Abstract
This chapter provides a review of the characteristics of essential oils of Baccharis species with special emphasis on their chemical composition and biological activities. Species of Baccharis show a great diversity in their morpho-anatomical and ecological features and also exhibit a wide range of chemical diversity in their essential oils. Several medicinally beneficial properties and biological activities, including anti-inflammatory, antimicrobial, antiulcerogenic, antimalarial, antioxidant, antitrypanosomal, cytotoxic, insecticidal, leishmanicidal, schistosomicidal, sedative, and adjuvant properties, have been reported for the essential oils of Baccharis species. Essential oils of Baccharis species and their major compounds have been reported to have medicinal properties and have shown significant activities against a range of insect pests, microorganisms, and parasites. Also, we illustrate the micromorphic features of various secretory structures that are responsible for biosynthesizing and storing the essential oils in Baccharis species, including glandular trichomes and secretory ducts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chemical composition
- Medicinal properties
- Insecticidal activity
- Parasiticidal activities
- Secretory structures
1 Introduction
Currently, it is estimated that more than 3000 essential oils (EOs) are known, and 300 of them have commercial value. EOs from a number of species are commonly used in pharmaceutical industries, aromatherapy and aromachology, household cleaning products, as flavoring and antimicrobial agents in food products, and as flavoring agents in cigarettes, drinks, perfumeries, and cosmetics. EOs are also used in air fresheners and deodorizers as well as in balneology and massage therapy. Isolated compounds of the EOs are obtained by extraction from the plant or synthesized (Calo et al. 2015; Schmidt 2016).
The genus Baccharis has been widely studied mainly on the chemical composition of EOs since the early 1900s. The genus has provided valuable biomolecules in the discovery of new medicinal natural products (Abad and Bermejo 2007). The most important classes of organic compounds present in the EOs are phenolics and terpenoids. Considering the terpenoids, monoterpenoids, sesquiterpenoids, and diterpenoids especially neo-clerodane, labdane, and kaurene are found in the EOs of Baccharis (Campos et al. 2016).
Species of Baccharis produce EOs composed mainly of monoterpenoids and sesquiterpenoids. Sesquiterpenoids are generally more abundant in the majority of the species. However, EOs of some species contained more monoterpenoids than sesquiterpenoids (Budel et al. 2018b). A bibliographic review stated that about 60 compounds (concentrations above 10%) identified in the EOs of 16 species of Baccharis have shown important biological activities (Campos et al. 2016).
In Baccharis, there are two economically important groups of species occurring in South America, namely, carquejas and vassouras. Carquejas is the folk name referring to the plants possessing cladodes. The most important and well-studied carqueja is B. crispa Spreng. (syn. B. trimera (Less.) DC.), which has been included in the latest edition of the Brazilian Pharmacopeia. The major compound of its EO is carquejyl acetate (Minteguiaga et al. 2018a). In Argentina, the medicinal carquejas recognized by the 6th Argentinean Pharmacopeia are B. crispa and B. articulata (Lam.) Pers. B. crispa is in traditional medicine used as stomachic and diuretic (Budel et al. 2008). Vassouras is the common name given to species without cladodes, having leaves and normal stems. The main example is B. dracunculifolia DC., which is used in folk medicine to treat gastric disorders and largely employed in the fragrance industry due to the high content of E-nerolidol in its EO (Budel et al. 2008).
Several medicinal properties defined for Baccharis are attributed to their EOs. In that context, the EOs of species of Baccharis presented many beneficial properties, such as antibacterial, antifungal (Valarezo et al. 2015; Negreiros et al. 2016; Perera et al. 2017), antitrypanosomal (Budel et al. 2018b), antiviral, antioxidant (Sobrinho et al. 2016; Zuccolotto et al. 2019; Oliveira et al. 2019), anti-inflammatory (Florão et al. 2012), schistosomicidal (Oliveira et al. 2012), cytotoxic (Pereira et al. 2017), sedative (Ascari et al. 2012), larvicidal against Aedes aegypti (Botas et al. 2017), and insecticidal actions against bed bugs (Budel et al. 2018b).
2 Essential Oils
2.1 General Characteristics
Essential oils (EOs) are complex mixtures of mainly low-molecular-weight components biosynthesized and stored in specialized secretory structures of plants and are extracted by different methods from whole plants or plant parts. They can act as chemical signals in the plant kingdom and as chemical defense against the animal kingdom, presenting a biological function vital to the survival and adaptation of the plants to the environment. There is a great variation in the chemical composition of EOs from different taxa of plants.
EOs of aromatic plants present major volatile components biosynthesized through three different biosynthetic pathways, the methylerythritol pathway leading to mono- and diterpenoids, the mevalonate pathway leading to sesquiterpenoids, and the shikimic acid pathway leading to phenylpropanoids (Franz and Novak 2016).
EOs can be extracted by different processes, depending on the part of the plant, amount of the plant material, and the quality required. The usual methods used in EO extraction include steam distillation, hydrodistillation, cold mechanical processing, Soxhlet extraction, solvent extraction, microwave-assisted hydrodistillation, supercritical solvent, and headspace techniques (Elshafie and Camele 2017). They are usually analyzed by GC/MS and/or GC/FID, and the compounds are identified by comparison of their retention indexes (RIs) and mass spectra with literature data.
The yield and the chemical composition of the EOs can be influenced by the plant genotype, development stage, environmental conditions (day length, irradiance, temperature, and water supply), phenological factors, physiological variations inherent to the plant, genetic features of the cultivars, plant nutrition, stress during growth or maturity, application of fertilizers, drying conditions of the plant material, storage conditions, grinding method, and the EO extraction methods (Gobbo-Neto and Lopes 2007; Tischer et al. 2017). Considering all the factors that influence the chemical composition, chemotaxonomic reports and conclusions have to be based on comparable plant material, grown and harvested under analogous conditions (Budel et al. 2018b).
Also, phytochemical polymorphism is frequently the case between different plant organs. Even though several species store qualitatively similar compounds in each organ, some produce different components, resulting from the same biosynthetic pathway. At the same time, there are also species in which the chemical composition of different plant organs presents no close connection between their biosynthetic origins (Németh-Zámboriné 2016). It is important to highlight that EOs from different plant parts such as leaves, stems, flowers, roots, and fruits may exhibit different properties due to differences in their chemical compositions.
Polymorphisms can also be observed when comparing the chemical profiles of individual plants of the same species and are based on the genetic characteristics of the plants. Therefore, it is difficult to ascertain if the differences in the chemical compositions are related to specific chemotypes or due to the environmental conditions of the plant (Franz and Novak 2016).
2.2 Secretory Structures
EOs are produced by several species of plants. The ability to store EOs is not universal in plants, yet widely present in the plant kingdom, especially in some families, including Alliaceae, Apiaceae, Asteraceae, Lamiaceae, Myrtaceae, Piperaceae, Poaceae, Rutaceae, and Zingiberaceae. EOs are usually found in the leaves, flowers, and fruits and less frequently in the roots, rhizomes, corks, and seeds.
Independent of their chemical composition, EOs are usually stored in oil ducts, resin ducts, oil cells, glands, or glandular trichomes of the plants. In Asteraceae, EOs are biosynthesized and accumulated in different secretory structures, such as oil cavities, idioblast oil cells, secretory ducts, and glandular trichomes. In Baccharis, EOs can be found in the leaves, stems, flowers, and roots and are stored in glandular trichomes (Fig. 10.1a, b, c) and/or secretory ducts (Fig. 10.1d, e, f).
Secretory structures of Baccharis species [(a, b, d) light microscopy; (c, e, f) scanning electron microscopy)]. B. uncinella (a), B. punctulata (b), B. illinita (c, f), B. pauciflosculosa (d, e). [gt, biseriate trichome; cx, cortex; tc, C-shaped flagelliform trichome; ft., straight flagelliform trichome; ph, phloem; sd, secretory duct; xy, xylem]. Scale bar: 50 μm. (Figure 10.1d reproduced from Budel et al. 2018a)
In Baccharis, there are different types of glandular trichomes (Freire et al. 2007). The most common are the biseriate glandular trichomes (Fig. 10.1a, b) and flagelliform glandular trichomes (Fig. 10.1c). Biseriate glandular trichome comprises two pairs of basal cells and a head with up to four pairs of secretory cells containing dense cytoplasm and covered by a cuticle layer. This trichome occurs in solitary, in groups with similar trichomes or groups with other flagelliform trichomes. The flagelliform glandular trichome consists of a voluminous body of about 10 secretory cells and an apical cell. The apical cell is elongated, whip-like, tubular, and translucent and contains dense oil substances (Budel et al. 2018a, b). The secretory body can be straight as seen in B. illinita DC. (Fig. 10.1c) or C-shaped as in B. punctulata DC. (Fig. 10.1b).
Secretory ducts are elongated and have large extracellular storage spaces containing secretion. In general, all Baccharis species possess secretory ducts. They are usually associated with the endodermis in the conducting system and occur next to the phloem. These ducts have uniseriate epithelium of 6–12 cells containing dense cytoplasm and lipophilic substances as observed in B. pauciflosculosa DC. (Fig. 10.1d, e) and B. illinita (Fig. 10.1f).
2.3 Essential Oils in Baccharis Species
In general, EOs of Baccharis species are liquid, clear or colored, volatile (with strong and characteristic aroma), and generally of lesser density than water. EO of B. punctulata is green, whereas it is yellowish in B. microdonta DC. (Budel et al. 2018b). EO extracted from aerial parts of B. obtusifolia Kunth had relative density of 0.8742 (Valarezo et al. 2015) and 0.8834 (Arze et al. 2004), whereas the relative density of the oils extracted from the leaves was 0.9151 for B. dracunculifolia and 0.9147 for B. uncinella DC. (Fabiane et al. 2008).
The olfactive profile of B. anomala DC. EO collected in Rio Grande do Sul, Brazil, was analyzed by gas chromatography-olfactometry (GC-O), and it was described to have a sweet, resinous, and woody odor. The major components of the EO were β-selinene (40.8%), caryophyllene oxide (9.9%), and spathulenol (6.8%) (Xavier et al. 2013).
The yield of EOs in Baccharis ranged from 0.08% to 2.82%. B. obovata Hook. & Arn. collected in Argentina presented the highest yield (Malizia et al. 2005), whereas the lowest content was obtained from B. lateralis Baker (syn. B. schultzii Baker) collected in Brazil (Lago et al. 2008).
Pretreatment processes such as drying and grinding are frequently applied to the plant material before the extraction of EOs for greater homogeneity. These processes must be carefully evaluated to provide reproducible results in EO investigation. In a recent study, Tischer et al. (2017) applied different grinding methods and compared their efficiencies as well as EO yields. They used cryogenics, knife (with and without cooling), and ball mills for grinding the materials of B. articulata and determined the yield and chemical compositions of the EOs. This study showed that cryogenic milling was found to be more efficient than the other methods in achieving lower particle size by disrupting the secretory structures (secretory ducts and glandular trichomes). However, this method of grinding resulted in the lower yields of EOs in comparison with other grinding methods (Tischer et al. 2017).
Another important factor affecting the EO yield is the plant part used in the extraction. EO extracted from the leaves of B. microdonta showed yields of 0.06–0.35% (Sayuri et al. 2010) and 0.08–0.21% (Lago et al. 2008), whereas Budel et al. (2018b) achieved 0.93% (v/w) of EO from the same species by extracting mixed parts of leaves and stems. Budel et al. 2018a observed large secretory ducts in the cortex of the stem. This anatomical feature contributed to the higher yield of EO.
Considering the dioecious nature of Baccharis species, there may be differences in the chemical compositions of EOs extracted from male and female plants and at different phenological stages. Some authors have reported slight differences in the EOs extracted at different growing stages (Zunino et al. 2004; Lago et al. 2008; Ascari et al. 2019). However, Besten et al. (2012) observed clear similarities between the EOs extracted from male and female specimens, during as well as outside the flowering periods, in five taxa of Baccharis, namely, B. caprariifolia DC., B. dracunculifolia, B. coridifolia DC., B. semiserrata var. elaegnoides (Steud. ex Baker) G.M.Barroso, and B. pentaptera (Less.) DC.
Differences in the EO chemical compositions have been reported for B. punctulata collected from different geographical locations. The EOs from aerial parts of the plants collected from Uruguay have shown β-phellandrene (5.2%), bornyl acetate (5.2%), α-cadinol (4.2%), δ-elemene (3.7%), and the ketone shyobunone (3.5%) as the major compounds (Minteguiaga et al. 2018b), whereas the EO from the leaves sourced from Guaíba, Brazil, have comprised bicyclogermacrene (9.73%), cis-cadin-4-en-7-ol (6.77%), and (Z)-ocimene (6.33%) (Schossler et al. 2009). Recent studies have also shown differences in the chemical composition of B. punctulata EO. α-Bisabolol was found in higher concentration (23.63%) in the aerial parts of plants collected in Paraná, Brazil (Budel et al. 2018b), whereas EO obtained from leaves of male plants showed δ-elemene (14.29%), germacrene D (11.29%), and bicyclogermacrene (10.90%), and in female plants bicyclogermacrene (42.44%), germacrene D (21.18%), and β-caryophyllene (14.06%) were found as major compounds (Ascari et al. 2019). Even though the chemical composition of EOs is often associated with environmental and phenological influences, it is necessary to investigate whether these variations in B. punctulata are also possibly linked to different chemotypes.
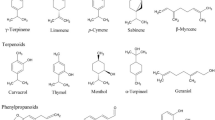
Chemically, EOs are generally composed of terpenoids and phenylpropanoids as the major compounds in addition to few aromatic and aliphatic constituents. Monoterpenes and sesquiterpenes, and their oxygenated derivatives, form the largest group of chemical entities in EOs. In Baccharis EOs, monoterpenes and sesquiterpenes are often found (Figs. 10.2 and 10.3). Sesquiterpenes appeared to be more plentiful in the majority of the species (Campos et al. 2016; Bogo et al. 2016; Zuccolotto et al. 2019; Oliveira et al. 2019; Tomazzoli et al. 2021), as observed in B. anomala, B. ochracea Spreng., B. megapotamica Spreng. (Budel et al. 2012), and B. punctulata (Ascari et al. 2019).
Sesquiterpenoid cyclic alcohols such as ledol, spathulenol, viridiflorol, and palustrol are not only important in the perfume industry due to their agreeable aromatic notes but also have taxonomic value (Minteguiaga et al. 2015).
Trombin-Souza et al. (2017) investigated EOs of 10 species of Baccharis, B. anomala, B. articulata, B. axillaris DC., B. calvescens DC., B. mesoneura DC., B. milleflora (Less.) DC., B. myriocephala DC., B. oblongifolia Pers., B. crispa, and B. uncinella. The major compound present in all species was limonene, whereas α- cadinol, α-thujene, α-pinene, β-pinene, p-cymene, (E)-β-ocimene, γ-terpinene, limonene, myrcene, sabinene, and spathulenol were identified in all samples. In addition, the chemical similarity was highest for B. anomala, B. articulata, B. calvescens, B. milleflora, B. myriocephala, B. crispa, B. oblongifolia, and B. uncinella.
The main components that were evidenced in a recent review that considered only EOs with biological activities were α-thujene (IV), β-caryophyllene (X), β-pinene (II), camphor (V), caryophyllene, caryophyllene oxide (VII), limonene (I), nerolidol (VIII), thymol (VI), thymol acetate, thymol methyl ether, sabinene (III), and spathulenol (IX) (Campos et al. 2016). According to Abad and Bermejo (2007), E-nerolidol, limonene, and spathulenol are the most abundant compounds in the EOs of Baccharis.
A recent investigation identified chemical markers for five Baccharis species with the major compounds spathulenol (22.74%) and kongol (22.22%) in B. microdonta; β-pinene (18.33%) and limonene (18.77%) in B. pauciflosculosa; α-bisabolol (23.63%) in B. punctulata; α-pinene (24.50%) in B. reticularioides Deble & A.S.Oliveira; and α-pinene (10.74%), β-pinene (15.24%), limonene (14.33%), and spathulenol (13.15%) in B. sphenophylla Dusén ex Malme. It is important to highlight that kongol was reported for Baccharis genus for the first time (Budel et al. 2018b).
2.4 Biological Activities of Baccharis Essential Oils
The chemical composition of the EOs generally shows a complex mixture of 20–60 compounds in different concentrations with 2 or 3 of the components in higher concentrations. In general, the major compounds present in the EOs are responsible for the biological activities of the oils (Guimarães et al. 2012). For example, nerolidol, a sesquiterpene found in many EOs, shows insecticidal and repellent activities, and the EOs containing this as a major compound exhibit these properties as well (Priestley et al. 2006).
Some plants possess major compounds characterizing the EOs. For example, B. tricuneata (L.f.) Pers. var. ruiziana presents more than 68% of E-nerolidol (Arze et al. 2004), making the correlation between the chemical composition and biological activities more evident. However, compounds present in lower concentration can act synergistically with other components, contributing to the action (Henriques et al. 2009).
2.4.1 Antioxidant Activity
The EO of B. trinervis Pers. showed significant antioxidant activity with IC50 values of 49.0 mg/mL and 28.87 mg/mL by the free radical DPPH• scavenging assay and β-carotene/linoleic-acid oxidation model system, respectively. The chemical profile showed α-phellandrene (27.79%), (Z)-lachnophyllum (14.04%), sabinene (13.03%), (Z)-β-ocimene (8.13%), and α-thujene (6.65%) as the main components (Sobrinho et al. 2016).
EO of B. milleflora also evidenced antioxidant activity by DPPH• methods with IC50 between 15.45 ± 0.52 and 21.06 ± 0.15 μg/mL, phosphomolybdenum with AAR 77.9 ± 0.90% – 79.81 ± 2.30%, TBARS with IA 12.60 ± 0.78% – 29.06 ± 1.47%, and ABTS•+ with IC50 between 3.85 ± 0.10 and 4.60 ± 0.07 μg/mL (Pereira et al. 2016b). The chemical profile of the EO collected in the four seasons was trans-caryophyllene (7.65–13.41%), germacrene-D (6.83–11.18%) and bicyclogermacrene (9.99–12.89%) (Pereira et al. 2016b).
EO extracted from leaves of B. oreophila Malme evidenced antioxidant capacity by three different methods, FRAP (4.09 μmol FeSO4 E/mL), ABTS•+ (1.45 μmol TE/mL), and DPPH• (1.04 μmol TE/mL).
2.4.2 Anti-inflammatory Effects
EOs of several species of Baccharis have been studied to assess their anti-inflammatory potentials. Florão et al. (2012) analyzed EOs extracted from the aerial parts of B. articulata, B. crispa, B. dracunculifolia, and B. gaudichaudiana DC. focusing on their immunomodulatory activities. All species except B. articulata inhibited expressively the proliferation of their phytohemagglutinin-stimulated counterparts. B. dracunculifolia showed the best anti-inflammatory effects, inhibiting significantly the casein-induced human granulocyte chemotaxis. The major compounds identified were spathulenol in B. articulata, B. dracunculifolia, and B. gaudichaudiana, as well as τ-gurjunene in B. gaudichaudiana and palustrol in B. articulata (Florão et al. 2012).
In a recent study, Ascari et al. (2019) subjected the leaf EOs of B. punctulata (male and female) to in vivo anti-inflammatory and in vitro antioxidant tests. Topical administration of both EOs was able to inhibit the formation of TPA-induced edema in the treated groups. Histological analysis evidenced that topical application of TPA promoted intense cellular infiltration. The results found in the ROS and DPPH• tests suggest that both samples were able to reduce the inflammatory cells influx and had in vitro antioxidant properties, respectively. The major compounds found in the EO of B. punctulata were δ-elemene (14.29%), germacrene D (11.29%), and bicyclogermacrene (10.90%) in the male sample and bicyclogermacrene (42.44%), germacrene D (21.18%), and β-caryophyllene (14.06%) in the female sample (Ascari et al. 2019).
2.4.3 Antiulcerogenic Activity
EO of B. dracunculifolia was subjected to a test for antiulcerogenic action. The treatment in the doses of 50, 250, and 500 mg/kg of EO expressively reduced the lesion index, the total lesion area, and the percentage of lesions in comparison with both positive and negative control groups. The major compounds of EO were nerolidol (23.58%), germacrene D (21.54%), bicyclogermacrene (19.24%), trans-caryophyllene (7.12%), and spathulenol (6.03%) (Massignani et al. 2009).
2.4.4 Cytotoxic Effects
Cytotoxic activity of EO sourced from the cladodes of B. milleflora was investigated in relation to Jurkat, Raji, and HL-60 cells, as well as the cell mechanisms. All the tumor cells showed IC50 values lower than 50 μg/mL at 24, 48, and 72 h by MTT assay. The decrease in cell DNA content was demonstrated due to the inhibition of the proliferation of Jurkat, Raji, and HL-60 cells. Raji cells evidenced the greatest inhibition of cell proliferation. The EO acted via both necrotic and apoptotic mechanisms. The chemical profile showed bicyclogermacrene (12.16%), germacrene D (11.18%), (E)-caryophyllene (9.28%), and α-humulene (8.05%) as the main components (Pereira et al. 2017).
2.4.5 Sedative Effects
B. uncinella is reported to be used by the Laklaño Indians in Santa Catarina State in Brazil for sedative purposes. Ascari et al. (2012) studied EO of B. uncinella collected from different locations in Paraná and Santa Catarina. Both of these samples significantly decreased locomotion and body temperature, as well as increased sleeping time. However, the hypnotic activity was sensitive to the differences in monoterpene composition. Sedative activity was observed better in B. uncinella that was collected in Santa Catarina because it presented a higher monoterpene/sesquiterpene ratio (0.31) in comparison to the other sample that showed a lower monoterpene/sesquiterpene ratio (0.004). The main compounds in the EO from Santa Catarina were caryophyllene (26.13%), spathulenol (13.39%), caryophyllene oxide (13.26%), limonene (7.21%), and α-pinene (6.42%), whereas the EO from Parana showed spathulenol (32.93%), caryophyllene oxide (27.78%), viridiflorol (5.29%), and α-cadinol (2.42%) as the major components (Ascari et al. 2012).
2.4.6 Antimicrobial Effects
EO of B. dracunculifolia was tested against Candida strains isolated from infants and their mothers during the lactation period to verify its enzymatic action and sensitivity. All strains were sensitive to EO of B. dracunculifolia with MIC between 0.2 and 6.25 mg/mL. The EO inhibited the growth of all strains, including the ones resistant to commercial antifungal agents (Pereira et al. 2011).
EO of B. tridentata Baker was observed in vitro to inhibit mycelial growth of the plant pathogens Fusarium oxysporum, Colletotrichum gloeosporioides, and Rhizoctonia solani. The chemical composition of the EO evidenced α-thujene (22.93%), β-pinene (20.33%), and β-felandrene (16.15%) as major compounds (Souza et al. 2011).
EO of B. darwinii Hook. & Arn. showed strong antifungal activity against yeast and dermatophytes of clinical relevance, including some fungi such as Candida spp. and Trichophyton spp. with MIC values between 62.5 and 125 μg/mL (Kurdelas et al. 2012).
EO sourced from the twigs of B. semiserrata DC. presented moderate antibacterial activity against Staphylococcus aureus, while EO from the leaves showed weak activity against S. aureus and Bacillus cereus. EOs from leaf and twig were active against Microsporum gypseum, Candida albicans, Epidermophyton floccosum, Trichophyton mentagrophytes, and Cryptococcus neoformans. The major compounds identified in the leaf EO included β-pinene (11.4%), spathulenol (9.8%), and E-nerolidol (9.6%), while the EO from twigs showed spathulenol (25.1%), limonene (9.1%), and caryophyllene oxide (8.0%) as major compounds. Baccharis semiserrata leaf EO was also active against Trichophyton rubrum (Vannini et al. 2012). This species and T. mentagrophytes also showed sensitivity when treated with EO from B. latifolia (Valarezo et al. 2013).
EO of B. coridifolia was tested for its antibiotic properties (ampicillin, cephalothin, chloramphenicol, gentamicin, and tetracycline) alone and in combination with the EO (4% v/v) through the disk diffusion susceptibility test. The occurrence of the synergistic or antagonistic effect was observed in both bacterial strains assessed, S. aureus and E. coli (Onofre et al. 2013).
The EO from the aerial parts of B. obtusifolia exhibited a moderate antibacterial effect against Klebsiella pneumoniae and Enterococcus faecalis and good antifungal activity against Trichophyton rubrum and T. mentagrophytes. The major compounds of the EO were limonene (28.3%), germacrene-D (9.8%), α-pinene (9.0%), β-pinene (8.2%), bicyclogermacrene (6.2%), and δ-cadinene (5.7%) (Valarezo et al. 2015).
Antimicrobial and antibiofilm activities of B. psiadioides (Less.) Joch.Müll. EO were tested against antibiotic-resistant E. faecalis strains. The oil inhibited the growth of the multidrug-resistant E. faecalis strains and was also effective when evaluated against biofilms. However, its activity was stronger when inhibiting the formation of a biofilm than when applied over established biofilms. The chemical profile showed β-pinene as the major component (33.13%), followed by D-3-carene (11.41%), limonene (5.97%), (E)-ocimene (3.82%), and α-pinene (2.58%) (Negreiros et al. 2016).
EO of B. dracunculifolia was studied to determine the minimal inhibitory concentration (MIC) against planktonic cultures of S. mutans and its antibacterial activity in biofilms formed in the discs of composite resin. The MIC of the B. dracunculifolia EO to planktonic growth of S. mutans was 6%. In biofilms of S. mutans clinical isolates, EO (6%) and chlorhexidine resulted in reductions of 53.3–91.1% and 79.1–96.6%, respectively. For the biofilm formed by the S. mutans reference strain, the reductions achieved with B. dracunculifolia EO and chlorhexidine were 39.3% and 88.1%, respectively (Pereira et al. 2016a).
EO from leaves of B. oreophila showed antimicrobial effects against S. aureus (10.33 ± 0.5 mm, MIC = 1250 μg/mL) and C. albicans (8.66 ± 0.5 mm, MIC >2500 μg/mL). The major compounds of the EO were khusimone (16.37%) and spathulenol (16.12%).
Although there are several EOs presenting antimicrobial activities, no activity was reported for the EO of B. dracunculifolia tested against methicillin-resistant S. aureus and Mycobacterium intracellulare (Parreira et al. 2010). Besides, EO of B. uncinella was inactive against all bacteria tested (Vannini et al. 2012).
EOs from B. organensis Baker, B. burchellii Baker, and B. aracatubaensis Malag. (male and female specimens) were tested against S. aureus, E. coli, P. aeruginosa, and C. albicans. They also did not exhibit any antimicrobial activity (Zuccolotto et al. 2019).
2.4.7 Insecticidal Activities
Allelochemical effects of EO of B. salicifolia (Ruiz & Pav.) Pers. and some isolated compounds were tested against adult red flour beetles, Tribolium castaneum. The EO showed toxicity and repellence activities. β-Pinene and pulegone were observed to be the most acutely toxic compounds after 3 days of treatment, whereas α-terpineol was identified as the most repellent compound (García et al. 2005).
EO of B. spartioides (Hook. & Arn. ex DC.) J. Rémy in Gay was evaluated for its repellency against Aedes aegypti. At concentrations of 12.5%, the EO showed the longest repellency. The major chemical compound of the EO was camphor (50.5%) (Gillij et al. 2008).
Baccharis salicifolia (Ruiz & Pav.) Pers. EO collected in Argentina showed α-pinene (21.7%) and spathulenol (14.4%) as the major compounds. EO was tested against A. aegypti and presented moderate repellency (Gleiser et al. 2011). This species was also collected in two other locations in San Luis, Argentina. In this study, the chemical composition of the EO was different; the major compounds were (Z)-b-ocimene, germacrene D, muuroladiene, and β-cubebene, with the addition of α-thujene and α-phellandrene, in location A and isoledene in location B. The EO from B. salicifolia from location A exhibited post-ingestive toxicity to Spodoptera littoralis larvae without antifeedant effects (Sosa et al. 2012).
EO of B. darwinii was tested in vitro to evaluate its insecticidal properties. It showed insecticidal activity against the Mediterranean fruit fly, Ceratitis capitata, with LD50 values of 19.9 and 31.0 μg/fly for males and females, respectively, at 72 h. The EO also displayed repellent activity against Chagas disease vector, Triatoma infestans, with an average repellence rate of 92%. The major components with recognized insecticidal and antimicrobial activities were limonene (47.1%), thymol (8.1%), and 4-terpineol (6.4%) (Kurdelas et al. 2012).
EO and its major compounds from aerial parts of B. dracunculifolia were tested against unengorged larvae and engorged females of the cattle tick Rhipicephalus microplus. In the larval packet test, the EO, as well as the pure compound nerolidol, exhibited high activity showing more than 90% mortality at concentrations from 15.0 and 10.0 mg/mL, respectively, whereas limonene did not show acaricidal activity. In the female immersion test, the EO and nerolidol also caused a reduction in the quantity and quality of eggs produced with a control rate of 96.3% and 90.3% at concentrations of 60.0 and 50.0 mg/mL, respectively (Lage et al. 2015).
EOs of B. milleflora collected during four different seasons showed repellency action IR 80.65 ± 6.69%, well above the IR 36.39 ± 21.00 found for the EO of Cymbopogon nardus, which was used as a standard. The fumigant activity showed a KT50 (min) of 10.63 ± 2.68 and 22.70 ± 3.40 for the EOs of B. milleflora and Melaleuca alternifolia, respectively (Pereira et al. 2016b).
Baccharis sphenophylla collected in South Brazil exhibited strong toxicity to Cimex lectularius (bed bug) in the fumigation bioassay, causing 66.67% mortality in “Bayonne” and 83.33% in “Ft.Dix” (Budel et al. 2018b).
2.4.8 Parasiticidal Activities
2.4.8.1 Antitrypanosomal Effects
EOs of five Baccharis species (B. microdonta, B. pauciflosculosa, B. reticularioides, B. punctulata, and B. sphenophylla) were submitted to test their antitrypanosomal activities against trypomastigotes cultures of T. brucei. All Baccharis species exhibited remarkable antitrypanosomal activities. B. pauciflosculosa demonstrated the highest effect, 0.31 μg/mL (IC50) and 0.52 μg/mL (IC90), followed by B. reticularioides at 0.96 μg/mL (IC50) and 2.49 μg/mL (IC90), and B. sphenophylla at 1.14 μg/mL (IC50) and 2.38 μg/mL (IC90) (Budel et al. 2018b).
2.4.8.2 Antimalarial Effects
EOs of five species of Baccharis were investigated against chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of Plasmodium falciparum. The results were, respectively, 10.90 μg/mL ± 0.98 and 14.20 μg/mL ± 1.08 for B. pauciflosculosa, 20.32 μg/mL ± 4.37 and 34.35 ± 10.15 for B. reticularioides, and 27.58 ± 1.64 and 32.53 ± 16.5 for B. sphenophylla, showing moderate antimalarial activities. B. microdonta and B. punctulata exhibited cytotoxicity to Vero cells (selectivity control) (Budel et al. 2018b).
EO of B. dracunculifolia was inactive in the antiplasmodial assay. Chloroquine and artemisinin were used as positive control. The IC50 values were 0.018 and 0.014 mg/mL, respectively, against P. falciparum (D6 clone) (Parreira et al. 2010).
2.4.8.3 Leishmanicidal Activity
EO of B. dracunculifolia showed activity against promastigote forms of Leishmania donovani with IC50 values of 42 μg/mL. The major compounds of the EO were E-nerolidol (33.51%) and spathulenol (16.24%). Pentamidine and amphotericin B were used as positive controls, which showed IC50 values of 1.9 mg/mL and 0.65 mg/mL, respectively (Parreira et al. 2010).
2.4.8.4 Schistosomicidal Activity
EO of B. dracunculifolia also displayed high schistosomicidal activity against the trematode parasite, Schistosoma mansoni, killing all pairs of adult worms after incubation with the EO (10, 50, and 100 μg/mL) (Parreira et al. 2010).
EO of B. crispa showed in vitro schistosomicidal activities. Its effects on the reproductive fitness and the tegumental alteration of adult worms were similar to those induced by praziquantel. The results evidenced a significant decline in the motility of the worms and a mortality rate of 100% 30 h after their exposure to the EO in the concentration of 130 μg/mL. As for the morphological changes, the EO of B. crispa induced a peeling on the tegument surface, as well as the destruction of tubercles and spines, which resulted in smooth areas on the body surface. The EO also caused tegument destruction in female worms, in addition to the destruction of the oral and acetabular suckers (Oliveira et al. 2012).
2.4.9 Adjuvant Property
Leaf EO of B. dracunculifolia was assessed for its inhibitory properties on the coagulating and fibrinogenolysis activities induced by Lachesis muta, Bothrops atrox, and Bothrops moojeni snake venoms. The EO caused 100% inhibition on the fibrinogenolysis induced by B. moojeni and L. muta venoms, evidencing that the EO can be used as adjuvants for the treatment of snakebites (Miranda et al. 2016).
3 Final Considerations
Essential oils of Baccharis species have numerous medicinally beneficial properties. Several pharmacological properties and biological activities, including anti-inflammatory, antimicrobial, antiulcerogenic, antimalarial, antioxidant, antitrypanosomal, cytotoxic, insecticidal, leishmanicidal, schistosomicidal, sedative, and adjuvant properties, have been reported for the EOs of Baccharis. EOs and their major compounds of many Baccharis species have shown significant activities against many insect pests, microorganisms, and human parasites.
Baccharis EOs mainly contain monoterpenes and sesquiterpenes, while the latter compounds are more plentiful in the majority of the species. The most abundant compounds in the EOs of Baccharis are E-nerolidol, limonene, and spathulenol. EOs of many of the species have unique compounds, which can be used as chemical markers for species identification and quality control.
The genus Baccharis comprises about 406 species and almost all species produce EOs of varying chemical compositions and medicinal properties. However, this review of the literature indicates that only less than 10% of the Baccharis oils have been studied. Even among these studied species, only very few, such as B. dracunculifolia, are studied in detail. A large portion of this medicinally important genus is still unexplored. Future research focusing on the taxonomy and chemical and biological aspects of the EOs and major compounds of different species of Baccharis could bring out the hidden wealth and usefulness of this important genus.
References
Abad MJ, Bermejo P (2007) Baccharis (Compositae): a review update. ARKIVOC 7:76–96. https://doi.org/10.3998/ark.5550190.0008.709
Arze JBL, Garneau FX, Collin G et al (2004) Essential oils from Bolivia. I. Asteraceae: Baccharis tricuneata (L.f.) Pers. var. ruiziana Cuatrecassas. J Essent Oil Res 16:429–431. https://doi.org/10.1080/10412905.2004.9698763
Ascari J, Sens SL, Nunes DS et al (2012) Sedative effects of essential oils obtained from Baccharis uncinella. Pharm Biol 50:113–119. https://doi.org/10.3109/13880209.2011.634423
Ascari J, Oliveira MS, Nunes DS et al (2019) Chemical composition, antioxidant and anti-inflammatory activities of the essential oils from male and female specimens of Baccharis punctulata (Asteraceae). J Ethnopharmacol 24:1–7
Besten MA, Jasinski VCG, Costa AGLC et al (2012) Chemical composition similarity between the essential oils isolated from male and female specimens of each five Baccharis species. J Braz Chem Soc 23:1041–1047. https://doi.org/10.1590/S0103-50532012000600007
Bogo CA, Andrade MH, Paula JP et al (2016) Comparative analysis of essential oils of Baccharis L.: a review. Rev Strict Sensu 1:1–11. https://doi.org/10.24222/2525-3395.2016v1n2p001
Botas GS, Cruz RAS, Almeida FB et al (2017) Baccharis reticularia DC. and limonene nanoemulsions: promising larvicidal agents for Aedes aegypti (Diptera: Culicidae) control. Molecules 22:1–14. https://doi.org/10.3390/molecules22111990
Budel JM, Matzenbacher NI, Duarte MR (2008) Genus Baccharis (Asteraceae): a review of chemical and pharmacological studies. In: Singh VK, Govil JN (eds) Recent progress in medicinal plants – phytopharmacology and therapeutic value. Studium Press LLC, Houston, pp 1–18
Budel JM, Duarte MR, Döll-Boscardin PM et al (2012) Composition of essential oils and secretory structures of Baccharis anomala, B. megapotamica and B. ochracea. J Essent Oil Res 24:19–24. https://doi.org/10.1080/10412905.2012.645634
Budel JM, Raman V, Monteiro LM et al (2018a) Foliar anatomy and microscopy of six Brazilian species of Baccharis (Asteraceae). Microsc Res Tech 81:832–842. https://doi.org/10.1002/jemt.23045
Budel JM, Wang M, Raman V et al (2018b) Essential oils of five Baccharis species: investigations on the chemical composition and biological activities. Molecules 23:1–19. https://doi.org/10.3390/molecules23102620
Calo JR, Crandall PG, O’Bryan CA et al (2015) Essential oils as antimicrobials in food systems – a review. Food Control 54:111–119. https://doi.org/10.1016/j.foodcont.2014.12.040
Campos FR, Bressan J, Jasinski VCG et al (2016) Baccharis (Asteraceae): chemical constituents and biological activities. Chem Biodivers 13:1–17. https://doi.org/10.1002/cbdv.201400363
Elshafie HS, Camele I (2017) An overview of the biological effects of some Mediterranean essential oils on human health. Biomed Res Int 27:1–14. https://doi.org/10.1155/2017/9268468
Fabiane KC, Ferronatto R, Santos AC, Onofre SB (2008) Physicochemical characteristics of the essential oils of Baccharis dracunculifolia and Baccharis uncinella DC. (Asteraceae). Rev Bras Farmacogn 18:197–203
Florão A, Budel JM, Duarte MR et al (2012) Essential oils from Baccharis species (Asteraceae) have anti-inflammatory effects for human cells. J Essent Oil Res 24:561–570. https://doi.org/10.1080/10412905.2012.728081
Franz C, Novak J (2016) Sources of essential oils. In: Baser KHC, Buchbauer G (eds) Handbook of essential oils: science, technology, and applications, 2nd edn. CRC Press, Boca Raton, pp 43–86
Freire SE, Urtubey E, Giuliano DA (2007) Epidermal characters of Baccharis (Asteraceae) species used in traditional medicine. Caldasia 29:23–38
García M, Donadel OJ, Ardanaz CE et al (2005) Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum. Pest Manag Sci 61:612–618. https://doi.org/10.1002/ps.1028
Gillij YG, Gleiser RM, Zygadlo JA (2008) Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour Technol 99:2507–2515. https://doi.org/10.1016/j.biortech.2007.04.066
Gleiser RM, Bonino MA, Zygadlo JA (2011) Repellence of essential oils of aromatic plants growing in Argentina against Aedes aegypti (Diptera: Culicidae). Parasitol Res 108:69–78. https://doi.org/10.1007/s00436-010-2042-4
Gobbo-Neto L, Lopes NP (2007) Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim Nova 30:374–381
Guimarães AG, Oliveira AP, Ribeiro EAN et al (2012) Atividade farmacológica de monoterpenos. In: Souza GHB, Melo JCP, Lopes NP (eds) Farmacognosia: Coletânea Científica. UFOP, Minas Gerais, pp 219–250
Henriques AT, Simões-Pires CA, Apel MA (2009) Óleos essenciais: importância e perspectivas terapêuticas. In: Yunes RA, Cechinel-Filho V (eds) Química de produtos naturais, novos fármacos e a moderna farmacognosia, 2nd edn. UNIVALI, Santa Catarina, pp 219–256
Kurdelas RR, López S, Lima B et al (2012) Chemical composition, anti-insect and antimicrobial activity of Baccharis darwinii essential oil from Argentina, Patagonia. Ind Crop Prod 40:261–267. https://doi.org/10.1016/j.indcrop.2012.03.024
Lage TCA, Montanari RM, Fernandes AS et al (2015) Chemical composition and acaricidal activity of the essential oil of Baccharis dracunculifolia De Candole (1836) and its constituents nerolidol and limonene on larvae and engorged females of Rhipicephalus microplus (Acari: Ixodidae). Exp Parasitol 148:24–29. https://doi.org/10.1016/j.exppara.2014.10.011
Lago JHG, Romoff P, Fávero OA et al (2008) Chemical composition of male and female Baccharis trimera (Less.) DC. (Asteraceae) essential oils. Biochem Syst Ecol 36:737–740. https://doi.org/10.1016/j.bse.2008.05.009
Malizia RA, Cardell DA, Molli JS et al (2005) Volatile constituents of leaf oils from the genus Baccharis. Part II: Baccharis obovata hooker et Arnott and B. salicifolia (Ruiz et Pav.) Pers. species from Argentina. J Essent Oil Res 17:194–197. https://doi.org/10.1080/10412905.2005.9698873
Massignani JJ, Lemos M, Maistro EL et al (2009) Antiulcerogenic activity of the essential oil of Baccharis dracunculifolia on different experimental models in rats. Phytother Res 23:1355–1360. https://doi.org/10.1002/ptr.2624
Minteguiaga M, Umpiérrez N, Xavier V et al (2015) Recent findings in the chemistry of odorants from four Baccharis species and their impact as chemical markers. Chem Biodivers 12:1339–1348. https://doi.org/10.1002/cbdv.201400329
Minteguiaga M, Mercado MI, Ponessa GI et al (2018a) Morphoanatomy and essential oil analysis of Baccharis trimera (Less.) DC. (Asteraceae) from Uruguay. Ind Crop Prod 112:488–498. https://doi.org/10.1016/j.indcrop.2017.12.040
Minteguiaga M, González A, Cassel E et al (2018b) Volatile constituents from Baccharis spp. L. (Asteraceae): chemical support for the conservation of threatened species in Uruguay. Chem Biodivers 15:e1800017. https://doi.org/10.1002/cbdv.201800017
Miranda CASF, Cardoso MG, Marcussi S et al (2016) Clotting and fibrinogenolysis inhibition by essential oils from species of the Asteraceae Family. Braz Arch Biol Technol 59:e16150775. https://doi.org/10.1590/1678-4324-2016150775
Negreiros MO, Pawlowski A, Zinic CA et al (2016) Antimicrobial and antibiofilm activity of Baccharis psiadioides essential oil against antibiotic-resistant Enterococcus faecalis strains. Pharm Biol 54:3272–3279. https://doi.org/10.1080/13880209.2016.1223700
Németh-Zámboriné É (2016) Natural variability of essential oil components. In: Baser KHC, Buchbauer G (eds) Handbook of essential oils: science, technology, and applications, 2nd edn. CRC Press, Boca Raton, pp 87–126
Oliveira RN, Rehder VLG, Oliveira ASS et al (2012) Schistosoma mansoni: in vitro schistosomicidal activity of essential oil of Baccharis trimera (Less.) DC. Exp Parasitol 132:135–143. https://doi.org/10.1016/j.exppara.2012.06.005
Oliveira CT, Maia BHLNS, Ferriani AP et al (2019) Chemical characterization, antioxidant capacity and antimicrobial potential of essential oil from the leaves of Baccharis oreophila Malme. Chem Biodivers 16:e1800372. https://doi.org/10.1002/cbdv.201800372
Onofre SB, Canton M, Pires PA (2013) Action of essential oils obtained from Baccharis coridifolia DC. (Asteraceae-Astereae) on the activity of antibiotics. Adv Microbiol 3:166–170. https://doi.org/10.4236/aim.2013.32026
Parreira NA, Magalhães LG, Morais DR et al (2010) Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia. Chem Biodivers 7:993–1001. https://doi.org/10.1002/cbdv.200900292
Pereira CA, Costa ACBP, Machado AKS et al (2011) Enzymatic activity, sensitivity to antifungal drugs and Baccharis dracunculifolia essential oil by Candida strains isolated from the oral cavities of breastfeeding infants and in their mothers’ mouths and nipples. Mycopathologia 171:103–109. https://doi.org/10.1007/s11046-010-9353-y
Pereira CA, Costa ACBP, Liporoni PCS et al (2016a) Antibacterial activity of Baccharis dracunculifolia in planktonic cultures and biofilms of Streptococcus mutans. J Infect Public Health 9:324–330. https://doi.org/10.1016/j.jiph.2015.10.012
Pereira CB, Farago PV, Borsato DM et al (2016b) Chemical composition and biological activities of Baccharis milleflora essential oil. Lat Am J Pharm 35:2225–2233
Pereira CB, Kanunfre CC, Farago PV et al (2017) Cytotoxic mechanism of Baccharis milleflora (Less.) DC. essential oil. Toxicol in Vitro 42:214–221. https://doi.org/10.1016/j.tiv.2017.04.031
Perera WH, Bizzo HR, Gama PE et al (2017) Essential oil constituents from high altitude Brazilian species with antimicrobial activity: Baccharis parvidentata Malag., Hyptis monticola Mart. ex Benth. and Lippia origanoides Kunth. J Essent Oil Res 29:109–116. https://doi.org/10.1080/10412905.2016.1210039
Priestley CM, Burges IF, Williamson EM (2006) Lethality of essential oil constituents towards the human louse, Pediculus humanus, and its eggs. Fitoterapia 77:303–309. https://doi.org/10.1016/j.fitote.2006.04.005
Sayuri VA, Romoff P, Fávero OA et al (2010) Chemical composition, seasonal variation, and biosynthetic considerations of essential oils from Baccharis microdonta and B. elaeagnoides (Asteraceae). Chem Biodivers 7:2771–2782. https://doi.org/10.1002/cbdv.201000083
Schmidt E (2016) Production of essential oils. In: Baser KHC, Buchbauer G (eds) Handbook of essential oils: science, technology, and applications, 2nd edn. CRC Press, Boca Raton, pp 127–164
Schossler P, Schneider GL, Wunsch D et al (2009) Volatile compounds of Baccharis punctulata, Baccharis dracunculifolia and Eupatorium laevigatum obtained using solid phase microextraction and hydrodistillation. J Braz Chem Soc 20:277–287. https://doi.org/10.1590/S0103-50532009000200012
Sobrinho ACN, Souza EB, Rocha MFG et al (2016) Chemical composition, antioxidant, antifungal and hemolytic activities of essential oil from Baccharis trinervis (Lam.) Pers. (Asteraceae). Ind Crop Prod 84:108–115. https://doi.org/10.1016/j.indcrop.2016.01.051
Sosa ME, Lancelle HG, Tonn CE et al (2012) Insecticidal and nematicidal essential oils from Argentinean Eupatorium and Baccharis spp. Biochem Syst Ecol 43:132–138. https://doi.org/10.1016/j.bse.2012.03.007
Souza SP, Cardoso MG, Souza PE et al (2011) Óleo essencial de Baccharis tridentata Vahl: composição química, atividade antioxidante e fungitóxica, e caracterização morfológica das estruturas secretoras por microscopia eletrônica de varredura. Rev Bras Pl Med 13:456–466. https://doi.org/10.1590/S1516-05722011000400011
Tischer B, Vendruscolo RG, Wagner R et al (2017) Effect of grinding method on the analysis of essential oil from Baccharis articulata (Lam.) Pers. Chem Pap 71:753–761. https://doi.org/10.1007/s11696-016-0052-0
Tomazzoli MM, Amaral W, Cipriano RR et al (2021) Chemical composition and antioxidant activity of essential oils from populations of Baccharis dracunculifolia DC. in southern Brazil. Braz Arch Biol Technol 64: e21190253. https://doi.org/10.1590/1678-4324-202119025
Trombin-Souza M, Trombin-Souza M, Amaral W et al (2017) Chemical composition of the essential oils of Baccharis species from southern Brazil: a comparative study using multivariate statistical analysis. J Essent Oil Res 29:400–406. https://doi.org/10.1080/10412905.2017.1322007
Valarezo E, Rosillo M, Cartuche L et al (2013) Chemical composition, antifungal and antibacterial activity of the essential oil from Baccharis latifolia (Ruiz & Pav.) Pers. (Asteraceae) from Loja, Ecuador. J Essent Oil Res 25:233–238. https://doi.org/10.1080/10412905.2013.775679
Valarezo E, Rosales J, Morocho V et al (2015) Chemical composition and biological activity of the essential oil of Baccharis obtusifolia Kunth from Loja, Ecuador. J Essent Oil Res 27:212–216. https://doi.org/10.1080/10412905.2015.1007217
Vannini AB, Santos TG, Fleming AC et al (2012) Chemical characterization and antimicrobial evaluation of the essential oils from Baccharis uncinella DC and Baccharis semiserrata DC. (Asteraceae). J Essent Oil Res 24:547–554. https://doi.org/10.1080/10412905.2012.728092
Xavier VB, Vargas RMF, Minteguiaga M et al (2013) Evaluation of the key odorants of Baccharis anomala DC. essential oil: new applications for known products. Ind Crop Prod 49:492–496. https://doi.org/10.1016/j.indcrop.2013.05.011
Zuccolotto T, Bressan J, Lourenço AVF et al (2019) Chemical, antioxidant, and antimicrobial evaluation of essential oils and an anatomical study of the aerial parts from Baccharis species (Asteraceae). Chem Biodivers 64:e1800547. https://doi.org/10.1002/cbdv.201800547
Zunino MP, Lopez ML, Zygadlo JA et al (2004) Essential oil composition of Baccharis articulata (Lam.) Pers. J Essent Oil Res 16:29–30. https://doi.org/10.1080/10412905.2004.9698643
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Manfron, J., Raman, V., Khan, I.A., Farago, P.V. (2021). Essential Oils of Baccharis: Chemical Composition and Biological Activities. In: Fernandes, G.W., Oki, Y., Barbosa, M. (eds) Baccharis. Springer, Cham. https://doi.org/10.1007/978-3-030-83511-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-83511-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83510-1
Online ISBN: 978-3-030-83511-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)