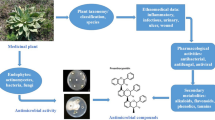
Abstract
In recent years, there has been a continuous increase in resistance to traditional antibiotics developed by pathogenic bacteria. However, in many parts of the world, several medicinal plants are traditionally used to control infectious microorganisms. Because of this, antimicrobial agents derived from natural products have received a lot of attention, both for their effectiveness and also for being more economically accessible. Therefore, the research discussed in this chapter aims to conduct a systematic review on the use of medicinal plants and isolated compounds as a potential antimicrobial agent. The study focuses on an investigation of several electronic databases: Scopus, Web of Science, Academic Google, SciELO, PubMed, SciFinder, and ScienceDirect. The results may contribute to the increase of strategies for the treatment of infections caused by microorganisms. Since medicinal plants play an essential role in health, they may represent a significant source of new antimicrobial drugs to combat microorganisms resistant to multidrug.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Starting from the twentieth century, there were significant advances in the field of science with the use of antimicrobial drugs, such as penicillin which was discovered in 1928 by Sir Alexander Fleming, intending to improve human life expectancy and control microbial infections (Aminov 2010). Antimicrobials can be chemotherapy (synthetic) or antibiotics (natural substances) that inhibit the development of microorganisms or even destroy them. Antimicrobial drugs are considered the second-most used drug class globally (Saez-Llorens et al. 2000; Abushaheen et al. 2020).
As these drugs are more widely used, microorganisms develop resistance, quickly causing a global problem (Anand et al. 2020). The use of antimicrobial drugs without a medical prescription is a problem reported at least half a century ago (Scheckler and Bennett 1970; Kunin et al. 1973). This is due to the misdiagnosis between a bacterial infection and a viral infection, the absence of educational programs for the rational use of antimicrobials or the wide distribution of antimicrobials. In this way, the use of these drugs and improper self-medication exacerbate antimicrobial resistance (Mota et al. 2010).
This resistance to antibiotics has become one of the main problems of humanity since the end of the twentieth century. Therefore, the search for new antimicrobials effective in eliminating microbes becomes necessary, given that the traditional approaches to find new antimicrobial drugs are no longer effective due to the rapid resistance developed against them (Abreu et al. 2012).
One of the strategies applied for the treatment of infectious diseases would be natural products. For several years, medicinal plants derived from these natural products play an essential role in health. They could represent a significant source of new antimicrobial drugs to combat multidrug-resistant microorganisms (Aleksic Sabo and Knezevic 2019). Besides, antimicrobial resistance to plant extracts would be less likely, since a wide variety of their active compounds are found in these extracts that could reverse antibiotic resistance and minimize man’s exposure to resistant bacteria (Gupta and Birdi 2017).
Bioactive compounds called natural products come from the biosynthesis of plants, lichens, fungi, bacteria, and microorganisms. Antibiotics, terpenoids, alkaloids, and polyketides are the main microbial natural products (Gunatilaka and Wijeratne 2012; Huang and Lin 2017; Newman and Cragg 2016).
It is noteworthy that approximately 50% of approved drugs are related to plants or natural products, and 10% of all antimicrobial agents are from natural plant products (Al-Marzoqi et al. 2016; Cowan 1999; Veeresham 2012). In this way, natural products are still used as a powerful therapy against pathogenic bacteria and are considered the pillars of discovering new antibiotics (Wright 2017).
According to Moore et al. (2017), there is an evident lack of new antimicrobial drugs. They are emphasizing the need to search for new bioactive therapeutic agents as treatment strategies for infections caused by microorganisms. In addition to medicinal plants, critical antimicrobial properties have been evidenced, demonstrating the enormous potential that does not control infectious diseases or that suggests as a valuable source of research for the removal of antimicrobial compost.
2 Antimicrobial Resistance
Antimicrobial resistance is a global public health problem, which has worsened over time, as infections caused by resistant microorganisms, in many cases, do not respond to treatment, which can increase hospital costs and increase the risk of death (Fair and Tor 2014). Some studies report that if there are no measures to neutralize this problem, there may be a beginning of a post-antibiotic era, with a shortage of effective treatments based on antimicrobials (Vasoo et al. 2015; Vanegas-Múnera and Jiménez-Quiceno 2020).
As these antimicrobials are indiscriminately used, microorganisms were developed by creating different forms of resistance, which led to a global problem (Davies and Davies 2010). Alexander Fleming, who received the Nobel Prize in Medicine in 1945, warned about the severe problem of antimicrobial resistance. Still, human being did nothing, and thus resistant bacteria emerged as a result of natural selection (Ventola 2015). This problem worsened over time due to the inappropriate use of antibiotics, was also favored by the lack of standards and control of their service, such as the ease of acquiring these drugs without a prescription, in addition to false marketing of antimicrobials (Organización de las Naciones Unidas para la Alimentación y la Agricultura 2016).
The first reports of antimicrobial resistance were published in 1944, three years after starting the use of penicillin. The isolates of Staphylococcus aureus were reported, these being the first isolates resistant to this antibiotic. Since then, the situation has only worsened; at times in the 1980s, there were few options of antibiotics available (Tang et al. 2014). Currently, the resistance rate is so high that some doctors use antibiotics that were previously discarded, because they have specific toxicity, such as colistin (Loho and Dharmayanti 2015).
Antimicrobial resistance can be of two types: natural or acquired. Natural or intrinsic, occur when there are bacteria of the same species naturally resistant to some families of antibiotics, which leads these bacteria to have a competitive advantage over other strains; therefore, the antibiotic has no effect. The acquired resistance can be attributed to mutations in chromosomes (stable, spontaneous, and transmitted vertically from generation to generation) or to changes in resistance genes (conjugation, translation or transformation and transmitted horizontally) (Guimarães et al. 2010; Pérez 2017).
The increase in antimicrobial resistance has severe consequences for public health, limiting the therapeutic variability of antimicrobial in combating pathologies in humans (Semret and Haraoui 2019; Costa et al. 2020). This increase in antimicrobial resistance is due to the misuse of these agents to resistant strains, such as methicillin to Staphylococcus aureus, vancomycin-resistant enterococci, resistance to drugs Streptococcus pneumoniae and Mycobacterium tuberculosis, enterobacteria, which produce beta-lactamase, extended-spectrum, and resistant to carbapenems, Pseudomonas aeruginosa, resistant to multiple drugs and Acinetobacter baumannii (Sabo and Knezevic 2019).
Thus, fungi became resistant to polyols, azoles, and echinocandins, which contributed to the emergence of drug-resistant strains and were found in all species of fungi (Robbins et al. 2017). An aggravating factor given the remarkable emergence of resistant protozoa and viruses is a limited number of antiprotozoal and antiviral agents (El-Taweel 2015; Irwin et al. 2016).
Plants are of great importance in modern medicine. Thus, the plant kingdom can be an alternative to combat antimicrobial resistance, so there is a search for new antimicrobial drugs that can combat resistant microorganisms. This can play an essential role in combating microbial resistance (Sabo and Knezevic 2019). However, there is little incentive from the pharmaceutical industries to produce new antimicrobial, mainly due to the low financial return, thus limiting research for this type of medicine (Aslam et al. 2018).
With the current antimicrobial resistance, a post-antibiotic era is getting closer and more concrete every day. This problem is due to not only the evolution and adaptation of these microorganisms but also by those who prescribe these drugs, by those who use them, by those responsible for the control and distribution of these drugs (Vanegas-Múnera and Jiménez-Quiceno 2020). Thus, public policies are needed to raise awareness and educate the population about the use of antimicrobial , as well as health professionals can limit the unnecessary use of antimicrobial (Hu et al. 2020).
3 Classification and Mechanism of Action
Antimicrobials are classified according to their chemical structure (derived from amino acids, sugars, acetates, propionates, among others), types of microorganisms on which they act, or even the effect of the organism (Manrique and Galvão 1997). Besides, they are classified through factors, such as the mechanism of action, bacterial activity, susceptible microorganisms, the spectrum of action, and mainly through the assessment of their natural or synthetic origin (Anvisa 2020).
The mechanism of action of an antimicrobial starts when it reaches an ideal concentration at the infection site, then actively or passively passes through the cell wall, showing an affinity for the binding site inside the bacteria and remaining long enough to exercise its inhibitory activity. Antibiotics can have bactericidal action, killing microorganisms directly, acting in strong reactions to the infecting cell, or just inhibiting bacterial growth through a bacteriostatic action, keeping the bacteria in a stationary phase. In bacteriophage, the host triggers its defenses, such as phagocytosis and the production of antibodies to control the invading microorganism. In this case, the inhibition can be reversible, as the bacteria can continue to produce toxins or become resistant to the drug if the host’s defenses are not efficient Fig. 9.1. (Katzung 2007; Lago 2011; Brunton et al. 2012; Tortora et al. 2012; Pankey and Sabath 2004; Engleberg et al. 2013).
Regarding natural antibiotics, together with their semi-synthetic derivatives, they comprise most of the antibiotics used clinically and can be classified into β-lactams (penicillins, cephalosporins, carbapenins, oxapenins, and monobactams), tetracyclines, aminoglycosides, glycopeptides, lipodepsipeptides, streptogramins, licosamines, chloramphenicol, among others. Antibiotics of synthetic origin are classified as sulfonamides, fluoroquinolones, and oxazolidinones (Abraham 2003; Patrick 2005; Pupo et al. 2006).
Other important examples of antimicrobial classes are Macrolides (Bologa et al. 2013), Glycopeptides (Guimarães et al. 2010), Amphenicols (Von 2004), and Quinolones (Emmerson and Jones 2003).
4 Herbal Medicines with Antimicrobial Action
The first antimicrobial drugs used successfully against potentially fatal infectious diseases were Salvarsan (arsphenamine) and its derivative neoarsphenamine, used against syphilis (infection with Treponema pallidum) in 1911 (Mann 1984; Gaynes 2017). During the late 1930s and early 1940s, sulfamide antibiotic derivatives (e.g., prontoil, sulfamethazine) effective against lobar pneumonia (caused mainly by Streptococcus pneumoniae), meningococcal meningitis (caused by Neisseria meningitidis), and gonorrhea (caused by Neisseria gonorrhoeae) were introduced to the market (Kumar and Clark 1998; Gaynes 2017). However, until the discovery of penicillin (USA: 1945; United Kingdom 1946), these synthetic agents remained the only chemotherapeutic agents used for bacterial infections (Mann 1984).
However, the widespread use of antibiotics created an evolutionary adaptation of bacteria, leading to the so-called resistance to multiple drugs (Neu 1992). The consequence of the increased resistance of bacterial strains to biocidal agents is an increased risk of chronic infections and difficulties in their treatment (wound infections, osteomyelitis, septic arthritis, endocarditis, etc.) (Karam et al. 2016). However, there is a strong need to develop new compounds that are not only highly efficient but also that should not cause resistance to development in bacteria (Kyzioł et al. 2020).
In search of these new antibiotics, research on herbal medicines may prove promising since these medicines have been used for centuries to treat infectious diseases (Landers et al. 2012). They are readily available for patients to purchase themselves and appear to be increasingly popular, especially in the United Kingdom. This growing public interest in herbs can reduce dependence on antibiotics, especially for self-limited infections (Hu et al. 2020). A recent WHO report on traditional medicines noted that most of the world’s population depends on traditional medicines for health care, including treatment of infections (WHO 2002).
Thus, compounds isolated from medicinal plants are preferable to synthetic compounds due to their use in conventional medicine (Rakholiya et al. 2013). Therefore, these compounds are considered a substituted source of antimicrobial drugs (Savoia 2012).
5 Plant Antimicrobial Activity
5.1 Extracts with Antimicrobial Action
Natural products are used since ancient times for the treatment and, or control of infectious diseases among them from fungi, bacteria, or pathogenic microorganisms (Dkhil et al. 2020; Frassinetti et al. 2020; Silva 2006). These products include extracts, fractions, essential oils, isolated compounds, among other medicinal plant derivatives, which may be promising for the advancement of new studies, therapies, and treatments for pathologies (Frassinetti et al. 2020; Mehlhorn 2014; Hayek et al. 2013). Thus, some tasks related to the development and progress of studies on prominent plant extracts, in potential, with antimicrobial action will be addressed.
Antimicrobial activities of the ethanolic extracts of the flowers and leaves of ten species of the genera Senna and Cassia were evaluated against seven bacteria and three strains of fungi, oral aerobic and anaerobic bacteria, and Candida spp. by the microdilution broth method. This investigation was due to the use of these species in treatments of infections in the Brazilian traditional medicine, used as laxative agents, analgesics, and antifungals for mycosis and other fungal infections of the skin. Nascimento et al. (2020) developed this work, showing that among the tested species, Cassia fistula L., Senna macranthera (Collad), Cassia bakeriana Craib, and Senna spectabilis DC., showed moderate activity against two bacterial strains with MIC values (minimum inhibitory concentration) varying between 200.0 and 400.0 μg.mL−1, while the ethanolic extract of S. macranthera flowers showed very low values of MIC (23.4, 11.7, and 5.9 μg mL−1) in the antifungal test.
Thus, the ethanol extract of S. macranthera flowers was subjected to liquid-liquid extraction with solvents of different polarities, which include n-hexane, dichloromethane and ethyl acetate. The ethyl acetate fraction of S. macranthera flowers showed better antifungal activity (MIC values of 5.9 μg mL−1 for Candida glabrata, 23.4 μg mL−1 for Candida albicans, and (0.1 − 0.2 μg mL−1) for Candida tropicalis using amphotericin B as a positive control, so this fraction indicated potential antifungal action and selectivity against strains evaluated. It is noteworthy that the MIC represents the lowest concentration of the extract capable of preventing microbial growth. From this fraction, analysis by UHPLC-ESI-MS/MS (High-Efficiency Liquid Chromatography Coupled by Tandem Mass Spectrometry) were performed, which identified different types of phenolic compounds, in particular, proanthocyanidins in several isomeric forms.
This class of secondary metabolites, proanthocyanidins (condensed tannins), are phenolic compounds (Kyraleou et al. 2020; Monteiro et al. 2005), active against species of Candida and thus may be associated with the antifungal action of the fraction cited (Piccinelli et al. 2016; Freitas et al. 2018). This study has collaborated to strengthen the traditional use of the species from these genera Senna and Cassia, particularly the flowers of S. macranthera, as a good and promising source of discoveries of compounds as antifungal agents.
Weremczuk-Jeŝyna et al. (2019) evaluated the anti-pathogenic potential of the hydromethanolic extract of Dracocephalum forrestii sprouts cultivated in nutrient-sprinkled bioreactors. These antimicrobial evaluations were performed against selective strains of bacteria, six Gram-pathogenic, and four Gram-negative, and three pathogenic strains of fungi, which showed moderate activity limiting the growth of pathogens. The minimum bactericidal concentration (MBC) and MIC activity of the extract were analyzed, where most of the strains tested were within the range of 2.5–5 mg.mL−1.
A greater effect was observed on Bacillus cereus, Escherichia coli, and Pseudomonas aeruginosa with MIC 2.5 mg mL−1. The hydromethanolic extract at a concentration of 2.5 mg mL−1. showed antifungal activity against Candida albicans and Candida glabrata. They were determined by the microdilution broth method according to the European Committee for Antimicrobial Susceptibility Testing (EUCAST). Rosmarinic acid, gentamicin, and fluconazole (all from Sigma-Aldrich) were used as reference antimicrobials with values lower than 1–5 mg.mL−1.
In this work, the phenolic compounds present in the cultivated extract were evaluated qualitatively by UPLC-PDA-ESI-MS (ultra-performance liquid chromatography (UPLC) coupled to photodiode array detection (PDA) and electrospray ionization (ESI) mass spectrometry) and quantitatively by UPLC-PDA. Rosmarinic acid (17.90 mg g−1 DW) and salvianolic acid B (6.50 mg g−1 DW) were verified in larger quantities. Deba et al. (2008) and Estevinho et al. (2008) reported that plant material rich in polyphenols, bioactive phenolic compounds, may be associated with anti-pathogenic effects due to the interaction between these constituents. The author suggests further studies to improve the research and thus to estimate a possible synergistic effect of D. forrestii extract on a likely antibiotic therapy against multi-resistant bacteria.
Fei et al. (2018), in their studies, reveal that the potent antioxidant activity of two polyphenols can be considered as one of the ways this class of natural products inhibits the development of pathogenic microorganisms.
5.2 Phytoconstituents with Antimicrobial Action
The use of antibiotics and/or synthetic or natural compounds to treat infectious diseases is growing widely (Hemaiswarya et al. 2008; Bimani and Hossain 2020). Thus, scientists are increasingly improving their search for finding natural compounds bioactive that may be able to exercise biological activities to treat, improve, or resolve the resistance of microorganisms to synthetic drugs (Bimani and Hossain 2020; Vidhya et al. 2020).
Andrade et al. (2020) evaluated the antimicrobial activity of two isolated natural molecules, Braquidines BR-A and BR-B, against S. aureus, E. coli, and C. albicans. The isolation and analysis were performed from the purification of the dichloromethane fraction of Arrabidaea brachypoda flowers, by HPLC-PCDA (High-Efficiency Liquid Chromatography with photodiode arrangement detection) and UV-VIS (Ultraviolet Spectroscopy-visible) revealing and identifying phenolic compounds, dimeric flavonoids differentiating only in the aromatic C ring by the presence of a hydroxyl group in BR-A, already in BR-B there is a methoxyl group, giving purity of 95 and 97%, respectively. BR-A showed no antimicrobial activity against the microbial strains tested, since MIC values above 1000 μg.mL−1, presenting a MIC of 1024 μg.mL−1, were determined by microdilution assay. Thus, according to the literature, these are considered clinically irrelevant results (Houghton et al. 2007). However, the BR-B showed antifungal activity against C. albicans of 161 μg.mL−1, showing promising future studies and improvements.
A phytochemical study on Trianthema decandra leaves was performed by Geethalakshmi and Sarada (2018), where two compounds of chloroform extract from the leaf were isolated and characterized using HPLC, UV, FT-IR, RMN, LC-MS, and CHNS techniques. Identifying a new sterol named as 17-(5-ethyl-6-methylheptan-2-yl) – 4, 4, 10, 13-tetramethyl-hexadecahydro-1H-cyclopenta (α) phenanthren-3-ol and the flavonoid as 2-(3´,4’dihydroxyphenyl) 3,5,7-trihydroxychromen-4-one. The antimicrobial activity of the sterol and flavonoid isolates were analyzed by disc diffusion and broth dilution assays, showing good results against the tested microorganisms, in particular sterol presented an MIC of 39.05 μg mL−1 against the strain Salmonella typhi and the flavonoid presented MICs of 78.10 and 39.05 μg mL−1 against Vibrio cholerae and P. aeruginosa, respectively.
5.3 Essential Oils with Antimicrobial Action
A variety of oils essential (OEs), derived from aromatic plants, are used as insecticide, antiparasitic, fungicide, virucidal, bactericide, cosmetics, food, and agricultural industries (Dhifi et al. 2016; Atif et al. 2020). These OEs also act as preservatives, sedatives, antimicrobial, and spasmolytic analgesics. The chemical composition of OEs includes sesquiterpenes, monoterpenes, and their oxygenated compounds, such as oxides, phenols, ketones, ethers, esters, aldehydes, molecules containing nitrogen or sulfur (Ahmed et al. 2019).
With the presence of secondary metabolisms, OEs play a protective role against various microbes in plants. The actions generated by these metabolites can inhibit or slow the growth of bacteria, yeasts, and molds, whose components have a variety of targets, particularly on the microbial membrane and cytoplasm, and in some situations, they drastically alter cell morphology (Chorianopoulos et al. 2008; De Martino et al. 2009).
Because they have antimicrobial activity against several microorganisms, OEs are considered an alternative to conventional antibiotics (Silva-Santos et al. 2017).
Herbs such as cloves, cinnamon, oregano, peppers, rosemary, and thyme have OEs that generate intense antibacterial activity against S. aureus, S. typhi, and P. aeruginosa (Conner and Branen 1993). Among all the essential oils tested, clove oil was the most effective. In addition, it was found that carvacrol has antimicrobial activity against a broad spectrum of bacterial strains (Fernández-Pan et al. 2015; Kristo et al. 2008).
Mohamed et al. (2013) reported effective inhibition in pathogenic bacterial strains such as N. gonorrhoeae, E. coli, Bacillus subtilis, S. aureus, and P. aeruginosa, developed by Syzygium cumini OE, containing 1,3,6 limonenoene, trans-carcinogenic-octatriene, β-pinene, δ-3-carene, α-pinene and α-caryophyllene; these compounds are responsible for the bactericidal action.
Because they come from plant sources, OEs can also develop synergistic activity, which effectively combat the growth of various microorganisms. Some constituents such as carvacrol, γ-terpinene, and p-cymene are more effective when combined (Elshafie and Camele 2017).
OEs are mentioned as potent antimicrobial agents, as they have substantial antibacterial activities against Gram-positive and Gram-negative pathogens (Yap et al. 2014). For example, OEs derived from medicinal aromatic plants, such as peppermint (Mentha piperita), thyme (Thymus vulgaris), and fennel (Foeniculum vulgare), are cataloged as effective against viruses, Gram-negative and Gram-positive bacteria, fungi, and yeast (Reichling 2018).
Various volatile, terpenic, and phenolic substances present in OEs show remarkable antimicrobial activity (Marchese et al. 2016). And according to the literature, OEs that contain these terpenes, phenolic, and aldehydes have an excellent application in biomedicine for properly eliminating many viral, fungal, and bacterial pathogens (Swamy et al. 2016).
6 Perspectives with the Use of Secondary Plant Metabolites
Antimicrobials have played an essential role in human health since their discovery. This relevance is reflected in the results obtained using antimicrobial pre-agents, which are also among the drugs most used by medicine. The discriminated and illicit use of antibiotics has resulted in the emergence of drug resistance among pathogens, which in many parts of the world, especially in developing countries, have reached critical levels (Ayukekbong et al. 2017).
This practical and negligent use of antibiotics is a growing concern worldwide, fueling antimicrobial resistance. In the clinical context, particularly in the health care sector, it is vital to validate a prescription for antibiotics before administering the medication. Besides, the dosage of antibiotics must be such that pathogenic bacteria are eliminated in a complete cycle of antibiotics. However, there are few effective techniques for a quick discovery of susceptibility to drugs. Continuous unintended exposure of bacteria to antibiotics can promote antimicrobial resistance that can increase in the body for longer in most living organisms. The development of technologies to detect antibiotics (mainly used in the therapy of human infection) is essential to containing the threat of antimicrobial resistance to guarantee its ideal use in the treatment of human bacterial infection (Nag et al. 2020).
Antimicrobial products characterized as drugs have a shelf life, changes in the metabolic and genetic level, a faster rate of evolution such as variable global temperature, a catalog of widely documented side effects. However, the main concerns of doctors and scientists are the prolonged use of antimicrobials, the high cost of clinical research and drug development. The main obstacle to antibiotic resistance is developing effective classes of antibiotics with a new mode of action (Anand et al. 2020).
It should be noted that more than 80% of the world population depends on conventional pharmaceutical products made up of medicinal plants to meet different conditions of human medicine (Ekor 2014; Oyebode et al. 2016; Pan et al. 2014; Mahomoodally 2013). Worldwide interest in research aimed at discovering new drugs from plant sources is growing (Anand et al. 2020).
The natural products are characterized as a diverse group with different bio-activities for therapeutic purposes. Natural sources are privileged source in searching for new antimicrobial molecules (Agrawal et al. 2017). More than half of medicines in clinical use approved by the US Food and Drug Administration (FDA) are derived from natural products (Newman and Cragg 2016).
Therefore, the work appears with the perspective of contributing to the development of new antimicrobial drugs to combat microorganisms resistant to multidrug drugs, in addition to enabling positive actions about natural compounds derived from plants, which may later be the basis of pre-clinical studies in humans and in a future perspective, which will contribute to implementation of these compounds of natural origin in the treatment of pathologies.
7 Conclusions
This chapter pointed out that phytochemicals, extracts, and derivatives of natural compounds could be effective in the treatment of resistant microorganisms, since they have antimicrobial activity against microorganisms, especially in the therapeutic approach or acting as drugs. In this way, natural products may serve as a promising therapeutic source for various pathogenic microorganism, and therefore are pillars for discovering new antibiotics. However, the pharmacological utility of these products will require further studies. Thus, this review becomes extremely important, as it shows medicinal plants as a source of new therapies that can contribute to the discovery of potential candidates for the treatment of resistant microorganisms.
Abbreviations
- CHNS:
-
Carbon, hydrogen, nitrogen, sulfur
- DW:
-
Dry weight
- EUCAST:
-
European Committee for Antimicrobial Susceptibility Testing
- FDA:
-
Food and Drug Administration
- FT-IR:
-
Fourier transform-infrared
- HPLC:
-
High-performance liquid chromatography
- HPLC-PCDA:
-
High-efficiency liquid chromatography with photodiode arrangement detection
- LC-MS:
-
Liquid chromatography-mass spectrometry
- MBC:
-
Minimum bactericidal concentration
- MIC:
-
Minimum inhibitory concentration
- NMR:
-
Nuclear magnetic resonance
- OEs:
-
Oils Essenciais Naturais
- UHPLC-ESI-MS/MS:
-
High-efficiency liquid chromatography coupled with tandem mass spectrometry
- UPLC-PDA:
-
Ultra-performance liquid chromatography coupled with photodiode array
- US:
-
United States
- UV-VIS:
-
Ultraviolet spectroscopy-visible
- WHO:
-
World Health Organization
References
Abraham DJ (2003) Burger’s medicinal chemistry & drug discovery: chemotherapeutic agents, 6th edn. Willey, Hoboken
Abreu AC, McBain AJ, Simoes M (2012) Plants as sources of new antimicrobials and resistance-modifying agents. Nat Prod Rep 29:1007–1021
Abushaheen MA, Fatani AJ, Alosaimi M, Mansy W, George M, Acharya S, Rathod S, Divakar DD, Jhugroo P (2020) Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon 66:1–21
Agrawal S, Acharya D, Adholeya A, Barrow CJ, Deshmukh SK (2017) Nonribosomal peptides from marine microbes and their antimicrobial and anticancer potential. Front Pharmacol 8:1–26
Ahmed AF, Attia FA, Liu Z, Li C, Wei J, Kang W (2019) Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci Hum Well 8:299–305
Aleksic AS, Knezevic P (2019) Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: a review. Ind Crop Prod 132:413–429
Al-Marzoqi AH, Al-Khafaji NMS, Hussein HJ (2016) In vitro antibacterial activity assessment of the crude phenolic, alkaloid and terpenoid compounds extracts of Lepidium sativum L. on human pathogenic bacteria. Int J ChemTech Res 9:529–532
Aminov RI (2010) A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 1:1–7
Anand U, Nandy S, Mundhra A, Das N, Pandey DK, Dey A (2020) A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist Updat 51:1–8
Andrade LMS, Oliveira ABM, Leal ALAB, Oliveira FAA, Portela AL, Neto JDSL, Siqueira-Júnior JP, Kaatz GW, Rocha CQ, Barreto HM (2020) Antimicrobial activity and inhibition of the NorA efflux pump of Staphylococcus aureus by extract and isolated compounds from Arrabidaea brachypoda. Microb Pathog 140:2–7
ANVISA. Agência Nacional de Vigilância Sanitária. Bulário Eletrônico da Anvisa. Disponível em: http://bulario.bvs.br. Access in: Set/2020
Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisae MA, Alvi RF, Aslam MA, Qamar UM, Salamat MKF, Baloch Z (2018) Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist 11:1645–1658
Atif M, Ilavenil S, Devanesan S, AlSalhi MS, Choi KC, Vijayaraghavan P, Alfuraydi AA, Alanazi NF (2020) Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind Crop Prod 147:1–9
Ayukekbong JA, Ntemgwa M, Atabe AN (2017) The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control 6:1–8
Bimani BMH, Hossain MA (2020) A new antimicrobial compound from the leaves of Dodonaea viscosa for infectious diseases. Bioact Mater 5:602–610
Bologa CG, Ursu O, Oprea TI, Melançon CE III, Tegos GP (2013) Emerging trends in the discovery of natural product antibacterials. Curr Opin Pharmacol 13:678–687
Brunton LL, Chabner BA, Knollmann BC (2012) As Bases Farmacológicas da Terapêutica, 12th edn. McGraw-Hill, Brazil
Chorianopoulos NG, Giaouris ED, Skandamis PN, Haroutounian SA, Nychas GJ (2008) Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid–base sanitizers. J Appl Microbiol 104:1586–1596
Conner DE, Davidson P, Branen AL (1993) Naturally occurring compounds, 1st edn. Marcel Dekker, New York
Costa MDC, Cruz AIC, Bispo ASDR, Ferreira MA, Costa JA, Evangelista-Barreto NS (2020) Occurrence and antimicrobial resistance of bacteria in retail market spices. Cienc Rural 50:1–7
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74:417–433
De Martino L, De Feo V, Nazzaro F (2009) Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 14:4213–4230
Deba F, Xuan TD, Yasuda M, Tawata S (2008) Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control 19:346–352
Dhifi W, Bellili S, Jazi S, Bahloul N, Mnif W (2016) Essential oils’ chemical characterization and investigation of some biological activities: a critical review. Medicines 3:1–16
Dkhil MA, Zreiq R, Hafiz TA, Mubaraki MA, Sulaiman S, Algahtani F, Abdel-Gaber R, Al-Shaebi EM, Al-Quraishy S (2020) Anthelmintic and antimicrobial activity of Indigofera oblongifolia leaf extracts. Saudi J Biol Sci 27:594–598
Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 4:1–10
Elshafie HS, Camele I (2017) An overview of the biological effects of some mediterranean essential oils on human health. Biomed Res Int 2017:1–14
El-Taweel HA (2015) Understanding drug resistance in human intestinal protozoa. Parasitol Res 114:1647–1659
Emmerson A, Jones A (2003) The quinolones: decades of development and use. J Antimicrob Chemother 51:13–20
Engleberg NC, Dirita V, Dermody T (2013) Schaechter's mechanisms of microbial disease, 5th edn. Lippincott Williams & Wilkins, Philadelphia
Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E (2008) Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol 46:3774–3779
Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 6:25–64
Fei P, Ali MA, Gong S, Sun Q, Bi X, Liu S, Guo L (2018) Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control 94:289–294
Fernández-Pan I, Maté JI, Gardrat C, Coma V (2015) Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll 51:60–68
Frassinetti S, Gabriele M, Moccia E, Longo V, Gioia DD (2020) Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT Food Sci Technol 124:109–149
Freitas ALD, Kaplum V, Rossi DCP, Silva LBR, Melhem MSC, Taborda CP, Mello JCP, Nakamura CV, Ishida K (2018) Proanthocyanidin polymeric tannins from Stryphnodendron adstringens are effective against Candida spp. isolates and for vaginal candidiasis treatment. J Ethnopharmacol 216:184–190
Gaynes R (2017) The discovery of penicillin—new insights after more than 75 years of clinical use. Emerg Infect Dis 23:849–853
Geethalakshmi R, Sarada VLD (2018) In vitro and in silico antimicrobial activity of sterol and flavonoid isolated from Trianthema decandra L. Microb Pathog 121:77–86
Guimarães DO, Momesso LS, Pupo MT (2010) Antibióticos: importância terapêutica e perspectivas para a descoberta e desenvolvimento de novos agentes. Quim Nova 33:667–679
Gunatilaka A, Wijeratne E (2012) Natural products from bacteria and fungi. J Pharmacogn Phytochem 3:1–27
Gupta PD, Birdi TJ (2017) Development of botanicals to combat antibiotic resistance. J Ayurveda Integr Med 8:266–275
Hayek SA, Gyawali R, Ibrahim S (2013) Antimicrobial natural products, 1st edn. Formatex, Spain: Badajoz
Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15:639–652
Houghton PJ, Howes MJ, Lee CC, Steventon G (2007) Uses and abuses of in vitro tests in ethnopharmacology: visualizing an elephant. J Ethnopharmacol 110:391–400
Hu XY, Logue M, Robinson N (2020) Antimicrobial resistance is a global problem–a UK perspective. Eur J Integr Med 36:1–4
Huang T, Lin S (2017) Microbial natural products: a promising source for drug discovery. Appl Microbiol Biochem 1:1–3
Irwin KK, Renzette N, Kowalik T, Jensen JD (2016) Antiviral drug resistance as an adaptive process. Virus Evol 2:1–10
Karam G, Chastre J, Wilcox MH, Vincent JL (2016) Antibiotic strategies in the era of multidrug resistance. Crit Care 20:1–9
Katzung BG (2007) Farmacologia Básica e Clínica, 9th edn. McGraw, Brasil
Kristo E, Koutsoumanis KP, Biliaderis CG (2008) Thermal, mechanical and water vapor barrier properties of sodium caseinate films containing antimicrobials and their inhibitory action on Listeria monocytogenes. Food Hydrocoll 22:373–386
Kumar P, Clark M (1998) Clinical medicine, 4th edn. W.B. Saunders, Edinburgh
Kunin CM, Tupasi T, Craig WA (1973) Use of antibiotics: a brief exposition of the problem and some tentative solutions. Ann Intern Med 79:555–560
Kyraleou M, Kallithraka S, Gkanidi E, Koundouras S, Mannion DT, Kilcawley KN (2020) Discrimination of five Greek red grape varieties according to the anthocyanin and proanthocyanidin profiles of their skins and seeds. J Food Compost Anal 92:1–9
Kyzioł A, Khan W, Sebastian V, Kyziol K (2020) Tackling microbial infections and increasing resistance involving formulations based on antimicrobial polymers. Chem Eng J 385:1–18
Lago J (2011) Mecanismos de Resistência e Selecção de Antibióticos, 1st edn. Jornadas bioMérieux, Lisboa
Landers TF, Cohen B, Wittum TE, Larson EL (2012) A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep 127:4–22
Loho T, Dharmayanti AC (2015) Um antibiótico e seu papel em infecções Gram-negativas multirresistentes. Acta Med Indones 47:157–168
Mahomoodally MF (2013) Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evid Based Complement Alternat Med 2013:2–14
Mann RD (1984) Modern drug use: an enquiry on historical principles, 1st edn. MTP Press Limited, London
Manrique EI, Galvão LL (1997) Racionalização e controle de antimicrobianos. Infecções hospitalares Prevenção e Controle 6:117–130
Marchese A, Orhan IE, Daglia M, Barbieri R, Lorenzo AD, Nabavi SF, Gortzi O, Izadi M, Nabavi SM (2016) Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem 210:402–414
Mehlhorn H (2014) Encyclopedic reference of parasitology, 6th edn. Springer Press, Cham
Mohamed AA, Ali SI, El-Baz FK (2013) Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS One 8:1–7
Monteiro JM, Albuquerque UP, Araújo EL, Amorim ELC (2005) Taninos: uma abordagem da química à ecologia. Quim Nova 28:892–896
Moore BS, Carter GT, Brönstrup M (2017) Are natural products the solution to antimicrobial resistance? Nat Prod Rep 34:685–686
Mota LM, Vilar FC, Dias LBA, Nunes TF, Moriguti JC (2010) Uso racional de antimicrobianos. Medicina (Ribeirão Preto) 43:164–172
Nag P, Sadani K, Mukherji S, Mukherji S (2020) Beta-lactam antibiotics induced bacteriolysis on LSPR sensors for assessment of antimicrobial resistance and quantification of antibiotics. Sens Actuators B Chem 311:2–9
Nascimento MNG, Martins MM, Cunha LCS, Santos PS, Goulart LR, Silva TS, Martins CHG, Morais SAL, Pivatto M (2020) Antimicrobial and cytotoxic activities of Senna and Cassia species (Fabaceae) extracts. Ind Crop Prod 148:1–11
Neu HC (1992) The crisis in antibiotic resistance. Science 257:1064–1073
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661
Organización de las Naciones Unidas para la Alimentación y la Agricultura (2016) El Plan de acción de la FAO sobre la resistencia a los antimicrobianos 2016–2020. Organización de las Naciones Unidas para la Alimentación y la Agricultura, Roma. Disponible en: http://www.fao.org/publications
Oyebode O, Kandala NB, Chilton PJ, Lilford RJ (2016) Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Policy Plan 31:984–991
Pan SY, Litscher G, Gao SH, Zhou SF, Yu ZL, Chen HQ, Zhang SF, Tang MK, Sun JN, Ko KM (2014) Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Alternat 2014:1–20
Pankey G, Sabath L (2004) Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram positives bacterial infections. Clin Infect Dis 38:864–870
Patrick GL (2005) An introduction to medicinal chemistry, 1st edn. Oxford University Press, New York
Pérez DQ (2017) Resistencia antimicrobiana: evolución y perspectivas actuales ante el enfoque" Una salud". Rev Cubana Med Trop 69:1–17
Piccinelli AL, Pagano I, Esposito T, Mencherini T, Porta A, Petrone AM, Gazerro P, Picerno P, Sansone F, Rastrelli L, Aquino RP (2016) HRMS profile of a hazelnut skin proanthocyanidin-rich fraction with antioxidant and anti-candida albicans activities. J Agric Food Chem 64:585–595
Pupo MT, Guimarães DO, Furtado NAJC, Borges WS (2006) Microbial natural products: a promising source of bioactive compounds, 2nd edn. Research Signpost, Thiruvananthapuram
Rakholiya K, Kaneria M, Desai D, Chanda S (2013) Microbial pathogens and strategies for combating them: science, technology and education. Microbiol Book Ser 2:850–856
Reichling J (2018) Plant-microbe interactions and secondary metabolites with antiviral, antibacterial and antifungal properties. Ann Plant Rev 39:189–279
Robbins N, Caplan T, Cowen LE (2017) Molecular evolution of antifungal drug resistance. Annu Rev Microbiol 71:753–775
Sabo VA, Knezevic P (2019) Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: a review. Ind Crop Prod 132:413–429
Saez-Llorens X, Wong MMC, Castaño E, Suman OD, Moros DD, Atencio ID (2000) Impact of an antibiotic restriction policy on hospital expenditures and bacterial susceptibilities: a lesson from a pediatric institution in a developing country. Pediatr Infect Dis J 19:200–206
Savoia D (2012) Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol 7:979–990
Scheckler WE, Bennett JV (1970) Antibiotic usage in seven community hospitals. JAMA 213:264–267
Semret M, Haraoui LP (2019) Antimicrobial resistance in the tropics. Infect Dis Clin 33:231–245
Silva MB (2006) Controle alternativo de pragas e doenças, 1st edn. EPAMIG-CTZM/UFV
Silva-Santos C, Piccoli RH, Tebaldi VMR (2017) Atividade antimicrobiana de óleos essenciais e compostos isolados frente aos agentes patogênicos de origem clínica e alimentar. Rev Inst Adolfo Lutz 76:17–19
Swamy MK, Akhtar MS, Sinniah UR (2016) Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat 2016:1–21
Tang SS, Apisarnthanarak A, Hsu LY (2014) Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community-and healthcare-associated multidrug-resistant bacteria. Adv Drug Deliv Ver 78:3–13
Tortora GJ, Funke BR, Case CL (2012) Microbiologia, 10th edn. Artmed Editora
Vanegas-Múnera JM, Jiménez-Quiceno JN (2020) Resistencia antimicrobiana en el siglo XXI:¿ hacia una era postantibiótica? Rev Fac Nac Salud Pública 38:1–6
Vasoo S, Barreto JN, Tosh PK (2015) Emerging issues in gram-negative bacterial resistance: an update for the practicing clinician. Mayo Clin Proc 90:395–403
Veeresham C (2012) Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res 3:200–201
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. Pharmacol Ther 40:277–283
Vidhya E, Vijayakumar S, Rajalakshmi S, Kalaiselvi S, Pandiyan P (2020) Antimicrobial activity and phytochemical screening of Ocimum americanum L extracts against pathogenic microorganisms. Acta Ecol Sin 40:214–220
Von DH (2004) Protein science: a publication of the protein society. Sci Phitochem 13(1–25):2004
Weremczuk-Jeŝyna I, Kochan E, Szymczyk P, Lisiecki P, Kuzma L, Grzegorczk-karolak I (2019) The antioxidant and antimicrobial properties of phenol-rich extracts of Dracocephalum forrestii W. W. Smith shoot cultures grown in the nutrient sprinkle bioreactor. Phytochem Lett 30:254–260
WHO (2002) WHO traditional medicine strategy 2002–2005. http://www.who.int/medicines/publications/traditionalpolicy/en/index.html
Wright GD (2017) Opportunities for natural products in 21st century antibiotic discovery. Nat Prod Rep 34:694–701
Yap PSX, Yiap BC, Ping HC, Lim SHE (2014) Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J 8:6–14
Acknowledgments
We appreciate the support from UFPI.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alves, P.e.S. et al. (2022). Natural Products from Plants with Antimicrobial Action. In: Rai, M., Kosalec, I. (eds) Promising Antimicrobials from Natural Products. Springer, Cham. https://doi.org/10.1007/978-3-030-83504-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-83504-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83503-3
Online ISBN: 978-3-030-83504-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)