Abstract
Enlarged vestibular aqueduct (EVA) is described when its horizontal dimensions are greater than 1.5 mm at the midpoint on the axial plane. It can be isolated as a separate group of inner ear malformations (IEM) in the presence of normal modiolus and cochlea or it may be accompanying incomplete partition type II (IP-II) malformation. They do not have a characteristic audiological configuration. Hearing loss is usually fluctuating and progressive throughout lifetime, with occasional sudden SNHL. There may be an air-bone gap present at the lower frequencies. They may become a candidate for cochlear implantation during follow-up. Cerebrospinal fluid gusher and facial nerve anomaly are not expected during cochlear implantation. In this chapter, radiological, audiological features, and surgical considerations of EVA along with case presentations are provided.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Inner ear malformations
- Cochleovestibular anomalies
- Enlarged vestibular aqueduct
- Cochlear implantation
- Fluctuating hearing loss
- Progressive hearing loss
-
1.
Enlarged vestibular aqueduct can be isolated or accompany cochlear deformities.
-
2.
No typical audiological configuration. Hearing loss usually fluctuating and progressive throughout lifetime, with occasional sudden SNHL. Air-bone gap is usually present at the lower frequencies.
-
3.
Usually they become a candidate for cochlear implantation during follow-up.
-
4.
The reason for the progressive SNHL may be CSF pressure transmission into the inner ear.
27.1 Definition
Vestibular aqueduct (VA) is a bony canal transmitting the endolymphatic duct between endolymphatic sac and vestibule. Normally it is less than 1.5 mm in diameter at the midpoint and also less than the diameter of the posterior semicircular canal (Fig. 27.1a, b).
Enlarged Vestibular Aqueduct (EVA): This entity describes the presence of an enlarged vestibular aqueduct (i.e. the midpoint between posterior labyrinth and operculum is larger than 1.5 mm on axial HRCT of the temporal bone) in the presence of a normal cochlea (Fig. 27.2a, b).
EVA was discovered by Carlo Mondini [1] during temporal bone dissection for the first time. Radiologically EVA was defined by Valvassori and Clemis [2] in 1978 when its horizontal dimensions are greater than 1.5 mm at the midpoint and 2 mm at the external aperture of the vestibular aqueduct on the axial plane. It can be isolated as a separate group of inner ear malformations (IEM) in the presence of normal modiolus and cochlea (Figs. 27.2 and 27.3) or it may be accompanying incomplete partition type II (IP-II) malformation (Fig. 27.4).
Computerized tomography (a) and magnetic resonance imaging (MRI) (b) findings in an incomplete partition type II patient with enlarged vestibular aqueduct (white arrow), with defective modiolus (thin white arrow) and defective interscalar septa (dotted white arrow). MRI demonstrates a very large endolymphatic sac (white star)
In the past, the term “Large vestibular Aqueduct Syndrome” was used to describe this entity. A syndrome is defined as “a group of signs and symptoms that occur together and characterize a particular abnormality or condition.” However, EVA can be present as an isolated finding or in association with cochlear abnormalities as in IP-II. Sometimes it is seen in association with congenital hypothyroidy (Pendred Syndrome). Therefore, it is more appropriate to accept this entity as “enlarged vestibular aqueduct” rather than “large vestibular aqueduct syndrome.”
According to a recent paper by Sennaroglu [3], etiology of enlarged vestibular aqueduct (EVA) appears to be the result of a genetic abnormality based on literature findings. It is usually bilateral. Rarely there may be asymmetry between the two sides. Depending on the time of the pathology and extent of CSF pressure transmission into the inner ear, other cochleovestibular findings may accompany EVA (see Chap. 24 IP-II for further details).
The difference between EVA and IP-II is the presence of additional findings in the latter. IP-II is characterized by a triad of anomalies, which were first described by Mondini [1]:
-
1.
EVA.
-
2.
Minimally dilated vestibule.
-
3.
Cochlear modiolar defects resulting in cystic apex.
EVA appears to be responsible for the changes observed in IP-II. High CSF pressure transmitted into inner ear via EVA may cause different modiolar defects resulting in IP-II. Thus in the setting of an EVA, the cochlea should be evaluated carefully for additional anomalies and if the cochlea is completely normal on imaging the term EVA should be used.
In the past EVA was diagnosed more often. However, with the development of better scanners it has become possible to detect smaller cochlear changes with the present day HRCT and MRI (Fig. 27.3). MRI particularly can demonstrate even scala vestibuli dilatation [4]. Therefore, many of the pathologies may be in fact IP-II rather that EVA.
It is appropriate to accept EVA as a separate IEM group because the cochlea and vestibule are normal on imaging in spite of EVA. However, audiological findings are similar to IP-II cases.
27.2 Anatomy of Vestibular Aqueduct (VA), Endolymphatic Duct (ED), and Sac (ES)
According to Emmet [5], Valvassori and Clemis [2] described VA as an inverted “J” shaped structure. It has two segments: short descending proximal segment and a longer descending distal segment:
-
1.
Proximal segment or isthmus, which appears as the short limb of “J”; it is approximately 1.5 mm in length, with a mean diameter of 0.3 mm.
-
2.
Distal segment which appears as the long limb of “J”; it is triangular in shape with the apex at the isthmus and base at the opening into the posterior fossa. It increases from 0.5 mm at the isthmus towards the outer opening.
Vestibular aqueduct houses endolymphatic duct (ED) and the endolymphatic sac (ES) and the anatomy of the latter structures were described in detail by Lo et al. [6]. The ED and ES are filled with endolymph and are the non-sensory components of the membranous labyrinth. The ED forms from the confluence of the utricular and saccular ducts. It passes through the VA to the ES, which extends through the distal VA out of the external aperture of the aqueduct to terminate in the epidural space of the posterior cranial fossa. Therefore, ED and ES consist of components both inside and outside the otic capsule connected by a narrow passageway through the capsule.
Lo et al. [6] indicated that its proximal segment, the sinus, lies in a groove on the posteromedial surface of the vestibule, while its major portion is contained within the short, slightly upwardly arched, horizontal segment of the VA. After entering the VA, the sinus tapers to its intermediate segment within the horizontal segment of the VA and then narrows at its isthmus of the VA. Endolymphatic sac begins distal to the isthmus and has a proximal, intraosseous portion, lying within the transversely widening, vertical segment of the VA, which is covered posteriorly by a thin scale of bone, the operculum. The distal, extra-osseous portion of the sac rests on a fovea on the posterior wall of the petrous bone, between layers of dura.
27.3 Histopathology and Pathophysiology
EVA appears to be responsible for the changes observed in IP-II. High CSF pressure transmitted into inner ear via EVA may cause different modiolar defects resulting in IP-II. The mildest deformity observed in the cochlea is dilatation of scala vestibuli and the most significant deformity is the complete modiolar absence. It is also possible that patency of the EVA is minimal or EVA occurs after the development of the inner ear is complete and no pathology is observed in the cochlea. If the cochlea is completely normal on imaging it is appropriate to use the term EVA. In Chap. 24 on IP-II, histopathology and pathophysiology are described in more detail.
27.3.1 Literature Findings
Pritchett et al. [7] evaluated the results of cochlear implantation in 55 individuals diagnosed with EVA. They reported that the severity of IEM affected the time of the implantation. They found that the age of CI was approximately 6 years in EVA due to the progressive nature of hearing loss. They also emphasized that individuals diagnosed with isolated EVA could benefit from hearing aids for a longer period without implantation than those diagnosed with IP-II malformation.
Ahadizadeh et al. [8] reported the audiological outcomes (word recognition scores, speech reception thresholds, and pure tone audiometry) in patients with EVA and IP-II in their retrospective longitudinal study. They mentioned that the severity of the hearing loss in patients with EVA had no correlation with the presence of the IP-II. The main reason for the severity of the hearing loss was thought to be the EVA.
In the paper by the authors [9], relationship between the size of the vestibular aqueduct and air-bone gap in patients with EVA was evaluated. It was found out that the size of the vestibular aqueduct is not associated with air-bone gap. Due to the progressive nature of the hearing loss in EVA, it is not possible to find a correlation between the size of the vestibular aqueduct and air-bone gap. While hearing changes over the time, the size of the vestibular aqueduct remains same.
Madden et al. [10] evaluated the audiometric thresholds and the configuration of the hearing loss and compared them with the size of the VA in patients with EVA. They found no correlation between the degree of the hearing loss and the size of the VA.
In contrast to these findings, a study by Ascha et al. [11] in 2017 identified a direct relationship between the size of the VA and the audiometric thresholds in the repeated measures of hearing test results. They reported an increase of 17.5 dB in speech reception threshold per each millimeter increase of the size of the VA. They emphasized that the size of the VA is directly associated with the hearing loss in patients with EVA.
Aimoni et al. [12] described the clinical and genetic characteristics of 14 adolescents with EVA depending on the pendrin gene mutations (SLC26A4) in their study. Participants were divided into three subgroups: Group 1 non-syndromic EVA; Group 2 EVA with SLC26A4 gene mutation without thyroid dysfunction; and Group 3 EVA with Pendred syndrome with two pathological mutations of the SLC26A4 gene. They defined that degree of the hearing loss changes from mild to profound in patients with non-syndromic EVA. Conversely, severe to profound hearing loss was identified in most of the patients (%70–75) with SLC26A4 gene mutation (for more details see Chaps. 6 and 12).
In the literature many papers showed that the audiological performance of the patients with EVA are similar to the patients without any cochlear malformation [13,14,15]. Isaiah et al. [14] retrospectively analyzed the speech perception and radiological characteristics of the pediatric cochlear implant users with IEMs. They found out that most of the children with EVA are able to achieve open set speech perception and perform as good as others with normal anatomy. Manzoor et al. [15] also compared the performance of the patients with isolated EVA and EVA with IP-II and could not find any significant difference between two the groups.
In addition to audiological findings, vestibular symptoms in EVA were also reported in a recent study by Song et al. [16]. In their series vestibular complaints were reported in more than half of the patients with EVA and surprisingly, the incidence of benign paroxysmal positional vertigo (BPPV) was 18.2%. Although the percentage of vestibular symptoms are less than cochlear findings, it was recommended to evaluate vestibular symptoms particularly BPPV, in EVA.
27.3.2 Clinical Findings
Clinical and audiological presentation is similar to IP-II patients. Hearing may be normal at birth. Usual presentation is progressive mixed or SNHL. There may be sudden hearing loss attacks. In case of sudden hearing loss, there is a strong possibility for hearing recovery if the treatment (steroids and vasodilators) is started immediately. Usually with more attacks hearing progresses to a point with no benefit from hearing aids and they become candidates for CI surgery. Meningitis and facial nerve anomaly are extremely rare in EVA.
27.3.3 Audiological Findings
Audiological presentation and management is similar to that of IP-II. There is no typical audiological configuration for EVA. A heterogeneous hearing pattern, such as progressive sensorineural hearing loss, mixed type hearing loss, and fluctuating hearing loss, is encountered in EVA malformation. At birth they may obtain pass-on hearing screening with otoacoustic emission and automatic auditory brainstem response tests. Hearing loss is usually progressive. At the beginning they benefit from hearing aids. With progressive hearing loss or sudden hearing loss attacks they become candidates for CI. A study by Govaerts et al. [17] emphasized that initial diagnosis of the hearing loss in EVA is between the age of 3.5 and 5 years.
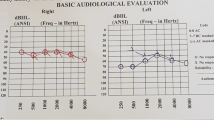
Hearing loss can be symmetric or asymmetric (Fig. 27.5a, b). The most characteristic audiological finding is air-bone gap (ABG) particularly present at low frequencies, but sometimes ABG can be seen in all frequencies. These patients should not be operated for ossicular fixation. The reason for air-bone gap is the third window phenomenon caused by the EVA which creates a bony defect in the labyrinth.
The pathological third window effect of EVA malformation causes the acoustic energy to go in a different direction from the cochlea, unlike the normal third window effect. This increases the compressive mechanism of bone conduction and results in better bone conduction hearing thresholds and lower air conduction hearing thresholds. Third window effect refers to selective stimulation of bone pathway in the presence of EVA [18].
27.3.4 Radiology
According to the IEM database of Hacettepe University Department of Otolaryngology, out of 776 patients with various IEMs, 69 of 1652 ears had EVA (4.2%).
Vestibular aqueduct enlargement is diagnosed when the midpoint between posterior labyrinth and operculum is larger than 1.5 mm in the presence of a normal cochlea (Fig. 27.2a, b). The external size of the cochlea as well as internal architecture should be normal. Higher field strength scanners with dedicated sequences such as three-dimensional constructive interference in steady-state (CISS), fast imaging employing steady-state acquisition (FIESTA), or driven equilibrium (DRIVE) allow higher resolution imaging of the cochlea with a better demonstration of modiolus, interscalar septa, scala tympani, and vestibule, allowing differentiation of EVA from IP-II (Figs. 27.3 and 27.4) [19,20,21]. In some cases of minor EVA, it may be difficult to diagnose EVA with only MRI. CT has been reported to have a higher accuracy compared to MRI in the depiction of enlargement (Fig. 27.2 and 27.3).
There are some controversies in the literature about the dimensions of VA to be accepted as enlarged. Vijayasekaran et al. [22] reported that VAs with midpoint or opercular widths of 1.0 and 2.0 mm or greater, respectively, are enlarged. Mohammed El Badry et al. [23] supported these measurements for diagnosis of EVA.
MRI always reveals normally developed cochlear nerves (Fig. 27.6). Therefore, ABI is not indicated in EVA.
EVA was mainly diagnosed on axial sections. Ozgen et al. [24] reported that the 45° oblique reformate provides better visualization and more accurate measurement of the VA compared to the routine images in the axial plane (Fig. 27.1b). It is very important to evaluate coronal sections as well (Fig. 27.6). In a recent paper by Sennaroglu and Bajin [25], the authors added the vertical dimension as well in the diagnosis of EVA. We have made the observation that EVA is present in a number of axial cuts (Fig. 27.7). This means that it can be enlarged in superior-inferior or oblique dimensions as well. It is advisable to use the same measurement of 1.5 mm at the midpoint on coronal sections for the diagnosis of EVA as well. Sometimes VA is enlarged in an oblique position; when axial measurements are taken into consideration, it may not be enlarged but it may be enlarged when oblique measurements are used (Fig. 27.7).
EVA is frequently bilateral and symmetric; rarely, it can be asymmetric.
27.4 Management
In patients with EVA, head trauma should be avoided. This may cause increase in CSF pressure and this may be transmitted into the inner ear resulting in sudden or progressive hearing loss. Helmets are advised during sports. It is advisable to avoid contact sports. In a recently published review study of Brodsky and Choi [26], it was reported that despite the possible association between EVA and sudden HL, head traumas are not a risk factor for overall progression of HL in EVA.
As hearing loss shows a progressive pattern, hearing aids are sufficient at the beginning. Language development in EVA is excellent.
With bilateral profound SNHL CI is indicated. There may be cases with asymmetric hearing loss with one side moderate, and other side profound SNHL. In this situation, CI is indicated on the side with profound HL.
27.5 Surgery
During CI surgery, all kinds of electrodes can be used as the modiolus and basal part of the cochlea is normal. Dimensions of cochlea and semicircular canals are normal; therefore, no facial nerve abnormality or difficulty during surgery is expected.
27.6 Hacettepe Experience
In our department between 1997 and September 2018, 2646 patients underwent CI and ABI operations. 279 of CI cases had IEMs. 30 had EVA deformity. During this period 30 patients with isolated EVA, out of 279 patients with IEMs, were implanted with CI. In this particular group of patients with EVA, minimum age of CI was 12 months and maximum age was 30 years. Mean age at CI was 9.7 years for our series of EVA. These findings suggest two important audiological characteristics of EVA malformation:
-
1.
Patients with EVA can benefit from their hearing aids for a longer period due to progressive nature of hearing loss.
-
2.
Patients with EVA may have sudden hearing loss at any time in their life.
Therefore, in patients with EVA, CI may be required at any time in their life.
During surgery, a facial recess approach was successfully used in all 30 patients. While pulsation was frequently observed, none of them had gusher. Six had oozing during surgery. This was easily controlled with any kind of electrode using soft tissue around the electrode.
Cochlear nerve is always present in EVA. None of the patients with EVA received an ABI.
27.7 Cases
Case 27.1: YY, 17-Year-Old Male Patient, Operated October 2016
He had progressive hearing loss necessitating a CI (Fig. 27.8a). HRCT of the patient revealed bilateral EVA. Despite a large air-bone gap especially at the low frequencies, no middle ear surgery was planned because of EVA. He received a CI on the right side in October 2016 (Fig. 27.8b).
Case 27.2: EÇ, 9-Year-Old Male Patient, Follow-Up with Hearing Aids
He had bilateral EVA with severe mixed type hearing loss (Fig. 27.9). His follow-up was done with bilateral hearing aids. Family was informed about the possibility of progressive hearing loss and necessity of a CI during follow-up.
Case 27.3: MI, 31-Year-Old Male Patient, Operated November 2017
History revealed sudden hearing loss on the right ear at the age of 4. His hearing on the left side was normal and he did not use any hearing aids on the right ear. He experienced sudden hearing loss on the left ear in February 2016 (Fig. 27.10a). Despite medical therapy, his hearing did not recover. An audiogram 10 months later showed another attack of hearing loss on the left ear (Fig. 27.10b). He started to use hearing aids on both sides. After the last attack, he applied for cochlear implantation in May 2017 (Fig. 27.10c). His left ear was operated with CI (Fig. 27.10d).
Present day approach with radiological imaging techniques and cochlear implantation was not present 30 years ago when his hearing loss on the right side occurred. At that time appropriate rehabilitation option was not planned for the right ear. At present time when a hearing loss occurs unilaterally, in a child of 4 years of age with bilateral EVA, cochlear implantation should be planned if there is no recovery after medical treatment. Only in this way bilateral hearing can be restored in a patient with EVA.
Case 27.4: MS, 3-Year-Old Male Patient, Operated December 2016
He was diagnosed with profound mixed type hearing loss (Fig. 27.11) and bilateral EVA. He was implanted with CI at the age of 3.
In contrast to Case 27.3, at present time CI is provided at the time of diagnosis of profound hearing loss. Because of the nature of the EVA, hearing loss is usually progressive and is expected on the left side as well. Therefore, in cases of EVA, CI should be performed in unilateral profound hearing loss.
References
Mondini C. Minor works of Carlo Mondini: the anatomical section of a boy born deaf. Am J Otol. 1997;18(3):288–93.
Valvassori GE, Clemis JD. The large vestibular aqueduct syndrome. Laryngoscope. 1978;88(5):723–8.
Sennaroglu L. Histopathology of inner ear malformations: do we have enough evidence to explain pathophysiology? Cochlear Implants Int. 2016;17(1):3–20.
Sennaroglu L. Another evidence for pressure transfer mechanism in incomplete partition two anomaly via enlarged vestibular aqueduct. Cochlear Implants Int. 2018;19(6):355–7.
Emmett JR. The large vestibular aqueduct syndrome. Am J Otol. 1985;6(5):387–415.
Lo WW, Daniels DL, Chakeres DW, Linthicum FH Jr, Ulmer JL, Mark LP, et al. The endolymphatic duct and sac. AJNR Am J Neuroradiol. 1997;18(5):881–7.
Pritchett C, Zwolan T, Huq F, Phillips A, Parmar H, Ibrahim M, et al. Variations in the cochlear implant experience in children with enlarged vestibular aqueduct. Laryngoscope. 2015;125(9):2169–74.
Ahadizadeh E, Ascha M, Manzoor N, Gupta A, Semaan M, Megerian C, et al. Hearing loss in enlarged vestibular aqueduct and incomplete partition type II. Am J Otolaryngol. 2017;38(6):692–7.
Batuk MÖ, Çınar BÇ, Özgen B, Sennaroğlu G, Sennaroğlu L. Audiological and radiological characteristics in incomplete partition malformations. J Int Adv Otol. 2017;13(2):233.
Madden C, Halsted M, Benton C, Greinwald J, Choo D. Enlarged vestibular aqueduct syndrome in the pediatric population. Otol Neurotol. 2003;24(4):625–32.
Ascha MS, Manzoor N, Gupta A, Semaan M, Megerian C, Otteson TD. Vestibular aqueduct midpoint width and hearing loss in patients with an enlarged vestibular aqueduct. JAMA Otolaryngol Head Neck Surg. 2017;143(6):601–8.
Aimoni C, Ciorba A, Cerritelli L, Ceruti S, Skarżyński P, Hatzopoulos S. Enlarged vestibular aqueduct: audiological and genetical features in children and adolescents. Int J Pediatr Otorhinolaryngol. 2017;101:254–8.
Clarós P, Fokouo JVF, Clarós A. Cochlear implantation in patients with enlarged vestibular aqueduct. A case series with literature review. Cochlear Implants Int. 2017;18(3):125–9.
Isaiah A, Lee D, Lenes-Voit F, Sweeney M, Kutz W, Isaacson B, et al. Clinical outcomes following cochlear implantation in children with inner ear anomalies. Int J Pediatr Otorhinolaryngol. 2017;93:1–6.
Manzoor NF, Wick CC, Wahba M, Gupta A, Piper R, Murray GS, et al. Bilateral sequential cochlear implantation in patients with enlarged vestibular aqueduct (EVA) syndrome. Otol Neurotol. 2016;37(2):e96–e103.
Song J-J, Hong SK, Lee SY, Park SJ, Kang SI, An Y-H, et al. Vestibular manifestations in subjects with enlarged vestibular aqueduct. Otol Neurotol. 2018;39(6):e461–e7.
Govaerts P, Casselman J, Daemers K, De Ceulaer G, Somers T, Offeciers F. Audiological findings in large vestibular aqueduct syndrome. Int J Pediatr Otorhinolaryngol. 1999;51(3):157–64.
Merchant SN, Nakajima HH, Halpin C, Nadol JB Jr, Lee DJ, Innis WP, et al. Clinical investigation and mechanism of air-bone gaps in large vestibular aqueduct syndrome. Ann Otol Rhinol Laryngol. 2007;116(7):532–41.
Dahlen RT, Harnsberger HR, Gray SD, Shelton C, Allen R, Parkin JL, et al. Overlapping thin-section fast spin-echo MR of the large vestibular aqueduct syndrome. AJNR Am J Neuroradiol. 1997;18(1):67–75.
Simons JP, Mandell DL, Arjmand EM. Computed tomography and magnetic resonance imaging in pediatric unilateral and asymmetric sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 2006;132(2):186–92.
Kachniarz B, Chen JX, Gilani S, Shin JJ. Diagnostic yield of MRI for pediatric hearing loss: a systematic review. Otolaryngol Head Neck Surg. 2015;152(1):5–22.
Vijayasekaran S, Halsted MJ, Boston M, Meinzen-Derr J, Bardo DM, Greinwald J, et al. When is the vestibular aqueduct enlarged? A statistical analysis of the normative distribution of vestibular aqueduct size. AJNR Am J Neuroradiol. 2007;28(6):1133–8.
El-Badry MM, Osman NM, Mohamed HM, Rafaat FM. Evaluation of the radiological criteria to diagnose large vestibular aqueduct syndrome. Int J Pediatr Otorhinolaryngol. 2016;81:84–91.
Ozgen B, Cunnane ME, Caruso PA, Curtin HD. Comparison of 45 degrees oblique reformats with axial reformats in CT evaluation of the vestibular aqueduct. AJNR Am J Neuroradiol. 2008;29(1):30–4.
Sennaroğlu L, Bajin MD. Classification and current management of inner ear malformations. Balkan Med J. 2017;34(5):397.
Brodsky JR, Choi SS. Should children with an enlarged vestibular aqueduct be restricted from playing contact sports? Laryngoscope. 2018;128(10):2219–20.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sennaroglu, L., Ozbal Batuk, M., Mocan, B.O. (2022). Enlarged Vestibular Aqueduct. In: Sennaroglu, L. (eds) Inner Ear Malformations. Springer, Cham. https://doi.org/10.1007/978-3-030-83674-0_27
Download citation
DOI: https://doi.org/10.1007/978-3-030-83674-0_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83673-3
Online ISBN: 978-3-030-83674-0
eBook Packages: MedicineMedicine (R0)