Abstract
Plants belonging to Baccharis genus (Asteraceae) have been used in folk medicine since ancient times. Usually, different Baccharis species are used in folk medicine as infusion or tea for gastrointestinal diseases, inflammation, ulcers, as an analgesic, spasmolytic, and antimicrobial, among others. Examples of medicinal plants from Baccharis are B. dracunculifolia D.C., B. illinita D.C., and B. trimera (Less.) DC., among many others. Over the years, these plants have been more studied: both chemical composition and biological activities. There are approximately 500 Baccharis species spread across the American continent, especially in South and Central America, which are important sources of bioactive compounds. The chemistry of these plants is characterized mainly by the presence of monoterpenes and sesquiterpenes in their essential oils. The nonvolatile fraction is characterized by diterpenes, triterpenes, and phenolic compounds, among others. Phenolic compounds are represented by phenylpropanoids, prenylated phenylpropanoids, flavonoids, flavonoid glycosides, coumarins, and simple phenolic compounds. In Baccharis spp. luteolin, chlorogenic acid, apigenin, acacetin, quercetin, kaempferol, p-coumaric acid derivatives, and coumarins have also been found. Many Baccharis spp. crude extracts and some of their isolated compounds were correlated with several biological activities. One example is the antioxidant effect of Brazilian Green Propolis, which is composed mainly of B. dracunculifolia compounds, such as flavonoid aglycones and p-coumaric acid derivatives, like artepillin C, baccharin, and drupanin. Baccharis spp. extracts display trypanocidal, antimicrobial, and anti-inflammatory activities, corroborating many folk medicinal uses. Therefore, in this chapter, an overview of the chemical composition is presented, highlighting the phenolic compounds of Baccharis spp., as well as its ethnopharmacological uses, in the light of many published scientific studies, focusing on the corroboration of folk uses. Furthermore, the toxicity of Baccharis species is discussed, which is a very important issue that is not well discussed in folk medicine: B. coridifolia, for example, is a poisonous plant responsible for necrosis of gastrointestinal tissue of rabbits and horses. Also, the chromatographic analyses of these plant extracts are addressed due to the importance of their chemical composition, the content of active compounds, and certainty of the correct botanical identification. In the final part, we conclude and discuss future perspectives for Baccharis extracts and their isolated compounds in the development of efficacious and safe medicines.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Ethnopharmacology of Baccharis spp.
Baccharis is one of the largest genus belonging to the Asteraceae family (formerly known as Compositae), which is formed mainly by shrubs (Verdi et al. 2005). According to The Plant List database (theplantlist.org), there are more than 440 accepted Baccharis species names and several of them have synonyms. Furthermore, there are more than 100 Baccharis species with unresolved names. Plants from this genus can be found especially in South American countries, such as Argentina, Bolivia, Brazil, and Colombia. Plant heights vary from 0.5 to 4.0 meters high. Most of these species, around 120, are found in Brazil, especially in the South and Southeast regions, in the states of Santa Catarina, Paraná, São Paulo, and Rio Grande do Sul. In Brazil, they are known by the population under the names “carqueja,” “vassoura,” “vassourinha,” “alecrim,” and “vassourinha do campo” (Verdi et al. 2005; Abad and Bermejo 2006).
From the numerous Baccharis species already identified, many of them have been reported in folk medicine for their medicinal properties, such as Baccharis anomala, which is used in the treatment of general infections and as a wound-washing agent (De Souza et al. 2004). These plants are widely used in folk medicine to treat gastrointestinal and liver disorders, anemia, inflammation, infections, and diabetes. Most of the time, they are consumed as an infusion or decoction of the plant (Abad and Bermejo 2006). Some selected Baccharis species and their ethnopharmacological uses are summarized in Table 12.1.
On the one hand, these plants are popularly used for treating several types of pathologies and many of these biological effects have been proved and reported in the scientific literature. They can also be used as ornamental plants, as hedges and to avoid erosion. Some bees are attracted to Baccharis spp. and from collecting their resins and nectar, the bees produce high-quality honey and propolis, which are important to the apicultural economy. The essential oil of Baccharis spp. is used in the cosmetic industry. On the other hand, some species are invasive and others biosynthesize toxic metabolites, like trichothecenes, which can cause dizziness, tremors, diarrhea, and even death to cattle, for example. The Baccharis species that produce these metabolites are generally toxic to human beings too. (Martinez et al. 2005; Verdi et al. 2005). Therefore, the use of these plants in folk medicine should be done carefully, considering that according to their chemical composition, it may display a pharmacological desired effect, a toxic effect, or be innocuous.
2 Chemical Composition
2.1 Phenolic Compounds
In Baccharis spp. different classes of secondary metabolites can be identified, and among them, phenolic compounds are present in high amounts (Abad and Bermejo 2006). Compounds belonging to this class can also be found in several plant species and as a matter of fact, it is widespread throughout the plant kingdom. These phytochemicals are biosynthesized in plants by the shikimate pathway and are undoubtedly important to plant development, contributing toward the defense against herbivore insects and pathogens, as well by giving color and scent (Balasundram et al. 2006). Considering that phenolic compounds display important biological effects, like gastroprotective, anti-inflammatory, antioxidant, antimicrobial, and antiparasitic effects (Arruda et al. 2017; Berretta et al. 2017; Costa et al. 2018; Ribeiro et al. 2018), in the past few years, several studies were performed aiming to discover promising compounds for treating many diseases that the available medicines are not satisfactory. Examples of these diseases are the ones known as “neglected diseases,” such as leishmaniasis, Chagas, malaria, and schistosomiasis. These diseases are usually caused by parasites or infectious agents, and most pharmaceutical companies do not support the development of novel medicines for their treatment. Therefore, the discovery of new compounds more effective and less toxic to treat these parasitic diseases is very important (Abad and Bermejo 2006; Grecco et al. 2010b; de Oliveira et al. 2012a, b, 2014). Due to their antioxidant activities, phenolic compounds have been correlated to the health effects of eating fruits and vegetables, as well as functional foods and beverages, whose consumption has increased in the last few years (Berretta et al. 2017).
Chemically, these compounds bear hydroxyl group(s) attached to a benzene ring, as a simple phenolic compound or phenolic polymers. Other phenols isolated from plants are the ones bearing one or more sugar moieties, along with esters and methyl ester derivatives. Some classes of phenolic compounds naturally occurring in plants are simple phenolics, quinones, hydroxybenzoic acids, phenylpropanoids, xanthones, stilbenes, lignans, lignins, tannins and flavonoids: flavonoids, tannins, and simple phenolic compounds are the most abundant in plants. Their chemical differences are in how many phenyl groups are present and the carbon side chain; for example, phenylpropanoids have an aromatic ring with a three-carbon side chain. The hydroxybenzoic acid derivatives have the aromatic ring attached to one-carbon side chain. Another class of phenolics are flavonoids, which have a mixed biosynthetic pathway and are formed by a C6-C3-C6 unit. Flavonoids can be found in most plant species and are subdivided into subclasses, depending on the ring C substitution: flavonols, flavones, flavanols, flavanones, isoflavones, flavanonols, and anthocyanidins (Balasundram et al. 2006).
Regarding Baccharis spp. phenolic compounds, several flavonoids, prenylated phenolics, simple phenolics, and phenolic compounds attached to sugar moieties can be found: B. dracunculifolia is probably the Baccharis species most studied from chemical and pharmacological point of view, and some of its major phenolic compounds are the prenylated phenolics baccharin, artepillin C, and drupanin (Lemos et al. 2007; De Sousa et al. 2011; Costa et al. 2018). Kupchan et al. (1976) described baccharin as a trichothecene triepoxide isolated from B. megapotamica, but more recently papers had named baccharin one of the prenylated phenolic compounds biosynthesized from p-coumaric acid (da Silva Filho et al. 2008; Cestari et al. 2011; De Sousa et al. 2011; Oliveira et al. 2011; Costa et al. 2018). Besides, p-coumaric, ferulic, trans-cinnamic, chlorogenic, and caffeic acids had also been identified in this plant species. The flavonoids, kaempferol, kaempferide, isosakuranetin, pinobanskin, chrysin, aromadendrin-4′-O-methyl ether, 11-hydroxy-10,11-dihydro-euparin, acacetin, and ermanin had also been detected in B. dracunculifolia extracts. Caffeoylquinic acids like 3,4-di-O-caffeoylquinic acid and 3,5-di-O-caffeoylquinic acid were reported, as well as the other phenolics, such as 6-hydroxy-tremetone, dihydrocumaric acid, 2,2-dimethyl-6-carboxyethenyl-2H-1-benzopyran acid, viscidone, protocatechuic acid, sinapic acid and (E)-3-(E)-3-hydroxy-3-methyl-1-butenyl-4-(2,3-dihydrocinnamoyloxy)-cinnamic acid (Abad and Bermejo 2006; Lemos et al. 2007; Nakajima et al. 2007; Barros et al. 2008; Chang et al. 2008; De Sousa et al. 2011; Costa et al. 2018).
Other well-studied Baccharis species is B. trimera, which also contains caffeoylquinic acids such as 5′-O-caffeoylquinic acid, 4-O-(E)- caffeoyl-1-methyl-quinic acid, 1′-5′-O-dicaffeoylquinic acid, 1,3-di-(E)-caffeoylquinic acid, 5-O-(E)-caffeoylquinic acid, 3,4-O-(E)-dicaffeoylquinic acid, 3,5-O-(E)-dicaffeoylquinic acid, 4,5-O-(E)-dicaffeoylquinic acid, and tricaffeoylquinic acid, along with the flavonoids eupafolin, hispidulin, quercetin, luteolin, and apigenin in its chemical composition (Aboy et al. 2012; Lívero et al. 2016a, b; de Araújo et al. 2017). Luteolin, acacetin, and quercetin, along with chlorogenic acid and 4′-O-β-D-gluco- pyranosyl-3′,5′-dimethoxybenzyl-caffeate, were found in B. articulata (Cariddi et al. 2012).
The phytochemical analyses of B. chilco, B. darwinii, and B. dentata revealed the presence of 5-O-[(E)-caffeoyl-quinic acid, 3,5-di-O-[(E)- caffeoyl]quinic acid and rosmarinic acid (Argoti et al. 2013), anisocoumarin, 7-geranyloxycoumarin and diversinin (Kurdelas et al. 2010), as well as caffeic acid, rutin, quercetin, apigenin, and kaempferol (Sartor et al. 2013), respectively. In B. genistelloides and B. illinita, several flavonoids have also been found, such as luteolin, identified in both species and eupatrin, cirsimaritin, cirsiliol, apigenin, genkwanim, eridictyol, hispidulin, quercetin, nepetin, rutin and eupatorin in B. genistelloides, and nobiletin, tangeretin, kaempferol, and naringenin in B. illinita. In the same way, only chlorogenic acid was reported in B. oxyodonta (Toyama et al. 2014).
B. pentladii, B. retusa, and B. spicata have revealed several flavonoids in their chemical composition: B. pentladii: 5,4′- dihydroxy-6,7,8,3′-tetramethoxyflavone, 8-Methoxycirsilineol, 5,4′-dihydroxy-6,7,8-trimethoxyflavone, xanthomicrol, 5,3′,4′-trihydroxy-6,7,8-trimethoxyflavone and sideritoflavone (Tarqui et al. 2012); B. retusa: sakuranetin, 5,6,7- trihydroxy-4′-methoxyflavanone and naringenin (Grecco et al. 2010b, 2012); and rutin in B. spicata (Agudelo et al. 2016). In B. retusa it was also found (7E, 18′Z)-hexacos- 18′-enyl coumarate and (7Z,18′Z)-hexacos-18′-enyl coumarate; and in B. spicata 3,5-dichlorogenic acid, 3,4-dichlorogenic acid, and 4,5-dichlorogenic acid (Agudelo et al. 2016; Ueno et al. 2018).
Regarding B. trinervis, five flavonoids were detected: rutin, luteolin, 5,7-Dihydroxy-6,4′-dimethoxyflavone, 5-Hydroxy-6,7,4′- trimethoxyflavone and 5,4′-dihydroxy-3,6,7-trimethoxyflavone, as well as caffeic, ellagic, and rosmarinic acids (Sharp et al. 2001; Jaramillo-García et al. 2018). Several compounds were identified in B. uncinella as well, including caffeic acid, ferulic acid, pectolinaringen, hispidulin, and dihydrooroxylin (Grecco et al. 2010a; Bocco et al. 2016). In B. incarum, chlorogenic acid, 3′,4′,5,7-tetrahydroxyflavone, dicaffeoyl quinic acid and 3′,4′,5,7-tetrahydroxy-3,6-dimethoxyflavone, 3′,4′,5,7-tetrahydroxy-3,6,8-trimethoxyflavone, 4′,5,7- trihydroxy-3′,3,6,8-tetramethoxyflavone, 4′,5-dihydroxy-3′,3,6,7,8-pentamethoxyflavone, chlorogenic acid, dicaffeoylquinic acid, and quercetin diglycoside were reported, as well (Zampini et al. 2009). The chemical compounds found in several Baccharis species and the biological activities displayed by these plant extracts are shown in Table 12.2.
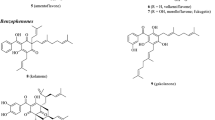
By comparing the phenolic compounds present in different Baccharis species, it is possible to observe that some compounds occur in several species, like chlorogenic acid, which is found in B. spicata, B. dracunculifolia, B. articulata, B. oxyodonta, and B. incarum. In the same way, luteolin and quercetin were identified in B. trimera, B. articulata, B. dentata, and B. genistelloides. Hispidulin, apigenin, and kaempferol are also described in different Baccharis species: hispidulin in B. uncinella, B. genistelloides, B. trimera and B. uncinella; apigenin in B. trimera, B. dentata, and B. genistelloides; and kaempferol in B. illinita, B. dracunculifolia, and B. articulata. Caffeic acid is reported in more than three Baccharis species, as well. These compounds, besides occurring in several Baccharis species, are widespread through the plant kingdom and are reported in high amounts, especially in edible plants. For example, chlorogenic acid, a hydroxycinnamic acid, is found in many sources, including coffee, apples, pears, berries, aubergines, etc. (Olthof et al. 2001). The flavonoids quercetin, kaempferol, luteolin, and apigenin are found in vegetables and fruits as well and, in most cases, are detected as a glycoside, although kaempferol, luteolin, and apigenin may be found as aglycones too (Miean and Mohamed 2001). Flavonoids and phenolic compounds usually display different biological effects, mainly antioxidants (De Oliveira et al. 2012a, b). Baccharis dracunculifolia also contains prenylated compounds, such as artepillin C, baccharin, and drupanin that are usually found in good amounts only in this plant species. Some of these compounds are important, because they are related to several biological effects, such as gastroprotective, anti-inflammatory, and cytotoxic against cancer cell lines (Lemos et al. 2007; Paulino et al. 2008; De Sousa et al. 2011; Szliszka et al. 2012; Costa et al. 2018). The chemical structures of these compounds, along with some flavonoids and other phenolics widespread in the Baccharis genus, are displayed in Fig. 12.1.
3 Biological Activities
In the past few decades, numerous scientific publications have reported the biological activities displayed by Baccharis spp., and several of their isolated phenolic compounds for the treatment of miscellaneous diseases. Examples are the anti-angiogenesis effect displayed by artepillin C (Ahn et al. 2007), the insecticidal by B. dracunculifolia ethanolic extract (Da Silva et al. 2017), hepatoprotective by B. dracunculifolia leaves extract (Rezende et al. 2014), B. trimera and some isolated phenolic compounds (Soicke and Leng-Peschlow 1987; Lívero et al. 2016a, b), the anti-emphysema by sakuranetin, a flavonoid isolated from B. retusa (Taguchi et al. 2015), and antiarthritic by B. genistelloides aqueous extract (Coelho et al. 2004). Another activity reported is the antivirus of B. trinervis aqueous extract (Palomino et al. 2002). Taking that into account, in this chapter, some of the important biological effects displayed by Baccharis spp. extracts and its isolated phenolic compounds are discussed. The biological effects and chemical composition of several Baccharis species are displayed in Table 12.2.
3.1 Gastroprotective
Several Baccharis species have been described in folk medicine regarding their effects in the treatment of gastrointestinal disorders, including ulcer. Therefore, many researchers have evaluated the pharmacological potential of plants belonging to this genus: Baggio et al. (2003) found that the stems and leaves ethanolic extracts and the roots and flowers aqueous extracts of B. illinita by the oral route were able to furnish gastroprotection for lesions caused by ethanol from 0.3 to 1.0 g/kg of body-weight. These authors also found that the hydroalcoholic extracts did not show toxic effects up to 6 g/kg of body weight, which indicates the pharmacological potential of this plant. Freitas et al. (2008), considering the previous report of the gastroprotective effect of B. illinita extracts and the importance of determining its mechanism of action, evaluated the possible pathways that B. illinita flowers chloroform extract could be acting. Their studies indicated that this effect occurs by decreasing the gastric secretion through inhibition of the H+/K+ ATPase, and consequently reducing the acid secretion. Besides this, the flavonoid luteolin, present in this extract, was able to act on H+/K+ ATPase, as well.
The hydroalcoholic extract of B. dracunculifolia , the botanical source of Brazilian green propolis, displays a significant gastroprotective activity too: from 50 to 500 mg/kg of body-weight, the extract decreased in a significant manner the gastric lesion in comparison with the negative control, by reducing the gastric juice volume and increasing the stomach pH. The major compounds found in this extract were caffeic acid, ferulic acid, p-coumaric acid, aromadendrin-4′-O-methyl-ether, isosakuranetin, baccharin, and artepillin C (Lemos et al. 2007). Therefore, this effect is probably due to the presence of these phenolic compounds in the extract. To prove this hypothesis, Barros et al. (2008) assessed the potential of caffeic, ferulic, p-coumaric, and cinnamic acids for ulcer treatment and in furnishing gastroprotection. They found that these compounds from 50 to 250 mg/kg were able to decrease the gastric lesion caused by NSAID, ethanol, and stress by diminishing the gastric juice and increasing the stomach pH, despite being less effective than the positive control omeprazole. On the other hand, these authors have demonstrated that up to 2000 mg/kg, these compounds were not toxic. Artepillin C, drupanin and the flavonoids aromadendrin-4′-O-methyl ether and kaempferide also display gastroprotective activity: at doses from 0.3 to 3 mg/kg, these phytochemicals displayed an antiulcer effect and were capable of preventing ulcer induced by ethanol/HCl or indomethacin. They promote this effect by different mechanisms of actions (Costa et al. 2018). These phenolic compounds showed a potent gastroprotective effect considering that at lower doses (0.3–3 mg/kg), they displayed the pharmacological effect, whereas the positive control (omeprazole and carbenoloxone) doses were considerably higher: 20 and 200 mg/kg, respectively. It shows that these compounds indeed contribute to the gastroprotective effect of B. dracunculifolia extracts. Therefore, these compounds and, B. dracunculifolia extracts and Brazilian green propolis present pharmacological potential for the possible development of novel phytomedicines and phytotherapeutic agents.
B. trimera has potential for the treatment of gastrointestinal disorders as well: its aqueous extract displays antacid and antiulcer effects at 1 and 2 g/kg by reducing the gastric volume and acid secretion, as well as it shows protection against lesion caused by restraint at 4 °C. It was found that the extract is composed of chlorogenic acid, flavonoids, and compounds from other secondary metabolite classes, such as ent-clerodane diterpene and a dilactonic neo-clerodane diterpene. The main mechanism of action of B. trimera extract is by modifying the cholinergic pathway (Biondo et al. 2011). The hydroalcoholic extract of this plant was also evaluated: when orally administered, it decreased the stomach lesion and oxidative stress, and yet stimulated the healing effect on chronic ulcer. It acts by inhibiting free radicals production and, therefore, lipid peroxidation too. This extract has flavonoids and caffeoylquinic acid derivatives in its chemical composition, which may be responsible for at least part of the curative and protective effect of ulcer lesions (Lívero et al. 2016a, b).
3.2 Cytotoxic
Considering the importance of cancer, several researchers have been assessing natural products against cancer cells aiming to discover novel drugs or plants that have cytotoxic potential without significant side effects: B. dracunculifolia hydroalcoholic extract displayed a GI50 value (50% of cells growth inhibition) of 5.5 μg/mL in human prostate’s primary malignant tumor cell lines. It also promoted a decrease in the cell viability of prostate’s metastasis cells, although higher doses were necessary to achieve optimum efficacy. This extract displays this effect probably by affecting the cell’s S phase arrest, and by regulating the expression of cyclins D1, CDK4, and B1 (Li et al. 2007). Artepillin C, one of the major compounds from B. dracunculifolia, possibly contributes to the cytotoxicity of this plant against cancer prostate cells and is capable of sensitizing the tumor necrosis factor–related apoptosis-inducing ligand, an important pathway of apoptosis in cancer cells (Szliszka et al. 2012).
Therefore, considering that promising extracts are the ones that show IC50 values (or ED50/GI50) lower than 20 μg/mL (Vijayarathna and Sasidharan 2012), B. dracunculifolia extract should be further studied for the development of new phytotherapeutic medicines to treat cancer. Furthermore, Baccharis’ phenolic compounds, such as artepillin C, display anticancer potential as well.
3.3 Antiparasitic
In an attempt to overcome the problem of low investments of the pharmaceutical industry in the development of innovative drugs to treat neglected diseases, like the ones caused by parasites, several researchers, especially from universities, have evaluated the potential of plants against many parasites. Da Silva Filho et al. (2009) found that B. dracunculifolia dichloromethanic extract displays IC50 values of 45 μg/mL against Leishmania donovani and approximately 20 μg/mL against Plasmodium falciparum. B. trimera showed significant in vivo and in vitro effects against the juvenile and adult Schistosoma mansoni worms: dichloromethane and aqueous fraction at 130 μg/mL inhibited 100% of the female’s oviposition and induced the death of S. mansoni worms by many morphological changes. In vivo, the samples at 40 mg/kg decreased by 75% (aqueous fraction) and 68% (dichloromethane fraction) the juvenile female worms, and by almost 100% the eggs in the feces. Studies like this are relevant due to the resistance, the numerous side effects, and low efficacy against the juvenile Schistosoma spp. of the drugs currently in the market, such as praziquantel. B. trimera. It is also a promising plant to be further studied regarding its antiparasitic effect (De Oliveira et al. 2014). Likewise, the aqueous extract of this species is effective against Rhipicephalus microplus , an ectoparasite that causes anemia and is responsible for the transmission of lethal diseases in cattle. Besides, the resistance to synthetic acaricides has been increasing, and B. trimera leaves aqueous extract at 150 and 200 mg/mL was able to reduce 100% of R. microplus egg hatching. Thus, it could be a new approach to the discovery of new acaricidal agents (Lázaro et al. 2013).
Regarding the antileishmanial and trypanocidal potential of Baccharis spp., the flavonoid 5,6,7- trihydroxy-4′-methoxyflavanone from B. retusa leaves methanolic extract was isolated, which displays a significant antiparasitic effect: the IC50 value against T. cruzi trypomastigotes found was 20.39 μg/mL, while benznidazole’s was 47.54 μg/mL. Therefore, this flavonoid is more potent than benznidazole, a standard drug currently used to treat Chagas disease (Grecco et al. 2010b). Passero et al. (2011) evaluated the effect of caffeic acid and the flavonoid pectolineragenin against L. amazonensis and L. braziliensis, which displayed IC50 values of 190 ng/μL for caffeic acid against L. amazonensis promastigotes and 110 μg/μL for pectolineragenin against L. braziliensis promastigotes. Although, since amphotericin B has IC50 of 0.30 and 0.07 ng/μL against L. amazonensis and L. braziliensis, respectively, the concentrations of Baccharis isolated compounds able to inhibit the growth of 50% of the parasites are considerably high in comparison with the positive control.
3.4 Anti-inflammatory and Antinociceptive
Several reports have described the anti-inflammatory and antinociceptive potential of Baccharis spp. and their isolated phenolic compounds: the hexane, hydroalcoholic and aqueous fractions of B. illinita aerial parts decreased the nociceptive response in vivo at doses of 30–1000 mg/kg, presenting a dose-related response (Freitas et al. 2009). Moreover, B. illinita leaves crude extract is a topical anti-inflammatory agent capable of inhibiting ear edema caused by 12-O-tetradecanoilforbol acetate and arachidonic acid. It decreased the polymorphonuclear cells migration as well, showing a similar effect in comparison with dexamethasone (Boller et al. 2010).
B. dracunculifolia, which contains caffeic acid, p-coumaric acid, aromadendrin-4′-O-methyl ether, drupanin, artepillin C, and 2,2-dimethyl-6-carboxyethenyl-2H-1-benzopyran as major compounds, display in vivo anti-inflammatory and antinociceptive effects at doses ranging from 50 to 400 mg/kg of its leaves hydroalcoholic extract. It reduced the number of abdominal constrictions caused by acetic acid, glutamate of complete Freund adjuvant, and decreased the nociceptive induced by formalin. Moreover, it was effective as anti-hypernociceptive in the acute inflammation pain caused by carrageenan and inhibited the enzyme COX-2 (dos Santos et al. 2010). Therefore, considering that many phenolic compounds are found in B. dracunculifolia hydroalcoholic extract, they probably contribute in a significant way to the anti-inflammatory and antinociceptive effects displayed by this extract.
B. dracunculifolia ethyl acetate extract, which shows a similar chemical profile (major compounds: baccharin, artepillin C, drupanin, caffeic acid, p-coumaric acid and aromadendrin-4-O-methyl ether), displays intestinal anti-inflammatory activity: from 5 to 50 mg/kg, it reduced significantly the ulcerative colitis caused by trinitrobenzenesulfonic acid by avoiding glutathione depletion, inhibiting lipid peroxidation, and decreasing myeloperoxidase effect (Cestari et al. 2011). Paulino et al. (2008) reported the in vivo anti-inflammatory effect of artepillin C, which inhibited the paw edema by 38% at 10 mg/kg. At 1 mg/kg, it displayed a similar effect in comparison with the positive control, indomethacin, at 1 mg/kg. Its activity comes from reducing the neutrophils numbers and prostaglandin E2. In vitro, this compound reduces nitric oxide generation and NF-kβ. Besides this, these authors found that artepillin C is orally absorbed in vivo, which is important to biological activity. Therefore, artepillin C may be considered a promising anti-inflammatory natural compound and, since artepillin C is one of the phenolic compounds present in high amounts in B. dracunculifolia, it corroborates with the hypothesis that the phenolic compounds found in Baccharis spp. have a relevant contribution to this biological effect. To increase the potency of B. dracunculifolia leaves hydroalcoholic extract, low diameter and biocompatible liposomes with the sample were developed: the free extract had reduced the swelling, the leucocytes and neutrophil migration; and the levels of TNF-α and interleukins 6 and 1β. The liposomes containing the extract increased the anti-inflammatory activity in vivo by reducing the effective dose by almost six times. Caffeic acid liposomes also had their anti-inflammatory effect improved (de Figueiredo-Rinhel et al. 2018).
A phenolic fraction of B. trimera aerial parts ethanolic extract at 15 mg/kg reduced the acute inflammation caused by carrageenan in comparison with the negative control (De Oliveira et al. 2012a, b). B. trimera aqueous extract also displayed a significant anti-inflammatory effect on carrageenan-induced edema at intraperitoneal doses of 400 and 800 mg/kg, respectively. This extract promoted a decrease in inflammatory parameters, such as cell migration, edema, polymorphonuclear leukocytes, and proteins (Paul et al. 2009). Although the aqueous extract was able to act as an anti-inflammatory agent, its doses are considerably higher than the phenolic fraction of the ethanolic extract. It probably demonstrates that the phenolic fraction has more active and/or potent compounds than the aqueous extract, which may contain several compounds with no anti-inflammatory effect, such as sugars, for example. Likewise, most of B. pentladii, B. obtusifolia, B. latifolia, and B. subulata extracts (hexanic, dichloromethanic, ethanolic and aqueous) in concentrations ranging from 12.5 to 200 μg/mL were capable of reducing the inflammatory parameters as well, such as COX-2, nitric oxide and TNF-α production (Abad et al. 2006).
3.5 Antidiabetic and Antiobesity
Diabetes is a disorder that affects millions of people worldwide and is caused by low production or resistance to insulin, consequently increasing blood glucose levels. Because of that, the body organs and tissues like the liver and muscle cannot use glucose or store it in glycogen form. Once it becomes a chronic disease, several other consequences take place, such as damage to the eyes, blood vessels, and many other body parts. B. articulata butanolic fraction of the crude extract, which has mainly flavonoids, when administered orally in vivo, was able to improve the insulin production, with an effect similar to glipizide, a standard drug; and the liver and muscle glycogen levels were increased (Kappel et al. 2012). Artepillin C, one of the major prenylated phenolic compounds from B. dracunculifolia and Brazilian green propolis, shows high affinity to the nuclear receptor peroxisome proliferator-activated receptor, known as PPAR, and by activating this receptor, genes like aP2, adiponectin, and glucose transporter are expressed, increasing the body response to insulin in type 2 diabetes. Besides, it stimulated adipocytes differentiation, increasing the glucose uptake by the mature adipocytes (Choi et al. 2011). A clinical trial using 16 healthy people aged around 20-year-old was performed with B. dracunculifolia extracts at 20 mg/kg. The sample intake led to a 25% decrease in glucose blood content and no significant alterations in cardiovascular parameters, such as blood pressure and heart rate. Since the extracts have phenolic compounds as the ones in higher concentrations, they can probably be associated with the antidiabetic effect of this plant (Oliveira et al. 2014). The activity on glucose homeostasis and on insulin regulation reflects the antidiabetic potential of Baccharis spp. and the phenolic compounds isolated from them. Therefore, they can possibly be sources for new antidiabetic drugs or for the development of new phytotherapeutic medicines.
Considering that obesity and type 2 diabetes are related, the discovery of new agents anti-obesity is important, and B. dracunculifolia extract, after oral administration to rats, induced the secretion of serum insulin at 30% in obese rats (Hocayen et al. 2016). B. trimera aqueous extract showed potential in treating obesity by reducing the lipids and adipogenic transcriptional factors by 90% (Do Nascimento et al. 2017). The methanol extract of this plant also inhibited the enzyme pancreatic lipase by 78%, which is responsible for hydrolyzing triacylglycerols. Therefore, its inhibition contributes to the antiobesity effect of B. trimera (de Souza et al. 2011). Caffeic and ferulic acids, found in many Baccharis species, including in B. uncinella aerial parts, in a mixture containing the two of them, showed to be effective in decreasing biochemical parameters associated with obesity, like hyperglycemia, high cholesterol, and triglycerides levels and avoided the gain of body weight (Bocco et al. 2016).
These reports show that Baccharis spp. and many of their secondary metabolites can modulate biochemical parameters related to obesity and/or diabetes and, therefore, represent a possible plant material source of bioactive compounds. These plants could be used for the development of functional foods and/or herbal medicines too.
3.6 Antimicrobial and Antifungal
Due to the increasing resistance of bacteria and fungi strains to the antibiotics currently in the market, compounds isolated from plants and the plant extracts have been evaluated for their antimicrobial and antifungal effects: diversinin, a coumarin isolated from B. darwinii at 15.6 μg/mL, inhibited Microsporum gypseum, Trichophyton rubrum, and Trichophyton mentagrophytes strains. Although the MIC value of diversinin is not high, the isolated compounds considered to be promising are the ones with MIC below 10 μg/mL, and in the case of extracts, the ones with MIC below 100 μg/mL (Kurdelas et al. 2010). Da Silva Filho et al. (2008) described the antifungal and antimicrobial effect of B. dracunculifolia leaves extract in comparison with Brazilian green propolis, which is made by bees from B. dracunculifolia: the leaf extract showed IC50 values of 65 μg/mL for C. krusei and 40 μg/mL for C. neoformans, while propolis extract displayed better effects, with IC50 of 9 μg/mL for C. krusei. It shows that although the bioactive compounds are present in the B. dracunculifolia extract, they are probably more concentrated in the propolis, increasing its potency.
Taking into account the antibiotic resistance, Nuño et al. (2012) tested B. incarum extracts at ethanol 60% and 80% against clinic isolated methicillin-resistant S. aureus and E. faecalis, and found that their MIC values were promising, ranging from 40 to 80 μg GAE/mL. Therefore, using the 60% tincture, a topical formulation was developed, which also displayed an antimicrobial effect. The in vitro drug-releasing experiments revealed that the phenolic compounds chlorogenic acid and 4′,5-dihydroxy-3′,3,6,7,8-pentamethoxyflavone were the major ones found in the receptor solution, showing that probably, these compounds were responsible for the biological effect. Therefore, this formulation, after additional studies, may be useful for the development of new anti-acne agents or for topical treatment of tissues infected by Propionibacterium acnes, as well as by S. aureus and/or E. faecalis methicillin resistant.
4 Toxicology
In folk medicine, usually many people think that all natural products are safe for consumption, although several studies show that it is not true. For example, B. pteronioides contains the toxic compound trichothecenes (Stegelmeier et al. 2009). Therefore, it is important to assess the toxicity of plants before stating their safety. Da Silva et al. (2016) evaluated the acute toxicity of B. trimera tinctures in wistar rats by administration of a single dose of 2000 mg/kg and found that it was not able to induce significant hematological or biochemical changes (lipid peroxidation, δ-aminolevulinate dehydratase, and catalase); neither showed other signs of toxicity nor increased the animals mortality. The subchronic toxicity was assessed by oral administration of the sample at 100, 200, and 400 mg/kg for 28 consecutive days. B. trimera tincture, besides not showing any toxicity, decreased the liver enzymes alanine and aspartate aminotransferases, which are related to hepatic cells damage. Therefore, in the subchronic treatment, it additionally promoted a hepatoprotective effect. Considering that B. trimera contains many phenolic compounds in its chemical composition, such as gallic acid, ellagic acid, rutin, quercitrin, and quercetin, they are probably not toxic and responsible for the hepatoprotective activity. Therefore, B. trimera can probably be considered safe at these doses.
The aqueous extract of B. genistelloides at doses of 4.2 and 42 mg/kg, after 37 days of oral treatment, did not show genotoxicity to liver and kidney. Moreover, it did not induce alterations in the aspects of kidneys, liver, and lungs, like color and weight. However, it reduced the body and thymus weights and glucose and triglyceride levels. Therefore, this extract is not toxic at these doses and displays hypoglycemic and hypotriglyceridemic effects (Coelho et al. 2004). On the other hand, B. dracunculifolia aqueous extract at 500, 1000, and 2000 mg/kg for 3 days induced genotoxic and mutagenic effects by increasing blood and liver DNA damage and the frequency of micronucleus in bone marrow (Rodrigues et al. 2009). On the other hand, one of the major compounds found in B. dracunculifolia, artepillin C at 0.4, 0.8, and 1.6 mg/kg, was not genotoxic and presented a protective effect against liver cells DNA damage induced by methyl methanesulfonate (Monteiro Neto et al. 2011). In a similar way, baccharin at 0.12, 0.24, and 0.48 mg/kg was also able to reduce DNA damage in liver cells and the frequency of micronucleated polychromatic erythrocytes in mice (Oliveira et al. 2011). Caffeic, cinnamic, and ferulic acids, three major compounds from B. dracunculifolia, increased the frequency of micronucleated cells, demonstrating a clastogenic effect of these compounds, despite presenting a not genotoxic effect to rat hepatoma tissue cells (Maistro et al. 2011). Therefore, these compounds, probably more concentrated in the aqueous extract, may have contributed to the mutagenic effect in vivo found by Rodrigues et al. (2009). On the other hand, artepillin C and baccharin were not genotoxic in these tested doses and are more likely to be found in the ethanolic extract.
5 Chromatographic Analyses
The pharmacological potential of plant extracts is usually related to their chemical composition, since the presence of bioactive compounds and their concentrations are the most important parameters for displaying the biological effect. To assure plant extracts quality, many analytical methods were developed for the chromatographic analyses of Baccharis extracts: by using thin-layer chromatography; De Oliveira et al. (2006) reported a simple method to differentiate B. articulata, B. cylindrica, B. spicata, B. trimera, and B. usterii, by using the aqueous extract of the leaves and its butyl alcohol fraction, obtained by the partition of the aqueous extract. The samples are applied on silica gel plates of 20 × 20, and the chromatographic elution is performed using chloroform:ethanol:acetic acid in a proportion of 60:40:6 v/v as mobile phase. The detection is undertaken by using two colorimetric reagents: anisaldehyde: H2SO4 plus heating to 100 °C and diphenylboryloxy-ethylamine 1% methanol, PEG 400 (5% w/v). After spraying the colorimetric reagent on the plates, they were observed under long-wave UV and visible lights. According to the chromatographic profile, considering the retention factor, number and color of the spots on silica plates, these Baccharis species can be differentiated. Lonni et al. (2003), by using HPLC coupled to a photodiode array detector and chemometric, were able to differentiate between different Baccharis species as well. By using as stationary phase a C18 column, methanol as mobile phase, and detection at 254 nm, the obtained chromatographic profile of the ethanolic extracts allowed distinguishing among B. genistelloides var. trimera, B. milleflora, and B. articulata.
A validated RP-HPLC method using a C-18 reversed-phase column and a gradient consisting of acidified water and acetonitrile was developed to perform analyses of 5-O-[E]-caffeoylquinic acid, 3,4-O-[E]-dicaffeoylquinic acid, 3,5-O-[E]-dicaffeoylquinic acid, 4,5-O-[E]-dicaffeoylquinic acid, and a tricaffeoylquinic acid in B. trimera hydroalcoholic extracts. The quantification of these compounds is important, because they are related to the digestive effect of B. trimera. The limits of quantification for the compounds were below 12.5 μg/mL and the method presented suitable selectivity, linearity, robustness, precision, and recovery according to ICH validation guidelines (Aboy et al. 2012).
Regarding B. dracunculifolia, aiming at proving the botanical origin of Brazilian green propolis, Kumazawa et al. (2003), by using liquid chromatography coupled to a mass spectrometer, compared the chemical constituents identified in both samples and concluded that there was no significant difference in the chemical composition of B. dracunculifolia and Brazilian green propolis. Although qualitative methods are important to determine each compound present in the samples, the quantitative analysis is also relevant to perform, because the biological effect of the plant extracts usually relies on both the presence and amount of bioactive compounds. Therefore, for the development of phytotherapeutic agents, analytical methods able to perform both qualitative and quantitative analyses are necessary. Taking that into account, de Sousa et al. (2009) developed and validated a reversed-phase HPLC method to analyze 10 phenolic compounds in B. dracunculifolia: caffeic acid, coumaric acid, ferulic acid, cinnamic acid, aromadendrin-4-O-methyl ether, isosakuranetin, drupanin, artepillin C, baccharin, and 2,2-dimethyl-6- carboxyethenyl-2H-1-benzopyran acid. The method parameters adjusted were: stationary phase a C18 column, mobile phase: nonlinear gradient of acetonitrile and water with mobile phase modifiers, and since the standards are all phenolic compounds, the wavelength of detection was set at 280 nm. Considering that it is a validated method, it presented selectivity, linearity, accuracy, precision, and robustness.
Some analytical methods to perform Baccharis spp. phenolic compounds quality control had been reported in the literature, although they cover mainly qualitative analysis. Only for B. dracunculifolia, the most studied Baccharis species, was developed a RP-HPLC method able to furnish both qualitative and quantitative results. It is really important from the pharmacological potential point of view to have reliable validated analytical methods to quantify the active compounds in medicinal plants, such as the ones belonging to Baccharis genus.
6 Conclusion
Baccharis spp. are used in folk medicine for the treatment of several diseases, such as gastrointestinal disorders, fever, inflammation, type-2 diabetes, parasitoses, and arthritis and many researchers have been undertaking scientific studies to corroborate many of the plants folk uses. Some examples of diseases that Baccharis spp. have proven effects against are diabetes, obesity, gastric ulcer, parasites, bacteria, fungi, arthritis, and inflammation, among others. The mechanisms of action of many extracts and/or their isolated phenolic compounds have been already reported. Analytical methods to perform chromatographic analyses of the plant material have been developed too due to the importance of the presence and amount of bioactive compounds in the samples. Only one validated quantitative method for analyzing phenolic compounds in Baccharis species was found, which was developed for B. dracunculifolia, the botanical source of Brazilian green propolis. It shows that Baccharis quality control field is still lacking analytical methods for the other Baccharis species. Even though these plants display important biological effects, some Baccharis species have compounds that present some toxicity. Therefore, Baccharis spp. may be potential plants for the development of novel phytotherapeutic medicines and/or be sources of bioactive compounds. However, more studies should be performed to validate the pharmacological activities and to better assess their toxicity.
References
Abad MJ, Bermejo P (2006) Baccharis (Compositae): a review update. ARKIVOC 2007:76–96
Abad MJ, Bessa AL, Ballarin B et al (2006) Anti-inflammatory activity of four bolivian Baccharis species (Compositae). J Ethnopharmacol 103:338–344
Aboy AL, Apel MA, Debenedetti S et al (2012) Assay of caffeoylquinic acids in Baccharis trimera by reversed-phase liquid chromatography. J Chromatogr A 1219:147–153
Agudelo IJ, Isolabella SA, Filip R et al (2016) Baccharis spicata (Lam) Baill: polyphenol screening, determination of their antioxidant activity and their main polyphenolic metabolites. J Pharmacogn Phytochem 5:278–285
Ahn MR, Kunimasa K, Ohta T et al (2007) Suppression of tumor-induced angiogenesis by Brazilian propolis: major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Lett 252:235–243
Argoti JC, Linares-Palomino PJ, Salido S et al (2013) On-line activity screening for radical scavengers from Baccharis chilco. Chem Biodivers 10:189–197
Arruda C, Eugênio D de S, Moreira MR et al (2017) Biotransformation of (-)-cubebin by Aspergillus spp. into (-)-hinokinin and (-)-parabenzlactone, and their evaluation against oral pathogenic bacteria. Nat Prod Res 6419:1–14
Baggio CH, Freitas CS, Rieck L, Marques MCA (2003) Gastroprotective effects of a crude extract of Baccharis illinita DC in rats. Pharmacol Res 47:93–98
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Barros MP, Lemos M, Maistro EL et al (2008) Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J Ethnopharmacol 120:372–377
Berretta AA, Arruda C, Miguel FG et al (2017) Functional properties of Brazilian propolis: from chemical composition until the market technology & medicine. In: Waisundara V, Shiomi N (eds) Superfood and functional food – an overview of their processing and utilization. Intech, London, pp 56–98
Biondo TMA, Tanae MM, Coletta ED et al (2011) Antisecretory actions of Baccharis trimera (Less.) DC aqueous extract and isolated compounds: analysis of underlying mechanisms. J Ethnopharmacol 136:368–373
Bocco BM, Fernandes GW, Lorena FB et al (2016) Combined treatment with caffeic and ferulic acid from Baccharis uncinella C. DC. (Asteraceae) protects against metabolic syndrome in mice. Braz J Med Biol Res 49:3–9
Boller S, Soldi C, Marques MCA et al (2010) Anti-inflammatory effect of crude extract and isolated compounds from Baccharis illinita DC in acute skin inflammation. J Ethnopharmacol 130:262–266
Borgo J, Xavier CAG, Moura DJ et al (2010) Influência dos processos de secagem sobre o teor de flavonoides e na atividade antioxidante dos extratos de Baccharis articulata (Lam.) pers., Asteraceae. Braz J Pharmacogn 20:12–17
Cariddi L, Escobar F, Sabini C et al (2012) Apoptosis and mutagenicity induction by a characterized aqueous extract of Baccharis articulata (Lam.) Pers. (Asteraceae) on normal cells. Food Chem Toxicol 50:155–161
Cestari SH, Bastos JK, Di Stasi LC (2011) Intestinal anti-inflammatory activity of Baccharis dracunculifolia in the trinitrobenzenesulphonic acid model of rat colitis. Evidence-Based Complement Altern Med 524349:1–9
Chang R, Piló-Veloso D, Morais SAL, Nascimento EA (2008) Analysis of a brazilian green propolis from Baccharis dracunculifolia by HPLC-APCI-MS and GC-MS. Braz J Pharmacogn 18:549–556
Choi SS, Cha BY, Iida K et al (2011) Artepillin C, as a PPARγ ligand, enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Biochem Pharmacol 81:925–933
Coelho MGP, Reis PA, Gava VB et al (2004) Anti-arthritic effect and subacute toxicological evaluation of Baccharis genistelloides aqueous extract. Toxicol Lett 154:69–80
Costa P, Almeida MO, Lemos M et al (2018) Artepillin C, drupanin, aromadendrin-4′-O-methyl-ether and kaempferide from Brazilian green propolis promote gastroprotective action by diversified mode of action. J Ethnopharmacol 226:82–89
Da Silva Filho AA, de Sousa JPB, Soares S et al (2008) Antimicrobial activity of the extract and isolated compounds from Baccharis dracunculifolia DC. (Asteraceae). Z Naturforsch C 63:40–46
Da Silva Filho AA, Resende DO, Fukui MJ et al (2009) In vitro antileishmanial, antiplasmodial and cytotoxic activities of phenolics and triterpenoids from Baccharis dracunculifolia D. C. (Asteraceae). Fitoterapia 80:478–482
da Silva ARH, Reginato FZ, Guex CG et al (2016) Acute and sub-chronic (28 days) oral toxicity evaluation of tincture Baccharis trimera (Less) Backer in male and female rodent animals. Regul Toxicol Pharmacol 74:170–177
Da Silva EM, Roel ARRR, Porto KRA et al (2017) Insecticidal effect of the ethanol extract of Baccharis dracunculifolia (Asterales: Asteraceae). Rev Biol Trop 65:517–523
De Araújo GR, Rabelo ACS, Meira JS et al (2017) Baccharis trimera inhibits reactive oxygen species production through PKC and down-regulation p47phoxphosphorylation of NADPH oxidase in SK Hep-1 cells. Exp Biol Med 242:333–343
De Figueiredo-Rinhel ASG, de Andrade MF, Landi-Librandi AP et al (2018) Incorporation of Baccharis dracunculifolia DC (Asteraceae) leaf extract into phosphatidylcholine-cholesterol liposomes improves its anti-inflammatory effect in vivo. Nat Prod Res 6419:1–5
De Oliveira SQ, Dal-Pizzol F, Moreira JCF et al (2004) Antioxidant activity of Baccharis spicata, Baccharis trimera and Baccharis usterii. Acta Farm Bonaer 23:365–368
De Oliveira SQ, Barbon G, Gosmann G, Bordignon S (2006) Differentiation of south Brazilian Baccharis species by TLC. J Liq Chromatogr Relat Technol 29:2603–2609
De Oliveira CB, Comunello LN, Lunardelli A et al (2012a) Phenolic enriched extract of Baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules 17:1113–1123
De Oliveira RN, Rehder VLG, Santos Oliveira AS et al (2012b) Schistosoma mansoni: in vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp Parasitol 132:135–143
De Oliveira RN, Rehder VLG, Oliveira ASS et al (2014) Anthelmintic activity in vitro and in vivo of Baccharis trimera (Less) DC against immature and adult worms of Schistosoma mansoni. Exp Parasitol 139:63–72
De Sousa JPB, Leite MF, Jorge RF et al (2011) Seasonality role on the phenolics from cultivated Baccharis dracunculifolia. Evidence-Based Complement Altern Med 464289:1–8
De Souza GC, Haas APS, Von Poser GL et al (2004) Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J Ethnopharmacol 90:135–143
De Souza SP, Pereira LLS, Souza AA, dos Santos CD (2011) Inhibition of pancreatic lipase by extracts of Baccharis trimera: evaluation of antinutrients and effect on glycosidases. Braz J Pharmacogn 21:450–455
Do Nascimento DSM, Oliveira RM, Camara RBG et al (2017) Baccharis trimera (Less.) DC exhibits an anti-adipogenic effect by inhibiting the expression of proteins involved in adipocyte differentiation. Molecules 22:1–16
Dos Santos DA, Fukui MJ, Nanayakkara DNP et al (2010) Anti-inflammatory and antinociceptive effects of Baccharis dracunculifolia DC (Asteraceae) in different experimental models. J Ethnopharmacol 127:543–550
Freitas CS, Baggio CH, Finau J et al (2008) Inhibition of H + /K + ATPase in the gastroprotective effect of Baccharis illinita DC. J Pharm Pharmacol 60:1105–1110
Freitas CS, Baggio CH, Dos Santos AC et al (2009) Antinociceptive properties of the hydroalcoholic extract, fractions and compounds obtained from the aerial parts of Baccharis illinita DC in Mice. Basic Clin Pharmacol Toxicol 104:285–292
Grecco SS, Gimenes L, Ferreira MJP et al (2010a) Triterpenoids and phenolic derivatives from Baccharis uncinella C.DC. (Asteraceae). Biochem Syst Ecol 38:1234–1237
Grecco SS, Reimão JQ, Tempone AG et al (2010b) Isolation of an antileishmanial and antitrypanosomal flavanone from the leaves of Baccharis retusa DC. (Asteraceae). Parasitol Res 106:1245–1248
Grecco SS, Reimão JQ, Tempone AG et al (2012) In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae). Exp Parasitol 130:141–145
Guimarães NSS, Mello JC et al (2012) Baccharis dracunculifolia, the main source of green propolis, exhibits potent antioxidant activity and prevents oxidative mitochondrial damage. Food Chem Toxicol 50:1091–1097
Hennig L, Garcia GM, Giannis A, Bussmann RW (2011) New constituents of Baccharis genistelloides (Lam.) Pers. ARKIVOC 2011:74–81
Herrerias T, Oliveira AAA, Belem ML et al (2010) Effects of natural flavones on membrane properties and citotoxicity of HeLa cells. Rev Bras Farmacogn 20:403–408
Hocayen PAS, Grassiolli S, Leite NC et al (2016) Baccharis dracunculifolia methanol extract enhances glucose-stimulated insulin secretion in pancreatic islets of monosodium glutamate induced-obesity model rats. Pharm Biol 54:1263–1271
Jaramillo-García V, Trindade C, Lima E et al (2018) Chemical characterization and cytotoxic, genotoxic, and mutagenic properties of Baccharis trinervis (Lam, Persoon) from Colombia and Brazil. J Ethnopharmacol 213:210–220
Kappel VD, Pereira DF, Cazarolli LH et al (2012) Short and long-term effects of Baccharis articulata on glucose homeostasis. Molecules 17:6754–6768
Kumazawa S, Yoned M, Shibata I et al (2003) Direct evidence for the plant origin of Brazilian Propolis by the observation of honeybee behavior and phytochemical analysis. Chem Pharm Bull 51:740–742
Kupchan SM, Jarvis BB, Dailey RG et al (1976) Baccharin, a novel potent antileukemic trichothecene triepoxide from Baccharis megapotamica. J Am Chem Soc 98:7092–7093
Kurdelas RR, Lima B, Tapia A et al (2010) Antifungal activity of extracts and prenylated coumarins isolated from Baccharis darwinii Hook & Arn. (Asteraceae). Molecules 15:4898–4907
Lázaro SF, Fonseca LD, Martins ER et al (2013) Effect of aqueous extracts of Baccharis trimera on development and hatching of Rhipicephalus microplus (Acaridae) eggs. Vet Parasitol 194:79–82
Lemos M, De Barros MP, Sousa JPB et al (2007) Baccharis dracunculifolia, the main botanical source of Brazilian green propolis, displays antiulcer activity. J Pharm Pharmacol 59:603–608
Li H, Kapur A, Yang JX et al (2007) Antiproliferation of human prostate cancer cells by ethanolic extracts of Brazilian propolis and its botanical origin. Int J Oncol 31:601–606
Lívero FAR, da Silva LM, Ferreira DM et al (2016a) Hydroethanolic extract of Baccharis trimera promotes gastroprotection and healing of acute and chronic gastric ulcers induced by ethanol and acetic acid. Arch Pharmacol 389:985–998
Lívero FAR, Martins GG, Telles JEQ et al (2016b) Hydroethanolic extract of Baccharis trimera ameliorates alcoholic fatty liver disease in mice. Chem Biol Interact 260:22–32
Lonni ASG, Scarminio IS, Silva LMC, Ferreira DT (2003) Differentiation of species of the Baccharis genus by HPLC and chemometric methods. Anal Sci 19:1013–1017
Maistro EL, Angeli JP, Andrade SF, Mantovani MS (2011) In vitro genotoxicity assessment of caffeic, cinnamic and ferulic acids. Genet Mol Res 10:1130–1140
Martinez MJA, Bessa AL, Benito PB (2005) Biologically active substances from the genus Baccharis L. (Compositae). Stud Nat Prod Chem 30:703–759
Miean KH, Mohamed S (2001) Apigenin content of edible tropical plants. J Agric Food Chem 49:3106–3112
Missima F, Da Silva Filho AA, Nunes GA et al (2007) Effect of Baccharis dracunculifolia D.C (Asteraceae) extracts and its isolated compounds on macrophage activation. J Pharm Pharmacol 59:463–468
Monteiro Neto MAB, de Souza Lima IM, Furtado RA et al (2011) Antigenotoxicity of artepillin C in vivo evaluated by the micronucleus and comet assays. J Appl Toxicol 31:714–719
Nakajima Y, Shimazawa M, Mishima S, Hara H (2007) Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci 80:370–377
Nuño G, Zampini IC, Ordoñez RM et al (2012) Antioxidant/antibacterial activities of a topical phytopharmaceutical formulation containing a standardized extract of Baccharis incarum, an extremophile plant species from argentine Puna. Phyther Res 26:1759–1767
Oliveira PF, Monteiro Neto MAB, Leandro LF et al (2011) In vivo antigenotoxicity of baccharin, an important constituent of Baccharis dracunculifolia DC (Asteraceae). Basic Clin Pharmacol Toxicol 109:35–41
Oliveira TA, Michel RG, Snak AL et al (2014) Baccharis dracunculifolia with high levels of phenol compounds reduces blood glucose in healthy human. Afr J Pharm Pharmacol 8:670–673
Olthof MR, Hollman PCH, Katan MB (2001) Human nutrition and metabolism chlorogenic acid and caffeic acid are absorbed in humans. J Nutr 131:66–71
Palomino SP, Abad MJ, Bedoya LM et al (2002) Screening of south american plants against human immunodeficiency virus: preliminary fractionation of aqueous extract from Baccharis trinervis. Biol Pharm Bull 25:1147–1150
Passero LFD, Bonfim-Melo A, Corbett CEP et al (2011) Anti-leishmanial effects of purified compounds from aerial parts of Baccharis uncinella C. DC. (Asteraceae). Parasitol Res 108:529–536
Paul EL, Lunardelli A, Caberlon E et al (2009) Anti-inflammatory and immunomodulatory effects of Baccharis trimera aqueous extract on induced pleurisy in rats and lymphoproliferation in vitro. Inflammation 32:419–425
Paula JT, Sousa IMO, Foglio MA, Cabral FA (2017) Selective fractionation of supercritical extracts from leaves of Baccharis dracunculifolia. J Supercrit Fluids 127:62–70
Paulino N, Abreu SRL, Uto Y et al (2008) Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur J Pharmacol 587:296–301
Prasad NR, Jeyanthimala K, Ramachandran S (2009) Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes. J Photochem Photobiol B Biol 95:196–203
Rezende TP, Corrêa JODA, Aarestrup BJV et al (2014) Protective effects of Baccharis dracunculifolia leaves extract against carbon tetrachloride and acetaminophen-induced hepatotoxicity in experimental animals. Molecules 19:9257–9272
Ribeiro VP, Arruda C, Abd El-Salam M, Bastos JK (2018) Brazilian medicinal plants with corroborated anti-inflammatory activities: a review. Pharm Biol 56:253–268
Rodrigues CRF, Dias JH, Semedo JG et al (2009) Mutagenic and genotoxic effects of Baccharis dracunculifolia (D.C.). J Ethnopharmacol 124:321–324
Sartor T, Xavier VB, Falcão MA et al (2013) Seasonal changes in phenolic compounds and in the biological activities of Baccharis dentata (Vell.) G.M. Barroso. Ind Crop Prod 51:355–359
Sharp H, Bartholomew B, Bright C et al (2001) 6-Oxygenated flavones from Baccharis trinervis (Asteraceae). Biochem Syst Ecol 29:105–107
Soicke H, Leng-Peschlow E (1987) Characterisation of flavonoids from Baccharis trimera and their antihepatotoxic properties. Planta Med 53:37–39
Sousa JPB, Da Silva Filho AA, Bueno PCP et al (2009) A validated reverse-phase HPLC analytical method for the quantification of phenolic compounds in Baccharis dracunculifolia. Phytochem Anal 20:24–32
Stegelmeier BL, Sani Y, Pfister JA (2009) Bacchavis pteronioides toxicity in livestock and hamsters. J Vet Diagn Investig 21:208–213
Szliszka E, Zydowicz G, Mizgala E, Krol W (2012) Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid) sensitizes LNCaP prostate cancer cells to TRAIL-induced apoptosis. Int J Oncol 41:818–828
Taguchi L, Pinheiro NM, Olivo CR et al (2015) A flavanone from Baccharis retusa (Asteraceae) prevents elastase-induced emphysema in mice by regulating NF-κB, oxidative stress and metalloproteinases. Respir Res 16:1–15
Tarqui ST, Segura YF, Vega GRA (2012) Polyoxygenated flavonoids from Baccharis Pentlandii. Rev Boliv Quim 29:10–14
Toyama DO, Ferreira MJP, Romoff P et al (2014) Effect of chlorogenic acid (5-Caffeoylquinic Acid) isolated from Baccharis oxyodonta on the structure and pharmacological activities of secretory phospholipase A2 from Crotalus durissus terrificus. Biomed Res Int 726585:1–10
Ueno AK, Barcellos AF, Grecco SS et al (2018) Sesquiterpenes, diterpenes, alkenyl p -coumarates, and flavonoid from the aerial parts of Baccharis retusa (Asteraceae). Biochem Syst Ecol 78:39–42
Verdi LG, Brighente IMC, Pizzolatti MG (2005) Gênero Baccharis (Asteraceae): Aspectos químicos, econômicos e biológicos. Quim Nova 28:85–94
Vijayarathna S, Sasidharan S (2012) Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pac J Trop Biomed 2:826–829
Zalewski CA, Passero LFD, Melo ASRB et al (2011) Evaluation of anti-inflammatory activity of derivatives from aerial parts of Baccharis uncinella. Pharm Biol 49:602–607
Zampini IC, Isla MI, Schmeda-Hirschmann G (2009) Antimicrobial and antioxidant compounds from the infusion and methanolic extract of Baccharis incarum (WEDD.) Perkins. J Chil Chem Soc 54:477–481
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bastos, J.K., Arruda, C. (2021). Chemistry and Biological Activities of Phenolic Compounds from Baccharis Genus. In: Fernandes, G.W., Oki, Y., Barbosa, M. (eds) Baccharis. Springer, Cham. https://doi.org/10.1007/978-3-030-83511-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-83511-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83510-1
Online ISBN: 978-3-030-83511-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)