Abstract
Intersexual differences in resource allocation between host plant sexes have been the most proposed mechanism to explain variation in herbivory in dioecious plants. In this sense, the sex-biased herbivory hypothesis predicts that male plants have higher growth rates and should be more susceptible to herbivores than females. Most studies testing this hypothesis were conducted in temperate regions and focused on a few host plant genera. Currently, the male-biased herbivory as a rule for dioecious species has been questioned. In this study, we reviewed the aforementioned hypotheses for Baccharis, performed a meta-analysis contrasting herbivory and resource allocation in Baccharis versus other dioecious systems, and addressed two case studies: (1) intersexual comparisons in plant architectural traits and herbivory for 12 species of Baccharis and (2) a fine-scale analysis for the mountaintop endemic Baccharis concinna in long- and short-term studies. In general, most Baccharis species showed no intersexual differences for vegetative resource allocation, except plant biomass that exhibited a positive trend to increase in male individuals. Most Baccharis species do not support the sex-biased herbivory hypothesis, likely due to an absence of intersexual differences in resource allocation. Although host plant sex was a weak predictor explaining galling insect abundance in this host plant genus, the plant traits evaluated here were important drivers of gall attack. Due to the lack of differential resource allocation and sex-biased herbivory in both short- and long-term sampling events, we have consistent support to reject the male-biased attack hypothesis on Baccharis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Selection Theory: Male and Female Functions
The theory of sexual selection was originally proposed by Charles Darwin (1871) to explain the evolution of different secondary sexual characteristics between male and female animals, and he argued that any trait that differed between the sexes would be the result of selection by one of the sexes and such trait would have evolved as a result of differential mating success. The theory of sexual selection predicts different energetic investments between the sexes (see Emlen and Oring 1977; Arnold and Wade 1984; Jaenicke and Morrow 2018). Males would have a lower energetic investment in reproduction because they only produce sperm, whereas females would have a higher energetic investment having to produce the larger eggs as well as, in the case of plants, investing in the development and protection of fruits and seeds (Stanton et al. 1986). This differential investment in reproduction would result in different reproductive success limitations between the sexes. A male’s reproductive success would be limited by how much sperm a male could spread to females, while a female’s reproductive success would be limited by the number of eggs that a female could produce (Jaenicke and Morrow 2018). Although the theory of sexual selection was originally developed to explain reproductive patterns observed in animals, it also applies to plants, and the selection of secondary traits in plants can be mediated via pollinators by selecting flowers that offer more resources (Stanton et al. 1986; Knauer and Schiesti 2017; Delph 2019).
The ancestral condition for plants is the presence of hermaphroditic flowers, with female and male reproductive structures within the same flower (Bawa 1980, Charlesworth 2018; Henry et al. 2018). In hermaphroditic flowers, pollinators are attracted to food resources such as nectar and pollen grains, but these monoecious flowers incur greater inbreeding reducing the plant’s fitness (Barrett 2002). Plants evolved dioecy, or male and female flowers in different plants, probably as a result of pressures to promote outcrossing and reduce inbreeding depression (Barret 2002), but herbivory has also been suggested as a potential selective pressure (Herms and Mattson 1992; Ashman 2002; Cornelissen and Stiling 2005; Moritz et al. 2017; Moreira et al. 2019; LeRoy et al. 2020). Although dioecy is not as common as hermaphroditism, approximately 37 plant families out of a total of 51 families encompass dioecious species (Bawa 1980; Henry et al. 2018), with some large genera containing almost exclusive dioecious species, such as Baccharis (Asteraceae).
Dioecy created challenges to pollination as male flowers provide resources to pollinators as pollen or nectaries, but female flowers compete with male flowers for pollinators and provide no resources in exchange for the pollinator’s visit. Female flowers already have a high energetic cost of producing ovaries containing eggs, which after fertilization become fruits containing seeds. Also, in female flowers, seed production (fitness) is frequently limited by resources and not by pollinator visits (Sutherland and Delph 1984; Vaughton and Ramsey 1998; Barrett 2002). Therefore, many female flowers mimic male flowers in color, shape, and size as closely as possible to trick pollinators into visiting them (Sutherland and Delph 1984; Vaughton and Ramsey 1998; Vega-Frutis et al. 2013).
1.2 Trade-Offs in Growth, Reproduction, and Defense Against Herbivores
In general, female plants allocate more resources into sexual reproduction, while male plants allocate more resources into vegetative growth (e.g., Loyd and Webb 1977; Wallace and Rundel 1979; Ågreen 1988; Popp and Reinartz 1988; Obeso 2002; Harris and Pannel 2008; Pfeiffer et al. 2019). The greater allocation of resources to growth by male plants results in the exhaustion of the pool of carbon molecules available to form secondary defensive compounds (e.g., Herms and Mattson 1992; Cornelissen and Fernandes 2001; Imaji and Seiwa 2010). Consequently, male plants should represent a food resource of high nutritional quality and low chemical defenses to insect herbivores.
This differential allocation of resources within the host plant represents an important source of variation in plant quality for insect herbivores (e.g., Boecklen et al. 1990; Boecklen and Hoffman 1993; Multikainen and Delph 1996; Espírito-Santo et al. 2003; Barret and Hough 2013; Erb 2018). Indeed, several studies have recorded male-biased herbivory, in which male plants sustain higher herbivore densities or greater levels of herbivory when compared with female plants (e.g., Lovett-Doust et al. 1987; Boecklen et al. 1990; Jing and Coley 1990; Pearson et al. 1990; Åhman 1997; Cornelissen and Stiling 2005; Rivkin et al. 2018). Nevertheless, exceptions to this pattern exist where insect herbivores preferentially attack female plants in the genus Baccharis (e.g., Espírito-Santo and Fernandes 1998; Faria and Fernandes 2001) or do not show attack preferences driven by plant gender (e.g., Boecklen et al. 1994; Åhman 1997; Espírito-Santo et al. 1999, 2012; Araújo et al. 2006; Marques and Fernandes 2016) (see Table 4.1). Boecklen et al. (1994) did not detect intersexual variation in herbivory in three herbivorous species on Salix lasiolepis (Salicaceae) and argued that the herbivore species were probably utilizing resources that were not sexually dimorphic in this host plant, such as leaves. On the other hand, Avila-Sakar and Romanow (2012) argued that the lack of sex preference by insect herbivores may be common and indicate that male and female plants could be allocating resources in a similar manner. These authors have pointed some methodological bias in male-biased herbivory studies, such as: (a) taxonomic bias, e.g., research efforts have focused on few species within a few orders and families, and (b) failure to make the connection between sex-biased herbivore damage and intersexual differences in growth rate and reproduction. Finally, Avila-Sakar and Romanow (2012) recommend a standard protocol for evolutionary-ecological studies, suggesting an increase in the taxonomic breadth of the studies of herbivory in dioecious species because only 2% of the dioecious species have been studied, mostly from the Salicaceae family. Moreover, other studies have focused on herbaceous dioecious species.
In temperate plants, resource allocation is concentrated in the Spring and Summer months, so herbivores have a small window of time to attack the host plant. Therefore, sampling insects only in a couple of months may be adequate to obtain a realistic picture of herbivory patterns in temperate areas. Furthermore, most of these studies only measured herbivore damage inflicted by one or a few herbivore species. This is not the scenario found in tropical regions where plants may grow for a much longer period of time and in which they are challenged by a much richer herbivore fauna. One study conducted in a tropical plant species by Wolfe (1997) found higher herbivore attack rates on male plants of Neea psychotrioides (Nyctaginaceae) in Costa Rica. This study showed that male plants had more and larger flowers when compared to female plants and that two galling insect species and a free-living lepidopteran species attacked flowers on male plants more often. But these data related to flower herbivory which is a resource with a differential allocation between the sexes, and the study did not address the sex-biased herbivory hypothesis which predicted greater herbivory on vegetative portions of the male plants. In this way, more studies in tropical regions are necessary to provide a realistic global pattern on intersexual differences in herbivore attack.
1.3 The Genus Baccharis as a Study System for Sex-Biased Herbivory
Baccharis represents an excellent model system to the study of plant-animal interactions because of the tremendously high number of insects of different guilds that feed upon them and its occurrence in temperate and tropical regions along gradients of salinity, humidity, altitude, and temperature (see Fernandes et al. 2014). Furthermore, this genus has more than 440 species, with most of them being dioecious (Heiden et al. 2019). Most of the studies on herbivory in dioecious tropical systems have been conducted by our research group on the component communities of galling insects mainly on B. concinna and B. dracunculifolia but also in other species in this genus (e.g., Marques 1997; Madeira et al. 1997; Wolfe 1997; Espírito-Santo and Fernandes 1998; Marques et al. 2002; Espírito-Santo et al. 2007, 2012; Marques and Fernandes 2016). So far, no clear patterns of differential resource allocation between plants of different sexes and sex-biased attack by herbivores have been detected in this system (see Table 4.1 and Box 4.1). Therefore, we set out to design studies that included several measures of resource allocation such as shoot growth, height, number of meristems, plant architecture, and aboveground biomass, as well as intensive samples of their associated herbivore communities. Sex-biased herbivory and intersexual differences in resource allocation have been studied mostly on B. concinna, B. dracunculifolia, and B. ramosissima (Espírito-Santo et al. 2012; Fernandes et al. 2014; see Table 4.1 for more details). In the present study, we bring new data involving intersexual comparisons for several species of Baccharis, aiming to detect a clearer picture of how the communities of insect herbivores respond to resource allocation patterns of their host plants.
Box 4.1: The Influence of Plant Gender on Plant-Insect Interactions and Baccharis as a Study System: An Integrative Review
We quantitatively reviewed the effects of plant gender on insect abundance and damage on dioecious plants, by systematically reviewing the published literature. Searches were conducted on Web of Science and Scopus databases, using as keywords “plant dioecy,” “dioecy,” “plant gender,” “gender,” “insect*,” “herbivor*,” and their combinations. We also used the database of Cornelissen and Stiling (2005), which meta-analytically reviewed the evidence for sex-biased herbivory. From a total of 127 studies found, 47 papers (Appendix 1) met the criteria of language (English) and statistics clearly reported (data of means and a measurement of variance reported separately for males and females) and were included in our review. To compare insect abundance and plant damage (i.e., herbivory) on male and female plants of dioecious systems, response mean values (Xmale, Xfemale), standard deviations (Smale, Sfemale), and sample size (Nmale, Nfemale) were gathered from the text, tables, and/or figures in each study. Insect abundance encompassed data on counts, density, number of eggs laid, number of galls and damage including leaf area removed, and number of feeding holes and/or leaf scars. Insect survivorship on both sexes was excluded from the analyses due to the low number of comparisons (n = 3). To address the effects of plant gender on insect data, we used the standardized mean difference between male plants and females, calculating Hedge’s d, and the cumulative Hedge’s d was calculated using a weighing method with the reciprocal of the sampling variance. For statistical purposes only, male plants were considered an experiment group (male-biased herbivory has been previously reported in the literature; see Cornelissen and Stiling 2005), and female plants were used as control. We ran analyses for each parameter (abundance, damage) separately using a random model and ran further analyses using only Baccharis species. All analyses were conducted on OPEN MEE (Wallace et al. 2017).
Forty-seven published studies, between 1978 and 2018, were included in our analysis, enabling 120 independent comparisons. These studies evaluated the effects of plant sex on 49 different plant species in 21 botanical families. Asteraceae and Salicaceae were the most represented families, encompassing almost half of the plants studied. Almost 50 species of insects were studied, and gall-formers and folivores were the most common guilds on the plants (almost 78% of all independent comparisons), and other guilds less represented were florivores, stem borers, and suckers.
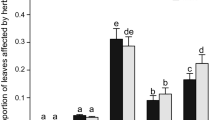
Our analysis for all plants indicates male-biased herbivory, with male plants supporting almost 50% more insects than female plants (insect abundance, E++ = 0.4946, CI = 0.2271 to 0.7622, df = 69) and 20% higher damage (E++ = 0.203, CI = 0.1657 to 0.4718, df = 4) than female plants (Fig. 4.1). These results were strongly influenced by gall-formers for data on abundance (E++ = 0.3369) and for folivores for data on damage (E++ = 0.4430), and these guilds were significantly different from each other (QB = 12.09, P = 0.05).
Standardized mean difference in insect abundance and herbivory (Hedge’s d) between male and female plants. Circles represent the cumulative effect size with associated confidence intervals, and the triangle represents the effect size for Baccharis species only. Numbers in parentheses indicate the number of comparisons for each response variable, and effects are significant if confidence intervals do not encompass zero
Species of Baccharis represented almost 30% of the species found in our database (14 out of 49 species, 39 independent comparisons), and we ran the same analyses using Baccharis only. Most studies with Baccharis addressed gall abundance on leaves and twigs (85% of the comparisons), and a few studies evaluated abundance of folivores (beetles), florivores (thrips), and sap-suckers (aphids).
Differently from the entire dataset, Baccharis species do not show evidence for male-biased herbivory (E++ = 0.1261, CI = −0.05 to 0.309, df = 33, Fig. 4.1), as shown by the confidence intervals around the mean effect size that encompasses the zero value. Therefore, although our dataset shows evidence for male-biased herbivory in terms of insect abundance and plant damage, the same does not hold true for Baccharis species here evaluated. Reasons for the absence of male-biased herbivory for these plants are discussed along the text.
An analysis of the available literature on the sex-biased herbivore hypothesis suggests that most studies were conducted in a few plant species (see Box 4.1), in short sampling periods, often involving one species of insect herbivore, and mostly in temperate latitudes. We set out to address these weaknesses by designing studies that addressed several host plant species, all herbivore species of a guild present on the host plant, and long-term studies up to 11 months, over a broad geographical range. Below, we address two cases of sex-biased herbivory in the genus Baccharis. The first one relates to a study across the genus in which the intersexual differences in resource investment and herbivory are evaluated in 12 Baccharis species. The second case evaluates resource allocation between plant sexes in B. concinna in a short-term destructive sampling and a long-term (12 months) study while also recording herbivory by galling insect species.
2 Study 1. Intersexual Differences on Resource Investment and Herbivory for 12 Baccharis Species
We investigated intersexual differences in resource allocation and gall attack in 12 species of Baccharis along the southern portion of the Espinhaço Mountain Range in Minas Gerais, Brazil, (see Table 4.2) during one rainy season in 2002–2003 (see Espírito-Santo et al. 2007 for further details on locations and sampling design). We sampled 30 individuals (15 males and 15 females) of each Baccharis species that were arbitrarily marked in the field. Plant sex was determined by analysis of floral morphology. Five traits related to plant architecture were evaluated in the study: plant height, number of fourth-level shoots, average ramification level, number of ramifications, and biomass (Table 4.2). For each plant, we recorded gall species and their abundance on leaves, flowers, and stems. Details on sampling design and statistics conducted and study sites can be found in Espírito-Santo et al. (2007). Fourth-level shoots were considered an optimal indicator of the amount of active meristems in a given individual (see Espírito-Santo et al. 2012; Fernandes et al. 2014).
In relation to resource investment, only one species showed difference in number of ramifications (B. minutiflora), where resource investment was found to be higher in female plants, contrary to the prediction of the differential resource allocation hypothesis (Ågreen 1987; Rivkin et al. 2018) (Table 4.2). No Baccharis species exhibited intersexual differences in the average ramification level. Only two species showed intersexual difference in height (B. minutiflora and B. medullosa), both being higher in female plants. Five Baccharis species showed intersexual differences in the number of fourth-level shoots, with B. aphylla, B. ramosissima, and B. dracunculifolia being female-biased and B. cognata and B. trinervis male-biased (see Table 4.2). Nine species showed intersexual differences in biomass, with three being female-biased (B. aphylla, B. minutiflora, and B. medullosa) and six male-biased (B. ramosissima, B. cognata, B. serrulata, B. dracunculifolia, B. concinna, and B. medullosa). In general, most Baccharis species showed no intersexual differences for vegetative resource allocation (Tables 4.2 and 4.3), except for plant biomass that showed a trend to be greater in male individuals (see Table 4.3). The biomass was the best predictor variable to verify the differential sex resource investment in Baccharis (Table 4.3). Other studies also have not detected differential allocation of resources between the sexes, even when total plant biomass was considered (Delph et al. 1993; Hemborg and Karlsson 1999; Espírito-Santo et al. 2012).
The 12 species of Baccharis were attacked by 55 different gall morphotypes (see Espírito-Santo et al. 2007 for a complete species list). We detected intersexual variations in herbivory for only five species (Tables 4.2 and 4.3), four male-biased (B. aphylla, B. truncata, B. minutiflora, and B. dracunculifolia) and one female-biased (B. ramosissima). Hence, most Baccharis species seem not to support the sex-biased herbivory hypotheses (see Table 4.3), likely due to lack of differences in resource allocation between the host sexes (Espírito-Santo et al. 2012; Fernandes et al. 2014; Marques and Fernandes 2016). Indeed, the total reproductive investment may not differ between male and female individuals for most Baccharis species. Although we did not quantify reproductive effort here, Espírito-Santo et al. (2012) found no differences in inflorescence number for two out of three Baccharis species in 1 year. For the species considered in the present study, inflorescences have a similar size in both sexes, and female flowers do not invest in pollinator rewards (authors’ personal observations). Seeds are tiny and wind-dispersed, and although their maturation may represent a high relative cost to female individuals, this investment may be compensated by male’s pollen production. Considering that we did not observe any marked difference in spatial distribution among sexes, it is likely that the lack of differences in reproductive investment is reflected in similar vegetative characteristics that regulate gall densities. In this way, both sexes of Baccharis would be equally susceptible to herbivore attack.
2.1 Relationship Between Plant Architectural Traits and Gall Attack
Although there was no clear intersexual difference in resource allocation and herbivory, we tested the effects of plant architectural traits on gall abundance for each of the 12 Baccharis species studied here. To summarize all architectural traits into a unique variable, we performed a principal component analysis (PCA) for each Baccharis species. We used PCA scores instead of the raw data because they were not orthogonal (i.e., not independent); therefore, the use of multiple regressions would not be recommended. The scores of the first axes from each PCA were chosen to indicate architectural gradients because they summarized the patterns observed in the data and explained most of the data variation, being suitable for use in the regression models described below.
We found a significant statistical relationship between gall insect abundance and the first axis of the PCA in 6 out of 12 Baccharis species (Fig. 4.2). For all significant relations, the first axis of the PCA was positively correlated with the number of fourth-level shoots, followed by three species which had the first axis of the PCA positively correlated with biomass and ramification level. Finally, only two species had the first axis of the PCA positively correlated with height (Fig. 4.2). These results reinforce the role of fourth-level shoots as the best proxy for architectural traits leading to gall attack in Baccharis species, as this variable is a good indicator of plant meristem number (Espírito-Santo et al. 2007, 2012). Indeed, meristematic activity influences the plant’s availability of young tissue, which is key to gall development (see Weis et al. 1988; Rohfritsch 1992; Carneiro et al. 2017) and highly attractive even to free-feeding herbivores (Boege 2005; Silva et al. 2012). In our study, intersexual differences in the number of fourth-level shoots were found for five Baccharis species, but no clear trend was observed in the gall abundance for these species (Table 4.1). Considering all species together, the lack of sex-related differences in gall attack is consistent with the general architectural similarity among male and female individuals of Baccharis.
Galling insect attack in relation to host architectural traits (summarized by the scores of the first axes of principal component analysis – PCA) for 12 species of Baccharis. The curves were adjusted based on parameters estimated from the analysis of generalized linear models (n = 30). Arrow below the x axis indicates the variables that positively correlated with the PCA axis-1
3 Case Study 2. A Fine-Scale Study for Baccharis concinna
The sex-biased herbivory hypothesis has not been supported in any of the studies conducted on several species of Baccharis in tropical areas of Brazil (Box 4.1), although differential resource allocation may have been detected within the host plant (see Table 4.1). Therefore, we set out to test the differential resource allocation and sex-biased herbivory hypotheses at both a short- and a long-term sampling event on B. concinna to observe whether these would show consistent results. We included several measures of resource allocation such as shoot growth and total aboveground biomass, and we also sampled entire galling insect communities over 11 months. The hope was that we would be able to detect a clearer picture of how galling insect communities are impacted by the resource allocation patterns of this host plant. Baccharis concinna is a perennial shrub species with continuous production of very small flowers and growth meristems throughout the year. It harbors 15 species of gall-inducing insects that attack leaves, flowers, and stems as described by Fernandes et al. (1996, 2014).
3.1 Long-Term Study
In the long-term study , one population of Baccharis concinna occurring along Geraldinho creek at 1100 m in elevation at the Reserva Vellozia, in Minas Gerais, Brazil, was studied for 12 months. Forty individuals of each sex were haphazardly chosen and marked. Plant size was estimated by measuring plant height and two measures of crown width. We measured the growth of lateral shoots as they became available for colonization by gall-inducing insect species throughout the 11 months. Lateral shoots are here defined as shoots growing along a main stem and with determinate growth. To assess the lateral shoot growth rates of the 40 previously marked plants, 10 haphazardly chosen lateral shoots were marked with bird tags and labeled 1 through 10 (see Marques and Fernandes 2016).
Our findings revealed that male and female plants did not differ in plant height and were 1.15 m tall (mean female height = 115.25 ± 4.05 cm; mean male height = 114.9 ± 3.89 cm; P > 0.05). Nevertheless, when total crown area was considered (crown width*crown length), male plants had, on average, twice the crown area of female plants (mean male area = 6726.37 ± 628.4 cm2; mean female area = 3220.25 ± 335.13 cm2; P < 0.01), suggesting that male plants have a greater number of shorter branches when compared to female plants. Overall, monthly growth rates of lateral shoots were greater on male plants with a slow but steady increase from March (the end of the rainy season) throughout May and September (dry season) and showing a greater increase in November, December, and January (rainy season) (Fig. 4.3). Cumulative growth rates of lateral shoots varied between sex and months with a non-significant interaction term – plant sex and months (LN) (sex: F = 4.16, df = 1, P < 0.042; months: F = 498.12, df = 9, P < 0.01; sex*month: F = 2.82, df = 9, P < 0.01, N = 5175; Fig. 4.3). The lateral shoots marked in February of 1998 were 1 year old and on average 0.6 cm longer in male plants. By the end of the second growing season, lateral shoots in male plants were on average almost 2 cm longer than those in female plants. The differential resource allocation hypothesis that predicted male plants would allocate more resources towards growth was corroborated in this study.
Cumulative mean lateral shoot growth rates (average of 10 shoots per plant) on 40 female (solid line and circles) and 40 male plants (dashed line and squares) of Baccharis concinna, monthly measured between 1998 and 1999, at the Reserva Vellozia, in Minas Gerais, Brazil (standard error for all points was below 0.002 cm)
Sex-biased herbivory – The community of galling insects in this population over 11 months consisted of 11 species out of the 15 known species to be associated with Baccharis concinna (Fernandes et al. 1996). Only five of these species had abundances above the hundreds, and only four species were common throughout the study period (Table 4.4). Male plants accumulated 11 gall-inducing insect species, while female plants had 10 species over the 12 months of study. Otherwise, gall-inducing insect species accumulated at a faster rate on male plants as opposed to female plants. Male plants had accumulated all 11 species by August (after 6 months of sampling), while female plants had all 10 species by December (after 10 months). Four galling insect morphospecies (A, B, F, and G) were present and abundant year-round, while most other species were common in a few months or were rare (low abundances) throughout the study. Gall-inducing insect richness showed an initial peak in March (the end of the rainy season) through July (dry season), with a sharp decrease in November (beginning of the rainy season). The difference between galling insect species between male and female plants was driven by rare species, which took longer to be detected on female plants. Mean gall-inducing insect abundance on male and female plants showed a very similar seasonal pattern to that of insect richness. Mean galling insect abundance differed between months but not between plant sex (sex: F = 0.057, df = 1, P > 0.813; months: F = 5.5, df = 8, P < 0.01; sex*month: F = 1.88, df = 8, P > 0.064, N = 355). Similarly, cumulative galling insect abundance differed between months but not between plant sex (natural logarithm +1) (sex: F = 0.17, df = 1, N = 48, P > 0.17; months: F = 178.9, df = 8, N = 368, P < 0.01; sex*month: F = 0.83, df = 8, N = 368, P > 0.05). In some months male plants would have a greater abundance of galling insects associated, while the opposite pattern was seen in other months where female plants had a greater abundance of galling insects. A few abundant species directed the patterns observed (Table 4.4). The only clear pattern observed was the seasonal distribution of gall-inducing insects on B. concinna. Gall-inducing insect species richness and abundance were higher in the drier months decreasing towards the rainy season. Therefore, the sex-biased herbivory hypothesis was not supported in this study.
3.2 Short-Term Study
We performed a more precise evaluation of intersexual differences in resource allocation by assessing the biomass of resources and shoot length on several populations of B. concinna . We further tested the sex-biased herbivory hypothesis by recording the galling insect species richness and abundance on the entire plant in one destructive sampling event at several locations and in the same region where the long-term study was conducted. All mature plants from both sexes were collected from one population at each of seven dry sites. The numbers of male and female plants collected at each site were I (28 M and 31 F), II (25 M and 35 F), III (18 M and 29 F), IV (24 M and 25 F), V (25 M and 25 F), VI (24 M and 25 F), and VII (22 M and 26 F), totalling 362 plants. Plants were cut at ground level and frozen at −10 °C until the analyses were performed. Plant sex was determined by analyzing the external morphology of flowers, which are always present in mature plants. To evaluate resource allocation to growth between plants of different sexes, three parameters were measured: (i) the current year’s growth of lateral shoots (cm), (ii) total plant dry biomass (g), and (iii) proportion of soft tissues (leaves and flowers) and hard tissues (stems) (%). Current year’s growth was evaluated by measuring the green portion of ten haphazardly chosen lateral shoots per plant. The number and abundance of new (green) as well as old (brown) galls per plant were counted under stereoscopic microscopes in the laboratory and were identified following Fernandes et al. (1996).
The data on plant biomass, shoot length, richness, and abundance of galling insects were log-transformed to meet the assumptions of normality of the tests utilized (Sokal and Rohlf 2012). Statistical analyses were conducted in two stages. First, two-way analyses of variance (ANOVAs) were used to check for differences in current year’s growth of lateral shoots and plant biomass between male and female plants. Second, multiple linear regressions were used to test the relationship between plant biomass, shoot length, plant sex, and galling species richness. Multiple linear regressions with backward stepwise procedure were also used to test the relationship between the variables mentioned above and the abundance of insect galls on B. concinna. The model included the variables site, sex, shoot length, biomass, and respective interactions on the richness and abundance of galling insect species.
Differential resource allocation for lateral shoot length varied between plant sex and sites with a significant interaction between site and plant sex (Table 4.5). Male plants produced longer lateral shoots when compared to female plants at three different sites, but at the other three sites no differences in shoot length were evident (Tables 4.6 and 4.7). Total plant biomass did not differ between plant sexes in B. concinna, but there were differences in plant biomass between sites (Tables 4.5 and 4.7). Similar results were observed when the resources were broken down into leaves and flowers and stems. No differences were observed on leaf and flower (soft tissues) and stem (woody tissue) dry biomass between male and female plants, while differences were evident between sites. The significance of the interaction terms suggests a sex-by-site interaction (leaf and flower site: F = 15.11, df = 1, n = 359, P < 0.05; sex: F = 2.44, df = 1, n = 359, P > 0.119; site*sex: F = 2.22, df = 1, n = 359, P < 0.05) (site*stem: F = 11.85, df = 1, n = 357, P < 0.05; sex: F = 1.861, df = 1, n = 357, P > 0.18; site*sex: F = 2.92, df = 1, n = 357, P < 0.05).
Resource allocation and plant growth varied between sites suggesting that B. concinna is strongly affected by environmental conditions. This becomes evident due to the significant interaction between site and sex suggesting a sex-by-site interaction where male plants growing in certain sites – maybe less stressful environmental conditions – would be able to produce longer shoots. Although all sites were dry and contained the same soil types, it is possible that soil conditions could differ between sites (see Marques et al. 2002). The differential growth of lateral shoots observed on male plants did not result in greater biomass of leaves and flowers or stems on male plants (data not shown). Since resource allocation for growth did not differ between the sexes, the differential resource allocation hypothesis was not corroborated in this study.
Sex-biased herbivory hypothesis was not corroborated in this study when the totality of all galling insect species was considered. A total of 11 galling insect morphospecies were found associated with B. concinna in this study. Galling morphospecies B, D, H, K, and L attacked new stems, whereas the morphospecies A, C, F, G, and I attacked apical buds; morphospecies E and N attacked leaves, and morphospecies M attacked flowers (Table 4.6). Galling insect richness differed between sites, but not between male and female plants (Table 4.7). Also, galling insect richness was not evenly distributed across sites. A total of 9 morphospecies were found at sites I and II, 6 morphospecies at site III, 8 morphospecies at site IV, 3 morphospecies at site V, 11 morphospecies at site VI, and 10 morphospecies at site VII (Table 4.6). Three morphospecies were common to all seven sites, three morphospecies occurred at six sites, and the five remaining morphospecies were rare (Table 4.6). Common galling morphospecies, those that occurred at more sites, were also more abundant. Mean gall-inducing insect richness differed between sites but not between plant sexes, with a non-significant interaction term – plant sex and sites (Tables 4.7 and 4.8). Male and female plants of B. concinna showed similar richness of galling insect species although richness varied between sites. Galling insect abundance differed between sites and plant sexes (Tables 4.6 and 4.7). Female plants supported greater galling insect abundance at site II, while male plants supported greater galling insect abundance at sites III and VI (Tables 4.6 and 4.7). Shoot length did not show a clear pattern with galling insect species abundance (Table 4.8), and neither did plant total biomass and species richness (Table 4.9).
In conclusion, lateral shoot length varied between plant sex and sites with a significant interaction between site and plant sex. Male plants produced longer lateral shoots when compared to female plants at three different sites, but at the other three sites no differences in shoot length were evident (Tables 4.7 and 4.8). Total plant biomass did not differ between plant sexes, but there were differences in plant biomass between sites (Tables 4.7, 4.8, and 4.9).
Resource allocation in B. concinna has been studied by measures of size and biomass for the entire plant (this study) and for apical and lateral shoots (Marques 1997; Madeira et al. 1997; Carneiro et al. 2005, 2006). Although in general no differential resource allocation pattern was seen for most of the resources studied in B. concinna, male plants had on average longer lateral shoots at some of the populations studied when compared to female plants (short-term study). This suggests the existence of architectural differences between male and female plants, where male plants probably compensate for the shorter apical shoots by investing on longer lateral shoots as shown in this study and that of Madeira et al. (1997). Longer apical shoots in female plants and lateral shoots in male plants did not translate into greater total plant, stem, or leaf biomass on plants of different sexes at these sites, as would be expected if any sex were allocating more resources into growth. Nevertheless, the end result is that there is no difference in leaf, stem, or total plant biomass between male and female plants as shown by this study.
In the long-term study involving one site, we found evidence to suggest that male plants of B. concinna had greater average lateral shoot length over 11 months. But these findings again suggest that plants at different sites reflect different growth patterns. The long-term study of gall-inducing insect richness and abundance between male and female plants differed only in 3 months but with no clear trends (Table 4.4). Galling richness was greater on female plants in March but greater on male plants in May and August. Although male plants accumulated morphospecies at a faster rate when compared with female plants, the most abundant morphospecies were present on both plant sexes, and the rare species, with very few occurrences, were responsible for driving this pattern. Gall-inducing insect abundance was greater on male plants only for the month of August with no difference observed between sexes for the remaining 10 months.
Nevertheless, an equivalent short-term sampling study conducted in the second case study across seven sites and which assessed total plant biomass in B. concinna did not detect any intersexual differences in this species but reflected differences in plant biomass at different sites. When plant biomass was broken down into its separate components of soft tissues and hard tissues, still no difference was detected between the host plant sexes. These findings further support other studies conducted by our laboratory which did not detect differential resource allocation in Baccharis. Delph et al. (1993) did not detect differential allocation of resources for growth or reproduction in Carex picta (Cyperaceae) and argued that the energetic cost of reproduction did not differ between the sexes because C. picta had dry, energetically inexpensive fruits much like B. concinna.
Plant quality in this tropical host species might also be affected by the extremely nutrient-poor soils of the rupestrian grasslands, at Serra do Cipó (Marques 1997), suggesting the existence of sex-by-site interaction where under such stressful environmental conditions male and female plants will perform differently (Boecklen and Hoffman 1993). For example, we know that male plants of B. concinna are more susceptible to aluminum in acidic soil conditions, such as those common to soils of rupestrian grasslands, when compared to female plants (Marques 1997).
4 Concluding Remarks
The two case studies outlined in this chapter provide a broad picture of all aspects of resource allocation towards growth over 12 species of Baccharis, over several sites, and a closer look at plant growth in B. concinna over 11 months. The first case study detailed the single sampling event of 12 species of Baccharis in Brazil, with no clear pattern of differential resource allocation being detected. When lateral shoot growth was considered in B. concinna in the second case study, the single destructive sampling event at seven sites revealed that male plants produced longer lateral shoots at three different sites, but at three other sites no differences were observed. In the long-term study involving one site, we found evidence to suggest that male plants of B. concinna had greater average lateral shoot length over 11 months. But these findings again suggest that plants at different sites reflect different growth patterns. These findings further support other studies conducted by our laboratory which did not detect differential resource allocation in Baccharis (Table 4.2).
The lack of consistency in differential resource allocation patterns suggests that resource allocation in Baccharis varies greatly with abiotic conditions including soil type, aluminum content, pH levels, precipitation, and temperature (Marques et al. 2002). Baccharis species are pioneer and colonizing plant species known to grow in disturbed habitats such as the sites of roads and clearings (Marques 1997). Their male and female flowers are abundant throughout the year in tropical species but are similar in size and small suggesting very similar energetic investment; their dry fruits suggest that female plants do not allocate much energy to the fruit and there is no investment in attracting dispersers because seeds are wind-dispersed.
In spite of the many studies done on the differential allocation of resources and resulting species distribution here reviewed, the understanding of the evolutionary ecology of Baccharis is still rudimentary. There is an enormous gap in the knowledge on Baccharis phenological trends, ecological niche, population, and reproductive ecology and genetics, which altogether builds the basis to understand its relationship with the associated fauna and biodiversity. Since male and female plants allocated most resources equally, we did not expect to find differences in the richness and abundance of the galling species associated with Baccharis species. Indeed, we conducted meta-analyses to determine the occurrence of sex-biased herbivory in general dioecious systems and for the genus Baccharis in particular (Box 4.1). Unlike the general pattern of higher herbivory on male plants (Boecklen et al. 1990; Åhman 1997; Cornelissen and Stiling 2005; Rivkin et al. 2018), no intersexual differences were found in Baccharis.
We selected to study gall-inducing insects because they utilize meristematic tissues that are still growing and differentiating (Mani 1964). That was indeed the case for most studies conducted by our group (Table 4.1), with one exception (Carneiro et al. 2005). However, these studies did not corroborate the differential resource allocation and the plant sex-biased herbivory hypotheses. We suggest that both sexes of these species, which grow slowly and produce vegetative and reproductive meristems continuously throughout the year, might be better chemically defended against herbivores when compared to temperate plants that experience one flush of growth in a year (see Herms and Mattson 1992; Sagers and Coley 1995). The second point was raised by Boecklen et al. (1994), who inquired if these studies were actually measuring the resources used by the insects or if, in reality, the resources utilized by these species were not sexually dimorphic. From the perspective of galling insects, it would be more realistic if studies measured the allocation of the resources which are actually utilized by the insects.
Furthermore, true patterns in nature should become stronger when more populations of the host plant are studied. That did not seem to be the case in this study, since as more populations were studied the less clear the patterns became. As seen, patterns of abundance could be attributed to one sex if only a few populations were studied, but by adding more populations the pattern became weaker. In addition, we must be very careful when selecting the scale (apical meristems, lateral meristems, or whole plant) used to search for patterns in galling species diversity/density for they can address different adaptive strategies of both host plants and galling insects. To further clarify the relationship between galling species richness, abundance, and plant sex in this system, future studies should consider the points mentioned above as well as measures of availability of resources throughout the year as meristems become available and galls colonize their host plants.
Herbivores are affected by multiple top-down and bottom-up forces that vary both spatially and temporally. As such, detecting general patterns through field studies in complex tropical environments is a quite complicated task. In the case of Baccharis, our results indicate that galling insects do not select for plant sexes, but long-term studies under controlled environmental conditions are necessary to confirm such a pattern. The genus Baccharis has the greatest number of galling insect species in any genus so far studied (Fernandes et al. 1996) and presents a great model to study these mainstream hypotheses on plant-animal interactions.
References
Ågreen J (1988) Sexual differences in biomass and nutrient allocation in the dioecious Rubus chamemorus. Ecology 69:962–973
Ågren J (1987) Intersexual differences in phenology and damage by herbivores and pathogens in dioecious Rubus chamaemorus L. Oecologia 72:161–169
Åhman I (1997) Growth, herbivory, and disease in relation to gender in Salix viminalis L. Oecologia 111:61–68
Araújo APA, Carneiro MAA, Fernandes GW (2003) Efeitos do sexo, do vigor e do tamanho da planta hospedeira sobre a distribuição de insetos indutores de galhas em Baccharis pseudomyriocephala Teodoro (Asteraceae). Rev Bras Entomol 47:483–490
Araújo APA, De Paula JD, Carneiro MAA, Schoereder JH (2006) Effects of host plant architecture on colonization by galling insects. Austral Ecol 31:343–348
Arnold SJ, Wade MJ (1984) On the measurement of natural and sexual selection: theory. Evolution 38:709–719
Ashman TL (2002) The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology 83:1175–1184
Avila-Sakar G, Romanow CA (2012) Divergence in defence against herbivores between males and females of dioecious plant species. Int J Evol Biol 12. ID 897157. https://doi.org/10.1155/2012/897157
Barrett SCH (2002) The evolution of plant sexual diversity. Nature 3:274–284
Barrett SCH, Hough J (2013) Sexual dimorphism in flowering plants. J Exp Bot 64:67–82. https://doi.org/10.1093/jxb/ers308
Bawa KS (1980) Evolution of dioecy in flowering plants. Annu Rev Ecol Syst 11:15–39
Boecklen WJ, Hoffman MT (1993) Sex-biased herbivory in Ephedra trifurca: the importance of sex-by-environment interactions. Oecologia 96:49–55
Boecklen WJ, Price PW, Mopper S (1990) Sex drugs and herbivores: sex-biased herbivory in arroyo willow (Salix lasiolepis). Ecology 71:581–588
Boecklen WJ, Mopper S, Price PW (1994) Sex-biased herbivory in arroyo willow: are there general patterns among herbivores? Oikos 71:267–272
Boege K (2005) Herbivore attack in Casearia nitida influenced by plant ontogenetic variation in foliage quality and plant architecture. Oecologia 143:117–125
Carneiro MAA, Fernandes GW, De Souza OFF (2005) Convergence in the variation of local and regional galling species richness. Neotrop Entomol 34:547–553
Carneiro MAA, Fernandes GW, De Souza OFF, Souza WVM (2006) Sex-mediated herbivory by galling insects on Baccharis concinna (Asteraceae). Rev Bras Entomol 50:394–398
Carneiro RGS, Isaias RMS, Moreira ASFP, Oliveira DC (2017) Reacquisition of new meristematic sites determines the development of a new organ, the cecidomyiidae gall on Copaifera langsdorffii Desf. (Fabaceae). Front Plant Sci 8:1622. https://doi.org/10.3389/fpls.2017.01622
Charlesworth D (2018) Does sexual dimorphism in plants promote sex chromosome evolution? Environ Exp Bot 146:5–12. https://doi.org/10.1016/j.envexpbot.2017.11.005
Cornelissen TG, Fernandes GW (2001) Defence, growth and nutrient allocation in the tropical shrub Bauhinia brevipes (Leguminosae). Austral Ecol 26:246–253
Cornelissen T, Stiling P (2005) Sex-biased herbivory: a meta-analysis of the effects of gender on plant-herbivore interactions. Oikos 111:488–500
Darwin C (1871) The descent of man, and selection in relation to sex. Murray, London
Delph LF (2019) Pollen competition is the mechanism underlying a variety of evolutionary phenomena in dioecious plants. New Phytol 224:1075–1079. https://doi.org/10.1111/nph.15868
Delph LF, Lu Y, Jayne LD (1993) Patterns of resource allocation in a dioecious Carex (Cyperaceae). Am J Bot 80:607–615
Emlen ST, Oring LW (1977) Ecology, sexual selection and the evolution of mating systems. Science 197:215–223
Erb M (2018) Plant defenses against herbivory: closing the fitness gap. Trends Plant Sci 23:187–194. https://doi.org/10.1016/j.tplants.2017.11.005
Espírito-Santo MM, Fernandes GW (1998) Abundance of Neopelma baccharidis (Homoptera:Psyllidae) galls on the dioecious shrub Baccharis dracunculifolia (Asteraceae). Environ Entomol 27:870–876
Espírito-Santo MM, Fernandes GW, Allain LR, Reis TRF (1999) Tannins in Baccharis dracunculifolia: effects of seasonality, water availability and plant sex. Acta Bot Bras 13:167–174
Espírito-Santo MM, Madeira BG, Neves FS, Faria ML, Fagundes M, Fernades GW (2003) Sexual differences in reproductive phenology and their consequences for the demography of Baccharis dracunculifolia (Asteraceae), a dioecious tropical shrub. Ann Bot 91:13–19
Espírito-Santo MM, Neves FS, Andrade-Neto FR, Fernandes GW (2007) Plant architecture and meristem dynamics as the mechanisms determining the diversity of gall-inducing insects. Oecologia 153:353–364
Espírito-Santo MM, Neves FS, Fernandes GW, Silva JO (2012) Plant phenology and absence of sex-biased gall attack on three species of Baccharis. PLoS One 7(10):e46896. https://doi.org/10.1371/journal.pone.0046896
Faria ML, Fernandes GW (2001) Vigour of a dioecious shrub and attack by a galling herbivore. Ecol Entomol 26:36–45
Fernandes GW, Carneiro MAA, Lara ACF, Allain LR, Julião GR, Reis TR, Andrade GI, Silva IM (1996) Galling insect on neotropical species of Baccharis (Asteraceae). Trop Zool 9:315–332
Fernandes GW, Silva JO, Espírito-Santo MM, Fagundes M, Oki Y, Carneiro MMA (2014) Baccharis: a Neotropical model system to study insect plant interactions. In: Fernandes GW, Santos JC (eds) Neotropical insect galls. Springer, Dordrecht, pp 193–220
Harris MS, Pannell JR (2008) Roots, shoots and reproduction: sexual dimorphism in size and costs of reproductive allocation in an annual herb. Proc R Soc B Biol Sci B 275:2595–2602. https://doi.org/10.1098/rspb.2008.0585
Heiden G, Antonelli A, Pirani JR (2019) A novel phylogenetic infrageneric classification of Baccharis (Asteraceae: Astereae), a highly diversified American genus. Taxon 68:1048–1081. https://doi.org/10.1002/tax.12128
Hemborg AM, Karlsson PS (1999) Sexual differences in biomass and nutrient allocation of first-year Silene dioica plants. Oecologia 118:453–460
Henry IM, Akagi T, Tao R, Comai L (2018) One hundred ways to invent the sexes: theoretical and observed paths to dioecy in plants. Annu Rev Plant Biol 69:553–575
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or to defend. Q Rev Biol 67:283–335
Imaji A, Seiwa K (2010) Carbon allocation to defense, storage and growth in seedlings of two temperate broad-leaved tree species. Oecologia 162:361–369
Jaenicke T, Morrow EH (2018) Operational sex ratio predicts the opportunity and direction of sexual selection across animals. Ecol Lett 23:384–391
Jing SW, Coley PD (1990) Dieocy and herbivory: the effect of growth rate on plant defense in Acer negundo. Oikos 58:369–377
Knauer AC, Schiesti FP (2017) The effect of pollinators and herbivores on selection for floral signals: a case study in Brassica rapa. Evol Ecol 31:285–304. https://doi.org/10.1007/s10682-016-9878-8
Krischik VA, Denno RF (1990) Patterns of growth, reproduction, defense, and herbivory in the dioecious shrub Baccharis halimifolia (Compositae). Oecologia 83:182–190
Leroy CJ, Hobbs JMR, Claeson SM, Moffett J, Garthwaite I, Criss N, Walker L (2020) Plant sex influences aquatic-terrestiral interactions. Ecosphere 11:1–10. https://doi.org/10.1002/ecs2.2994
Lovett-Doust J, O’Brien G, Doust LL (1987) Effect of density on secondary sex characteristics and sex ratio in Silene alba (Caryophyllaceae). Am J Bot 74:40–46
Loyd DG, Webb CJ (1977) Secondary sex characters in plants. Bot Rev 43:177–216
Madeira BG, Cornelissen TG, Faria ML, Fernandes GW (1997) Insect herbivore preference for plant sex and modules in Baccharis concinna, Barroso (Asteraceae). In: Raman A (ed) Ecology and evolution of plant-feeding insects in natural and man-made environments. International Scientific Publications, New Delhi, pp 135–143
Mani MS (1964) The ecology of plant galls. Junk, The Hague
Marques AR (1997) Sexo, distribuição espacial e alocação de recursos em Baccharis concinna, uma espécie dióica e endêmica do Espinhaço. MSc Dissertation Universidade Federal de Minas Gerais. Belo Horizonte-MG, Brazil
Marques ESA, Fernandes GW (2016) The gall inducing insect community on Baccharis concinna (Asteraceae): the role of shoot growth rates and seasonal variations. Lundiana 12:17–26
Marques AR, Fernandes GW, Reis IA, Assunção RM (2002) Distribution of adult male and female Baccharis concinna (Asteraceae) in the rupestrian fields of Serra do Cipó, Brasil. Plant Biol 4:94–103
Moreira X, Romero-Perez A, Luna-Chaparro E, Orona-Tamayo D, Quintana-Rodirguez E, Reyes-Chilpa R, Abdala-Roberts L, Cano-Santana Z, Hernandez-Cumplido J (2019) Effects of plant sex on insect abundance across three trophic levels in the perennial shrub Buddleja cordata. Entomol Exp Appl 167:950–956. https://doi.org/10.1111/eea.12845
Moritz KK, Bjorkman C, Parachnowitsch AL, Stenberg JA (2017) Plant sex effects on insect herbivores and biological control in a Short Rotation Coppice willow. Biol Control 115:30–36
Multikainen P, Delph LF (1996) Effects of herbivory on male reproductive success in plants. Oikos 75:353–358
Obeso JR (2002) The cost of reproduction in plants. New Phytol 155:321–348. https://doi.org/10.1046/j.1469-8137.2000.00571.x
Pearson JT, Sparrow AD, Lange RT (1990) Prolonged exposure to sheep grazing reduces palatability on Australian saltbush populations. Aust J Ecol 35:337–344
Pfeiffer T, Schmidt L, Roschanski AM, Schnittler M (2019) A battle of the sexes? Sex ratio and performance at small scales in dioecious Mercurialis perennis. Acta Oecol 100:103462. https://doi.org/10.1016/j.actao.2019.103462
Popp JW, Reinartz IA (1988) Sexual dimorphism in biomass allocation and clonal growth of Xanthoxylum americanum. Am J Bot 75:1732–1741
Ribeiro-Mendes HN, Marques ESA, Silva IM, Fernandes GW (2002) Influence of host-plant sex and habitat on survivorship of insect galls within the geographical range of the host plant. Trop Zool 15:5–15
Rivkin LR, Barrett SCH, Johnson MTJ (2018) Effects of plant sexual system and latitude on resistance to herbivores. Am J Bot 105:977–985. https://doi.org/10.1002/ajb2.1098
Rohfritsch O (1992) Patterns in gall development. In: Shorthouse JD, Rohfritsch O (eds) Biology of insect-induced galls. Oxford University Press, Oxford, pp 60–86
Sagers CL, Coley PD (1995) Benefits and costs of defense in a Neotropical shrub. Ecology 76:1835–1843
Silva JO, Espírito-Santo MM, Melo GA (2012) Herbivory on Handroanthus ochraceus (Bignoniaceae) along a successional gradient in a tropical dry forest. Arthropod-Plant Interact 6:45–57
Sokal RR, Rohlf FJ (2012) Biometry: the principles and practice of statistics in biological research. Freeman, New York
Stanton ML, Snow AA, Handel SN (1986) Floral evolution: attractiveness to pollinator increases male fitness. Science 232:1625–1627
Sutherland S, Delph LF (1984) On the importance of male fitness in plants: patterns of fruit set. Ecology 65:1093–1104
Vaughton G, Ramsey M (1998) Floral display, pollinator visitation and reproductive success in the dioecious perennial herb Wurmbea dioica (Liliaceae). Oecologia 115:93–101
Vega-Frutis R, Munguía-Rosas MA, Varga S, Kytöviita MM (2013) Sex-specific patterns of antagonistic and mutualistic biotic interactions in dioecious and gynodioecious plants. Perspect Plant Ecol Evol Syst 15:45–55
Wallace CS, Rundel PW (1979) Sexual dimorphism and resource allocation in male and female shrubs of Simondsia chinensis. Oecologia 44:34–39
Wallace BC, Lajeunesse MJ, Dietz G, Dahabreh IJ, Trikalinos TA, Schmid CH, Gurevitch J (2017) OpenMEE: intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol Evol 8:941–947
Weis AE, Walton R, Greco CL (1988) Reactive plant tissue sites and the population biology of gall makers. Annu Rev Entomol 33:467–486
Wolfe LM (1997) Differential flower herbivory and gall formation on males and females of Neea psychotrioides, a dioecious tree. Biotropica 29:169–174
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Frazier, E.M., Silva, J.O., Espírito-Santo, M.M., Cornelissen, T., Fernandes, G.W. (2021). Intersexual Differences in Demography, Resource Investment, and Herbivory in Baccharis. In: Fernandes, G.W., Oki, Y., Barbosa, M. (eds) Baccharis. Springer, Cham. https://doi.org/10.1007/978-3-030-83511-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-83511-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83510-1
Online ISBN: 978-3-030-83511-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)