Abstract
In this chapter, we present and discuss information regarding biological invasions by species in the genus Baccharis L. around the world: in native, expansive, and introduced distributional ranges. Baccharis halimifolia L. is the invasive species par excellence of this genus. Therefore, we dedicate a great part of the chapter to describe (1) its distribution and introduction history; (2) abiotic and biotic factors that affect its invasion; (3) types of ecosystems invaded and environmental, economic, and social impacts; and (4) management of the species. Lastly, we collate all the available information in the literature regarding other species of this genus that are considered invasive or potentially invasive in both native and introduced areas. Those species are Baccharis coridifolia DC., Baccharis dracunculifolia DC., Baccharis neglecta Britton., Baccharis pilularis DC., Baccharis pteronioides DC., Baccharis salicifolia (Ruiz & Pav.) Pers., Baccharis salicina Torr. & A.Gray, Baccharis sarothroides A.Gray, Baccharis spicata (Lam.) Baill., and Baccharis ulicina Hook. & Arn.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Invasive Character of the Genus Baccharis
Multiple species of the genus Baccharis share life-history characteristics common to many invasive species, including effective dispersal mechanisms, adaptations to pioneer stages of succession, high competitive ability, and production of allelopathic compounds (Westman et al. 1975; Ibáñez and Zoppolo 2011; Caño et al. 2013a). Female shrubs produce numerous seeds that can travel long distances via several vectors (Panetta 1977; Weber 2003; USDA 2018) and rapidly colonize disturbances that occur over multiple scales. Seeds germinate under a wide range of environmental conditions and, as time passes, dense stands develop that prevent other species from establishing (Fried and Panetta 2016). While plants in this genus reproduce mainly by seeds, several species have the capacity to reproduce vegetatively by sprouting after cutting or burning (Westman et al. 1975; Hobbs and Mooney 1985; Grace et al. 2001). Thus, these species respond well to any form of disturbance, such as fire, animal activity (e.g., grazing and burrowing), or the biomass removal pursuant to targeted control.
Another characteristic that makes some species of this genus invasive is their generalist behavior, based upon morphological and physiological plasticity (Panetta 1977; Caño et al. 2016), which allows them to thrive in a wide variety of environmental conditions and endure rapid changes in the environment (Westman et al. 1975; Tucat 2015; Haque et al. 2008). However, the species of this large genus have evolved to invade different types of ecosystems. For instance, B. halimifolia sometimes forms dense monospecific stands in places with relatively high salinity levels (Caño et al. 2016), B. salicifolia is commonly found growing along waterways (Dimmit 2000), and B. sarothroides dominates desert regions partly because of its long root system which allows it to reach water and nutrients stored in deep parts of the soil (Dimmit 2000; Haque et al. 2008). Although the genus is generally evergreen, some species can also be deciduous as an adaptation to withstand less favorable environmental conditions. For example, B. halimifolia is deciduous in the cooler parts of its distributional range (Sims-Chilton and Panetta 2011), and B. sarothroides loses its leaves under drought conditions during the summer period (Virginia Tech 2018). Allelopathy is another mechanism by which invasive plants displace other species (Orr et al. 2005). Several studies show that some species of this genus, such as B. dracunculifolia and B. ulicina, produce secondary metabolites that negatively impact neighboring plants (Tucat 2015; Ibáñez and Zoppolo 2011). Lastly, this plant genus generally has a low palatability for herbivores, and some of its species are even toxic to them (Boldt 1989; Jarvis et al. 1991; USDA 2018), which increases their potential to outcompete other plants that are preferred by herbivores.
All the abovementioned characteristics make multiple species of Baccharis particularly important from an ecological and an economic point of view, not only in the invaded regions of the world but also within their native distributional ranges. The major invasive Baccharis species are shown in Fig. 8.1.
Photos of the major invasive Baccharis species (with the exception of B. pteronioides): (a) B. coridifolia , (b) B. dracunculifolia , (c) B. halimifolia , (d) B. neglecta , (e) B. pilularis , (f) B. salicifolia , (g) B. salicina , (h) B. sarothroides , (i) B. spicata , and (j) B. ulicina . (Photos courtesy of G. Heiden)
2 Baccharis halimifolia L. (Eastern Baccharis, Groundsel Bush, Saltbush)
2.1 Distribution
2.1.1 Native Range
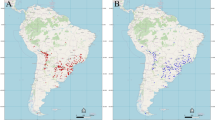
Baccharis halimifolia is native to the Atlantic and Gulf Coasts of North and Central America (Cronquist 1980; Sundberg and Bogler 2006; Fig. 8.2). It is widely distributed in areas of Nova Scotia (southeastern Canada), eastern and southern United States, eastern Mexico (especially northeastern, but it can also be found in areas near Veracruz, southeastern Mexico), the Bahamas, and Cuba (USDA 2018). This shrub occurs in areas from 0 to 100 m above sea level (Sundberg and Bogler 2006). During the last century, it has been expanding its native distributional range towards interior areas of the United States (Duncan 1954; Estes 2004), following human-induced disturbances such as reductions in tree canopy cover and increases in edge habitat (Ervin 2009).
Distributional ranges of B. halimifolia and earliest dates when this species was reported in the invasive regions. Regional maps correspond to (a) North America (occurrence points taken from Ervin (2009) and SERNEC Data Portal (2018)), (b) Europe (occurrence points taken from Fried et al. (2016) and dates from Caño et al. (2013a) and Fried et al. (2016)), and (c) Australia (occurrence points taken from Atlas of Living Australia website (2018) and dates from Bailey (1899), Sims-Chilton and Panetta (2011) , and Atlas of Living Australia website (2018)). In panel (b), records of B. halimifolia accompanied by “(C)” indicate regions where B. halimifolia is known only from cultivated populations
2.1.2 Introduced Range
This species has invaded multiple regions across the world, including western Europe (France, northern Spain, northern Italy, southern England, Belgium, and the Netherlands), eastern Australia (Queensland and New South Wales), New Zealand, and the Republic of Georgia, where it has been introduced for ornamental use, soil stabilization, windbreaks, or aesthetic purposes (Caño et al. 2013a; Fried et al. 2016). Caño et al. (2013a) and Fried et al. (2016) conducted a comprehensive study regarding the history of B. halimifolia introduction and spread throughout western Europe. They pointed out that B. halimifolia became naturalized in northern Spain at the end of the nineteenth century. In France, this species was considered as locally invasive in the 1940s, reached the Mediterranean coast in the 1980s, and rapidly increased in numbers during the 1990s. Fried et al. (2016) also indicated that this shrub is considered invasive in the Tuscany region of Italy, it is naturalized (meaning that the species forms self-sustaining populations) in multiple coastal areas of Belgium, and that scattered individuals have been reported from southern England and the Netherlands. In Australia, it is believed that the species was introduced in 1888 (Bailey 1899). Sims-Chilton and Panetta (2011) specified that it became a serious problem in coastal areas of southeastern Queensland by the 1930s and spread both northwards and southwards in the 1970s.
2.2 Factors That Affect Its Invasion
2.2.1 Seed Production and Dispersal
Female shrubs of B. halimifolia produce numerous viable seeds annually, beginning as early as 3 years after germination (USDA 2018). Westman et al. (1975) pointed out that each plant can produce up to 1.5 million seeds per year. Boldt (1989) found that 4-year-old plants produced 31% more seeds than plants that were 9 years old, indicating that seed production decreases as the plants age. Seeds can travel long distances carried by the wind, water, animals, or vehicles (Panetta 1977; Weber 2003); however, Parsons and Cuthbertson (1992) indicated that with a steady breeze of 16 kph, most seeds disperse less than 6 meters from their mother shrub. Diatloff (1964) recorded that 2-meter-high plants can disperse their seeds to distances of 140 m. Seeds germinate without undergoing a period of dormancy and under a wide range of environmental conditions (Westman et al. 1975; USDA 2018), which will be explained in more detail in subsequent sections of the chapter.
2.2.2 Abiotic Factors
Temperature
The most suitable areas for B. halimifolia in both the native and the invasive range occur in temperate to subtropical regions, including the Mediterranean areas in Europe and Australia (Sims-Chilton et al. 2010; Caño et al. 2013a; Fried et al. 2016). Modelling has shown a higher probability of presence when Maximum Temperature of Warmest Month is between 20 and 30 °C and Minimum Temperature of Coldest Month is above 0 °C (Fried et al. 2016). These data, along with the fact that the species is not present at high latitudes (up to 42° in North America and 51° in Europe), suggest that cold temperatures and frosts could limit its extension northwards in the Northern Hemisphere. However, the species has been also considered to be resistant to −15 °C (Huxley 1992). The cold resistance of the species has not been directly assessed in the field, neither has it been experimentally tested. Likewise, little is known about the physiological responses of B. halimifolia to temperature variations. Only at the seed stage Westman et al. (1975) and Panetta (1979a) demonstrated that optimal germination occurs between 15 and 20 °C. At the population level, Sims-Chilton et al. (2009) found that plant density increases with temperature, whereas plants’ size seems to be unaffected.
Light Availability
The fact that B. halimifolia colonizes open and disturbed habitats, often associated with other woody plants, as well as forests or pine plantations, demonstrates its high plasticity in response to light availability.
Experiments under controlled conditions have demonstrated that there is indeed a degree of shade tolerance during establishment (Panetta 1977). Plastic responses of leaf traits (e.g., increased specific leaf area) allow high shade tolerance (17% daylight) during the first stages of seedling growth, but older seedlings’ shade tolerance decreases, probably because the morphological response is no longer enough to compensate for the decrease in net assimilation rate (Panetta 1977). The plasticity of leaf traits, such as specific leaf area and stomatal conductance, has been also found to contribute to the shade tolerance of B. halimifolia in estuarine communities (Pivovaroff et al. 2015). However, viable seed production is highly reduced by canopy closure (Westman et al. 1975; Panetta 1979b). Interestingly, Lázaro-Lobo et al. (2020) found shade-adaptive effects of parental environment on B. halimifolia offspring. In their study, progeny from low maternal light conditions performed better in the shade treatment than did those offspring from maternal plants grown under high light conditions, whereas the opposite pattern was found in high light conditions.
In the field, colonization patterns might not only reflect the ability to respond to light conditions but also could reflect the outcome of different competitive interactions and responses to disturbance or stressors (see sections below). For instance, Panetta (1979c) indicated that, since canopy closure did not affect population size structure in pine stands, other factors such as litter accumulation or the occurrence of disturbances at the soil level might determine the survival or productivity of this species.
Nutrient Availability
The presence of B. halimifolia does not seem to be limited by nutrient availability since it is able to colonize different types of soils. In Australia, it is recorded from dry infertile forest soils to rich volcanic loams and low-lying clay soils with high moisture content (Winders 1937, cited in Sims-Chilton and Panetta 2011). In coastal Mississippi, USA, B. halimifolia occurrence was not affected by carbon-to-nitrogen ratio (Paudel and Battaglia 2015). Likewise, the species was found to colonize different sites in Queensland where soil nitrogen and phosphorus ranged, respectively, from 560 to 5500 ppm and from 4 to 73 ppm (Westman et al. 1975). In contrast, B. halimifolia’s productivity does respond positively to high levels of N availability under experimental conditions (Vick and Young 2013) and in the field (Connor and Wilson 1968). However, demand for P may increase under high levels of N concentration, moderating any potential increase in B. halimifolia’s growth (Westman et al. 1975; Vick and Young 2013). Also, recurrent flooding in coastal communities can reduce P assimilation and root growth (McKee et al. 2002). However, since, in its native range, the roots of B. halimifolia are colonized by arbuscular mycorrhizal fungi (AMF, Paudel et al. 2014), it is possible that mutualistic associations with AMF would enhance nutrient uptake or contribute to salt or flooding stress tolerance (Neto et al. 2006).
Salinity
Baccharis halimifolia is highly tolerant to salinity, but it is a facultative halophyte, i.e., its optimal growth occurs in the absence of salinity (Caño et al. 2016). For this reason, this species colonizes different types of soils where salinity values range from 0 to sea water salt concentrations (Westman et al. 1975; Young et al. 1994; Caño et al. 2013b, 2014; Frau et al. 2014), although the highest occurrence of B. halimifolia has been recorded under low to moderate levels of salinity (Young et al. 1994; Caño et al. 2014; Paudel and Battaglia 2015). On the other hand, threshold levels of tolerance to salt stress exposure can prevent colonization through massive mortality at the halophilous end of the gradient (Caño et al. 2013a). As in most non-obligate halophytic species, exposure of B. halimifolia to salt stress actually reduces root and shoot biomass and triggers a set of physiological responses affecting leaf traits, water relations, photosynthesis, and osmolyte accumulation, both in the field and under controlled conditions (Young et al. 1994; Tolliver et al. 1997; Zinnert et al. 2012; Fuertes-Mendizabal et al. 2014; Caño et al. 2013b, 2014, 2016).
Experiments under controlled conditions have demonstrated low mortality at high salinity levels (20 g NaCl/L) and an ability to germinate and reproduce under saline conditions (Tolliver et al. 1997; Paudel and Battaglia 2013; Caño et al. 2016). Paudel and Battaglia (2013) also reported that germination can be unaffected by salinity concentrations up to 20 g/L. However, others have shown that percent germination of B. halimifolia can be drastically reduced by salinity levels as low as 10 g/L (Young et al. 1994; Lázaro-Lobo et al. 2020). Environmental salinity of the maternal plants can highly affect progeny tolerance to salinity. For example, Lázaro-Lobo et al. (2020) found that seeds produced by maternal plants growing in saline areas had higher and faster germination in saline environments than seeds from plants growing in non-saline or subsaline areas. Moreover, seedlings are more likely to establish in saline environments if they are exposed to salinity since germination and, therefore, have more time to acclimate to this abiotic stressor (Lázaro-Lobo et al. 2020). Salinity levels at the upper end of the tolerance range of B. halimifolia are likely to delay or suppress flowering, but moderate salinity levels can trigger flowering, both in the greenhouse (Caño et al. 2016) and in the field (Caño et al. 2013b).
A set of physiological adaptations that characterize halophytic species and a degree of plasticity have been shown to underlie the high salt tolerance displayed by this species. B. halimifolia reduces specific leaf area and increases both leaf succulence and stomatal density under saline conditions, which may facilitate a better regulation of stomatal function and transpiration in order to avoid a greater NaCl concentration in tissues (Fuertes-Mendizabal et al. 2014). This shrub behaves as a salt-accumulating plant (Zinnert et al. 2012; Caño et al. 2016), and it has the ability to synthesize high levels of proline that likely act as an osmolyte or osmoprotectant (Fuertes-Mendizabal et al. 2014). It can resist high salt concentrations in its tissues and simultaneously maintain low photosynthetic activity without necrosis, although it shows no capacity for salt exclusion (Zinnert et al. 2012; Fuertes-Mendizabal et al. 2014).
Water Availability (Floods and Droughts)
Drought stress or anoxic stress by waterlogging might depend on precipitation and evapotranspiration in the upper zone of marshes and non-saline soils and on tidal influence in moderate-high salinity communities (Caño et al. 2013b; Pivovaroff et al. 2015). Pivovaroff et al. (2015) found that at the landward edge of B. halimifolia’s range, physiological adjustments to water stress were achieved through greater cavitation resistance. Concerning other hydrodynamic variables, Frau et al. (2014) showed that invasive estuarine populations in Spain occur in areas that are inundated <26% of the year, with water speed and water flow <0.1 m/s and <0.85 m3/s, respectively.
2.2.3 Biotic Factors
Competition with Other Plants
Despite the wide ecological amplitude of Baccharis halimifolia regarding light or nutrient availability and different stressors, establishment in the field is often determined by the competitive relations established with neighboring plants in different kinds of habitats. B. halimifolia can easily outcompete herbaceous species and take advantage of disturbance, but competition with woody species limits its establishment and reproduction (Ervin 2009; Caño et al. 2013a). In coastal communities in North America, interspecific differences in response to flooding and salinity underlie the zonation among different shrubs, and thus B. halimifolia typically inhabits the intermediate marsh zone, together with the salt-tolerant shrub Iva frutescens (Young et al. 1994; Tolliver et al. 1997). Although competition between these species can prevent the formation of monospecific stands at the subhalophilous zone of the marsh in North America, shrub and tree vegetation are absent in these communities in Europe. Here B. halimifolia outcompetes the dominant native herbaceous species (Juncus maritimus, Elytrigia spp., and eventually Phragmites australis), both in the Atlantic and Mediterranean coastal wetlands. It establishes in small gaps and spreads in these communities, even in almost undisturbed sites (Caño et al. 2013a; Fried et al. 2014; Fried and Panetta 2016). While this facultative halophyte could potentially perform better in upper marsh and non-saline sites, competition with highly productive estuarine alder forests (Alnus glutinosa, Salix atrocinerea) in such sites has been shown to prevent B. halimifolia’s colonization in coastal communities in Spain (Caño et al. 2013a).
Herbivory
In its native range, various species of Lepidoptera, Hemiptera, and Coleoptera feed on Baccharis halimifolia as larvae or adults. Surveys conducted in North America have identified up to 133 phytophagous insects feeding on B. halimifolia, of which 14 were considered restricted to the genus Baccharis and 11 specific to B. halimifolia (Palmer 1987; Palmer and Bennett 1988). Some of these insects have been introduced in Australia as biological control agents (Sims-Chilton et al. 2010; see management section). In Europe, natural enemies identified are mostly mealybugs (Ceroplastes sinensis and Saissetia oleae), aphids (Aphis fabae, A. spiraecola), and sooty molds, but the presence of an undetermined Agromyzidae has been also reported (Dauphin and Matile-Ferrero 2003; Fried et al. 2013; Caño et al. 2013b).
Despite the abundant phytophagous species found on B. halimifolia, the level of impact and consumption in both the native (Westman et al. 1975) and the invasive ranges does not seem to reduce the species’ performance (Westman et al. 1975; Fried et al. 2013; Caño et al. 2013b; Lovet 2015). However, Kraft and Denno (1982) reported that populations defoliated by the specialist beetle Trirhabda bacharidis failed to flower. Acetone soluble resins in the leaves of B. halimifolia have been found to act as deterrents for generalist herbivores, despite being tolerated by specialists such as the beetle T. bacharidis (Kraft and Denno 1982).
Finally, the trophic dynamics in B. halimifolia have been found to be affected in a qualitative and a quantitative way by plant sex (Krischik and Denno 1990b), plant and population characteristics, climatic variables (Sims-Chilton et al. 2009), environmental factors modifying leaf chemistry or quality (Younginger et al. 2009; Caño et al. 2013b), and mutualistic associations with mycorrhizal fungi (Moon et al. 2013).
Parasites, Fungi, Bacteria, and Viruses
There are almost no serious diseases affecting B. halimifolia in its native range (Gilman 1999). A rust fungus specific to the Baccharis genus (Puccinia evadens, Groundsel bush rust) causes defoliation during summer and winter, and, in extreme cases, stems can die back over summer (Sims-Chilton and Panetta 2011; F.D. Panetta, pers. obs.). Also, different species of nematodes have been found associated with B. halimifolia in Florida and Australia, but high variability across years and experimental conditions obscures any clear differences between the native and the exotic range (Porazinska et al. 2014). Although B. halimifolia is a natural host of the bacterium Xylella fastidiosa, the latter does not cause any disease in this species (Hopkins and Adlerz 1988).
2.3 Type of Ecosystems Invaded and Impacts
Baccharis halimifolia colonizes a great variety of ecosystems in both the native and the introduced distributional ranges. In the southern United States, it typically invades coastal prairies and marshes (Penfound and Hathaway 1938; Harcomb 1989; Bruce et al. 1995), overgrazed rangelands (USDA 2018), disturbed grasslands (Penfound and Hathaway 1938; Allen 1950), desert areas (Boldt 1989), hedgerows and fallow fields (Krischik and Denno 1990a), former industrial sludge basins (Olson and Fletcher 2000), and roadsides, pine plantations, and forest edges (Ervin 2009). In Australia, this shrub invades not only human-disturbed and human-managed areas such as pastures (McFadyen 1973) and exotic pine plantations (Panetta 1979c) but also Melaleuca swamps and dry eucalypt forests that experience periodic natural disturbances such as fire and flooding (Westman et al. 1975). In Europe, B. halimifolia colonizes human-disturbed areas such as road and rail networks, irrigation channels, and wastelands (Le Moigne and Magnanon 2009), as well as subhalophilous coastal communities, which are part of the protected habitat “Atlantic Salt Meadows” included in the Habitats Directive 92/43/EEC (Caño et al. 2013a). In Spain, B. halimifolia occurs in up to 18 sites of community importance (Campos et al. 2014), and multiple endangered halophilous species restricted to these areas are threatened by B. halimifolia invasion (Caño et al. 2013a, 2014).
In the invaded regions, this species causes negative effects on biodiversity, ecosystem functioning, and human activities in multiple ways. It has a direct impact on the surrounding vegetation due to its ability to form dense monospecific thickets that prevent other species from establishing (Fried and Panetta 2016). Baccharis halimifolia not only reduces the herbaceous diversity of the coastal prairies and estuarine communities that it invades but also converts the native herbaceous vegetation into a landscape of monospecific woody stands (Harcomb 1989; Campos and Herrera 2009; Caño et al. 2014; Fried et al. 2014; Fried and Panetta 2016). Fried et al. (2016) indicated more details about what plant communities are more affected by B. halimifolia invasions in their work “Monographs on Invasive Plants in Europe: Baccharis halimifolia L.”
Impacts that this species has on vegetation also have been shown to have consequences for some animal species. For example, Arizaga et al. (2013) showed that B. halimifolia causes perceptible changes on bird assemblages by promoting woodland species and potentially affects migrant species associated with ecosystems that have been invaded. Mallard (2008) pointed out that insect species richness and abundance were lower in stands of B. halimifolia than in native woody plant species. Furthermore, the impacts caused by this invasive species are aggravated by its potential ability to affect ecosystem processes such as sedimentation dynamics (Campos and Herrera 2009), fire regimen (Sinnassamy 2004), light interception, and succession (Campos et al. 2004). Thus, Campos and Herrera (2009) considered B. halimifolia as a “transformer” species (sensu Richardson et al. 2000) due to its potential ability to transform the structure and function of littoral ecosystems.
In addition to its impacts on natural ecosystems, this early successional species is also considered as a pest because it rapidly invades rangelands used for livestock grazing (Westman et al. 1975; Sims-Chilton and Panetta 2011). Hence, it reduces the productivity of the pasture and limits cattle movement (Palmer and Sims-Chilton 2012). Furthermore, B. halimifolia has a low palatability for herbivores and is even toxic to them (Boldt 1989; USDA 2018; see also Chap. 15 in this volume). Nevertheless, seedling establishment can be greatly affected by domestic cattle (Caño et al. 2013a; Fried et al. 2016). In Spain, the progressive reduction of cattle farming promotes the invasion of disturbed communities located in the salt marsh area. However, in grazed or managed meadows, B. halimifolia is totally absent due to periodic disturbance (Caño et al. 2013a). This shrub also causes problems in forestry plantations due to its capability to outcompete pine seedlings (Palmer and Sims-Chilton 2012) and in salt production areas by decreasing wind velocity and evaporation of water (Fried et al. 2016). Lastly, the pollen from this shrub can reach high concentrations in the air and potentially cause allergies in sensitive persons (Green et al. 2011).
2.4 Management
2.4.1 Mechanical Control
Mechanical control objectives in the management of B. halimifolia are basically twofold: (1) to kill individual plants and (2) to suppress flowering and thereby reduce seed production and spread. Young plants (less than 1 m in height) can be pulled up with little risk of sprouting, especially when the soil profile is moist. This approach may be feasible and cost-effective for small, incipient infestations. Larger plants often regrow from any parts of the root system that have not been removed. Such a strong vegetative regeneration capacity essentially limits the scope for mechanical control when used alone for this species, but methods that combine mechanical with chemical control are more effective (see below). Timely slashing of infestations can reduce seed production, and burning may also be an effective method of control, but rapid regrowth is common (Allain and Grace 2001). Flooding for several months during winter can eliminate adult plants; permanent flooding has been used effectively in Spanish estuarine environments (Campos et al. 2014; Fried et al. 2016).
2.4.2 Chemical Control
Given that B. halimifolia was first recognized as a serious weed in Australia (having been declared noxious in the 1950s), chemical control methods were first developed there and became a major component of its management. The plant was readily controlled by overall spray application of either 0.2% salts or esters of 2,4-D ((2,4-dichlorophenoxy) acetic acid) or 2,4,5-T ((2,4,5-trichlorophenoxy) acetic acid). Basal barking with esters in oil and cut-stumping using salts in water and esters in water or oil were also effective control methods (Sims-Chilton and Panetta 2011). Other work has reported effectiveness of dicamba plus MCPA, glyphosate, picloram plus 2,4-D, and triclopyr (Weber 2003). In hardwood forest plantations in southeastern Arkansas, Gann et al. (2012) found triclopyr to be more efficient in controlling B. halimifolia than imazamox, aminopyralid, and glyphosate.
During the 1950s in Australia, “brushing” was the most common method used, consisting of cutting plants and swabbing their stems with chemicals. Today cut-stumping, i.e., application of relatively concentrated herbicide solutions to the stumps of plants just after cutting, is a method commonly used in environmentally sensitive areas, such as nature reserves. When used properly this method presents less risk of off-target damage than foliar applications of more dilute solutions. Glyphosate is the most common herbicide used for cut-stumping in France and Spain (Fried et al. 2016). Follow-up treatment is essential because infestations of B. halimifolia develop persistent seed banks (Panetta 1979a). This will likely combine hand pulling of young plants with foliar spraying of regrowth from plants not killed by the previous treatment.
2.4.3 Biological Control
Surveys for potential biological control agents for B. halimifolia were initiated in the southern United States in the 1960s. These surveys, undertaken by researchers from the Queensland Government, continued for several decades. Overall, 35 agents were imported into Australia for testing, 14 were released, and 7 have established (Sims-Chilton et al. 2009). One of these was the pathogen Puccinia evadens, which has established over most of B. halimifolia distribution in Australia (Sims-Chilton et al. 2009). The other six were insects, including three species of Lepidoptera (Aristotelia ivae (Gelechiidae), Bucculatrix ivella (Bucculatricidae), and Hellinsia balanotes (Pterophoridae)); two species of Coleoptera (Megacyllene mellyi (Cerambycidae) and Trirhabda bacharidis (Chrysomelidae)); and a dipteran (Rhopalomyia californica (Cecidomyiidae)). All are native to North America, except for M. mellyi which is South American.
Biological control impacts on B. halimifolia vary in relation to several environmental factors (Sims-Chilton et al. 2009), but overall there has been a marked decline in the abundance of the weed throughout its Australian range. This decline is at least partially due to a long-term decrease in the climatic suitability of the invaded areas (Sims-Chilton et al. 2010). Regardless, the Australian experience reveals several potentially effective agents in the event that biological control is attempted elsewhere. In France, sheep have intentionally been used to control sprouting after application of physical methods on large areas (Fried et al. 2016).
3 Species of the Genus Baccharis That Are Invasive Within Their Native Distributional Range
3.1 Baccharis coridifolia DC. (Mio-mio)
Baccharis coridifolia is native to Paraguay, northern and central Argentina, Uruguay, and southern Brazil (Mongelli et al. 1997; Rizzo et al. 1997). This species can readily colonize abandoned fields (Sione et al. 2016) and thrives in pastures where livestock reduce the cover of palatable grasses (Berretta 2001). Baccharis coridifolia is primarily considered invasive due to its toxic effects in livestock, especially during the flowering season (April–May; Rizzo et al. 1997). In fact, it is one of the most important toxic plants within its native range (de Almeida et al. 2009), and several works have documented its negative effects in livestock (e.g., Tokarnia and Döbereiner 1975; Habermehl et al. 1985; Costa et al. 1995), as it is explained in another section of the book (Chap. 15).
3.2 Baccharis dracunculifolia DC. (White Chilca)
Baccharis dracunculifolia occurs in the southern region of South America, including Argentina, Paraguay, southern Brazil, Uruguay, and Bolivia (Lombardo 1964; Barroso 1976; cited in Ibáñez and Zoppolo 2011; Müller 2006). This shrub has high reproductive rates and dispersal capacity (Frenedozo 2004). Female plants produce large numbers of seeds that germinate readily (Gomes and Fernandes 2002). It has been recognized as an invasive and colonizing species on several occasions in some areas within its native range, partly because of its efficient establishment and growth in disturbed habitats (Dos Santos et al. 2008; Galindez et al. 2009). Frenedozo (2004) included B. dracunculifolia as one of the few colonizer species that appeared at limestone mining quarries. Ibáñez and Zoppolo (2011) examined the allelopathic properties of B. dracunculifolia and its phytotoxic effects on other species. They concluded that germination and growth of other plants were significantly inhibited by the essential oil of this shrub, which would explain the reduction of weeds near B. dracunculifolia. Even though B. dracunculifolia is an obligate seeder species and does not sprout after fire (Overbeck and Pfadenhauer 2007), its high seedling establishment after burning allows this shrub to compete with other vegetatively propagated species (Galindez et al. 2009). Furthermore, B. dracunculifolia is adapted to a wide range of soil conditions. It efficiently colonizes high fertility agriculture fields (Macedo et al. 2003) and degraded areas with low nutrient availability (Dos Santos et al. 2008). Negreiros et al. (2012) examined the survival and early growth of B. dracunculifolia seedlings grown across a gradient of nutrient availability. The results showed that seedlings growing on less fertile soils exhibited the highest survival rates. However, seedlings had a higher growth rate and accumulation of biomass on more fertile substrates. Finally, B. dracunculifolia has been proposed, among other species, as a helpful species to regenerate disturbed areas such as arsenic-contaminated areas and overburden piles produced by surface mining, as part of revegetation programs within its native range (Dos Santos et al. 2008; Gilberti et al. 2014).
3.3 Baccharis neglecta Britton. (Roosevelt Weed)
Baccharis neglecta is mainly found in open habitats from the southwestern and south-central United States to Coahuila, Chihuahua, and Durango, northern Mexico (Matuda 1957; Correll and Johnston 1979; cited in Boldt and Robbins 1987; Van Auken and Bush 1990). The species has been reported as invasive within its native distributional range on several occasions (Hamilton et al. 2004, U.S. Department of Homeland Security 2004), primarily in overgrazed or disturbed productive rangelands, where it causes negative economic effects (Everitt et al. 1978). According to Mutz et al. (1979), livestock can occasionally graze upon young plants, but the species has little or no nutritional value.
Van Auken and Bush (1990) conducted an experiment to evaluate the light requirements of B. neglecta seedlings, obtaining higher values of number of leaves, stem length, basal diameter, and above- and belowground biomass from the plants submitted to the highest light treatment (photon flux densities (PPFD) of 611 μM·m−2·sec−1). Furthermore, seedling mortality was very high under the lower light treatments (PPFD <1 and PPFD = 53 μM·m−2·sec−1). All deaths occurred within 3 weeks of the start of the experiment, and all the plants died under the lowest light level. The above results show that B. neglecta is a sun-tolerant plant, or heliophyte, which is favored by removal of native vegetation by disturbances such as heavy grazing (Van Auken and Bush 1990; Hamilton et al. 2004).
It appears that B. neglecta can be controlled by maintaining a solid grass cover, decreasing the water available in the upper soil profile (Van Auken and Bush 1990). Other alternatives to prevent the establishment and spread of B. neglecta are mechanical removal, chemical treatments, and biological control. Individuals can be temporarily controlled by shredding or burning, but they will sprout in a short period of time (Boldt and Robbins 1987). Herbicides such as 2,4-D, picloram, and tebuthiuron are very effective against this species, but expensive (Scifres 1980). Boldt and Robbins (1987) evaluated the potential of host-specific insects from South America for the biological control of B. neglecta. They found that 17 insect species used this invasive shrub as an alternate feeding host, but only 3 species consistently caused damage to individual plants. Finally, B. neglecta, as with B. sarothroides, has been considered as a potential candidate for phytoremediation of mine tailings (Mendez and Maier 2008) due to its arsenic (As) tolerance and accumulating capacity (Flores-Tavizon et al. 2003).
3.4 Baccharis pilularis DC. (Coyote Brush)
Baccharis pilularis is probably the second most studied shrub of this genus. Its native distributional range corresponds to northwestern Mexico and the western United States (Hickman 1993; Ross 2004; USDA 2018). It is commonly found in chaparral, coastal scrub, and foothill woodland communities (Hobbs and Mooney 1985; Underwood et al. 2007), but B. pilularis can also grow in harsh serpentine soils (Hickman 1993).
McBride and Heady (1968) described the invasive character of this species for the first time in annual grasslands of northern California, and they studied the influence of grazing and burning on its expansion process. The results suggested that the spread of B. pilularis was favored by the reduction of wildfire frequency and the elimination of grazing livestock since the establishment of Regional Parks in 1934. McBride (1974) also reported the widespread distribution of this shrub in the region and emphasized the pivotal role that livestock play in preventing its invasion, even though mature shrubs have a low palatability. Multiple later studies have deepened our understanding of the invasions by this species in coastal ranges of California. Keeley (2005) reviewed the fire history of the grasslands mentioned above and concluded that changes in the fire regime were not as important as cessation of grazing to explain B. pilularis invasion. Further studies showed that the invasion of annual grasslands by this species often fails because seedling roots cannot reach deep parts of the soil profile before the summer drought occurs (Williams and Hobbs 1989). However, unusually favorable temperatures and adequate soil moisture conditions during this period (in years of abundant spring rainfall) allow for successful colonization (Williams et al. 1987; Williams and Hobbs 1989). Laris et al. (2016) argued that the use of mechanical treatments for the practice of grazing, including bulldozing or disking, caused long-lasting impacts on the region’s vegetation dynamics, and, therefore, shrub advancement rates were lower in the least intensively disturbed sites, such as upper and steeper slopes.
Hobbs and Mooney (1986) studied the impacts of grassland colonization by B. pilularis and found that the abundances of all herbaceous species declined significantly after Baccharis stands formed a closed canopy at 2–3 years. They suggest that this result could be due to both the reduction of light penetrating the canopy and herbivory by small mammals, which are known to seek shelter in Baccharis stands. However, scattered B. pilularis individuals did not cause a great reduction of grassland species abundances. This shrub can also increase the fuel load of the invaded areas, thus altering their fire regimes (Russell and Tompkins 2005). Allelopathy was suggested to be another potential mechanism by which B. pilularis affects the surrounding vegetation (Hobbs and Mooney 1986).
In contrast to studies of managed grasslands, Cushman et al. (2011) showed, in a coastal foredune system in northern California (USA), that B. pilularis was the only native plant that dominated the site, along with the exotic species Ammophila arenaria and Carpobrotus edulis . However, herbaceous exotic species can greatly impact the growth of Baccharis seedlings, especially under dry conditions (Da Silva and Bartolome 1984).
Management of this species is very important to avoid changes from grassland to shrubland (Hobbs and Mooney 1985). Mechanical removal has proven to have low effectiveness at halting the spread of B. pilularis. The shrubs, especially 3–4-year-old plants, sprout readily from the base after cutting or burning, due to the development of an extensive root system for storage and water capture (Hobbs and Mooney 1985). However, seedlings and younger plants are susceptible to fire damage (Ross 2004). Biological methods could be used to control invasion of this species. For example, as described above, livestock grazing effectively decreases the establishment of new individuals (McBride and Heady 1968; McBride 1974). Seedling herbivory by slugs also has a considerable effect on seedling performance (Zavaleta 2006), and a fungal parasite called Diaporthopsis causes witches’-broom and dieback in mature shrubs (Bonar 1966). Chemical control is an effective but expensive control measure because it requires large quantities of herbicides (Elmore et al. 1968; Hyland and Holloran 2005; Ogden and Rejmánek 2005).
The effects of climate change on this shrub have also been studied by Zavaleta (2006), who suggested that seed germination could be increased by higher CO2 concentrations and accelerated by water addition.
3.5 Baccharis pteronioides DC. (Yerba de Pasmo)
Baccharis pteronioides is a drought deciduous shrub that dominates some semidesert areas of its native distributional range (Bock and Bock 1992; Stegelmeier et al. 2009), corresponding to southern United States (Texas, New Mexico and Arizona) and northern Mexico (Kearney and Peebles 1969; Lamb 1975, cited in Kenney et al. 1986). It is considered troublesome in Arizona because it invades grasslands used for livestock grazing (Kenney et al. 1986). An experiment conducted by Kenney et al. (1986) demonstrated that the exclusion of domestic cattle increased the population density of B. pteronioides, even though livestock do not feed on this shrub when other plants are present. Baccharis pteronioides , as many other species from this genus, is fire resistant (Tellman 2002), which decreases the effectiveness of burning to prevent its spread. Furthermore, several livestock poisoning incidents caused by this species due to lack of alternative forage have been reported in the southwestern United States (Stegelmeier et al. 2009), which is explained in more detail in another section of the book (Chap. 15).
3.6 Baccharis salicifolia (Ruiz & Pav.) Pers. (Mule-Fat)
Baccharis salicifolia is widely distributed from the southwestern United States to Patagonia (Boldt and Robbins 1990; Müller 2006). However, it is believed that this species originated along the Andes Mountains in Argentina (Cuatrecasas 1968; Benson and Darrow 1981; Nesom 1988, cited in Boldt and Robbins 1990). It commonly grows along waterways, forming dense stands (Dimmit 2000). Several studies have pointed out the dominant role of this shrub in some areas within its native range. A study conducted in southern California by Boland (2014) showed that seedlings of B. salicifolia were widely distributed throughout the area, but adults comprised the dominant shrub species in the upper elevations of riparian woodlands. Seedling survivorship was very poor in the low-elevation riparian zone during the first winter, and adult survivorship was poor in the intermediate riparian zone in later years. DiPietro et al. (2002) indicated that B. salicifolia dominated southern California riparian southern willow scrub habitats. In the North Andean-Patagonian region of Argentina, Serra et al. (2013) specified that terrestrial vegetation was dominated by this shrub, among others.
Baccharis salicifolia is a phreatophyte, and its deep root system allows the plant to draw groundwater from deeper zones; the resulting additional use of water in semiarid basins can become problematic for neighboring species (Gatewood et al. 1950; Fletcher and Elmendorf 1955). McGuire (2005) concluded that transpiration through the leaves and stems of B. salicifolia was greater than precipitation during the growing season and that transpiration rate was comparable to the overstory cottonwood. Parker (1972) included this shrub in his book An Illustrated Guide to Arizona Weeds. Furthermore, B. salicifolia has a moderate tolerance to salinity (Glenn et al. 1998), which allows for occurrence in a wider range of environments. Humans have also influenced the spread of this species, planting it along waterways to control erosion due to its rapid growth rate and deep root system (Boldt and Robbins 1990; USDA 2018).
As with other Baccharis species, B. salicifolia has a low palatability to livestock or wildlife (Boldt and Robbins 1990; USDA 2018) and can sprout after fire, which increases its potential to behave as an invasive species. Boldt and Robbins (1990) evaluated the possible biological control of B. salicifolia by insects and found that this shrub was the host or alternate host for 106 species of phytophagous insects, which fed on the plant and caused moderate damage in localized areas.
3.7 Baccharis salicina Torr. & A.Gray (Willow Baccharis)
Baccharis salicina is native to the south-central United States (Colorado, Kansas, Oklahoma, Texas, New Mexico, and Arizona) and northern Mexico (Boldt and Robbins 1994; USDA 2018). However, it was identified by Polacik and Maricle (2013) as a non-native species in the Cedar Bluff Reservoir (Kansas, USA). Baccharis salicina requires light to germinate, is adapted to numerous soil types, and grows in moist disturbed areas and along saline waterways, forming narrow riparian strips (Ungar 1968; Boldt and Robbins 1994). Skousen et al. (1990) sampled the vegetation growing in unreclaimed surface mine sites in east-central Texas, finding that B. salicina established soon after mining and was the dominant woody plant species on 5–30-year-old sites.
Currently, B. salicina is invading riparian areas and lake basins throughout Texas, along with Tamarix ramosissima , due to both species’ ability to outcompete other native vegetation in riparian areas (Muñoz et al. 2017). This species also is known to invade rangelands, where it has little or no value to livestock, making it undesirable in these systems. Managers of invaded rangelands have implemented different methods to control the rapid expansion of this shrub, including prescribed burning, herbicides, and mechanical treatments, with little success (Muñoz et al. 2017). There have also been attempts to promote the biological control of this species. Boldt and Robbins (1994) assessed the insects occurring and feeding on B. salicina in its native distributional range and found that the species was the host or alternate host for 61 species of phytophagous insects, of which only 19 occurred at densities greater than 1 per plant. Muñoz et al. (2017) studied the control of B. salicina and T. ramosissima with goats and hypothesized that exposure to the plants at weaning would improve acceptance and consumption of the plant species by these animals. They found that goats consumed both invasive plant species but preferred T. ramosissima over B. salicina. This selective browsing could leave B. salicina without competitors in rangeland areas, allowing it to become the dominant shrub. Muñoz et al. (2017) also mentioned the possible toxic effects of B. salicina on goats and observed that the animals would consume the plant until the consumption reached toxic levels.
3.8 Baccharis sarothroides A.Gray (Desert Broom)
Baccharis sarothroides is native to northwestern Mexico and southwestern United States. It is considered invasive in some areas of its native range due to its ability to grow in harsh environments and highly disturbed areas (Mendez and Maier 2008). This species, like others in the genus, can reach water and nutrients stored in deep parts of the soil with its long root system, and its rapid growth allows it to withstand partial burial by sand (Haque et al. 2008). All the above characteristics make B. sarothroides one of the most dominant plants of sandy floodplains in areas of its native distributional range (Dimmit 2000). Another adaptation of this shrub to semiarid regions is that it loses its leaves under drought conditions, allowing it to survive during the summer dry period (Virginia Tech 2018). Haque et al. (2008) examined the phytoremediation potential of this species on mine tailings in Arizona and found that it was not affected by the excessive metal and metalloid concentration in the soil. This result suggests that B. sarothroides possesses certain metal adaptability and resistance, which could allow the colonization of other inhospitable environments. As with other Baccharis species, in some areas B. sarothroides is considered a bothersome plant due to its aggressive, invasive nature (Dimmit 2000).
3.9 Baccharis ulicina Hook. and Arn. (Yerba de la Oveja)
The distributional range of B. ulicina includes portions of Argentina and Bolivia. The major study regarding the biology and management of this species was documented by Tucat (2015) in his doctoral dissertation. All the information included in this section was obtained from that work. Baccharis ulicina is not palatable by cattle, which has facilitated its presence and dominance in agricultural systems. In fact, it is widespread in areas used for domestic livestock grazing, especially in pastures of the semiarid zone of Argentina. On average, one plant can produce from 900 to 1300 capitula per year. Each capitulum contains an average of 24 seeds, giving this species high reproductive potential. Moreover, seeds germinate in a short period of time after dispersal, and germination occurs under a wide range of environmental conditions. Experimental results showed that the germination rate was very high between temperatures of 10 and 28 °C and under any light level. This species also seems to possess some allelopathic activity. Specifically, B. ulicina negatively affected the establishment of other plants, such as the cultivated species Avena sativa , Lolium perenne , and Raphanus sativus , as well as the native species Nassella clarazzi . Finally, Tucat (2015) concluded that mechanical removal efforts and burning did not effectively control the spread of this species, due to its ability to sprout after the disturbance from stem buds near the ground. However, chemical control with glyphosate proved to be a good management tool.
3.10 Other Species of the Genus Baccharis That Colonize Disturbed Areas and Regeneration Patches
There are scattered studies that show the high capacity of other species from the genus Baccharis to colonize disturbed areas, as well as their important role in the regeneration of the vegetation within their native distributional ranges. These traits suggest that the introduction of these species to other areas could have detrimental consequences for the native vegetation. However, the competitive abilities of these species are not well understood, nor are their abiotic requirements for growth.
Holmgren et al. (2000) analyzed the recolonization of shrub species in patches that were either burned or cleared in a coastal area of central Chile. They found that Baccharis linearis (Ruiz & Pav.) Pers. was one of the two dominant species after the clearing of vegetation, but seed availability in burned patches was very low, probably because its seeds were unable to survive even low-intensity fires. They also stated that B. linearis is frequently found in abandoned agricultural fields.
Safford (2001) studied the postfire vegetation development in the surroundings of Rio de Janeiro (Brazil) and concluded that Baccharis glaziovii Baker, Baccharis reticularia DC., and especially Baccharis platypoda DC. successfully colonized burned areas, due to evolutionary adaptations to fire. However, the regeneration and postfire colonization rates were highly influenced by biotic and physical variables, such as altitude, aspect, and slope.
Limited research has been published on another handful of species in this genus. Baccharis singularis (Vell.) G.M.Barroso, for example, was found to occur commonly in fallow areas in southeastern Brazil (Salimon and Negrelle 2001). Slocum et al. (2004) showed that Baccharis myrsinites (Lam.) Pers. colonized areas previously occupied by fern thickets in the Dominican Republic, but only after the fern species were mechanically removed. Baccharis punctulata DC., Baccharis notosergila Griseb., and Baccharis coridifolia were found to be the dominant invasive species in abandoned fields of northeastern Argentina (Sione et al. 2016). Baccharis punctulata also was shown to invade natural forests and degraded grasslands, also in northeastern Argentina (Casermeiro and Spahn 1999; Marchesini 2003; Sione et al. 2016). Mechanical treatments such as shrub removal had to be applied multiple times to successfully control the spread of this species (Sabattini et al. 2012). Finally, Lazarotto et al. (2017) considered Baccharis psiadioides (Less.) Joch.Müll. as a dominant species that forms dense stands within its native distributional range in southern Brazil and Uruguay (Deble et al. 2005). They further found that its essential oil affects seeds and seedlings of other plant species, such as Arabidopsis thaliana.
4 Sporadic Introductions
4.1 Baccharis spicata (Lam.) Baill
Baccharis spicata is native to southeastern South America (northeastern and central Argentina, Uruguay, Paraguay, and southern Brazil), where it occurs in grasslands, steppes, arable lands, river margins, disturbed coastal areas, abandoned paddy fields, and urbanized sites (Giuliano and Plos 2014; Verloove et al. 2018). Several localities in South America have recognized its potential invasive tendencies throughout its native distributional range and have organized campaigns to eradicate the species from susceptible areas. This species has recently invaded disturbed areas in Portugal but has not been reported in natural areas for the moment. However, considering its ecology in its native distributional range and its high capacity to disperse long distances by wind, it is likely that the species will spread to nearby natural areas (Verloove et al. 2018). Two naturalized populations were spotted in the surroundings of Porto (Matosinhos and Vila do Conde) in 2015. Verloove et al. (2018) provided details about those records of B. spicata and described the potential of this species to invade Europe. They stated that the species was especially abundant in Vila do Conde, where the stand probably consisted of 500–1000 individuals. This location was affected by an excavation in 2016, which favored the invasion of the area by B. spicata. They claimed that after the disturbance, the vegetation was dominated by this species due to its ability to sprout and germinate readily. Multiple hypotheses about this introduction are provided, but accidental introduction and subsequent naturalization seem to be the most likely. Moreover, it is likely that this species produces allelopathic compounds that affect negatively other plant species growing nearby (Damasceno et al. 2010).
4.2 Other Sporadic Introductions
Bartoli et al. (2008) reported the presence of Baccharis pingraea DC. and Baccharis articulata (Lam.) Pers. in southern Spain. The latter species was eradicated with herbicide treatments. The introduction of both species was associated with timber imported from South America (Verloove et al. 2018).
5 Use of Baccharis as Bioherbicide to Control Other Weeds
Extracts from a few species of the genus Baccharis have been used as an alternative bioherbicide to control the establishment and spread of weeds in agroecosystems. For example, the allelopathic compounds of Baccharis trimera (Less.) DC. and Baccharis uncinella DC. were used to control the invasive species Eragrostis plana and Bidens pilosa , respectively, in southern Brazil (Gonçalvez 2014; Dias et al. 2017).
6 Future Research on the Invasiveness of Baccharis
Further research is needed to deepen our understanding of the mechanisms of invasion by the genus Baccharis. There are multiple potential studies that would provide pivotal pieces of information about the invasiveness of Baccharis. For example, little is known about the importance of human activities for expansion of most of the species mentioned in this chapter. It is also uncertain whether there are differences in plant traits and demographic stages among the native, expansive, and invasive distributional ranges of B. halimifolia. In this regard, two of the authors of this chapter (AL-L and GNE) are investigating possible variations in early demographic stages, physiological tolerance, and plasticity among the abovementioned distributional ranges of B. halimifolia. Nothing is known about genetic diversity in B. halimifolia, either within or between its populations. Another potential field of study would be the evaluation of the effects of climate change on future distributions and invasiveness of Baccharis species. Furthermore, research could explore whether other congeners have characteristics like those of the known invasive Baccharis species in order to identify other high-risk plants. Lastly, although it is well known that the genus Baccharis produces essential oils composed mainly of monoterpenoids and sesquiterpenoids, which have multiple biological activities (e.g., antibacterial, antifungal, antiprotozoal, repellent, and cytotoxic properties; see Chap. 9 in this volume), there is little information on the ecological consequences in natural ecosystems of those compounds, such as chemical defense against natural enemies or allelopathic effects against plant competitors.
References
Allain L, Grace JB (2001) Changes in density and height of the shrub Baccharis halimifolia following burning in coastal tallgrass prairie. In: Bernstein NP, Ostrander LJ (eds) Proceedings of the 17th North American Prairie Conference. North Iowa Area Community College, Mason City, pp 66–72
Allen PF (1950) Ecological bases for land use planning in gulf coast marshlands. J Soil Water Conserv 5:57–85
Arizaga J, Unamuno E, Clarabuch O et al (2013) The impact of an invasive exotic bush on the stopover ecology of migrant passerines. Anim Biodivers Conserv 36:1–11
Atlas of Living Australia website (2018). http://www.ala.org.au. Accessed 8 Nov 2018
Bailey FM (1899) The Queensland flora, part 1. Diddams, Brisbane
Barroso G (1976) Compositae-Subtribo Baccharinidae Hoffmann. Estudo das espécies ocorrentes no Brasil. Rodriguésia 40:2–273 (original work not seen, see instead Ibáñez and Zoppolo 2011)
Bartoli A, Sánchez Gullón E, Weickert P et al (2008) Plantas americanas nuevas para la flora adventicia del sur de España [New American plants for the adventive flora of southern Spain]. Acta Bot Malacit 32:276–282
Benson L, Darrow R (1981) Trees and shrubs of the southwestern deserts. University of Arizona Press, Tucson (original work not seen, see instead Boldt and Robbins, 1990)
Berretta EJ (2001) Ecophysiology and management response of the subtropical grasslands of Southern South America. In: Gomide JA, Mattos WRS, Silva SC (eds) Proceedings of the XIX International Grassland Congress. Piracicaba, Brazil
Bock JH, Bock CE (1992) Vegetation responses to wildfire in native versus exotic Arizona grassland. J Veg Sci 3:439–446
Boland JM (2014) Factors determining the establishment of plant zonation in a southern Californian riparian woodland. Madrono 61:48–63
Boldt PE (1989) Baccharis (Asteraceae): a review of its taxonomy, phytochemistry, ecology, economic status, natural enemies and the potential for its biological control in the United States. Texas Agricultural Experiment Station, Texas A&M University, College Station
Boldt PE, Robbins TO (1987) Phytophagous and pollinating insect fauna of Baccharis neglecta (Compositae) in Texas. Environ Entomol 6:887–895
Boldt PE, Robbins TO (1990) Phytophagous and flower-visiting insect fauna of Baccharis salicifolia (Asteraceae) in the southwestern United States and Northern Mexico. Environ Entomol 19:515–523
Boldt PE, Robbins TO (1994) Phytophagous insect faunas of Baccharis salicina, B. pteronioides, and B. bigelovii (Asteraceae) in the south-western United States and northern Mexico. Environ Entomol 23:47–57
Bonar L (1966) A new Diaporthopsis causing brooming in Baccharis. Am J Bot 53:181–184
Bruce KA, Cameron GN, Harcombe PA (1995) Initiation of a new woodland type on the Texas Coastal Prairie by the Chinese tallow tree (Sapzum sebiferum (L.)) Roxb. Bull Torrey Bot Club 122:215–225
Campos J, Herrera M (2009) Invasive exotic flora assessment in the Basque Country (In Spanish). Dirección de Biodiversidad y Participación Ambiental. Departamento de Medio Ambiente y Ordenación del Territorio. Gobierno Vasco. Bilbao
Campos JA, Herrera M, Biurrun I et al (2004) The role of alien plants in the natural coastal vegetation in central-northern Spain. Biodivers Conserv 13:2275–2293
Campos JA, Caño L, Herrera M (2014) La invasión de Baccharis halimifolia en la costa cantábrica. Ambienta 109:78–91
Caño L, Campos JA, García-Magro D et al (2013a) Replacement of estuarine communities by an exotic shrub: distribution and invasion history of Baccharis halimifolia in Europe. Biol Invasions 15:1183–1188
Caño L, García-Magro D, Herrera M (2013b) Phenology of the dioecious shrub Baccharis halimifolia along a salinity gradient: consequences for the invasion of Atlantic subhalophilous communities. Plant Biosyst 147:1128–1138
Caño L, Campos JA, García-Magro D et al (2014) Invasiveness and impact of the non-native shrub Baccharis halimifolia in sea rush marshes: fine-scale stress heterogeneity matters. Biol Invasions 16:2063–2077
Caño L, Fuertes-Mendizabal T, García-Baquero G et al (2016) Plasticity to salinity and transgenerational effects in the nonnative shrub Baccharis halimifolia: insights into an estuarine invasion. Am J Bot 103:808–820
Casermeiro AL, Spahn E (1999) Caracterización de los recursos forrajeros nativos del norte entrerriano. In: Casermeiro J, Spahn E (eds) Sistemas agroforestales para pequeños productores de zonas húmedas. UNER-UNC-Gobierno de Entre Ríos-CERIDE, Paraná, pp 77–82
Connor DJ, Wilson GL (1968) Response of a coastal Queensland heath community to fertilizer application. Aust J Bot 16:117–123
Correll DS, Johnston MC (1979) Manual of the vascular plants of Texas. University of Texas, Dallas (original work not seen, see instead Boldt and Robbins 1987)
Costa ER, Costa JN, Armién AG, Barbosa JD, Peixoto PV (1995) Intoxicação experimental por Baccharis coridifolia (Compositae) em eqüinos. Pesqui Vet Bras 15:19–26
Cronquist A (1980) Vascular flora of the Southeastern United States. University of North Carolina Press, Chapel Hill
Cuatrecasas J (1968) Notas adicionales, taxonómicas y corológicas, sobre Baccharis. Rev Acad Colomb Cienc Exact Fis Nat 13:201–226 (original work not seen, see instead Boldt and Robbins, 1990)
Cushman JH, Lortie CJ, Christian CE (2011) Native herbivores and plant facilitation mediate the performance and distribution of an invasive exotic grass. J Ecol 99:524–531
Da Silva PG, Bartolome JW (1984) Interaction between a shrub, Baccharis pilularis subsp. consanguinea (Asteraceae) and an annual grass, Bromus mollis (Poaceae), in coastal California. Madrono 31:93–101
Damasceno FC, Nicolli KP, Caramão EB et al (2010) Changes in the volatile organic profile of Schinus polygamus (Anacardiaceae) and Baccharis spicata (Asteraceae) induced by galling psyllids. J Braz Chem Soc 21:556–563
Dauphin P, Matile-Ferrero D (2003) Présence de Ceratoplastes sinensis Del Guercio (Homoptera Coccidae) sur Baccharis halimifolia L. (Astéracées) en Gironde. Bull Soc Linn Bordx 31:261–263
de Almeida MB, Schild AL, Brasil ND, de Souza Quevedo P, Fiss L, Pfister JA, Riet-Correa F (2009) Conditioned aversion in sheep induced by Baccharis coridifolia. Appl Anim Behav Sci 117:197–200
Deble LP, Oliveira AS, Marchiori JNC (2005) O gênero Baccharis Lessing e táxones afins. Balduinia 1:1–20
Dias MP, Nozari RM, Santarem ER (2017) Herbicidal activity of natural compounds from Baccharis spp. on the germination and seedlings growth of Lactuca sativa and Bidens pilosa. Allelopath J 42:21–36
Diatloff G (1964) How far does groundsel seed travel? Queensland Agric J 90:354–356
Dimmit M (2000) Sunflower family. In: Steven JP, Patricia WC (eds) A natural history of the Sonoran Desert. Arizona-Sonora Desert Museum Press, Tucson, pp 174–175
DiPietro D, Ustin SL, Underwood EC (2002) Mapping the invasive plant Arundo donax and associated riparian vegetation using AVIRIS. In: Proceedings 11th Airborne visible/infrared image spectrometer (AVIRIS) Workshop: Jet Propulsion Laboratory, Pasadena
Dos Santos R, Citadini-Zanette V, Leal-Filho LS et al (2008) Spontaneous vegetation on overburden piles in the Coal Basin of Santa Catarina, Brazil. Restor Ecol 16:444–452
Duncan WH (1954) More and more weeds in Georgia. Bull GA Acad Sci 12:99–103
Elmore CL, Humphrey WA, Kretchum T (1968) Preemergence weed control in ground cover plantings. Calif Turfgrass Cult 18:9–10
Ervin GN (2009) Distribution, habitat characteristics, and new county-level records of Baccharis halimifolia L. on a portion of its present US range boundary. Southeast Nat 8:293–304
Estes D (2004) Noteworthy collections: middle Tennessee. Castanea 69:69–74
Everitt JH, Gerbermann AH, Akers DG (1978) Chemical control of dryland willow in the Lower Rio Grande Valley of Texas. J Rio Grande Val Hortic Soc 32:89–93
Fletcher H, Elmendorf H (1955) Phreatophytes – a serious problem in the West. In: Yearbook of agriculture. USDA, Washington, DC, pp 423–429
Flores-Tavizon E, Alarcon-Herrera MT, Gonzalez-Elizondo S et al (2003) Arsenic tolerating plants from mine sites and hot springs in the semi-arid region of Chihuahua, Mexico. Acta Biotechnol 23:113–119
Frau F, Ondiviela Eizaguirre B, Galván Arbeiza C et al (2014) The role of the hydrodynamic regime in the distribution of the invasive shrub Baccharis halimifolia (Compositae) in Oyambre Estuary (Cantabria, Spain). Limnetica 33:1–12
Frenedozo RC (2004) Plant reproductive phenology and dispersal patterns after natural regeneration in a limestone mining spoil banks. Braz Arch Biol Technol 47:261–271
Fried G, Panetta FD (2016) Comparing an exotic shrub’s impact with that of a native life form analogue: Baccharis halimifolia L. versus Tamarix gallica L. in Mediterranean saltmarsh communities. J Veg Sci 27:812–823
Fried G, Balmès V, Germain JF (2013) A contribution to the inventory and study of the impacts of phytophagous insects found on Baccharis halimifolia in its introduced range in France. EPPO Bull 43:285–289
Fried G, Laitung B, Pierre C et al (2014) Impact of invasive plants in Mediterranean habitats: disentangling the effects of characteristics of invaders and recipient communities. Biol Invasions 16:1639–1658
Fried G, Caño L, Brunel S et al (2016) Monographs on invasive plants in Europe: Baccharis halimifolia L. Bot Lett 163:127–153
Fuertes-Mendizabal T, Caño L, González-Murua C et al (2014) Plasticity in the physiological response to salinity in the invasive species Baccharis halimifolia. Plant Biology Europe FESPB/EPSO Congress, Dublin
Galindez G, Biganzoli F, Ortega-Baes P et al (2009) Fire responses of three co-occurring Asteraceae shrubs in a temperate savanna in South America. Plant Ecol 202:149–158
Gann B, Thompson L, Schuler JL (2012) Control and management of eastern Baccharis in a recently established bottomland hardwood plantation. In: Butnor JR (ed) Proceedings of the 16th biennial southern silvicultural research conference e-general technical report SRS-156, United States Department of Agriculture Forest Service, Southern Research Station, Asheville
Gatewood J, Robinson T, Colby B et al (1950) Use of water by bottom-land vegetation in lower Safford Valley, Arizona. Geological survey water supply paper, vol 1103. U.S. Department of the Interior, Washington, DC
Gilberti LI, Menezes AL, Rodrigues AC, Fernandes GW, Berbara RL, Marota HB (2014) Effects of arsenic on the growth, uptake and distribution of nutrients in the tropical species Baccharis dracunculifolia DC (Asteraceae). J Toxicol Sci 2014:1–18
Gilman EF (1999) Baccharis halimifolia Salt Bush, Groundsel Bush. University of Florida, Gainesville
Giuliano DA, Plos A (2014) Género Baccharis [The genus Baccharis]. In: Zuloaga FO, Belgrano MJ, Anton AM (eds) Flora Argentina: flora vascular de la República Argentina, vol 7. Instituto de Botánica Darwinion, San Isidro, pp 43–123
Glenn E, Tanner R, Mendez S et al (1998) Growth rates, salt tolerance and water use characteristics of native and invasive riparian plants from the delta of the Colorado River, Mexico. J Arid Environ 40:281–294
Gomes V, Fernandes GW (2002) Germinação de aquênios de Baccharis dracunculifolia DC. (Asteraceae). Acta Bot Bras 16:421–427
Gonçalvez CEP (2014) Alelopatia de carqueja (Baccharis trimara Less) e ação de fungos em capim-annoni (Eragrostis plana Ness). Dissertação de Mestrado, Universidade Federal de Santa Maria
Grace JB, Smith MD, Grace SL et al (2001) Interactions between fire and invasive plants in temperate grasslands of North America. In: Galley KEM, Wilson TP (eds) Proceedings of the invasive species workshop: the role of fire in the control and spread of invasive species. Tall Timbers Research Station, Tallahassee, pp 40–65
Green B, Simpson R, Dettmann M (2011) Assessment of airborne Asteraceae pollen in Brisbane, Australia. Aerobiologia 28:295–301
Habermehl GG, Busam L, Heydel P, Mebs D, Tokarnia CH, Döbereiner J, Spraul M (1985) Macrocyclic trichothecenes: cause of livestock poisoning by the Brazilian plant Baccharis coridifolia. Toxicon 23:731–745
Hamilton WT, McGinty A, Ueckert DN et al (2004) Brush management: past, present, future. A & M University Press, College Station
Haque N, Peralta-Videa JR, Jones GL et al (2008) Screening the phytoremediation potential of desert broom (Baccharis sarothroides Gray) growing on mine tailings in Arizona, USA. Environ Pollut 153:362–368
Harcomb PA (1989) Reports progress of three prairie restoration/management projects in Houston area (Texas). Restor Manag Notes 7:35
Hickman JC (ed) (1993) The Jepson manual: higher plants of California. University of California Press, Berkeley
Hobbs RJ, Mooney HA (1985) Vegetative regrowth following cutting in the shrub Baccharis pilularis ssp. consanguinea (DC.) Wolf. Am J Bot 72:514–519
Hobbs RJ, Mooney HA (1986) Community changes following shrub invasion of grassland. Oecologia 70:508–513
Holmgren M, Segura AM, Fuentes ER (2000) Limiting mechanisms in the regeneration of the Chilean matorral. Plant Ecol 147:49–57
Hopkins DL, Adlerz WC (1988) Natural hosts of Xylella fastidiosa in Florida. Plant Dis 72:429–431
Huxley A (1992) The new RHS dictionary of gardening. MacMillan/Stockton Press, London
Hyland T, Holloran P (2005) Controlling European beachgrass (Ammophila arenaria) using prescribed burns and herbicide. In: California Invasive Plant Council Symposium 2005. Chico, CA
Ibáñez F, Zoppolo R (2011) Assessment of allelopathic properties of Baccharis dracunculifolia DC in laboratory and field conditions. Allelopath J 28:77–86
Jarvis BB, Mokhtari-Rejali N, Schenkel EP et al (1991) Trichothecene mycotoxins from Brazilian Baccharis species. Phytochemistry 30:789–797
Kearney TH, Peebles RH (1969) Arizona flora. University California Press, Berkeley (original work not seen, see instead Kenney et al. 1986)
Keeley JE (2005) Fire history of the San Francisco East Bay region and implications for landscape patterns. Int J Wildland Fire 14:285–296
Kenney WR, Bock JH, Bock CE (1986) Responses of the shrub, Baccharis pteronioides, to livestock exclosure in southeastern Arizona. Am Midl Nat 116:429–431
Kraft SK, Denno RF (1982) Feeding responses of adapted and non-adapted insects to the defensive properties of Baccharis halimifolia L (Compositae). Oecologia 52:156–163
Krischik VA, Denno RF (1990a) Differences in environmental response between the sexes of the dioecious shrub, Baccharis halimifolia (Compositae). Oecologia 83:176–181
Krischik VA, Denno RF (1990b) Patterns of growth, reproduction, defense, and herbivory in the dioecious shrub Baccharis halimifolia (Compositae). Oecologia 83:182–190
Lamb SH (1975) Woody plants of the Southwest. The Sunstone Press, Santa Fe (original work not seen, see instead Kenney et al. 1986)
Laris P, Brennan S, Engelberg K (2016) The coyote brush invasion of Southern California grasslands and the legacy of mechanical disturbance. Geogr Rev 107:640–660
Lázaro-Lobo A, Herrera M, Campos JA, Caño L, Goñi E, Ervin GN (2020) Influence of local adaptations, transgenerational effects and changes in offspring's saline environment on Baccharis halimifolia L. under different salinity and light levels. Environ Exp Bot 177:104134
Lazarotto DC, Da Silva ER, Pawlowski A et al (2017) Phytotoxic effects of Baccharis psiadioides (Asteraceae) volatiles on different phases of plant development. J Essent Oil Res 29:313–319
Le Moigne G, Magnanon S (2009) Le séneçon en arbre (Baccharis halimifolia). Conservatoire Botanique National de Brest, Brest
Lombardo A (1964) Flora Arbórea y Arborescente del Uruguay. Concejo Departamental de Montevideo, Montevideo (original work not seen, see instead Ibáñez and Zoppolo 2011)
Lovet J (2015) Lutte biologique contre un arbuste invasif en France: Données de biologie et impact de la cochenille Ceroplastes sinensis (Hem., Coccidae) sur Baccharis halimifolia (Asteraceae) en Camargue. MSc dissertation, Ecole des Métiers de l’Environnement (Rennes, France)
Macedo JF, Brandão M, Lara JFR (2003) Plantas daninhas na pós-colheita de milho nas várzeas do rio São Francisco, em Minas Gerais. [Weed survey on corn post-harvest under meadow holm conditions along São Francisco river, Minas Gerais, Brazil]. Planta Daninha 21:239–248
Mallard F (2008) Effet d’une espèce végétale introduite envahissante le Séneçon en arbre Baccharis halimifolia sur le peuplement d'arthropodes dans le Golfe du Morbihan. MSc dissertaion, Université de Rennes
Marchesini E (2003) Control de chilcas. Hoja Informativa Electrónica. Estación Experimental Agropecuaria INTA. Concepción del Uruguay, 86
Matuda E (1957) El género Baccharis en Mexico. Ann Inst Biol Mex 28:143–174. (original work not seen, see instead Boldt and Robbins 1987)
McBride JR (1974) Plant succession in the Berkeley Hills, California. Madrono 22:317–329
McBride JR, Heady HF (1968) Invasion of grassland by Baccharis pilularis DC. J Range Manag 21:106–108
McFadyen PJ (1973) Insects for groundsel bush control. Queensland Agric J 99:607–611
McGuire RD (2005) Quantifying consumptive water use by seep willow (Baccharis salicifolia) within the San Pedro Riparian National Conservation Area (SPRNCA). Master’s theses, The University of Arizona
McKee KL, Feller IC, Popp M et al (2002) Mangrove isotopic (δ15N and δ13C) fractionation across a nitrogen vs. phosphorus limitation gradient. Ecology 83:1065–1075
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments – an emerging remediation technology. Environ Health Perspect 116:278–283
Mongelli E, Desmarchelier C, Talou JR, Coussio J, Ciccia G (1997) In vitro antioxidant and cytotoxic activity of extracts of Baccharis coridifolia DC. J Ethnopharmacol 58:157–163
Moon DC, Barnouti J, Younginger B (2013) Context-dependent effects of mycorrhizae on herbivore density and parasitism in a tritrophic coastal study system. Ecol Entomol 38:31–39
Müller J (2006) Systematics of Baccharis (Compositae-Astereae) in Bolivia, including an overview of the genus. Syst Bot Monogr 76:1–341
Muñoz A, Garcia A, Scott C et al (2017) Consumption of salt cedar and willow Baccharis by Boer-cross goats. Rangel Ecol Manag 70:374–379
Mutz JL, Scifres CJ, Mohr WC et al (1979) Control of willow Baccharis and spiny aster with pelleted herbicides. Texas Agric Exp Stn Bull–1194
Negreiros D, Esteves D, Fernandes GW et al (2012) Growth-survival tradeoff in the widespread tropical shrub Baccharis dracunculifolia (Asteraceae) in response to a nutrient gradient. Trop Ecol 55:167–176
Nesom G (1988) Baccharis monoica (Compositae: Astereae), a monoecious species of the B. salicifolia complex from Mexico and Central America. Phytologia 65:160–164 (original work not seen, see instead Boldt and Robbins, 1990)
Neto D, Carvalho LM, Cruz C et al (2006) How do mycorrhizas affect C and N relationships in flooded Aster tripolium plants? Plant Soil 279:51–63
Ogden JAE, Rejmánek M (2005) Recovery of native plant communities after the control of a dominant invasive plant species, Foeniculum vulgare: implications for management. Biol Conserv 125:427–439
Olson PE, Fletcher JS (2000) Ecological recovery of vegetation at a former industrial sludge basin and its implications to phytoremediation. Environ Sci Pollut Res 7:195–204
Orr SP, Rudgers JA, Clay K (2005) Invasive plants can inhibit native tree seedlings: testing potential allelopathic mechanisms. Plant Ecol 181:153–165
Overbeck GE, Pfadenhauer J (2007) Adaptive strategies in burned subtropical grassland in southern Brazil. Flora 202:27–49
Palmer WA (1987) The phytophagous insect fauna associated with Baccharis halimifolia L. and B. neglecta Britton in Texas, Louisiana and northern Mexico. P Entomol Soc Wash 89:185–199
Palmer WA, Bennett FD (1988) The phytophagous insect fauna associated with Baccharis halimifolia L. in the eastern United States. P Entomol Soc Wash 90:216–228
Palmer WA, Sims-Chilton NM (2012) Baccharis halimifolia L. – groundsel bush. In: Biological control of weeds in Australia. CSIRO Publishing, Melbourne, pp 86–95
Panetta FD (1977) The effect of shade upon seedling growth in groundsel bush (Baccharis halimifolia L.). Aust J Agric Res 28:681–690
Panetta FD (1979a) Germination and seed survival in the woody weed, groundsel bush (Baccharis halimifolia L.). Aust J Agric Res 30:1067–1077
Panetta FD (1979b) The effects of vegetation development upon achene production in the woody weed, groundsel bush (Baccharis halimifolia L.). Aust J Agric Res 30:1053–1065
Panetta FD (1979c) Shade tolerance as reflected in population structures of the woody weed, groundsel bush (Baccharis halimifolia L.). Aust J Bot 27:609–615
Parker K (1972) An illustrated guide to Arizona weeds. University of Arizona Press, Tucson
Parsons WT, Cuthbertson EG (1992) Groundsel bush. In: Parsons WT, Cuthbertson EG (eds) Noxious weeds of Australia. Inkata Press, Melbourne, pp 256–259
Paudel S, Battaglia LL (2013) Germination responses of the invasive Triadica sebifera and two co-occurring native woody species to elevated salinity across a gulf coast transition ecosystem. Wetlands 33:527–535
Paudel S, Battaglia LL (2015) The role of light, soil and human factors on the probability of occurrence of an invasive and three native plant species in coastal transitions of coastal Mississippi, USA. J Plant Ecol 8:491–500
Paudel S, Baer SG, Battaglia LL (2014) Arbuscular mycorrhizal fungi (AMF) and success of Triadica sebifera invasion in coastal transition ecosystems along the northern Gulf of Mexico. Plant Soil 378:337–349
Penfound WT, Hathaway ES (1938) Plant communities in the marshlands of southeastern Lousiana. Ecol Monogr 8:3–56
Pivovaroff AL, Swift C, Battaglia LL et al (2015) Physiological profiles as indicators of response to hurricane disturbance for three coastal wetland species. J Coast Res 31:986–993
Polacik KA, Maricle BR (2013) Effects of flooding on photosynthesis and root respiration in saltcedar (Tamarix ramosissima), an invasive riparian shrub. Environ Exp Bot 89:19–27
Porazinska DL, Fujisaki I, Purcell MF et al (2014) Plant invasions from a belowground nematocentric perspective. Soil Biol Biochem 77:213–220
Richardson DM, Pysek P, Rejmanek M et al (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107
Rizzo I, Varsavky E, Haidukowski M, Frade H (1997) Macrocyclic trichothecenes in Baccharis coridifolia plants and endophytes and Baccharis artemisioides plants. Toxicon 35:753–757
Ross C (2004) Baccharis pilularis DC. In: Francis JK (ed) Wildland shrubs of the United States and its territories: thamnic descriptions GTR IITF–GTR–26. USDA Forest Service International Institute of Tropical Forestry and USDA Forest Service Rocky Mountain Research Station, San Juan, PR and Fort Collins, CO, pp 103–104
Russell W, Tompkins R (2005) Estimating biomass in coastal Baccharis pilularis dominated plant communities. Fire Ecol 1:20–27
Sabattini RA, Ledesma SG, Sione SMJ (2012) Dinámica de la cobertura de Melica macra Nees y Baccharis punctulata D.C en un bosque nativo sometido a desarbustado mecánico. Rev Fac Cienc Agrar 12:13–19
Safford HD (2001) Brazilian páramos. III. Patterns and rates of postfire regeneration in the Campos de altitude. Biotropica 33:282–302
Salimon CI, Negrelle RRB (2001) Natural regeneration in a quaternary coastal plain in southern Brazilian Atlantic rain forest. Braz Arch Biol Technol 44:155–163
Scifres CJ (1980) Brush management. Texas A&M University, College Station
SERNEC Data Portal (2018). https://sernecportal.org/portal/index.php. Accessed 16 Nov 2018
Serra MN, Albarino R, Diaz Villanueva V (2013) Invasive Salix fragilis alters benthic invertebrate communities and litter decomposition in northern Patagonian stream. Hydrobiologia 701:173–188
Sims-Chilton NM, Panetta FD (2011) The biology of Australian weeds 58. Baccharis halimifolia L. Plant Prot Q 26:114–123
Sims-Chilton NM, Zalucki MP, Buckley YM (2009) Patchy herbivore and pathogen damage throughout the introduced Australian range of groundsel bush, Baccharis halimifolia, is influenced by rainfall, elevation, temperature, plant density and size. Biol Control 50:13–20
Sims-Chilton NM, Zalucki MP, Buckley YM (2010) Long term climate effects are confounded with the biological control programme against the invasive weed Baccharis halimifolia in Australia. Biol Invasions 12:3145–3155
Sinnassamy JM (2004) Baccharis halimifolia. In: Plantes invasives en France. Coordinated by Muller, S. Muséum national d'Histoire naturelle, Paris
Sione SMJ, Ledesma SG, Rosenberger LJ et al (2016) Banco de semillas del suelo en un área de bosques nativos sujeta a cambio en el uso de la tierra (Entre Ríos, Argentina). FAVE Sección Ciencias Agrarias 15:87–104
Skousen JG, Call CA, Knight RW (1990) Natural revegetation of an unreclaimed lignite surface mine in East-Central Texas. Southwest Nat 35:434–440
Slocum MG, Aide TM, Zimmerman JK et al (2004) Natural regeneration of subtropical montane forest after clearing fern thickets in the Dominican Republic. J Trop Ecol 20:483–486
Stegelmeier BL, Sani Y, Pfister JA (2009) Baccharis pteronioides toxicity in livestock and hamsters. J Vet Diagn Investig 21:208–213
Sundberg SD, Bogler DJ (2006) Baccharis. In: Flora of North America Editorial committee 1993+ (ed) Flora of North America North of Mexico. New York/Oxford, pp 23–28
Tellman B (ed) (2002) Invasive exotic species in the Sonoran region. University of Arizona Press, Arizona
Tokarnia CH, Döbereiner J (1975) Intoxicação experimental em bovinos por “mio-mio”, Baccharis coridifolia. Pesqui Agropec Bras 10:79–97
Tolliver KS, Martin DW, Young DR (1997) Freshwater and saltwater flooding response for woody species common to barrier island swales. Wetlands 17:10–18
Tucat G (2015) Estudios sobre la biología de Baccharis ulicina Hook & Arn y su susceptibilidad a estrategias de manejo en el sur bonaerense. Doctoral dissertation, Universidad Nacional del Sur (Bahía Blanca, Argentina)
U.S. Department of Homeland Security (2004) Environmental impact statement for operation Rio Grande. CBP, Washington, DC
Underwood EC, Ustin SL, Ramirez CM (2007) A comparison of spatial and spectral image resolution for mapping invasive plants in coastal California. J Environ Manag 39:63–83
Ungar IA (1968) Species-soil relationships on the Great Salt Plains of northern Oklahoma. Am Midl Nat 80:392–406
USDA, NRCS (2018) The PLANTS database. http://plants.USDA.gov, 12 April 2018. National Plant Data Team, Greensboro, NC 27401–4901 USA
Van Auken OW, Bush JK (1990) Influence of light levels soil nutrients and competition on seedling growth of Baccharis neglecta Asteraceae. Bull Torrey Bot Club 117:438–444
Verloove F, Dana ED, Alves P (2018) Baccharis spicata (Asteraceae), a new potentially invasive species to Europe, Plant Biosyst 152:416–426
Vick JK, Young DR (2013) Comparative responses of a non-N-fixing shrub and an actinorhizal N-fixing shrub to N fertilization. Plant Soil 371:377–385
Virginia Tech (2018) Department of Forest Resources and Environmental Conservation. https://frec.vt.edu/
Weber E (2003) Invasive plant species of the world: a reference guide to environmental weeds. CABI Publishing, Oxon
Westman WE, Panetta FD, Stanley TD (1975) Ecological studies on reproduction and establishment of the woody weed groundsel bush (Baccharis halimifolia L.: Asteraceae). Aust J Agric Res 26:855–870
Williams K, Hobbs RJ (1989) Control of shrub establishment by springtime soil water availability in an annual grassland. Oecologia 81:62–66
Williams K, Hobbs RJ, Hamburg SP (1987) Invasion of an annual grassland in Northern California by Baccharis pilularis ssp. consanguinea. Oecologia 72:461–465
Winders CW (1937) Groundsel-bush in southeastern Queensland. Queensland Agric J 48:656-664. (original work not seen, see instead Sims-Chilton and Panetta 2011)
Young DR, Erickson DL, Semones SW (1994) Salinity and the small-scale distribution of three barrier island shrubs. Can J Bot 72:1365–1372
Younginger B, Barnouti J, Moon DC (2009) Interactive effects of mycorrhizal fungi, salt stress, and competition on the herbivores of Baccharis halimifolia. Ecol Entomol 34:580–587
Zavaleta E (2006) Shrub establishment under experimental global changes in a California grassland. Plant Ecol 184:53–63
Zinnert JC, Nelson JD, Hoffman AM (2012) Effects of salinity on physiological responses and the photochemical reflectance index in two co-occurring coastal shrubs. Plant Soil 354:45–55
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lázaro-Lobo, A., Ervin, G.N., Caño, L., Panetta, F.D. (2021). Biological Invasion by Baccharis. In: Fernandes, G.W., Oki, Y., Barbosa, M. (eds) Baccharis. Springer, Cham. https://doi.org/10.1007/978-3-030-83511-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-83511-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83510-1
Online ISBN: 978-3-030-83511-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)