like an energy source, and inorganic salts. Also, under specific conditions, they can grow in a heterotrophic medium using organic carbon source such as glucose or glycerol), and in a mixotrophic medium. The microalgae size cell is between 2 and 200 μm, exist almost 800,000 species of microalga, nonetheless, only 50,000 species have been defined (Sharif et al. 2017); they can be unicellular, multicellular, or colonial. In the open sea, microalgae can fix carbon and nitrogen inorganic to produce organic matter and oxygen, the latter is an essential gas for the life of other organisms. Microalgae are also part of the aquatic food, and they can grow in almost every environment and habitat on the earth. Some of them are planktonic because they float in the water body, and others grow attached to plant surfaces, macroalgae, rocks, grains of sand, or any other rigid surface (Borowitzka 2018).
The term microalgae involve the eukaryotic and prokaryotic domains. The eukaryotic cell comes from the group of green algae, and the prokaryotic cell is identified as cyanobacteria (Varfolomeev and Wasserman 2011). The cyanobacteria are gram-negative, and they are classified into five subgroups (Stanier et al. 1979). The use of photosynthetic pigments that microalgae use to obtain light energy and thus the assimilation of carbon dioxide is essential, resulting in the release of oxygen and the formation of complex molecules of high value for the physiological processes of the microalgae cell. According with the type of microalgae pigment, they are grouped into specific groups and classified in green algae, red algae, brown algae and golden algae (Borowitzka 2018).
The main advantages of the cultivation of microalgae are attributed to the fact that its growth is faster than macroalgae; it can be harvested at any season of the year. The cultivation of microalgae does not compete with food agriculture, it does not need pesticide application, and nowadays the water resources can be recycled (Yu et al. 2019), and take advantage of the nutrients in wastewater (Gouveia et al. 2016). Also, they can be stressed by abiotic parameters (e.g., temperature, salinity, nutrient starvation, pH, light, and UV radiation) to induce the maximum production of a specific valuable metabolite, it can be by accumulation (intracellular) or release (extracellular) of those compounds (Paliwal et al. 2017).
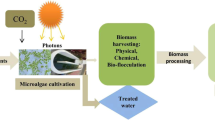
The microalgae biomass is a potential raw source for the production of biofuels (such as biodiesel, bioalcohol, biohydrogen, and biogas), bioproducts (such as pigments, essential fatty acids, proteins, carbohydrates, vitamins, minerals) and among others bioactive metabolites. They have been studied for various applications in foods, animal feed, bioremediation, biofertilizers, biostimulants, and bioplastics (Khan et al. 2018). Nowadays, microalgae are considered as a way of production of high added-value compounds, as illustrated in Fig. 10.1.
This chapter shows a complete overview on using microalgae as a high added value compounds factory, and their potential applications in biofuels, food, and feeds animals, bioremediation, bioplastics, biofertilizers and biostimulants. Secondly, it exposes the role of membrane technology in microalgae bioprocesses including microalgae cultivation, biomass harvesting, and its use for the recovery of high added-value compounds. Finally, the importance of protocols for membrane cleaning by microalgae fouling is also addressed.
2 Microalgae: The Latent Tool for Producing High Added-Value Compounds and Their Applications
2.1 Lipids and Carbohydrates for Biofuels
In a biorefinery, lipids and carbohydrates are among the main produced by microalgae, which can be extracted efficiently to biofuels production, however, other high added-value compounds can be extracted, such as polyunsaturated fatty acids, bioactive peptides, polysaccharides, pigments, vitamins, and minerals. Microalgae-based fuels are in fact recognized as the third generation of biofuels, with better environmental advantages compared with biofuels from crops. At this point, the oilseed microalgae are the most promising feedstock in biodiesel production. A microalgae strain is typically named oilseed when its oil content is between 20–70% of the dry weight of biomass. Some other promising strains for biodiesel production are Ankistrodesmus, Isochrysis, Nannochloris, and Nitzschia (Afzal et al. 2017), Chlorella vulgaris, Chaetoceros mulleri, among others. Still, oil production from microalgae is not highly competitive and it does not looks economically attractive in comparison with traditional oil crops, due to their high energy requirements for cultivation and processing of the biomass (Jez et al. 2017).
The main challenges are focused to explore new strains with higher capacity of oil production (Sadvakasova et al. 2019). For instance, the cultivation of known strains under stress conditions to achieve the maximum oil production can be handled through the starvation or limitation of nutrients (e.g., nitrogen, phosphorus, and sulfur), abiotic factors (light intensity/different wavelengths, carbon dioxide levels, salinity stress, temperature, stress to heavy metals, and the use of nanoparticles) (Luangpipat and Chisti 2017; Alishah Aratboni et al. 2019). Moreover, the optimization and design of bioreactors are priority (Lee et al. 2014), together with the improvement of biomass harvesting (Suparmaniam et al. 2019; Leam et al. 2020), extraction methods, to achieve the principles of biorefinery and circular bioeconomy (Naresh Kumar et al. 2019; Kumar et al. 2020). Here, the genetic and metabolic engineering coupled with nanotechnology offers a good strategy for enhancing microalgae oil production; in which microalgae models, such as Chlamydomonas rehinhardtii and species of Nannochloropsis, are the most explored (Alishah Aratboni et al. 2019).
On the other hand, microalgae cell walls are mainly based on cellulose but also have starch in the plastids. To some extent, these biopolymers can be converted into fermentable sugars. Interestingly, microalgae do not have lignin and a low content of hemicellulose, which are difficult to ferment or degrade by biological methods. In this way, microalgae represent a promising source of fermentable carbohydrates for biofuel production (Chen et al. 2013). In a recent study, 46 microalgae strains were evaluated about their carbohydrate content , the study showed that the carbohydrate content was ranged from 16.5% (Mychonastes sp. A313) to 71.6% (Porphyridium purpureum SAG 13801-1d). The main monosaccharide was glucose; in the second place were mannose, galactose, and rhamnose. Xylose and ribose were also found in several species. Fucose and arabinose were rarely found. The proportion and quantity of monosaccharides were dependent on the microalgae specie, and culture conditions (Schulze et al. 2017). Since fermentable carbohydrate-rich biomass is an important feedstock to biofuels production; such as bioethanol, butanol, acetone, hydrogen, and methane. Theoretically, a bioethanol production of around 0.26 gethanol/gbiomass is estimated from a carbohydrate-rich microalgae biomass, this biomass must contain approximately 50% of fermentable carbohydrates (de Farias Silva et al. 2019). To increase the microalgae carbohydrate content, the stress is generally done via nutrient starvation (mainly nitrogen), light energy supply, temperature variation, pH shift, and CO2 supplement. The goal is primarily to increase the carbohydrate content without compromising the cell growth rate (Chen et al. 2013). Towards the increase of biomass production, the scale up is the recommended way to make more economically profitable process (de Farias Silva et al. 2019).
2.2 Polyunsaturated Fatty Acids
Polyunsaturated fatty acids (PUFAs) are commonly obtained from selected seed plants, marine fish and certain mammals, nonetheless, their fast increasing demand has led to exploring alternative sources. Microalgae PUFAs production is increasing with special interest, even more for marine microalgae. Moreover, the nutritional value attributed to microalgae is for being a rich source of polyunsaturated fatty acids (PUFAs) or long-chain fatty acids. The nutritional value attributed to microalgae deals with a rich source of PUFAs, or long-chain fatty acids. Here, the nutritionally value from microalgae is Docosahexaenoic acid (DHA) and Eicosapentaenoic acid (EPA), among others (Ramesh Kumar et al. 2019). PUFAs are known by their role as an energy source and biological benefits in human health; they have been seen as influencing the inflammatory cascade, reducing oxidative stress, presenting neuroprotection, and cardiovascular protection. DHA in the human cell membrane (~50%) seems to contribute to the fast transport of rhodopsin on the two sides of the membrane, facilitating the initiation of the visual cascade, concurrently, the DHA rich membrane assures the differentials in Na+ and K+ necessary for signal transmission (Nagy 2017). Microalgae cultured under specific stress conditions can produce PUFAs reaching 40% of the total fatty acids produced, and its percentage varies from strain to strain (Ramesh Kumar et al. 2019). For example, the optimal EPA content of 34.6% (w/w) in Porphyridium purpureum biomass was found using for its growth a culture media with concentrations of 29.98 g/L of sodium chloride, 9.34 g/L of magnesium sulfate, and 1.86 g/L of sodium nitrate (Kavitha et al. 2016). Three main strategies have been suggested to improve DHA and EPA productivity: i) regulating the cultivation conditions to maximize the physiological potential for switching the cell metabolism to lipid and DHA + EPA synthesis, ii) escalate the biomass and lipid productivity through the breeding of strains, iii) genetic engineering approach for EPA and DHA production (Ramesh Kumar et al. 2019).
2.3 Bioactive Extracellular Polysaccharides
The production of extracellular polysaccharides has recently received interest. Microalgae species considered as potential source are Calothrix sp., Nostoc sp., Nostoc calcicola, Nostoc muscorum, Nostoc punctiformae, Nostoc carneum, Anabaena sp., Cylindropermum sp., Gleocapsa (Singh and Das 2011), Chlamydomonas reinhardtii (Bafana 2013), Porphyridium sp.(Geresh et al. 2002), Porphyridium cruentum (Patel et al. 2013), Rhodella maculata, Schizochlamydella capsulata, Chlorella stigmatophora (Gasljevic et al. 2008), Synechocystis aquatilis (Flamm and Blaschek 2014), just mention a few. These polysaccharides have immunomodulatory, antitumor, antiviral, antioxidant, anti-inflammatory, anticancer, antithrombotic, anticoagulant activity, biolubrication property (De Jesus Raposo et al. 2013) and emulsifying capacity (Morales-Jiménez et al. 2020). To date, it is known that Porphyridium cruentum polysaccharides are sulfated, and due to the complexity of its structure, its structural elucidation is still explored. An average molar mass of 2.39 × 105 g/mol is reported for Porphyridium cruentum exopolysaccharide (Patel et al. 2013), and 2.3 × 106 g/mol for Porphyridium sp.’s exopolysaccharide (Geresh et al. 2002).
2.4 Pigments
As mentioned previously, microalgae biomass is also a promising source for natural pigments, such as carotenoids (e.g., β-carotene, lutein, and astaxanthin), chlorophyll, and phycobiliprotein (e.g., phycoerythrins, phycocyanins, phycoerythrocyanins, and allophycocyanins). These pigments can act as a color agent for foods (as a natural healthy ingredient), and at the same time due to their biological activity, they can be used in the nutraceutical and pharmaceutical applications (Dufossé 2018). Haematococcus pluvialis is one of the most commercial microalgae strains due to its astaxanthin production (Bustos-Garza et al. 2013). These microalgae can accumulate astaxanthin between 3.8 and 5% of dry cell weight depending on the cultivation conditions and photobioreactor design (Khoo et al. 2019). Production of lutein is led by Chlorella species, and the halotolerant microalgae as Dunaliella salina, Scenedesmus almeriensis, Galdieria sulphuraria (Přibyl et al. 2015; Sun et al. 2015). Specific strategies have been done to increase yields including cultivation for long periods and under abiotic stress (e.g., nutrient stress, high light intensity, and high salinity) to led progress the carotenogenesis until getting cell of orange-red color at the end (Sun et al. 2015; Khoo et al. 2019). Also, Spirulina platensis can produce phycocyanin (Soni et al. 2017). β-phycoerythrin and R-phycocyanin can be obtained from Porphyridium cruentum, Nostoc sp., and other Rhodophyta species (Román et al. 2002; Johnson et al. 2014).
2.5 Proteins-Bioactive Peptides
Spirulina is the most successful source of protein for downstream applications, it has between 60 to 70% of its weight (Soni et al. 2017). Chlorella vulgaris cells contain approximately 42%, Nannochloropsis oculata 42%, Phaeodactylum tricornutum 39%, Porphyridium cruentum 35%, and Haematococcus pluvialis 25%. Owing to the high content of protein in microalgae has been attributed to them nutritive value, which supports its application in human food (Matos 2019). Within this characteristic, the content, proportion, and availability of amino acids, specifically essential amino acids, are the main parameter. Regarding the bioactive peptides are a short specific sequence of amino acids that display biological activity on human health, such as mineral binding, immunomodulatory, antimicrobial, antioxidant, antithrombotic, hypocholesterolemic, and antihypertensive. These kinds of molecules are obtained from Spirulina to a large degree (Ovando et al. 2018), nonetheless, some other strains are being studied (Alzahrani et al. 2018). The bioactive peptides are typically used as ingredients in health-promoting foods, dietary supplements, pharmaceutical and cosmetic applications (Castro-Muñoz and Fíla 2018; Apone et al. 2019).
2.6 Vitamins
Microalgae as a vitamin source is an important parameter to consider for the use of microalgae biomass as a food supplement, even more with promising benefits on human health. Nannochloropsis oceanica was able to produce vitamin D3 up to 1.0 μg/g underexposure ultraviolet-B light (Ljubic et al. 2020). Anabaena cylindrica was identified as a rich source of vitamin K1, around 200 μg/g on a dry weight basis, after an optimization of growth conditions, an increase of vitamin K1 was found and its biomass also showed 11.6 μg/g of riboflavin (B2), 5.8 μg/g of thiamine (B1), 1.5 μg/g of cobalamin (B12), and 0.18 μg/g of biotin (Tarento et al. 2018). A recent study evaluated commercial powders of microalgae, the study showed a riboflavin content between 21 to 41 μg/g, while niacin content varied from 0.13 to 0.28 mg/g in Chlorella sp., Spirulina (Arthrospira sp.) and Nannochloropsis gaditana powders. Chlorella powders showed 19.7 μg/g of total folate and Spirulina powders showed 3.5 μg/g. Chlorella sp., and N. gaditana powders contained active vitamin B12 up to 2.1 μg/g. Spirulina powers contained high amounts of pseudovitamin B12, a no bioactive form of vitamin B12 (Edelmann et al. 2019). In the case of vitamin B12 from microalgae, there are two scenarios, many eukaryotic microalgae require exogenous B vitamins for growth, while marine cyanobacteria can synthesize pseudovitamins; new findings explain that certain microalgae species can turn the inactive vitamin B12 into its biologically active form through a process named vitamin remodeling. This has been identified as an important criterion to survive in the photic environment (Grossman 2016). For vitamin E, commercial Spirulina has 1.3 mg of vitamin E (α-tocopherol)/100 g of dry mass (Gómez-Coronado et al. 2004).
2.7 Minerals
The essential minerals for human health and animal life are classified according to the level of requirement in two general categories, more importantly calcium, phosphorous, potassium, sodium, chlorine, magnesium, and sulfur; and secondly, trace minerals, such as iron, zinc, manganese, molybdenum, chromium, and copper. Most trace minerals usually act in the human body as cofactors for the correct functioning of enzymes (Costa-Pinto and Gantner 2020). Calcium and phosphorus are the main structural components in hard and soft tissues. These minerals are also implicated in osmoregulation process, nerve/muscle function, acid/base equilibrium, and cell membrane functionality, among others. Microalgae as a mineral source is also an important criterion to use in the biomass as a food supplement; its mineral content varies between marine or freshwater microalgae, and from one species to another (Fox and Zimba 2018). For example, Spirulina sp. biomass has 13,630 μg/g of potassium, 1950 μg/g of magnesium, 1200 μg/g of calcium, 10,480 μg/g of sodium, 285 μg/g of iron, and 20 μg/g of zinc. Anabaena cylindrical biomass has 9530 μg/g of potassium, 3840 μg/g μg/g of magnesium, 3140 μg/g of calcium, 2330 μg/g of sodium, 593 μg/g of iron, 16.3 μg/g of zinc (Tarento et al. 2018). Regarding marine microalgae, Biddulphia sp. is rich in calcium (18.23 g/Kg), Amphora sp. in phosphorus (12.39 g/Kg), Achnanthes sp. in potassium (20.88 g/Kg), Thalassiosira sp. in sodium (321.38 g/Kg) and zinc (39.55 mg/Kg), Phaeodactylum limnetica in magnesium (37.15 g/Kg), and Navicula sp. in iron (1912.98 mg/Kg), and copper (9.49 mg/Kg) (Silva et al. 2015).
2.8 Other Bioactive Compounds
Microalgae can also produce other interesting biomolecules, e.g., enzymes, such as cellulases, amylases, galactosidases, proteases, lipases, phytases, laccases, antioxidant enzymes, and enzymes involved in carbohydrate accumulation and carbon concentration (dos Brasil et al. 2017). Phytohormones including auxin, abscisic acid, cytokinin, ethylene and gibberellins (Lu and Xu 2015). Phytochemicals can be produced as well, such as flavonoids, alkaloids, saponins (El Semary and Abd El Naby 2010). While cyanotoxins, such as microcystin, nodularin, saxitoxin, anatoxins, cylindrospermopsin, lipopolysaccharides, have been reported and utilized for potential application as allelochemicals, herbicides, and insecticides. Cyanobacterial siderophores are recognized as iron chelators, and as photoprotective UV-screening compounds for mycosporine-like amino acids and scytonemin (Rastogi and Sinha 2009).
2.9 Microalgae for Food and Feed Animals
Microalgae biomass has nutritional value in terms of high content of protein, carbohydrates, fatty acids, and biologically active compounds. To date, there are a few microalgae that are commercialized as dietary supplements, such as the biomass of Chlorella, Spirulina, and Nostoc. Chlorella but displaying low digestibility. It is documented that Spirulina has been eaten by the Aztecs from Mexico, this strain is considered a superfood by its high protein content, fatty acids, vitamins, minerals, antioxidants, and others bioactive compounds (Soni et al. 2017). It is estimated that more than 12,000 tons of Spirulina biomass are produced every year (Matos 2019). Some studies have shown the application of its biomass and other high added-value compounds in food matrices. Chlorella vulgaris (green), Chlorella vulgaris (orange) and Haematococcus pluvialis (red) biomass have been used as coloring and antioxidant agent in emulsions (Gouveia et al. 2006). Chlorella vulgaris (green) biomass has been used as a color ingredient in butter cookies (Gouveia et al. 2007). Specific polyunsaturated fatty acids from Isochrysis galbana biomass were proposed to enrich the nutritional value and enhance the texture properties of biscuits (Gouveia et al. 2008), while Haematococcus pluvialis and Spirulina maxima biomass were employed for vegetarian food gels (Paula et al. 2012). Spaghetti enriched with Chlorella vulgaris and Spirulina maxima biomass were used to enhance the quality parameters of the product (Fradique et al. 2010).
To animal feed, the biomass of Porphyridium sp. was incorporated into chicken to reduce cholesterol in the egg (Ginzberg et al. 2000). Carotenoids from Chlorella vulgaris (orange) and Haematococcus pluvialis were blended in fish (rainbow trout and gilthead seabream) as a pigmentation supplement (Gouveia and Empis 2003). The oil extracted from microalgae was incorporated in the feed of pigs to partially replace corn and soybean meal (Foltz et al. 2016). Lambs were feeding in a dietary supplementation with docosahexaenoic acid-rich microalgae to produce an increase in polyunsaturated fatty acids in intramuscular fat (Díaz et al. 2017).
2.10 Bioremediation
When dealing with environmental matters, microalgae can assimilate carbon dioxide (CO2), which is a greenhouse gas (Castro-Muñoz et al. 2019a). Their cultivation can reduce the CO2 emissions produced by combustion; and also release oxygen. Microalgae can fix 1.83 Kg CO2 while generating 1.0 Kg of biomass (Gupta et al. 2019). To date, CO2 concentrations in the environment exceed 400 parts per million (Panchenko et al. 2020); therefore, there is today an interest in developing suitable and environmentally friendly capturing technologies of CO2 from the environment or flue gas (Martin-Gil et al. 2019; Ahmad et al. 2021), in which microalgae cultivation represents an alternative, the main goal here is to get pure CO2 without toxic gases, such as NOx and SOx (Rahaman et al. 2011).
Microalgae have been proven for wastewater treatment as bioremediation using this wastewater as a culture medium: basically, the aim is to recover nutrients from municipal, agricultural and industrial wastewaters for microalgae growth. In this way, the reduction of costs of microalgae cultivation, harvesting, and downstream of their bioproducts, simultaneous lipid, protein, and carbohydrates production are done. The most common nutrients to remove from wastewater are nitrate and phosphate, which represent a source of nitrogen and phosphorus, respectively. For the screening of microalgae strains suitable for the wastewater treatment, some criteria are established such as fast growth rate, high nutrient removal rate, strong adaptability to different types of wastewater, strong adaptability to local climate, and high biomass productivity (Li et al. 2019). At this purpose, Chlorella and Scenedesmus species are the most successful strains, nonetheless, many other strains have been proposed. For example, pig biogas slurry was mixed with municipal wastewater for Chlorella zofingiensis cultivation, an 8% of pig biogas slurry was found as the best to get 2.5 g/L biomass, and the removal 93% total nitrogen and 90% total phosphorus (Zhou et al. 2018). Nejayote, which is identified as the main by-product of Nixtamalization processes (Castro-Muñoz et al. 2019c), and swine wastewater have been evaluated for Arthrospira maxima and Chlorella vulgaris growth (López-Pacheco et al. 2019). Scenedesmus quadricauda and Tetraselmis suecica were cultivated using dairy wastewater (Daneshvar et al. 2019). Also, symbiotic growth may be possible, e.g., microalgae and bacteria were co-cultivated (Makut et al. 2019).
The biomass could also be a removal pollutant matrix. Immobilized Phormidium sp. was used to remove reactive dyes (such as remazol blue, Reactive Black B) from a model dye-rich wastewater (Ertu and Bak 2007). The toxic and heavy metals that result from industrial processes are priority pollutants to be removed due to their great damage to the environment (Castro-Muñoz et al. 2021). Chlorella vulgaris and Scenedesmus spinosus were tested due to their biosorbent capacity towards metal ions, C. vulgaris had a higher tolerance than S. spinosus, and it showed a capacity to remove Cu and Mo from metal mine tailings water (Urrutia et al. 2019). In particular, Synechocystis PCC6803 has shown its potential in the bioremediation of heavy metals via biosorption, such as Cr (VI), Cd (II), Cu (II), Pb (II), Ni (II), Mn (II), Mn (IV), As (III), As (V), Cs and Hg (Pembroke et al. 2015). The microalgae extracellular polymeric substances could be potentially useful too. The exopolysaccharide of Synechocystis sp. has shown antimony trapping capacity in concentrations of 5–100 mg/L, where 32.4–48.5% of the chemical was absorbed (Zhang et al. 2012).
Although the capacities of microalgae to reduce the impact of pollutants have been demonstrated, their application in bioremediation processes is scarce. Therefore, it is worth exploring this research topic further given the advantages of microalgae.
2.11 Biofertilizers and Biostimulants from Microalgae
The serious effects on the environment, animal , and human health caused by the use of chemical fertilizers in agriculture crops have been driven the research toward alternative sources, environmentally friendly and cost-effective strategies. In this sense, microalgae dry biomass has been explored as biofertilizer; Spirulina platensis and Chlorella vulgaris biomass were used separately and blended with cow dung as fertilizer in onion cultivation. The growth parameters, yield attributes, and biochemical composition were high in Spirulina platensis and cow dung mixes (Dineshkumar et al. 2020). De-oiled biomass waste (residual biomass) of Scenedesmus sp., was used to reduce chemical fertilizers in rice crops. The combined application of microalgae with chemical fertilizers resulted in an increased yield and quality of rice (Nayak et al. 2019).
Cyanobacteria are known for their potential to fix atmospheric nitrogen and make it available for plants; specifically, Diazotrophes, which are useful for eco-friendly biofertilizers. The most efficient nitrogen-fixing cyanobacteria are Nostoc linkia, Anabaena variabilis, Aulosira fertilisima, Calothrix sp., Tolipothrix sp., and Scytonema sp. Anabaena and Nostoc, which can fix up to 20–25 Kg/ha atmospheric nitrogen. In addition to biofertilizer, there are numerous advantages of using living cells of cyanobacteria, such as make porous soil and produce adhesive substances, excretion of phytohormones (growth-stimulating hormones), vitamins and amino acid, improve the water holding capacity of soil through their characteristic jelly structure, increase in biomass of soil after their death and decomposition decrease in soil salinity, controls weeds growth, availability of soil phosphate by excretion of organic acids, and efficient absorption of heavy metals on the microbial surface (bioremediation) (Chittora et al. 2020). Microalgae extracts are also a potential biostimulating of plants due to their content of bioactive compounds including polysaccharides, lipids, proteins, phenolic compounds, phytohormones, and polyamines (Chiaiese et al. 2018). The application of crude polysaccharides extracts from Arthrospira platensis, Dunaliella salina and Porphyridium sp. on tomato plants improved nodes number, shoot dry weight, shoot length, pigments content, proteins content, and change in fatty acids, sterol and alkanes profiles (Rachidi et al. 2020); however, it is still necessary to know the specific effect of microalgae biostimulating on plant metabolism.
2.12 Microalgae-Based Bioplastics
The biodegradable bio-based polymers are generally based on proteins (e.g., casein, collagen, albumin, and keratin), and polysaccharides (e.g., starch, cellulose, alginate, carrageenan, polylactic acid, and polyhydroxyalkanoates). Today, there a clear trend in producing bio-based polymers to replace the chemically synthesized ones that represent an important environmental issue (Castro-Muñoz and González-Valdez 2019; Díaz-Montes and Castro-muñoz 2021). It is known that the lipids are not polymers strictly, nonetheless, they are included in this group. Biopolymers can be produced from plants, animals, microorganisms and their derived metabolites. The bioplastics can be manufactured in different ways but always presenting biodegradable nature (Niaounakis 2015). Microalga biomass and their extracellular polymeric substances could be a potential raw source for bioplastics and bioactive film production due to their content of lipids, proteins, polysaccharides, and some other high added-value compounds. Antimicrobial edible films for food packaging were done using Arthrospira platensis protein and lysozyme (Benelhadj et al. 2016). Similarly, transparent and flexible bioactive films were obtained from extracellular biopolymers from Nostoc sp., and Porphyridium purpureum (Morales-Jiménez et al. 2020). Furthermore, biodegradable films were prepared with a blend of Heterochlorella luteoviridis and Dunaliella tertiolecta biomass/biomass extract and cassava starch (Carissimi et al. 2018). Another strategy was to get bioplastics made of wheat gluten and Spirulina platensis biomass like a filler (Ciapponi et al. 2019). Thermoplastic corn starch biocomposites were prepared with the addition of biomass from Nannochloropsis, Spirulina, and Scenedesmus (Fabra et al. 2018)., Nostoc muscorum can accumulate intracellularly poly-β-hydroxybutyrate under carbon stress (Haase et al. 2012), this polymer was proposed as a potential substitute for plastics.
3 The Role of Membrane Technology in Microalgae Bioprocessing
Membrane technologies for microalgae processing is currently gaining great interest due to their efficiencies in the separation of particles, macromolecules and micromolecules (Castro-Muñoz et al. 2016). Their main advantages concern to the non-chemical, biological or thermal change of the component (Cui et al. 2010). Some other advantages, such as ease of operation, high separation efficiency and scalability, increase the usefulness of the membrane-based technologies for microalgae bioprocessing (Kumar et al. 2020). Membrane processes have a wide range of applications, specifically pressure-driven processes can be divided by the pore size of the membrane used and the required transmembrane pressure (Castro-Muñoz et al. 2019b). In general, such processes can be classified as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO) (Cui et al. 2010). Figure 10.2 gives an overview of the pore size of the membranes and their application to separate specific molecules. Due to the complex nature of microalgae cultivation media, MF has been mainly studied for microalgae harvesting, it means, the dewatering of the biomass until the concentration stage, and this is possible due to cell sizes of microalgae (2–200 μm). The needing of the microalgae bioprocessing has led the application of membrane process in the microalgae cultivation, pretreatment of wastewater, recovery, and purification of CO2 to culture, and implementation of membrane photobioreactors. Moreover, the membrane processes are being useful in the recovery of high added-value compounds from microalgae within isolation and purification strategies. The following section provide a clear overview of the uses of membrane processes in these applications.
3.1 Membrane Technology for Microalgae Cultivation
3.1.1 Pretreatment of Wastewater as a Culture Medium for Microalgae Cultivation
Wastewater is a promising source of culture medium and represents an excellent alternative to reduce the costs of nutrients for the microalgae culture. Due to the complexity of wastewater, there are still many challenges to solve in these aspects, such as organic and inorganic nutrients concentration, pH, color, total dissolved solids, and microbial contaminants (Li et al. 2019). These parameters are generally extreme when compared with the optimum values for microalgae growth, making it inappropriate for direct application on the cultivation. In this case, the pretreatment of the wastewater is strictly necessary. To reduce the microbial contaminants, the wastewater is exposed to UV light or autoclaved, nonetheless, for a large-scale system the operation cost increases. MF and UF could be excellent alternatives. Sandefur et al. (2016) evaluated the potential use of UF technology to remove inorganic solids and bacteria as biological contaminant from swine wastewater, which was latest used for the cultivation of Porphyridium cruentum . A hollow fiber membrane cartridge of 50,000 MWCO was used to eliminate coliforms, and 75% of total solids; membrane system was optimized at 27 °C and a transmembrane pressure of 17.5 psi (Sandefur et al. 2016). After the microalgae biomass harvest, the spent culture medium still contents nutrients, these can be used to cultivate microalgae again under a water and nutrients recycling approach. To reuse spent microalgae culture medium from large-scale outdoor ponds, the presence of bacteria and some other organisms are identified as the main drawback due to the microbes consume nutrients and oxygen during their growth, moreover, their metabolites influence the physicochemical properties of the medium, including pH, along with the limitation of the available light for microalgae growth. Yu et al. (2019) showed that MF membranes were useful to remove microbial contaminants of the spent culture medium from Arthrospira platensis biomass harvesting process, in a large-scale outdoor raceway pond. At this point, polystyrene membrane of 0.1 μm of nominal pore size was used to reject bacteria and micro zooplankton from the residual medium (Yu et al. 2019).
3.1.2 Membrane Technologies Coupled to Photobioreactors
To simultaneously improve the cultivation, harvesting, and dewatering, a novel strategy has been recently implemented combining membrane processes and photobioreactors. When the membrane module is adapted inside of the photobioreactor, the membrane is useful for carbonation or biomass retention; also, biomass retention by membrane module can be installed outside the photobioreactor, as illustrated in Fig. 10.3. A carbonation membrane photobioreactor can act as a contactor system to enhance the fixation and delivery of CO2 into the culture media. On the other hand, a biomass retention in photobioreactor via membranes allows obtaining spent culture medium through permeate stream. This strategy has been especially useful for wastewater treatment and biomass production simultaneously. The factors affecting microalgae growth and nutrients removal rates in biomass retention are lightning conditions, CO2 carbonation (between 0.15–8.0 L/min), nutrients concentrations, hydraulic retention time, and biomass retention time (Zhang et al. 2019). Membrane photobioreactor processes have still many issues to be overcome and optimized, e.g., membrane fouling (Liao et al. 2018). It is important to highlight that the fouling phenomenon is identified as the main drawback of membrane technologies. In these applications, the membrane tend to be prone to “biofouling” (Pichardo-Romero et al. 2020), which is implies the adhesion of micro- or macro organisms as membrane foulant, and it is the “vulnerable” part of the microalgae harvesting by these technologies.
3.2 Membrane Technology for Microalgae Biomass Harvesting
Microalgae biomass harvesting is a process where the biomass is separated from the spent culture medium. This microalgae process is particularly complex due to the small size of the microalgae cell, suspended in a big volume of the medium. Their high cost, which is up to 20–30% of the total biomass production cost, is also a relevant factor (Fuad et al. 2018; Yin et al. 2020). Harvesting methods are classified as chemical (flocculation), physical (centrifugation, filtration, gravity sedimentation, flotation, electrophoretic separation), biological (bioflocculation and autoflocculation), and magnetic (Yin et al. 2020). In specific cases, the synergy of different methods offers better harvesting efficiency, for example, flocculation followed by membrane filtration (Zhao et al. 2020). Flocculation is the most useful harvesting method due to its ease of operation, recovery efficiency (70–99%), and cost, however, the use of chemical flocculants causes contamination to the biomass and the culture medium, it driven to limited chances to recycle the culture medium. Choosing the best microalgae harvesting method according to the objective is still a challenge. The harvesting method can be selected according to the microalgae species, type of products in the downstream process, the versatility of the method, and the possibility of re-use the spent culture medium (Yin et al. 2020).
Membrane technology for microalgae harvesting has been widely studied over 20 years. For microalgae harvesting, MF and UF have shown similar results in terms of permeate flux, under the same operating conditions at low transmembrane pressure (Sun et al. 2013). The membrane filtration allows biomass recovery efficiencies up to 100%, biomass free of chemical additives, and culture medium with the possibility to reuse. Also, they offer important characteristics for improving the quality in the downstream process, diminish the water footprint, reusing nutrients, reducing costs, and being environmentally friendly. Hwang and Rittman (2017) evaluated the effect of permeate recycling on the growth of Synechocystis sp. PCC 6803. Permeate was obtained by the MF process. The results showed that the highest biomass concentration was 0.63 g/L at the end of the experiment using a permeate ratio of 50% and complemented with 50% of the BG11 medium. The reuse of permeate two times (two runs) had no negative impact on Synechocystis sp. PCC 6803 growth. Growth inhibition was observed during run 3 probably caused by nutrient deficiency, presence of cell debris, and extracellular substances. A degradation process was necessary to reuse permeate after run 2 (Hwang and Rittmann 2017). For such a scenario, Monte et al. (2019) demonstrated the viability of recycling cultivation medium from Dunaliella salina after the membrane harvesting (ultrafiltration) of the biomass, the permeate stream was recovered and treated by advanced oxidation to degrade the organic compounds. Cultivation medium treated and no treated was used to Dunaliella salina growth and carotenoid production. The maximum volumetric productivity of carotenoids was 6.93 ± 0.48 mg/L·d for the untreated permeate and 5.98 ± 0.41 mg/L·d for the treatment with oxidation (Monte et al. 2019).
One of the determining criteria for microalgae harvesting using membrane technology is the energy consumption and cost analysis. Monte et al. (2018) compared the energy consumption using centrifuge and membrane unit to recover efficiently the biomass and consequently the cost involved, for the harvesting of Dunaliella salina at pilot scale. Harvesting with UF pre-concentration led to a 45% reduction in energy and a 52% reduction in the total cost of ownership when compared with only centrifugation (Monte et al. 2018). Nowadays, deeper cost analysis is mandatory according to microalgae strain, membrane equipment used, operation conditions, and energy consumption as done by Gerardo et al. (2015), who evaluated the influence of operating conditions and scale-up effect to diminish the cost through energy consumption. They have shown that by manipulating parameters such as transmembrane pressure, initial biomass concentration, energy intake, temperature, and membrane area, it is possible to reduce the total energy consumption around 56% in the pilot-scale cross-flow MF of Chlorella minutissima (Gerardo et al. 2015).
Other criteria for membrane microalgae harvesting are the optimized operating conditions and the membrane material selection. At this purpose, several membrane materials have been explored including polyvinylidene fluoride, polyacrylonitrile, polyethersulfone, cellulose acetate, and inorganic materials (see Table 10.1). The selection is based in biomass concentration, species characteristics, surface charge, hydrophobicity, and feed flow parameters (Leam et al. 2020). Rossi et al. (2004) evaluated 11 commercial membranes to harvest Arthrospira platensis. Polyacrylonitrile membrane of 40 kDa, having a neutral and hydrophilic nature, was the best in terms of permeation flux, and clean ability (Rossi et al. 2004). More lately, Marbelia et al. (2016) used polyacrylonitrile membranes to filter microalgae and they evaluated the influence of porosity, surface charge, and microalgae species on membrane fouling. They found that large pores were easily blocked by foulants, negatively charged membranes reduced membrane fouling, and also microalgae with non-spherical shape (Scenedesmus and Phaeodactylum), large size and rigid cell wall were easier to filter than microalgae without a cell wall (Isochrysis) and microalgae with a flexible cell wall (Pseudanabaena). Importanly, the filtration performance was determined by a cake layer formation (Marbelia et al. 2016).
To date, the main bottleneck concerns to solve the severe membrane fouling since conducts to a short useful life of the membrane, and consequently increases the cost of operation. The membrane fouling is promoted by operating conditions, membrane characteristics, algae species, and foulant compounds. The fouling can take places in several ways, such as internal fouling (including pore plugging and adsorption) and external fouling, which implies cake deposition. The fouling could be reversible or irreversible depending on the nature of the foulant (Rickman et al. 2012; Díaz-Montes et al. 2020). The foulants from microalgae are generally complete cells, cell debris and algogenic organic matter. The algogenic organic matter is released by algal metabolism into the extracellular medium, and its production strongly depends on the growing conditions, including temperature, pH, and nutrients content. It has been observed that changes in the nitrogen-phosphorus ratio may affect the composition of algogenic organic matter, which can be composed of protein, neutral and charged polysaccharides, nucleic acids, lipids, and small molecules (Huang et al. 2012). Zhang et al. (2016), for instance, studied the impact of the released algogenic organic matter from Microcystis aeruginosa and Chlorella sp. culture on the membrane fouling according to the growth phase; herein, a MF unit was proposed using a ceramic membrane. The results showed that the MF filtration stage of the algogenic organic matter from both algal species at the stationary phase (35 days) caused a more severe flux decline compared with the corresponding to the log phase (12 days). This fact was attributed to the stationary phase containing significantly greater amounts of high fouling potential components (e.g., protein and humic-like substances) (Zhang et al. 2016).
Various strategies have been developed to diminish the membrane fouling during the filtration process. Zhang and Fu (2018) pointed out strategies, such as pretreating the feed water (e.g., adding Ca+2 and changing the pH, adding coagulants, oxidants, or adsorbents), changing the membrane properties (e.g., pore size, hydrophilicity/hydrophobicity surface, and charge), and enhancing the hydrodynamic conditions (e.g., cross-flow rate, using aeration, and dynamic filtration systems near the membrane surface) (Zhang and Fu 2018), to obtain more efficient recovery efficiencies. The application of any of these strategies must be carefully selected according to the subsequent processes to avoid counterproductive effects that lead to more serious fouling of the membrane.
3.3 Membrane Technology for the Recovery of High Added Value Compounds from Microalgae
The recovery of high value compounds requires processes that guarantee maximum efficiency due to the costs that are involved. For microalgae processes, membrane technology is indeed a promising technique in the recovery of lipids, triacylglycerol, polysaccharides, glucose, proteins, and pigments (such as chlorophyll a, β-phycoerythrin, and phycocyanin) (see Table 10.2). Here, the membrane technology is implemented as a purification step, where the degree of purity is one of the most important parameters. Patel et al. (2013) showed in their study a comparison of three methods for the separation of extracellular polysaccharides from the Porphyridium cruentum culture media. Membrane separation in a diafiltration mode using a membrane of 300 kDa molecular weight cut off was the most efficient for a large scale process, and the extracellular polysaccharides were obtained with a high degree of purity (Patel et al. 2013). The diafiltration step allows the cleaning of the compound of interest through washing with deionized water (or a buffer) until it reaches its maximum purity, it is necessary to monitor the decrease in the contaminant. The diafiltration step is generally performing after the concentration step, especially in the recovery of compounds from marine microalgae having high content of salt. Continuous steps of filtration in a gradual reduction of molecular cut is an adequate strategy in the separation of high added-value compounds. As seen in Table 10.2, microalgae extract is performed before the membrane process to release the intracellular compounds; and therefore the extracellular compounds from the spent extracellular medium can be directly processed. The membrane pressure-driven processes used mainly in the pre-purification stage of high-added value compounds are microfiltration and ultrafiltration. For this purpose, a pretreatment must be developed to eliminate cells and cell debris of the extract obtained. Centrifugation is the most used together with conventional filtration using glass discs or gauze.
3.4 The Importance of Protocols for Membrane Cleaning by Microalgae Fouling
Despite the great advantages that membrane technology offers in microalgae processes, the main drawback is the membrane fouling, which causes a severe decline in the fluxes, growing transmembrane pressure, increase the cleaning frequency, and energy consumption, and consequently a short lifespan of the membrane. For the microalgae process, fouling is generally provoked by foulants such as microalgae cell debris, inorganic colloidal particles, natural organic matters, extracellular organic matters, and soluble microbial products (Liao et al. 2018). For microalgae biomass harvesting, several factors affecting membrane fouling include membrane pore size, pressure, and temperature (Leam et al. 2020). Also, some other factors are involved in the membrane fouling, e.g., Liao et al. 2018 have very recently explored and analyzed multiple factors involved in membrane photobioreactor process, however, some of these factors apply to the microalgae membrane process overall. They identified that the membrane fouling is caused by factors such as the hydrodynamic conditions (e.g., sparging intensity and cross flow-velocity), process operation conditions (e.g., hydraulic retention time and solids retention time), environmental conditions of the culture media (e.g., pH, temperature, and nutrients), microalgae concentration, and microalgae characteristics (e.g., specie, cell size, particle size distribution, surface properties such as extracellular polysaccharides and soluble microbial products content and composition, hydrophobicity and zeta potential)(Liao et al. 2018). Some strategies to diminish membrane fouling involving feed pretreatments, optimization of operational conditions, membrane surface modification, and intensive physical and chemical cleaning of the membrane (e.g., aeration, membrane relaxation, backflushing, and ultrasonication) (Leam et al. 2020). Membrane maintenance is mainly determined by the cleaning process, while each microalgae strain shows a particular behavior onto the membrane fouling, which drives complex strategies for cleaning up the membrane. At this point, it is likely that more efficient cleaning protocols need to be developed, where the selection of the physical method and variables, such as cleaning agent, concentration, and temperature, will require to be optimized. Particularly, Ahmad et al. (2014) investigated different chemical cleaning agents as an important criterion for the cleanup of membranes. A cellulose acetate membrane (1.2 um of the pore size) was used for harvesting Chlorella sp. The water flux recovery and the foulant removal were tested, showing that 0.75% of NaOCl exhibited the best cleaning performance, and a 98% of flux recovery was obtained while removing mostly major foulants (Ahmad et al. 2014).
4 Concluding Remarks
Over the course of this chapter, microalgae have proved to be a promising source of compounds with high-added value. The study and application of each strain depends on the scope of research and the production of compounds of interest. When dealing with the recovery of their metabolites, membrane technology is a potential tool for upstream and downstream microalgae processing thanks to their well-defined molecular sieving, however, severe membrane fouling issue, mainly in biomass harvesting, limit its long-term application and profitable use. To date, various strategies have been developed to lessen its ravages but it is still a bottleneck to solve.
Towards the recovery of high-added value compounds, the membrane technology has proven its ability as a purification step of the various compounds. Importantly, pretreatment steps seem to be required for a more efficient recovery to concurrently mitigate the membrane fouling. Today, there is a current trend in developing integrated membrane systems to fractionate a complex feed bulk solutions, along with decreasing the biofouling (Castro-Muñoz and Yáñez-Fernández 2015; Díaz-Montes and Castro-Muñoz 2019). Interestingly, these integrated membrane systems may also provide the possibility to implement continuous processes for possible large-scale applications.
References
Afzal I et al (2017) Chapter 3 – microalgae: a promising feedstock for energy and high-value products. In: Zia KM, Zuber M, Blends A, Composites MBT-ABP (eds) . Elsevier, pp 55–75. https://doi.org/10.1016/B978-0-12-812360-7.00003-3
Ahmad AL et al (2012) Crossflow microfiltration of microalgae biomass for biofuel production. Desalination 302:65–70. https://doi.org/10.1016/j.desal.2012.06.026
Ahmad AL et al (2014) Chemical cleaning of a cross-flow microfiltration membrane fouled by microalgal biomass. J Taiwan Inst Chem Engineers Taiwan Institute of Chemical Engineers 45(1):233–241. https://doi.org/10.1016/j.jtice.2013.06.018
Ahmad MZ et al (2021) Novel MMM using CO2 selective SSZ-16 and high-performance 6FDA-polyimide for CO2/CH4 separation. Sep Purif Technol 254:117582. https://doi.org/10.1016/j.seppur.2020.117582
Alishah Aratboni H et al (2019) Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Fact BioMed Central 18(1):1–17. https://doi.org/10.1186/s12934-019-1228-4
Alzahrani MAJ, Perera CO, Hemar Y (2018) Production of bioactive proteins and peptides from the diatom Nitzschia laevis and comparison of their in vitro antioxidant activities with those from Spirulina platensis and Chlorella vulgaris. Int J Food Sci Technol 53(3):676–682. https://doi.org/10.1111/ijfs.13642
Apone F, Barbulova A, Colucci MG (2019) Plant and microalgae derived peptides are advantageously employed as bioactive compounds in cosmetics. Front Plant Sci 10(June):1–8. https://doi.org/10.3389/fpls.2019.00756
Bafana A (2013) Characterization and optimization of production of exopolysaccharide from Chlamydomonas reinhardtii. Carbohyd Polymers Elsevier Ltd 95(2):746–752. https://doi.org/10.1016/j.carbpol.2013.02.016
Balti R et al (2018) Concentration and purification of Porphyridium cruentum exopolysaccharides by membrane filtration at various cross-flow velocities. Process Biochem 74(March):175–184. https://doi.org/10.1016/j.procbio.2018.06.021
Benelhadj S et al (2016) Properties of lysozyme/Arthrospira platensis (spirulina) protein complexes for antimicrobial edible food packaging. Algal Res Elsevier B.V 15:43–49. https://doi.org/10.1016/j.algal.2016.02.003
Bilad MR et al (2014) Coupled cultivation and pre-harvesting of microalgae in a membrane photobioreactor (MPBR). Bioresour Technol Elsevier Ltd 155:410–417. https://doi.org/10.1016/j.biortech.2013.05.026
Borowitzka MA (2018) Chapter 3 – Biology of microalgae, In Levine IA, Fleurence JBT-M, H. and D. P. (eds). Academic, pp 23–72. https://doi.org/10.1016/B978-0-12-811405-6.00003-7
Bustos-Garza, C., Yáñez-Fernández, J. and Barragán-Huerta, B. E. (2013) Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials Food Res Int, 54(1), pp. 641–649. https://doi.org/10.1016/j.foodres.2013.07.061.
Carissimi M, Flôres SH, Rech R (2018) Effect of microalgae addition on active biodegradable starch film. Algal Res Elsevier 32(January):201–209. https://doi.org/10.1016/j.algal.2018.04.001
Castro-Muñoz, R. and Fíla, V. (2018) Membrane-based technologies as an emerging tool for separating high-added-value compounds from natural products Trends Food Sci Technol, 82, pp. 8–20. https://doi.org/10.1016/j.tifs.2018.09.017.
Castro-Muñoz R, González-Valdez J (2019) New trends in biopolymer-based membranes for pervaporation. Molecules 24(19):1–17. https://doi.org/10.3390/molecules24193584
Castro-Muñoz R, Yáñez-Fernández J (2015) Valorization of Nixtamalization wastewaters (Nejayote) by integrated membrane process. Food Bioprod Process 95:7–18. https://doi.org/10.1016/j.fbp.2015.03.006
Castro-Muñoz R, Yáñez-Fernández J, Fíla V (2016) Phenolic compounds recovered from agro-food by-products using membrane technologies: an overview. Food Chem 213:753–762. https://doi.org/10.1016/j.foodchem.2016.07.030
Castro-Muñoz R et al (2019a) Enhanced CO2 permeability in Matrimid® 5218 mixed matrix membranes for separating binary CO2/CH4 mixtures. Sep Purif Technol 210:553–562. https://doi.org/10.1016/j.seppur.2018.08.046
Castro-Muñoz R, Conidi C, Cassano A (2019b) Membrane-based technologies for meeting the recovery of biologically active compounds from foods and their by-products. Crit Rev Food Sci Nutr Taylor & Francis 59(18):2927–2948. https://doi.org/10.1080/10408398.2018.1478796
Castro-Muñoz R, Fíla V, Durán-Páramo E (2019c) A review of the primary by-product (Nejayote) of the nixtamalization during maize processing: potential reuses. Waste Biomass Valorizat Springer Netherlands 10(1):13–22. https://doi.org/10.1007/s12649-017-0029-4
Castro-Muñoz R, González-Melgoza LL, García-Depraect O (2021) Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water. Chemosphere 270:–129421. https://doi.org/10.1016/j.chemosphere.2020.129421
Chaiklahan R et al (2011) Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour Technol Elsevier Ltd 102(14):7159–7164. https://doi.org/10.1016/j.biortech.2011.04.067
Chen CY et al (2013) Microalgae-based carbohydrates for biofuel production. Biochem Eng J Elsevier B.V 78:1–10. https://doi.org/10.1016/j.bej.2013.03.006
Chiaiese P et al (2018) Renewable sources of plant biostimulation: microalgae as a sustainable means to improve crop performance. Front Plant Sci 871(December):1–6. https://doi.org/10.3389/fpls.2018.01782
Chittora D et al (2020) Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem Biophys Rep Elsevier B.V 22(January):100737. https://doi.org/10.1016/j.bbrep.2020.100737
Ciapponi R, Turri S, Levi M (2019) Mechanical reinforcement by microalgal biofiller in novel thermoplastic biocompounds from plasticized gluten. Materials 12(9). https://doi.org/10.3390/ma12091476
Clavijo Rivera E et al (2020) Cross-flow filtration for the recovery of lipids from microalgae aqueous extracts: membrane selection and performances. Process Biochem Elsevier 89(November):199–207. https://doi.org/10.1016/j.procbio.2019.10.016
Costa-Pinto R, Gantner D (2020) ‘Macronutrients, minerals, vitamins and energy. Anaesthesia Intens Care Med Elsevier Ltd 21(3):157–161. https://doi.org/10.1016/j.mpaic.2019.12.006
Cui ZF, Jiang Y, Field RW (2010) Fundamentals of pressure-driven membrane separation processes. Membrane Technol Elsevier Ltd:1–18. https://doi.org/10.1016/B978-1-85617-632-3.00001-X
Daneshvar E et al (2019) Sequential cultivation of microalgae in raw and recycled dairy wastewater: microalgal growth, wastewater treatment and biochemical composition. Bioresour Technol Elsevier 273(October):556–564. https://doi.org/10.1016/j.biortech.2018.11.059
Das P et al (2019) The effect of culture salinity on the harvesting of microalgae biomass using pilot-scale tangential-flow-filter membrane. Bioresour Technol 293(August). https://doi.org/10.1016/j.biortech.2019.122057
de Farias Silva CE, Sforza E, Bertucco A (2019) Enhancing carbohydrate productivity in photosynthetic microorganism production: a comparison between cyanobacteria and microalgae and the effect of cultivation systems. In: Advances in feedstock conversion technologies for alternative fuels and bioproducts. Elsevier Inc. https://doi.org/10.1016/b978-0-12-817937-6.00003-5
De Jesus Raposo MF, De Morais RMSC, De Morais AMMB (2013) Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs 11(1):233–252. https://doi.org/10.3390/md11010233
Díaz MT et al (2017) Journal of food composition and analysis feeding microalgae increases omega 3 fatty acids of fat deposits and muscles in light lambs. J Food Comp Anal Elsevier Inc 56:115–123. https://doi.org/10.1016/j.jfca.2016.12.009
Díaz-Montes E, Castro-Muñoz R (2019) Metabolites recovery from fermentation broths via pressure-driven membrane processes. Asia Pac J Chem Eng 14(4). https://doi.org/10.1002/apj.2332
Díaz-Montes E, Castro-Muñoz R (2021) Edible films and coatings as food-quality preservers: An overview, Foods. https://doi.org/10.3390/foods10020249
Díaz-Montes E et al (2020) ‘Fractionation of Stevia rebaudiana aqueous extracts via two-step ultrafiltration process: towards rebaudioside a extraction. Food Bioprod Process 123:111–122. https://doi.org/10.1016/j.fbp.2020.06.010
Dineshkumar R et al (2020) Exploring the microalgae biofertilizer effect on onion cultivation by field experiment. Waste Biomass Valorizat Springer Netherlands 11(1):77–87. https://doi.org/10.1007/s12649-018-0466-8
dos Brasil BSAF et al (2017) Microalgae and cyanobacteria as enzyme biofactories. Algal Res Elsevier 25(April):76–89. https://doi.org/10.1016/j.algal.2017.04.035
Dufossé L (2018) Chapter 4 – Microbial pigments from bacteria, yeasts, fungi, and microalgae for the food and feed industries. In Grumezescu AM, Holban AMBT-N, AFA, FD (eds) Handbook of food bioengineering. Academic, pp 113–132. https://doi.org/10.1016/B978-0-12-811518-3.00004-1
Edelmann M et al (2019) Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J Food Composit Anal Elsevier 82(May):103226. https://doi.org/10.1016/j.jfca.2019.05.009
El Semary NA, Abd El Naby M (2010) Characterization of a Synechocystis sp. from Egypt with the potential of bioactive compounds production. World J Microbiol Biotechnol 26(6):1125–1133. https://doi.org/10.1007/s11274-009-0280-3
Ertu S, Bak M (2007) Treatment of dye-rich wastewater by an immobilized thermophilic cyanobacterial strain: Phormidium sp. 2:244–248. https://doi.org/10.1016/j.ecoleng.2007.11.011
Fabra MJ et al (2018) Structural and physicochemical characterization of thermoplastic corn starch films containing microalgae. Carbohyd Polym Elsevier 186(January):184–191. https://doi.org/10.1016/j.carbpol.2018.01.039
Flamm D, Blaschek W (2014) Exopolysaccharides of Synechocystis aquatilis are sulfated arabinofucans containing N-acetyl-fucosamine. Carbohyd Polymers Elsevier Ltd 101(1):301–306. https://doi.org/10.1016/j.carbpol.2013.09.036
Foltz KL, Smith DL, Moritz JS (2016) Porcine feed intake of corn–soybean based diets supplemented with oil-extracted microalgae and subsequent performance. Profess Anim Scientist Elsevier Masson SAS 32(6):849–853. https://doi.org/10.15232/pas.2016-01534
Fox JM, Zimba PV (2018) Minerals and trace elements in microalgae. Microalgae Health Dis Prevent Elsevier Inc. https://doi.org/10.1016/b978-0-12-811405-6.00008-6
Fradique Ḿ et al (2010) Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: preparation and evaluation. J Sci Food Agric 90(10):1656–1664. https://doi.org/10.1002/jsfa.3999
Fuad N et al (2018) Mass harvesting of marine microalgae using different techniques. Food Bioprod Process Institution of Chemical Engineers 112:169–184. https://doi.org/10.1016/j.fbp.2018.10.006
Gao F et al (2019) Lipid accumulation properties of Chlorella vulgaris and Scenedesmus obliquus in membrane photobioreactor (MPBR) fed with secondary effluent from municipal wastewater treatment plant. Renew Energy Elsevier Ltd 136:671–676. https://doi.org/10.1016/j.renene.2019.01.038
Gasljevic K et al (2008) Drag-reducing polysaccharides from marine microalgae: species productivity and drag reduction effectiveness. J Appl Phycol 20(3):299–310. https://doi.org/10.1007/s10811-007-9250-z
Gerardo ML, Zanain MA, Lovitt RW (2015) Pilot-scale cross-flow microfiltration of Chlorella minutissima: a theoretical assessment of the operational parameters on energy consumption. Chem Eng J Elsevier B.V 280:505–513. https://doi.org/10.1016/j.cej.2015.06.026
Geresh S et al (2002) Characterization of the extracellular polysaccharide of Porphyridium sp.: molecular weight determination and rheological properties. Carbohydr Polym 50(2):183–189. https://doi.org/10.1016/S0144-8617(02)00019-X
Ginzberg A et al (2000) Chickens fed with biomass of the red microalga Porphyridium sp. have reduced blood cholesterol level and modified fatty acid composition in egg yolk. J Appl Phycol 12(3–5):325–330. https://doi.org/10.1023/A:1008102622276
Giorno F, Mazzei R, Giorno L (2013) Purification of triacylglycerols for biodiesel production from Nannochloropsis microalgae by membrane technology. Bioresour Technol Elsevier Ltd 140:172–178. https://doi.org/10.1016/j.biortech.2013.04.073
Gómez-Coronado DJM et al (2004) Tocopherol measurement in edible products of vegetable origin. J Chromatogr A 1054(1–2):227–233. https://doi.org/10.1016/j.chroma.2004.08.072
Gouveia L, Empis J (2003) Relative stabilities of microalgal carotenoids in microalgal extracts, biomass and fish feed: effect of storage conditions. Innov Food Sci Emerg Technol 4(2):227–233. https://doi.org/10.1016/S1466-8564(03)00002-X
Gouveia L et al (2006) Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur Food Res Technol 222(3–4):362–367. https://doi.org/10.1007/s00217-005-0105-z
Gouveia L et al (2007) Chlorella vulgaris biomass used as colouring source in traditional butter cookies’, Innov Food Sci Emerg Technol 8(3):433–436. https://doi.org/10.1016/j.ifset.2007.03.026
Gouveia L et al (2008) Functional biscuits with PUFA-ω3 from Isochrysis galbana. J Sci Food Agric 88(5):891–896. https://doi.org/10.1002/jsfa.3166
Gouveia L et al (2016) Microalgae biomass production using wastewater: treatment and costs scale-up considerations. 16:167–176. https://doi.org/10.1016/j.algal.2016.03.010
Grossman A (2016) Nutrient acquisition: the generation of bioactive vitamin B12 by microalgae. Curr Biol Elsevier Ltd 26(8):R319–R321. https://doi.org/10.1016/j.cub.2016.02.047
Gupta S, Pawar SB, Pandey RA (2019) Science of the Total environment current practices and challenges in using microalgae for treatment of nutrient rich wastewater from agro-based industries. Science Total Environ Elsevier B.V 687:1107–1126. https://doi.org/10.1016/j.scitotenv.2019.06.115
Haase SM, Huchzermeyer B, Rath T (2012) PHB accumulation in Nostoc muscorum under different carbon stress situations. J Appl Phycol 24(2):157–162. https://doi.org/10.1007/s10811-011-9663-6
Huang W, Chu H, Dong B (2012) Characteristics of algogenic organic matter generated under different nutrient conditions and subsequent impact on microfiltration membrane fouling. Desalination Elsevier B.V 293:104–111. https://doi.org/10.1016/j.desal.2012.03.001
Hwang JH, Rittmann BE (2017) Effect of permeate recycling and light intensity on growth kinetics of Synechocystis sp. PCC 6803. Algal Res Elsevier 27(September):170–176. https://doi.org/10.1016/j.algal.2017.09.008
Jez S et al (2017) Comparative life cycle assessment study on environmental impact of oil production from micro-algae and terrestrial oilseed crops. Bioresour Technol Elsevier Ltd 239:266–275. https://doi.org/10.1016/j.biortech.2017.05.027
Johnson EM, Kumar K, Das D (2014) Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresour Technol Elsevier Ltd 166:541–547. https://doi.org/10.1016/j.biortech.2014.05.097
Kavitha MD et al (2016) Culture media optimization of Porphyridium purpureum: production potential of biomass, total lipids, arachidonic and eicosapentaenoic acid. J Food Sci Technol 53(5):2270–2278. https://doi.org/10.1007/s13197-016-2185-0
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial Cell Fact BioMed Central 17(1):1–21. https://doi.org/10.1186/s12934-018-0879-x
Khoo KS et al (2019) Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour Technol Elsevier 288(May):121606. https://doi.org/10.1016/j.biortech.2019.121606
Kim, D. et al. (2019) ‘Turbulent jet-assisted microfiltration for energy efficient harvesting of microalgae’, J Membr Sci, 575(December), pp. 170–178. doi: https://doi.org/10.1016/j.memsci.2018.12.069.
Kumar R, Ghosh AK, Pal P (2020) Synergy of biofuel production with waste remediation along with value-added co-products recovery through microalgae cultivation: a review of membrane-integrated green approach. Sci Total Environ Elsevier B.V 698:134169. https://doi.org/10.1016/j.scitotenv.2019.134169
Leam JJ et al (2020) Membrane Technology for Microalgae Harvesting. Microalgae Cult Biofuels Prod Elsevier Inc. https://doi.org/10.1016/b978-0-12-817536-1.00007-2
Lee E et al (2014) Design tool and guidelines for outdoor photobioreactors. Chem Eng Sci Elsevier 106:18–29. https://doi.org/10.1016/j.ces.2013.11.014
Li H et al (2011) Pilot-scale isolation of bioactive extracellular polymeric substances from cell-free media of mass microalgal cultures using tangential-flow ultrafiltration. Process Biochem 46(5):1104–1109. https://doi.org/10.1016/j.procbio.2011.01.028
Li K et al (2019) Bioresource technology microalgae-based wastewater treatment for nutrients recovery : a review. Bioresour Technol Elsevier 291(July):121934. https://doi.org/10.1016/j.biortech.2019.121934
Liao Y et al (2018) A review of membrane fouling and its control in algal-related membrane processes. Bioresour Technol Elsevier 264(April):343–358. https://doi.org/10.1016/j.biortech.2018.06.102
Ljubic A et al (2020) Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem Elsevier 320(November):126627. https://doi.org/10.1016/j.foodchem.2020.126627
López-Pacheco IY et al (2019) Combination of nejayote and swine wastewater as a medium for Arthrospira maxima and Chlorella vulgaris production and wastewater treatment. Sci Total Environ 676:356–367. https://doi.org/10.1016/j.scitotenv.2019.04.278
Lu Y, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci Elsevier Ltd 20(5):273–282. https://doi.org/10.1016/j.tplants.2015.01.006
Luangpipat T, Chisti Y (2017) Biomass and oil production by Chlorella vulgaris and four other microalgae – effects of salinity and other factors. J Biotechnol Elsevier B.V 257:47–57. https://doi.org/10.1016/j.jbiotec.2016.11.029
Makut BB, Das D, Goswami G (2019) Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: a sustainable approach. Algal Res Elsevier 37(November):228–239. https://doi.org/10.1016/j.algal.2018.11.020
Marbelia L et al (2016) Polyacrylonitrile membranes for microalgae filtration: influence of porosity, surface charge and microalgae species on membrane fouling. Algal Res Elsevier B.V 19:128–137. https://doi.org/10.1016/j.algal.2016.08.004
Marcati A et al (2014) Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Res Elsevier B.V 5(1):258–263. https://doi.org/10.1016/j.algal.2014.03.006
Martin-Gil V et al (2019) Economic framework of membrane technologies for natural gas applications. Separat Purif Rev Taylor & Francis 48(4):298–324. https://doi.org/10.1080/15422119.2018.1532911
Matos ÂP (2019) Chapter 3 – microalgae as a potential source of proteins. In CMBT-PSS (ed) Galanakis processing and applications. Academic, pp 63–96. https://doi.org/10.1016/B978-0-12-816695-6.00003-9
Monte J et al (2018) Harvesting of Dunaliella salina by membrane filtration at pilot scale. Sep Purif Technol Elsevier 190(April):252–260. https://doi.org/10.1016/j.seppur.2017.08.019
Monte J et al (2019) Recycling of Dunaliella salina cultivation medium by integrated membrane filtration and advanced oxidation. Algal Res Elsevier 39(July):101460. https://doi.org/10.1016/j.algal.2019.101460
Monte J et al (2020) Development of an integrated process of membrane filtration for harvesting carotenoid-rich Dunaliella salina at laboratory and pilot scales. Sep Purif Technol Elsevier 233(August):116021. https://doi.org/10.1016/j.seppur.2019.116021
Morales-Jiménez M et al (2020) Production, preparation and characterization of microalgae-based biopolymer as a potential bioactive film. Coatings. https://doi.org/10.3390/coatings10020120
Nagy K (2017) Importance of fatty acids in physiopathology of human body ( Chapter 1). In Catala I-DTE-A (ed). IntechOpen, Rijeka. https://doi.org/10.5772/67407.
Naresh Kumar A et al (2019) Deoiled algal biomass derived renewable sugars for bioethanol and biopolymer production in biorefinery framework. Bioresour Technol Elsevier 296:122315. https://doi.org/10.1016/j.biortech.2019.122315
Nayak M, Swain DK, Sen R (2019) ‘Strategic valorization of de-oiled microalgal biomass waste as biofertilizer for sustainable and improved agriculture of rice (Oryza sativa L.)crop. Sci Total Environ Elsevier B.V 682:475–484. https://doi.org/10.1016/j.scitotenv.2019.05.123
Niaounakis M (2015) Chapter 1 – introduction. In Niaounakis MBT-BP, P (ed). Andrew Publishing, Oxford, pp 1–77. https://doi.org/10.1016/B978-0-323-26698-7.00001-5.
Ovando CA et al (2018) Functional properties and health benefits of bioactive peptides derived from spirulina: a review. Food Rev Intl Taylor & Francis 34(1):34–51. https://doi.org/10.1080/87559129.2016.1210632
Padaki, M. et al. (2015) Membrane technology enhancement in oil-water separation. A review. Desalination Elsevier B.V., 357, pp. 197–207 doi: https://doi.org/10.1016/j.desal.2014.11.023.
Paliwal C et al (2017) Bioresource technology abiotic stresses as tools for metabolites in microalgae. Bioresour Technol Elsevier Ltd 244:1216–1226. https://doi.org/10.1016/j.biortech.2017.05.058
Panchenko MV et al (2020) Carbon dioxide in the atmosphere-water system and biogenic elements in the littoral zone of Lake Baikal during period 2004–2018. J Great Lakes Res Int Assoc Great Lakes Res 46(1):85–94. https://doi.org/10.1016/j.jglr.2019.10.016
Patel AK et al (2013) Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour Technol Elsevier Ltd 145:345–350. https://doi.org/10.1016/j.biortech.2012.12.038
Paula A et al (2012) Novel foods with microalgal ingredients – effect of gel setting conditions on the linear viscoelasticity of Spirulina and Haematococcus gels. J Food Eng Elsevier Ltd 110(2):182–189. https://doi.org/10.1016/j.jfoodeng.2011.05.044
Pembroke J, Naveena B, Armshaw P (2015) The potential of the photoautotroph Synechocystis for metal bioremediation. https://doi.org/10.5772/60514
Pichardo-Romero D et al (2020) Current advances in biofouling mitigation in membranes for water treatment: an overview. PRO. https://doi.org/10.3390/pr8020182
Přibyl P et al (2015) Elevated production of carotenoids by a new isolate of Scenedesmus sp. Algal Res 11:22–27. https://doi.org/10.1016/j.algal.2015.05.020
Rachidi F et al (2020) Microalgae polysaccharides bio-stimulating effect on tomato plants: growth and metabolic distribution. Biotechnol Reports Elsevier B.V 25:e00426. https://doi.org/10.1016/j.btre.2020.e00426
Rahaman MSA et al (2011) A review of carbon dioxide capture and utilization by membrane integrated microalgal cultivation processes. Renew Sustain Energy Rev Elsevier Ltd 15(8):4002–4012. https://doi.org/10.1016/j.rser.2011.07.031
Ramesh Kumar B et al (2019) Microalgae as rich source of polyunsaturated fatty acids. Biocatalysis Agricult Biotechnol Elsevier Ltd 17(January):583–588. https://doi.org/10.1016/j.bcab.2019.01.017
Rastogi RP, Sinha RP (2009) Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv Elsevier Inc 27(4):521–539. https://doi.org/10.1016/j.biotechadv.2009.04.009
Rickman M, Pellegrino J, Davis R (2012) Fouling phenomena during membrane filtration of microalgae. J Membrane Sci Elsevier 423–424:33–42. https://doi.org/10.1016/j.memsci.2012.07.013
Román RB et al (2002) Recovery of pure b-phycoerythrin from the microalga Porphyridium cruentum. J Biotechnol 93(1):73–85. https://doi.org/10.1016/S0168-1656(01)00385-6
Rossi N et al (2004) Harvesting of cyanobacterium Arthrospira Platensis using organic filtration membranes. Food Bioproducts Process 82(3):244–250. https://doi.org/10.1205/fbio.82.3.244.44177
Sadvakasova AK et al (2019) Search for new strains of microalgae-producers of lipids from natural sources for biodiesel production. Intl J Hydr Energy Elsevier Ltd 44(12):5844–5853. https://doi.org/10.1016/j.ijhydene.2019.01.093
Sandefur, H. N. et al. (2016) ‘Recovery of nutrients from swine wastewater using ultrafiltration: applications for microalgae cultivation in photobioreactors. Ecol Eng Elsevier B.V., 94, pp. 75–81 doi: https://doi.org/10.1016/j.ecoleng.2016.05.066.
Schulze C et al (2017) Carbohydrates in microalgae: comparative determination by TLC, LC-MS without derivatization, and the photometric thymol-sulfuric acid method. Algal Res Elsevier 25(April):372–380. https://doi.org/10.1016/j.algal.2017.05.001
Sharif N et al (2017) Chapter 4 – origin of algae and their plastids. In Zia KM, Zuber M, Ali Blends, and Composites, MBT-A BP (eds) Elsevier, pp 77–113. https://doi.org/10.1016/B978-0-12-812360-7.00004-5
Silva BF et al (2015) Analysis of some chemical elements in marine microalgae for biodiesel production and other uses. Algal Res Elsevier B.V 9:312–321. https://doi.org/10.1016/j.algal.2015.04.010
Singh S, Das S (2011) Screening, production, optimization and characterization of cyanobacterial polysaccharide. World J Microbiol Biotechnol 27(9):1971–1980. https://doi.org/10.1007/s11274-011-0657-y
Soni RA, Sudhakar K, Rana RS (2017) Spirulina – from growth to nutritional product: a review. Trends Food Sci Technol Elsevier Ltd 69:157–171. https://doi.org/10.1016/j.tifs.2017.09.010
Stanier RY et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology Microbiol Soc 111(1):1–61. https://doi.org/10.1099/00221287-111-1-1
Sun X et al (2013) A comparative study of microfiltration and ultrafiltration for algae harvesting. Algal Res 2(4):437–444. https://doi.org/10.1016/j.algal.2013.08.004
Sun Z, Li T, Zhou Z-G (2015) Microalgae as a source of lutein: chemistry, biosynthesis, and carotenogenesis. Adv Biochem Eng Biotechnol 153. https://doi.org/10.1007/10_2015_331
Suparmaniam U et al (2019) Insights into the microalgae cultivation technology and harvesting process for biofuel production: a review. Renew Sustain Energy Rev Elsevier Ltd 115(August):109361. https://doi.org/10.1016/j.rser.2019.109361
Tarento TDC et al (2018) Microalgae as a source of vitamin K1. Algal Res Elsevier 36(June):77–87. https://doi.org/10.1016/j.algal.2018.10.008
Urrutia C, Yañez-mansilla E, Jeison D (2019) Bioremoval of heavy metals from metal mine tailings water using microalgae biomass. Algal Res Elsevier 43(August):101659. https://doi.org/10.1016/j.algal.2019.101659
Varfolomeev SD, Wasserman L a (2011) Microalgae as source of biofuel, food, fodder, and medicines. Appl Biochem Microbiol 47(9):789–807. https://doi.org/10.1134/S0003683811090079
Wicaksana F et al (2012) Microfiltration of algae (Chlorella sorokiniana): critical flux, fouling and transmission. J Memb Sci Elsevier B.V 387–388(1):83–92. https://doi.org/10.1016/j.memsci.2011.10.013
Yin Z et al (2020) A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions. Bioresour Technol 301(November). https://doi.org/10.1016/j.biortech.2020.122804
Yu, J. et al. (2019) Continuous cultivation of Arthrospira platensis for phycocyanin production in large-scale outdoor raceway ponds using microfiltered culture medium. ’, Bioresour Technol Elsevier, 287(May), p. 121420 doi: https://doi.org/10.1016/j.biortech.2019.121420.
Zhang Y, Fu Q (2018) Algal fouling of microfiltration and ultrafiltration membranes and control strategies: a review. Sep Purif Technol 203:193–208. https://doi.org/10.1016/j.seppur.2018.04.040
Zhang D, Pan X, Mu G (2012) Biosorption of Sb(III) to exopolymers from cyanobacterium Synechocystis sp.: a fluorescence and FTIR study. Pol J Environ Stud 21(5):1497–1503
Zhang X et al (2016) Impact of algal organic matter released from Microcystis aeruginosa and Chlorella sp. on the fouling of a ceramic microfiltration membrane. Water Res Elsevier Ltd 103:391–400. https://doi.org/10.1016/j.watres.2016.07.061
Zhang M et al (2019) Membrane technologies for microalgal cultivation and dewatering: recent progress and challenges. Algal Res Elsevier 44(April):101686. https://doi.org/10.1016/j.algal.2019.101686
Zhao Z et al (2020) Synergy between membrane filtration and flocculation for harvesting microalgae. Sep Purif Technol Elsevier 240:116603. https://doi.org/10.1016/j.seppur.2020.116603
Zhou W et al (2018) ‘Cultivation of microalgae Chlorella zofingiensis on municipal wastewater and biogas slurry towards bioenergy. J Biosci Bioeng Elsevier Ltd 126(5):644–648. https://doi.org/10.1016/j.jbiosc.2018.05.006
Acknowledgments
The authors gratefully acknowledge financial support by the Consejo Nacional de Ciencia y Tecnología (CONACYT) of Mexico through the scholarship to Mónica Morales-Jiménez, with the support of the project SIP-20201350, and SIP-20211141 from Instituto Politécnico Nacional. Rosalba Coronel Santiago by her support in Engineering Design to the image edition.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Morales-Jiménez, M., Yáñez-Fernández, J., Castro-Muñoz, R., Barragán-Huerta, B.E. (2021). Recovery of High Added Value Compounds from Microalgae Cultivation Using Membrane Technology. In: Jafari, S.M., Castro-Muñoz, R. (eds) Membrane Separation of Food Bioactive Ingredients. Food Bioactive Ingredients. Springer, Cham. https://doi.org/10.1007/978-3-030-84643-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-84643-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84642-8
Online ISBN: 978-3-030-84643-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)