Abstract
The regulation of nutrition and metabolism plays a pivotal role in regulating the aging process. The nutrition is a critical external factor influencing the development of aging and associated disorders. One of the well-known dietary interventions to slow aging and reduce mortality is calorie restriction. The results of the range of studies indicate that calorie restriction facilitates the increase in life expectancy and a decrease in the aging processes. The effect of calorie restriction on the aging process has been associated with a wide range of the reactions in different types of cells, particularly the stem cells. This Chapter discusses the role of the stem cells in aging processes and associated disorders in the context of calorie restriction strategies. It encompasses the analysis of the results of preclinical and clinical studies on the relationship between the calorie restriction and adult stem cell function in order to understand the effects of calorie restriction on the health and longevity. The Chapter highlights the role of stem cells in neurogenesis, alterations in stem cell function under the influence of calorie restriction. In addition, the potential of the calorie restriction mimetics as aging modulators has been discussed too.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
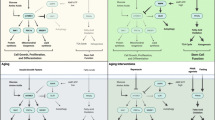
The results of numerous studies have not yet led to the universal concept of aging. During the last decades, several distinct, interrelated and interdependent, biological mechanisms underlying the process of aging and age-related diseases have been identified (Kennedy et al. 2014). These mechanisms encompass macromolecular damage, metabolism, stem cells and regeneration, proteostasis, adaptation to stress, inflammation and epigenetics (Kennedy et al. 2014). In the context of the current understanding of aging, it has been assumed that human body has developed adaptive strategies to recognize and neutralize the combination of the stress factors that affect the biological mechanisms described above and adaptive consequences of protective responses formed during the evolution process (Epel and Lithgow 2014; Santoro et al. 2020). In case the human body fails to adapt to the stressor, it leads to the disruption of the homeostasis and eventually may cause the development of age-related changes (Santoro et al. 2020).
One of the most important environmental factors associated with the vital activity of the body is the availability of nutrients (Speakman 2013). The lack of the nutrients is a possibly predictable event and in the course of evolution adaptive mechanisms for starvation have been formed, but excess of nutrients (especially some in particular) and associated obesity is non-adaptive, and facilitates metabolic dysfunction (Santoro et al. 2020).
The importance of the regulation of nutrition and metabolism is demonstrated in recent advances in proteomics and metabolomics indicate that metabolic signalling pathways play a pivotal role in regulating the aging process and nutrition can be a critical external factor influencing the development of aging (Schüler et al. 2020). Moreover, according to the hypothesis “metabolic age score” metabolic changes accumulate over time, and an estimate of metabolic age score is an informative measurement of the biological age (López-Otín et al. 2016; Hertel et al. 2016). In compliance with the numerous studies congenital defects in the metabolic signalling pathways accelerate aging, and the maintenance of longevity is closely related to the metabolic processes (López-Otín et al. 2016; Barzilai et al. 2012, Catic 2018).
The regulation of aging by the metabolic processes can be considered within the concept of hormesis, where a certain level of the metabolic disorders weakens the effects of aging due to the activation and upregulation of the protective cellular pathways. However, over-activation of the metabolism leads to an acceleration of the aging processes (Gems and Partridge 2008; Santoro et al. 2020). This concept agrees with the numerous data that indicate that calorie restriction (CR) from a normal diet, and not just a lack of overeating, facilitates the increase in life expectancy and a decrease in the aging processes of animals and humans (Heilbronn and Ravussin 2003; Flanagan et al. 2020; Franceschi et al. 2018). Along with the organismal level, CR promotes changes in the aging phenotypes at the tissue, cellular and molecular levels. (Ma et al. 2020).
The selection of the optimal CR program is a very urgent task in gerontology. Considering CR in the prism of hormesis, the use of the moderate (usually intermittent) stress is necessary to obtain a beneficial effect as it was noted in the pioneering works of Mattson (Mattson 2008). In addition, it was assumed that adaptive responses arising under the influence of the food stress in the aging organisms lose the intensity of the response and so-called “metabolic flexibility”, i.e. the ability to balance between the energy consumption and energy storage (Storlien et al. 2004). Perhaps this is one of the reasons for the change in the body's sensitivity to CR with age. In addition, other negative aspects of CR should be noted: acceleration of the sarcopenia in old organisms, possible malnutrition, difficulty in maintaining the diet in the elderly, etc. (Xie et al. 2020; Locher et al. 2016, Madeo et al. 2019). In this regard, a possible alternative is a chemical compound capable of imitating CR – calorie restriction mimetics (Shintani et al. 2018, Madeo et al. 2019).
The difficulty in assessing the effect of CR on the aging process is associated with a wide range of the reactions in different types of cells, tissues and organs caused by aging and the subsequent various adaptive and non-adaptive consequences of the calorie restriction (Ma et al. 2020). To improve the understanding of this processes we should focus on the impact of CR on the adult stem cells, which are the key in maintaining tissue homeostasis. Adult stem cells (ASC) (specific tissue stem cells, somatic stem cells) have been found in many human and animal tissues. ASCs are undifferentiated cells (relative to the functional tissue) capable of proliferation, self-renewal and differentiation into tissue-specific precursors to maintain the tissue homeostasis and tissue regeneration upon the illness or injury (Loeffler and Roeder 2002). The most studied adult stem cells are hematopoietic stem cells and mesenchymal stem cells (Clevers 2015, Gonzalez and Bernad 2012). Like normal somatic cells, somatic stem cells are exposed to various stress factors throughout the life, which leads to aging, (Jones and Rando 2011, Sharpless and DePinho 2007, Alt et al. 2012, Liu and Rando 2011, Rando 2006) and this can be one of the reasons of the overall organism aging (Fukada et al. 2014; Mimeault and Batra 2009). Taking into the account the key role of the stem cells in maintaining a dynamic balance in tissue and organ homeostasis, it is easy to assume that they play a central role in aging and the pathophysiology of the various age-associated diseases such as cardiovascular and cerebrovascular diseases, malignant tumors, diabetes, autoimmune diseases, recurrent infections, impaired wound healing and other diseases (Schultz and Sinclair 2016, Goodell and Rando 2015, Smith and Daniel 2012, Sharpless and DePinho 2007, Boyette and Tuan 2014).
One of the main mechanisms in aging of the adult stem cells is a disruption of proteostasis and autophagy (Chang 2020). At the same time, CR contributes to the restoration of these processes inside the cell (Chang 2020, Chung and Chung 2019) and therefore the CR mimetics should have a similar effect on the aging of the adult stem cells.
In recent years, much attention has been paid to the development of anti-aging strategies aimed at preventing and/or slowing down the aging process based on the use of the body's internal abilities for self-healing (Santoro et al. 2020), it is important to consider the potential of the CR mimetics in maintaining normal tissue homeostasis through the activation of the adult stem cells.
2 Calorie Restriction and Adult Stem Cells
The best known dietary intervention to slow aging and reduce mortality is calorie restriction (CR). Food intake is reduced below the energy requirements without malnutrition or deprivation of the essential nutrients. (Heilbronn and Ravussin 2003; Flanagan et al. 2020). In various preclinical models CR strikingly increase life expectancy (Heilbronn and Ravussin 2003; Flanagan et al. 2020). Data from controlled clinical trials in humans associates CR with prolonged life spanonaver age by 1–5 years (Flanagan et al. 2020). The specific mechanisms underlying CR are not clear, but existing evidence suggests that CR stimulates cellular defense mechanisms such as autophagy, mitochondrial efficacy, decreases ROS production, and decreases inflammatory cytokines (Flanagan et al. 2020). According to the preclinical studies CR is strongly dependent on the genotype and sex, and the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis (Komatsu et al. 2019). Given the importance of adult stem cells in aging, many researchers have studied the relationship between the calorie restriction and adult stem cell function to understand the effects of CR on the health and longevity. In a number of studies caloric restriction modulates the functionality of adult stem cells and may have positive effects on the biology of stem cells in various tissues (Mazzoccoli et al. 2014, Maharajan et al. 2020).
The most sensitive cells to caloric restriction are hematopoietic stem cells (Wilkinson and Yamazaki 2018). For example, restricting diet to 75% food intake for about 2 years in BALB/cByJ mice (BALB)inhibits haematopoiesis and prevents HSC senescence. Interestingly, HSC function in 25 month old CR mice was better than in 3 month old mice without CR (Chen et al. 2003). In addition, compared to the long-term exposure, 5 months calorie restriction meals improved function of HSC to a lesser extent. (Chen et al. 2003). In turn, Tangetal found that a long-term diet of 70% of food intake (30% CR) for 6 and 12 months positively affects the phenotypes of aging HSC (increased number of HSCs and bias towards myeloid HSC during aging.) and the resting state of HSC in C57BL/6 J mice, but at the same time negatively affects B-lymphopoiesis in mice, disrupting the differentiation of HSC down the lymphoid lineage. The negative effect of 30% CR on B-lymphopoaesis was mediated by a decrease in the level of IL-6, IL-7, while the effect of CR on the resting state of cell was associated with a decrease in the concentration of IGF1 (Tang et al. 2016).
On the other hand, a lifelong caloric restriction facilitated stabilization of the bone marrow cellularity, but the number of HSCs increased compared to the animals with aging without calorie restriction. Also, the lifelong calorie restriction did not improve the reduced HSC functionality (Lazare et al. 2017). The authors suggest that the inconsistency of the results is associated with different experimental design (different duration of the diet - fasting kinetics), and also point to the importance of the composition of the standard diet, especially the content of valine (Lazare et al. 2017).
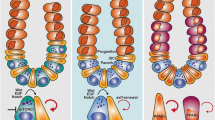
Intestinal stem cells are another attractive object of the research to assess the effect of CR on the adult stem cells (Bruens et al. 2020; Igarashi and Guarente 2016; Yilmaz et al. 2012). Early studies showed that a 60–70% CR-diet increased the number of stem cells in the intestinal crypts (Igarashi and Guarente 2016; Yilmaz et al. 2012) and reduced the incidence of the intestinal polyps by almost 60% (Mai et al. 2003). In addition, Yilmaz et al. revealed that Paneth cells in the intestinal stem cell nichemediate the effects of CRonISC. An important finding in the study of the effect of CR on ISC was made recently, where at 40% CR for 8 weeks in mice, the length and width of the small intestine did not change, but there was a reversible increase in crypt diameter, which was associated with an increase in the number of Lgr5 + stem cells and lysozyme + Paneth cells (niche expansion) (Bruens et al. 2020). Another significant results revealed that the increase in the number of stem cells associated with the CR diet leads to the displacement of the stem cells carrying pathological mutations (oncogenic APC mutations) from the niche as a result of competition of the stem cells on the basis of fitness and also leads to a lower retention of mutations in the intestine (Bruens et al. 2020).
In addition, CR affects not only Lgr5 + stem cells, but also reserve ISCs. CR expands the pool of the reserve ISCs and increases the regenerative capacity of the intestinal epithelium (Yousefi et al. 2018).
The mechanism of the intestinal stem cells regulation is based on the kinase-regulated signalling pathway, mammalian target of the rapamycin complex 1 (mTORC1) (Igarashi and Guarente, 2016; Yilmaz et al. 2012; Yousefi et al. 2018). This signalling pathway shows the opposite responses in the ISC compared to the niche cells (Maharajan et al. 2020). Thus, CR favours a decrease in mTOR signalling in Paneth cells and induce the bone stromal antigen 1 (Bst-1), which converts NAD1 into secreted cyclic ribose ADP (cADPR) and activates calcium signalling and this, in turn, stimulates the proliferation of neighbouring stem cells (Yilmaz et al. 2012). Suppression of mTORC1 by exposure to CR was also noted in reserve ISCs (Yousefi et al. 2018). In contrast, a diet associated with caloric restriction activates the mTORC1-p70 ribosomal S6 kinase (S6K1) axis and increases the number of Lgr5 + stem cells through the NAD-dependent protein deacetylase SIRT1(Igarashi and Guarente, 2016).
Interesting data was obtained in a recent study, when in a short-term (9 days) 60% CR diet the number of the stem cells increased, but the proliferation and size of the organelles obtained from the crypts diminished. At the same time, a similar diet in the germ-free (GF) mice did not cause changes in the size of organelles, which designate the role of the microbiome in the effect of CR on the intestinal stem cells (Glenny et al. 2020).
Adipose tissue is very sensitive to a calorie restriction, mitigating age-related adipocyte size increase and stimulating the production of the functional beige fat in the subcutaneous and visceral adipose tissue, and prevent aging of the white adipose tissue (Sheng et al. 2020, Fabbiano et al. 2016). However, the effect of CR on the adipose tissue derived mesenchymal stem cells is poorly understood. Keeping C57BL/6 mice at the age of 4 months or 21–29 months on a 60% CR diet for 9 months ensued a decrease in the age-related increase in the number of ASCs, but it also reduced cell clonality during aging and CR (Schmuck et al. 2011).
Thermoregulation studies in CR animals showed the importance of the ambient temperature and coat and skin for regulating the energy balance (Ravussin et al. 2012). 60% CR diet for 6 months in Swiss mice results in a decrease in subcutaneous fat reserves and thickening of the epidermis and an increase in the pool of the hair follicle stem cells. The authors of this work suggest that the thermoregulatory adaptive evolutionary mechanism compensates for the heat loss (Forni et al. 2017).
One of the most dangerous conditions associated with aging is dementia and other cognitive impairments that dramatically reduce the productivity and quality of life in elderly (Desai et al. 2010). Therefore, the regulation of the brain aging processes with diet, and in particular calorie restriction, draws in much consideration of the researchers. (Dias et al. 2020). Mild to moderate CR (≤ 40%) CR launched at an early stage improved neurovascular functions of the brain, cognitive function and memory in aging animals compared to the controls (Parikh et al. 2016; Kaptan et al. 2015). Considering the role of stem cells in neurogenesis, alterations in stem cell function under the influence of CR have called the increased attention in studying the effects of CR on cognitive function and brain neuroplasticity. (Apple et al. 2019; Dias et al. 2020). Several preclinical studies show that CR stimulates cell proliferation in the dentate gyrus in the hippocampus and subventricular zone (SVZ), where neurogenesis occur throughout life and slows down with aging (Park et al. 2013; Kaptan et al. 2015; Apple et al. 2019). On the other hand, long-term CR at the age of 3 to 11 months had no effect on neurogenesis in the granular cell layer (GCL), but stimulated the survival of the newly formed glial cells in the hilus of the dentate gyrus (Bondolfi et al. 2004).
A recent study by Apple et al. (2019) found out that calorie restriction has a differential effect on the proliferation and production of neurons by the neuronal stem cells in the SVZ, depending on the age at which the diet was initiated (Apple et al. 2019). If 60% of CR was launched at an early age (6 to 7 months) within 16 weeks, it supported the increase of proliferation of stem and progenitor cells in the SVZ. Moreover, if the CR diet was started at an old age (from 12 to 18 months), these animals did not have an increase in proliferation, but the neurogenesis was equivalent to that in the young mice. The authors suggest that perhaps caloric restriction may improve neonatal survival of neurons. In addition, in this study, CR was shown to contribute to the ability of the neural stem cells in aged mice to differentiate into neurons in vivo. A possible effect of CR on the preservation of the neural stem cell function in the old brain has been associated with the amelioration of the progressive inflammation (decreased number of the activated microglia and cytokine expression) (Apple et al. 2019).
One of the mechanisms involved in increasing the adaptiveness and resistance of the neurons in CR may be the induction of the neurotrophic factors, such as brain neurotrophic factor (BDNF) (Fontán-Lozano et al. 2008; Kishi et al. 2014). It should be noted that there is conflicting evidence regarding BDNF expression in CR, if short-term exposure to 15% CR for 4 weeks in young females resulted in increased levels of BDNF in the hippocampus and prefrontal cortex (PFC) in adulthood (Kaptan et al. 2015), than long-term exposure to 30% CR in male mice starting at 3 months of age for 9 months or over 17 months reduced BDNF levels compared to the animals fed ad libitum (Yang et al. 2014). Most studies suggest that one of the key mechanisms of regulation induced by CR might be the inhibition of the decrease in age-related autophagy, in particular through the mTOR signalling pathway (Maiese et al. 2013; Raman et al. 2013). Several studies revealed that aging-related neurodegeneration is accompanied by the increased activation of mTOR signalling (Maiese et al. 2013; Troca-Marín et al. 2011). On the other hand according to Yang et al. there is a parallel decrease in mTOR signalling and activity with age in the hippocampus of mice (Yang et al. 2014). Therefore, it is assumed that the mTOR signalling cascade play different roles in the hippocampus of young and old mice (Yang et al. 2014). However, the main mechanisms mediating the relationship between CR and neurogenesis are poorly understood. Interesting data were obtained in a recent study where short-term CR (overnight) activates new neurons of the olfactory bulb born in adult mice in a ghrelin-dependent manner (Ratcliff et al. 2019).
One of the main reasons of frailty in older people and their loss of independence is age-related degenerative muscle loss, deterioration of the muscle fibres, and a decrease in the strength of muscle contraction, which is called sarcopenia (Xie et al. 2020). Several animal studies have been known to show that CR leads to a reduction or delay in the age-related defects that occur in the skeletal muscle (Xie et al. 2020; Boldrin et al. 2017). Muscle stem cells are also sensitive to the effects of CR. Thus, an early study conducted in young C57BL/6 mice (2 months), and on old mice (18 months), which were exposed to CR (1 week at 20% restriction and 11 weeks at 40%) showed that CR promoted an increase in frequency of skeletal muscle stem cells in young and old mice. These cells had an increased number of the mitochondria and muscle after CR showed increased regeneration rate (Cerletti et al. 2012). Later studies also showed that 25 weeks exposure to 30% CR increased the skeletal muscle stem cells in aged mice (60 weeks old) (Sato et al. 2017). Also in this study, mice on CR diet showed the reorganization of the transcriptome in muscle stem cells, manifested in the regular transcription of genes associated with the self-renewal and decreased activity of the genes involved in inflammation or repair of the mitochondrial DNA (mtDNA). This might be an indirect evidence of the restoration of the stem cells and the slowing down of their aging.
More recently, a 40% CR short-term (3 months) or long-term (6 months) diet in 12-week-old adult male Sprague–Dawley rats sustained the clonal myogenic activity in muscle stem cells (Abreu et al. 2020). At the same time, interestingly, calorie restriction did not affect mitochondrial (oxygen consumption rates, including basal and physiological respiration, ATP-associated and dependent on proton leakage respiration, maximum and reserve respiration) or glycolytic function. (Abreu et al. 2020). Overall, these results showed that caloric restriction did not result in overt changes in the muscle stem cell metabolism.
In addition, the effects of CR on the muscle stem cells depend on many factors, such as lineage, sex and age of animals according to the findings of Boldrin et al. in 2017. (Boldrin et al. 2017). Researchers showed that short-term (3 months) and longer (9 and 19 months) CR have different effects on the skeletal muscle in male and female C57Bl/6 and DBA/2 mice (shorter-lived strain)36. The exposure to CR was shown to increase the number of the satellite cells by 6 months, but their number decreases by 12 months. In addition, CR increased fibrosis by increasing the collagen VI content in mouse muscle and decreased muscle regenerative response. Also CR makes satellite cells less proliferative in vitro (Boldrin et al. 2017).
Reducing oxidative stress, increasing mitochondrial function, suppressing inflammation and apoptosis, and activating autophagy are thought to play an important role in CR-mediated regulation of the sarcopenia, but the exact mechanism by which CR affects stem cells in the skeletal muscle is still a matter of debate.
It should be noted that restricting calories can have both positive and negative consequences. (Xie et al. 2020). Age has been shown to play an important role in the response to calorie restriction. For example, it was shown that short-term CR (4 weeks) led to the accelerated muscle degradation in mice in old SD rats (25 months) (Park et al. 2017). In this aspect, it is the effect of calorie restriction on sarcopenia in the elderly that is one of the main obstacles in calorie restriction therapy (Xie et al. 2020). Thus, based on the above results of the various studies, it could be concluded that calorie restriction has an impact on the functioning of the adult stem cells. At the same time, the degree of the impact depends on the duration of the calorie restriction diet, and in the safest and most effective diet option is a short-term diet. In addition, the age at which the diet is launched is an important parameter. Thus, the best effects were achieved with a diet started at a young age, whereas a diet in old age may not have positive results or even lead to the negative consequences. It should also be noted that the heterogeneity of the adult stem cells is responsible for the variability of the signalling pathways underlying the mechanisms of CR effect.
In addition, there is evidence that often the calorie restricted diet used in various models is not complete and often leads to malnutrition in both mice and rats (Cerqueira and Kowaltowski 2010). Another limitation is the loss of the muscle mass and a decrease in the body mass index in CR in the elderly, which carries potential risks associated with an increased risk of disability and mortality. (Xie et al. 2020). Taken together, these potential risks of CR are of concern that must be considered before it could be widely used. The establishment of standardized preclinical complete diet models is needed to study the mechanisms of CR in more detail.
Considering the risks of CR for the elderly, it is necessary to develop alternative approaches, one of which may be the use of strategies based on simulating the effects of CR, for example, the use of calorie restriction mimetics (Ingram et al. 2006).
3 Calorie Restriction Mimetics as Aging Modulators
However, despite the accumulated data on the effect of calorie restriction on slowing aging, the body responses in older adults differ from those in younger people. Thus the health benefits of calorie restriction in elderly remain uncertain (Locher et al. 2016). Changing the eating behaviour of older adults and, moreover, maintaining long-term calorie restriction is challenging (Madeo et al. 2019). Therefore, the idea to develop techniques or compounds that can reproduce the effect of calorie restriction without restricting the food intake seems very attractive (appealing). In 1998, Lane et al. found that feeding rats with 2-deoxy-d-glucose (2DG) for 24 weeks could mimic the metabolic effects of long-term CR intervention without significant toxicity or sustained alteration in food intake (Lane et al. 1998). Based on the obtained results, they proposed the concept of calorie restriction mimetics (CRMs), i.e. compounds that demonstrate the systemic effects of CR (Madeo et al. 2019).
Currently, in the broadest terms, CRMs are viewed as any intervention that demonstrates the systemic effects of CR and increases life expectancy and prevents aging. These include anorectic or nutrient absorption inhibiting agents such as caloric restriction mimetics, calorie-reducing drugs such as sodium glucose co-transporter 2 inhibitors (SGLT2i), and even bariatric surgery (Ingram et al. 2006).
An important debatable issue in this concept is the precise definition of the concept of CR mimetics as pharmacological agents (Shintani et al. 2018, Madeo et al. 2019). Over the past two decades, there has been no consensus on the biochemical and functional determination of CR mimetics (Ingram and Roth 2015). Pioneers of the mimetic concept, Ingram et al. suggested descriptors specific to CRM: (1) mimics the metabolic, hormonal, and physiological effects of the CR; (2) activates stress response pathways observed in CR and enhances the stress protection; (3) produces CR-like effects on longevity, reduces age-related disease, and maintains more youthful function; and (4) does not significantly reduce food intake, at least over the short-term (Ingram and Roth, 2015; Ingram et al. 2006, 2004). Based on these descriptors, the concept of mimetics is a broad view of the CR mimetics.
In a narrower sense, the basic properties of CR mimetics are thought to be associated with the regulation of autophagy and glucose metabolism as key mechanisms of calorie restriction within the cell (Shintani et al. 2018, Madeo et al. 2019). According to Madeo et al. CRms are compounds that activate autophagy by promoting the deacetylation of the cellular proteins, by (1) depleting acetyl coenzyme A (AcCoA), (2) inhibiting acetyltransferases, and/or (3) stimulating deacetylases (Madeo et al. 2019).
The question of the ability of CRMs to increase life expectancy is unclear. It is assumed that the overall cumulative effect of the CRM exposure is an increase in life expectancy and a decrease in age-related disorders (Ingram et al. 2006). However, the effectiveness of CRMs in this aspect is unclear and, for example, so far, only rapamycin has shown a steady effect on the increase in life expectancy as in rodents regardless of their sex (Miller et al. 2011; Harrison et al. 2009). But at the same time, in another study, rapamycin has a limited effect on aging in mammals and increases lifespan, possibly activating mechanisms of suppression of carcinogenesis (Neff et al. 2013). The lack of CRMs effectiveness to increase lifespan may be due to the fact, that the positive effects of the CR are mediated through the regulation of many intracellular signaling pathways, whereas CRMs are apparently involved in the activation of only some of the pathways and there is no single CR mimetic capable of mimicking CR alone. Therefore, some studies suggest the use of a combination of several CR mimetics (Ingram and Roth 2015). Thus, most likely CRM is a group of molecularly unrelated compounds capable of partially causing effects similar to CR on the cells, tissues, and organs.
However, despite the absence of the mimetics that completely mimick the effects of the calorie restriction, the currently known CRMs are able to positively influence the aging processes of the cardiovascular system, and also reduce acute ischemia in preclinical models (Sciarretta et al. 2020). Slowing down the aging of the neuromuscular junctions and muscle fibers has also been observed with the exposure to CRMs (Stockinger et al. 2018). What's more, CRMs have beneficial effects on the brain aging and prevent Alzheimer's disease (Van Cauwenberghe et al. 2016; Chiba et al. 2010). In addition, numerous studies indicate a protective role for CRMs in diabetes and obesity (Chiba et al. 2010).
4 Calorie Restriction Mimetics and Adult Stem Cell Aging
The previous section of the chapter described the relationship between CR and adult stem cell function, and reported studies showing that CR has a generally positive effect on the adult stem cells and inhibits aging. Accordingly, it is logical to assume that when exposed to CRMs, one can expect their influence on the functioning of the stem cells through the activation of the molecular pathways involved in the response to CR. Therefore, in this section of the chapter, we summarize the impact of CRMs on the adult stem cells in aging.
The intestinal tract is the first organ in contact with food, and at the same time is very sensitive to calorie restriction (Peña-Villalobos et al. 2019), therefore, first of all, it is necessary to pay attention to the reactions of the intestinal stem cells to the action of CRMs. It has been shown that the antidiabetic drug metformin, which is classified as CRMs (Martel et al. 2021), improve aging phenotypes (hyperproliferation, centrosome amplification, and accumulation of DNA damage) in the Drosophila intestinal stem cells through the down-regulation of the AKT / TOR signaling pathway (Na et al. 2015, 2018). It was recently found that alpha-lipoic acid (ALA; 1,2-dithiolane-3-pentanoic acid), also referred to as CRM, also had a positive effect on the aging phenotypes of the Drosophila intestinal stem cells, resulting in the suppression of the age-related hyperproliferation of the intestinal stem cells (Du et al. 2020). Interestingly, the effect of ALA on stem cells was achieved not due to the antioxidant capacity, but due to the activation of the expression of specific genes associated with the autophagy and endocytosis in old cells (Du et al. 2020).
Nicotinamide riboside (NR), a precursor of nicotinamide adenine dinucleotide (NAD + ), is a potent anti-inflammatory agent and an aging modulator (Mehmel et al. 2020) with CRMs properties (Madeo et al. 2019). A study by Igarashi et al. (2019) found that oral administration of NR at a concentration of 500 mg/kg/day in drinking water for 6 weeks resulted in ISC recovery in aged mice in vivo, while ex vivo studies showed improved colony formation in aged mice and the effect of NR was blocked by the mTORC1 inhibitor rapamycin or the SIRT1 inhibitor EX527 (Igarashi et al. 2019). At the same time, data on the effect of the mTORC1 inhibitor rapamycin, one of the most studied CRMs, on the intestinal stem cells is controversial (He et al. 2020; Igarashi and Guarente 2016; Yilmaz et al. 2012). On the one hand, a recent study showed that mTORC1 is strongly upregulated in the ISC cells in aged mice and mTORC1 inhibition resulted in a partial improvement in aging phenotypes in 16 diet-restriction-induced ISC expansion of one-month-old mice (He et al. 2020). In contrast, Igarashi et al. demonstrated that caloric restriction induces an increase in mTORC1 activity in ISCs leading to the increased cell proliferation, whereas rapamycin suppressed dietary restriction-induced ISC proliferation (Igarashi and Guarente 2016).
The functioning of the hematopoietic stem cells can also be altered by CRMs, especially by the compounds associated with the regulation of mTOR. The importance of mTOR in HSC aging is evidenced by the results of a study that showed that age-related functional decline in HSC is improved in long-lived mTOR mutant mice (Selman et al. 2016). And also in another study, mTOR activity increases in the HSC of mice with aging, and rapamycin at a dose of 4 mg/kg every other day for 6 weeks increased lifespan, restored self-renewal and hematopoiesis of HSC in old mice (22 months) (Chen et al. 2009). An ex vivo study showed that rapamycin treatment inhibited the cellular senescence, possibly through Bmi1 activation and p16 inhibition, and led to the stimulation of ex vivo expansion and long-term hematopoietic repair of HSCs (Luo et al. 2014).
Resveratrol also affects hematopoiesis in vitro and in vivo (Rimmelé et al. 2014; Zhang et al. 2010; Matsui et al. 2012). Rimmelé et al. demonstrated that daily administration of resveratrol (5 mg/kg) for three weeks increased the frequency and the total number of the Lin - Sca1 + c-Kit + (LSK) cells in the bone marrow of the C57BL/6 mice (Rimmelé et al. 2014). In addition, the treatment with the resveratrol improved the state of the LSK-HSC, positively influenced the microenvironment of the bone marrow, and partially corrected the abnormal status of the cell cycle in a mouse model of the Fanconi anemia (Zhang et al. 2010). A similar effect on the same Fanconi model of anemia was demonstrated by metformin, a therapy that led to an improvement in the hematopoiesis, increased the size of the hematopoietic stem cell compartment, and enhanced the rest of hematopoietic stem cells and progenitor cells. Moreover, metformin reduced the DNA damage and improved the spontaneous chromosome breakage in cells (Zhang et al. 2016).
The importance of the autophagy activation when exposed to CRMs on the skeletal mouse stem cells has been shown in a number of studies (Ramos et al. 2012). It was shown that in prematurely senescent mice, the muscle-derived stem/progenitor cells (MDSPCs) exhibit abnormalities in proliferation, chondrogenic, osteogenic, and myogenic differentiation and regenerative potential, and cultivation in the rapamycin-containing media resulted in the improved differentiation and decreased apoptosis and aging (Kawakami et al. 2019).
One of the key regulators of the autophagy is Sirtuin 1 (Sirt1), also known as a NAD-dependent deacetylase sirtuin-1, the nutrient sensor, so resveratrol as an activator of the Sirt1 can influence the functioning of the satellite cells (Alway et al. 2014). Resveratrol, for example, caused the restoration of muscle mass in the plantar muscle of old rats after hanging the hind limbs, which was associated with the improvement of the satellite cell proliferation in the hind limb muscles (Bennett et al. 2013). On the other hand, the 10-month consumption of the resveratrol had a protective effect against the aging-induced oxidative stress in the skeletal muscles, but did not attenuate sarcopenia in mice (Jackson et al. 2011). This is possible because the effect of resveratrol may depend on its concentration. Resveratrol at low doses (10 μM) caused the myoblast cell cycle stop, migration and promoted the muscle regeneration in vitro by attenuating ROS exposure, while higher doses (40–60 μM) suppressed these effects (Bosutti and Degens, 2015).
Metformin, as a regulator of autophagy, also caused the metabolic reprogramming in the fate of the skeletal muscle stem cells. Interestingly, in a recent study by Pavlidou et al. it was shown that unlike resveratrol, metformin did not lead to the activation of Sirt1 in the satellite cells, and contributed to the maintenance of the satellite cells at rest, caused a delay in their differentiation in vitro and slowed down the process of muscle regeneration after the cardiotoxin injury in vivo (Pavlidou et al. 2019). On the other hand, the metformin improved in vivo the regeneration after a burn injury by activating the proliferation of the Pax7-positive satellite cells via the AMPK signalling pathway (Yousuf et al. 2020). The authors suggest that the differences between the two studies are due to the fact, that the burn injury causes systemic inflammation that lasts 2 weeks, as opposed to the shorter exposure to cardiotoxin. In addition, after a burn, animals are more mobile and this affects the muscle regeneration (Yousuf et al. 2020). Moreover, conflicting results have been obtained with the myogenic cell differentiation. On the one hand, it was found that in myoblasts, the metformin-treated MyoD and p21cip1 are not activated, which negatively affects the myogenic differentiation (Pavlidou et al. 2017). In contrast, another study showed that metformin did not affect the proliferation but enhanced the myogenic cell differentiation (Senesi et al. 2016). These results demonstrate the complex nature of the effects of metformin on the muscle stem cells and the need for more detailed studies.
Several studies have shown an association between the changes in polyamine levels in the muscle fibers and the skeletal muscle atrophy and hypertrophy (Lee and MacLean, 2011). It was shown that in vitro polyamines (putrescine, spermidine and spermine) cause activation of the satellite cells and the expression of myogenic regulatory factors (Thornton et al. 2013). in vivo studies have shown that intraperitoneal injections of spermidine at a dose of 100 mg/kg of body weight every other day for 32 days induced the autophagy in the satellite cells and activated the resting satellite cells in mice (Zhang et al. 2018).
Simulating the effect of calorie restriction on the cardiovascular system is one of the most important properties of mimetics (Sciarretta et al. 2020). An interesting question is how much adult heart stem cells are involved in this process, which can play a central role in the age-related remodeling of the heart (Carresi et al. 2021).
Among the known mimetics, the most effective heart stem cell stimulant (CSC) is the resveratrol. The main results were obtained from the studies related to the transplantation of the CSCs for the treatment of myocardial infarction. A study in Sprague–Dawley rats demonstrated that preliminary systemic administration of resveratrol at a concentration of 2.5 mg/kg/day for 2 weeks improved the myocardial tissue environment and increased the survival and proliferation and the differentiation of transplanted CSCs in the area of the myocardial infarction (Gurusamy et al. 2010).
Pre-treatment with resveratrol for 60 min with the CSC before transplantation resulted in the improved cardiac function and enhanced engraftment of the implanted stem cells that had increased the expression of Nrf2, Ref-1 and NFοB (Gorbunov et al. 2012). Resveratrol at a concentration of 2.5 mg/kg per day for 4 weeks was also shown to activate the transplanted Sca-1 + CSC cells in the heart. The authors showed that the upregulation of the VEGF and SDF-1α is the likely mechanism underlying the resveratrol action (Ling et al. 2017). Resveratrol has been shown in several studies to promote the differentiation of the Human Induced Pluripotent Stem Cells into cardiomyocytes through inhibition of the canonical Wnt signaling pathway and the SRF-miR-1 (Liu et al. 2016). It was also shown that the resveratrol concentration of 10 μmol/L was optimal for stimulating the differentiation of the mouse embryonic stem cells into cardiomyocytes (Ding et al. 2016).
Rapamycin also alters the functional properties of the CSCs, which is associated with the importance of mTOR activity for the vital activity of the heart stem cells (Zheng et al. 2017). Several studies have shown that rapamycin effectively accelerates the differentiation of the embryonic stem cells into the cardiomyocytes (Qiu et al. 2017; Lu et al. 2017). Investigations that demonstrate the role of rapamycin regulation of the mTOR activity in the senescent CSCs are important. Studies of the effect of rapamycin on the CSC showed that in vitro the treatment of the cells with the rapamycin (10 nM) and resveratrol (0.5 μM) reduced the cellular senescence and IL1β secretion and increased the rate of cell proliferation, due to the mechanisms that were activated by the increased phosphorylation of AMPK. Moreover, this preliminary pharmacological treatment of the old CSCs in vitro led to an improvement in their reparative potential in vivo (Avolio et al. 2014).
A recent study showed that a treatment with rapamycin (100 nM) markedly improves the cellular functions, attenuates the replicative senescence, and promotes the proliferation of the senescent human cardiac progenitor cells (hCPCs) (Park et al. 2020). In addition, the long-term treatment with rapamycin improves the clonogenic potential and maintains the migration/differentiation capacity of the old hCPCs (Park et al. 2020). It was also shown that the rapamycin pretreatment of mesenchymal stem cells isolated from the rat bone marrow enhances the cardiogenesis and differentiation of the transplanted cells into the cardiomyocytes in a model of myocardial infarction (Li et al. 2020). However, metformin impaired the homing and survival of the MSCs in the heart in the diabetic streptozotocin-induced cardiomyopathy in rats (Ammar et al. 2021).
5 Calorie Restriction Mimetics for Neural Stem Cells and Neurogenesis
The previous section of the chapter summarized the results of the studies showing that the CR enhances the neurogenesis. Therefore, it is necessary to consider the effects of the mimetics on the neural stem cells and neurogenesis. The available data indicate that the resveratrol has different effects on neurogenesis of a young and healthy organism (Park et al. 2012) under various stress conditions (Madhyastha et al. 2013; Moriya et al. 2011; Shen et al. 2016) and aging (Kumar et al. 2016). Thus, the administration of the resveratrol (1–10 mg/kg) for 14 days inhibited the proliferation and survival of the neural progenitor cells (NPCs) in the dentate gyrus of the hippocampus of the young C57BL/6 mice and reduced the levels of the phosphorylated form of the cyclic AMP response element-binding protein (pCREB) and the BDNF in the hippocampus (Park et al. 2012). While in healthy animals resveratrol suppressed the neurogenesis, in rats exposed to embryonic stress, the resveratrol treatment increased the number of neonatal neurons in the hippocampus and the BDNF expression (Madhyastha et al. 2013).
Resveratrol was also shown to stimulate the neurogenesis and mRNA expression of the brain neurotrophic factor in the hippocampus of mice with chronic fatigue (Pavlidou et al. 2019). in vitro, the exposure to resveratrol on the neural stem cells after the oxygen–glucose deprivation/reoxygenation leads to the increased cell survival and proliferation, reduces apoptosis and the MDA levels, and also increases the SOD activity, GSH content, and the expression of Nrf2, HO- 1, and NQO1 proteins (Li et al. 2020). Resveratrol also exhibited a two-phase effect on NPCs under the culture conditions (Kumar et al. 2016). While low concentrations of resveratrol (10 μM) induced cell proliferation through the signal pathways associated with the signal-regulated extracellular kinases (ERK) and p38 kinases phosphorylation, the high concentrations (>20 μM) leveled these effects. The administration of resveratrol (20 mg/kg body weight) to old rats (15 months) enhanced the neurogenesis in the region of the dentate gyrus of the hippocampus (Kumar et al. 2016).
Numerous in vitro and in vivo studies have confirmed that metformin may be an important regulator of the metabolism and functioning of the brain cells, including the stem cells, and has a great potential in the treatment of the neurodegenerative diseases (Markowicz-Piasecka et al. 2017; Jiang and Liu, 2020). Several studies have shown the activation of the neural stem cells by metformin in the various injuries. It has been shown that metformin causes an activation of the neural stem cells in the spinal cord only in males, and the progenitor cells in both sexes. Exposure to metformin resulted in the improved functional outcomes after trauma in the thoracic spinal cord (Gilbert and Morshead, 2019).
On the other hand, in a study conducted by Ruddy et al. where it was shown that the effect of metformin on the NPCs depended on sensitivity to sex hormones, especially the estradiol, and only females increased the proliferation of progenitor cells. At the same time, the neural stem cells and the neurogenesis were activated to the same extent in both sexes in a model of neonatal stroke (Ruddy et al. 2019). In an another study, it was demonstrated that administration of metformin for 7 days activates the endogenous NPCs, expands the NPC pool, and aids migration and differentiation of the NPCs in the damaged neonatal brain in a hypoxia-ischemic injury model (Dadwal et al. 2015). Using the advanced glycosylation end products (AGEs) model of the neuronal damage, metformin was shown to increase the survival of the human neural stem cells (hNSC) and enhance the expression of the AMPK, PGC1α, NRF-1, and Tfam (Chung et al. 2015).
Metformin preconditioning of the human induced pluripotent stem cells of the neural stem cells prior to the brain transplantation damaged by stroke has also been shown to improve their engraftment and regeneration and recovery after (Ould-Brahim et al. 2018). Activation of the atypical PKC-CBP pathway, which enhances the neurogenesis in the brain of the adult mice, is assumed to be signalling pathways involved in the regulation of the response to the metformin action on NPCs (Wang et al. 2012). In addition, Fatt et al. revealed that the neuronal differentiation of the NPCs extracted from the subventricular zone was enhanced by metformin and depended on the activation of the AMPK-aPKC-CBP pathway (Fatt et al. 2015).
Also, the metformin treatment has recently been shown to result in rejuvenation of the oligodendrocyte progenitor cells (OPCs) and the improvement of remyelination in old animals after focal demyelination (Neumann et al. 2019).
The mTOR signaling pathway is a key in the neuronal and glial differentiation processes and the maintenance of the neural stem cell stemness (LiCausi and Hartman 2018). Intra-cerebro-ventricular infusion of rapamycin (0.5 mM) into the left ventricle of mice was shown to reduce the number of proliferating neural stem cells for almost two times within 7 days (Paliouras et al. 2012). In addition, this study showed that rapamycin suppresses the division and differentiation of the neural stem cells in vitro and reduces both the size and the number of the neural stem cells grown as neurospheres (Paliouras et al. 2012).
An interesting comparative study of the effect of rapamycin or metformin on the proliferating neural precursor SVZ and DG cells was carried out in mice (Kusne et al. 2014). A 9-week regimen of daily intraperitoneal (ip) injection of rapamycin at a dose of 75 μg/kg (low dose) or 2.5 mg/kg (high dose) has been shown to reduce the proliferation of the neural stem cells in the SVZ region of the brain of adult C57BL/6 J., while a similar regimen of metformin administration at a dose of 200 mg/kg per day did not cause such an effect. A decrease in the proliferation and differentiation was also found under the influence of rapamycin, but not metformin (Kusne et al. 2014).
The study of the mTOR system in the brain during aging showed that the total number of the neural stem cells and their proliferation in the hippocampus sharply decreases with age and this correlates with a decrease in the activity of the mTOR signalling in the old brain, including the NSC (Romine et al. 2015). It was shown that a single injection of rapamycin (10 mg/kg) led to a significant decrease in the number of proliferating cells in the hippocampus in young animals. Also, if activation of the mTOR system with ketamine improved the neurogenesis in the hippocampus, then the joint intraperitoneal injections of ketamine (10 mg/kg) and rapamycin (10 mg/kg) to 12 month old mice reduced the level of proliferation (Romine et al. 2015). Collectively, these data indicate that the use of rapamycin may exacerbate brain aging, and in this aspect the compound is not a calorie restriction mimetic.
Endogenous polyamines play an important role in the neural differentiation, learning and memory (Guerra et al. 2016) and the levels of polyamines in the aging brain gradually decrease as they age (Sigrist et al. 2014). Spermidine and spermin have recently been shown to induce autophagy and inhibit brain aging in the SAMP8 mice (Xu et al. 2020). Perhaps this deceleration is also associated with the effect of spermidine on the neural stem cells, as evidenced by another study, the cultivation of the NPC with spermidine, the cell migration was facilitated, the number of neurites increased and the BDNF expression increased 7 days after the addition of SPD (Signor et al. 2017).
Thus, some of the chemical compounds that are classified as the CRMs undoubtedly have the properties that allow them to mimic calorie restriction, including through the mechanisms associated with the activation of the adult stem cells. However, the impact of the CRMs on stem cells can be both positive and negative depending on the type of stem cells, which is associated with the heterogeneity of the adult stem cells in the body. Moreover, the effects of the CRMs on stem cells can be considered within the concept of hormesis, when the small doses of the CRMs induced the activation of endogenous stem cells, while the large doses suppressed. It should also be noted that the CRMs often have the antioxidant properties, and in high doses they presumably can cause the so-called antioxidant stress in healthy cells and disrupt a normal redox homeostasis, and this, on the contrary, can lead to an accelerated aging (Kornienko et al. 2019).
Apart from that, the responses of the adult stem cells to the mimetics are age and gender dependent, so that they vary greatly from positive to negative. In addition, the state of a niche or tissue microenvironment has a strong influence on the functioning of the adult stem cells, which, under the various stresses and diseases, can modulate the effects of mimetics. The role of the microbiome, which may be involved in mediating the effects of the CRMs on stem cells, remains unexplored. We believe that this direction is the very promising in the development of a further strategy for the use of mimetics. Therefore, further studies are required to determine the role of metabolic reprogramming in the fate of stem cells with the help of mimetics. It has to include more accurate and detailed characterization and understanding of the molecular and cellular mechanisms of the aging process. Moreover, there is a need to elucidate the interaction of the microbiome axis - stem cells and determine the optimal concentrations of mimetics and their combinations. Finally, the search for the new compounds with the most complete properties mimicking calorie restrictions are needed.
References
Abreu P, Serna JDC, Munhoz AC, Kowaltowski AJ (2020) Calorie restriction changes mu scle satellite cell proliferation in a manner independent of metabolic modulation. Mech Ageing Dev 192:111362
Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R (2012) Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res 8:215–225
Alway SE, Myers MJ, Mohamed JS (2014) Regulation of satellite cell function in sarcopenia. Front Aging Neurosci 6:246
Ammar HI, Shamseldeen AM, Shoukry HS, Ashour H, Kamar SS, Rashed LA, Fadel M, Srivastava A, Dhingra Sa (2021) Metformin impairs homing ability and efficacy of mesenchymal stem cells for cardiac repair in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol-Heart Circ Physiol 320(4):H1290–H1302. https://doi.org/10.1152/ajpheart.00317.2020
Apple DM, Mahesula S, Fonseca RS, Zhu C, Kokovay E (2019) Calorie restriction protects neural stem cells from age-related deficits in the subventricular zone. Aging 11:115–126
Avolio E, Gianfranceschi G, Cesselli D, Caragnano A, Athanasakis E, Katare R, Meloni M, Palma A, Barchiesi A, Vascotto C, Toffoletto B, Mazzega E, Finato N, Aresu G, Livi U, Emanueli C, Scoles G, Beltrami CA, Madeddu P, Beltrami AP (2014) Ex vivo molecular rejuvenation improves the therapeutic activity of senescent human cardiac stem cells in a mouse model of myocardial infarction. Stem Cells 32:2373–2385
Barzilai N, Huffman DM, Muzumdar RH, Bartke A (2012) The critical role of metabolic pathways in aging. Diabetes 61:1315–1322
Bennett BT, Mohamed JS, Alway SE (2013) Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLOS ONE 8:e83518
Boldrin L, Ross JA, Whitmore C, Doreste B, Beaver C, Eddaoudi A, Pearce DJ, Morgan JE (2017) The effect of calorie restriction on mouse skeletal muscle is sex, strain and time-dependent. Sci Rep 7:5160
Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M (2004) Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging 25:333–340
Bosutti A, Degens H (2015) The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci Rep 5:8093
Boyette LB, Tuan RS (2014) Adult stem cells and diseases of aging. J Clin Med 3
Bruens L, Ellenbroek SIJ, Suijkerbuijk SJE, Azkanaz M, Hale AJ, Toonen P, Flanagan DJ, Sansom OJ, Snippert HJ, van Rheenen J (2020) Calorie restriction increases the number of competing stem cells and decreases mutation retention in the intestine. Cell Rep 32:107
Carresi C, Scicchitano M, Scarano F, Macrì R, Bosco F, Nucera S, Ruga S, Zito MC, Mollace R, Guarnieri L, Coppoletta AR, Gliozzi M, Musolino V, Maiuolo J, Palma E, Mollace V (2021) The potential properties of natural compounds in cardiac stem cell activation: their role in myocardial regeneration. Nutrients 13:275
Catic A (2018) Cellular metabolism and aging. Prog Mol Biol Transl Sci 155:85–107
Cerletti M, Jang YC, Finley LWS, Haigis MC, Wagers AJ (2012) Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell 10:515–519
Cerqueira FM, Kowaltowski AJ (2010) Commonly adopted caloric restriction protocols often involve malnutrition. Ageing Res Rev 9:424–430
Chang NC (2020) Autophagy and stem cells: self-eating for self-renewal. Front Cell Dev Biol 8
Chen C, Liu Y, Liu Y, Zheng P (2009) mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Sign 2:ra75-ra75.
Chen J, Astle CM, Harrison DE (2003) Hematopoietic senescence is postponed and hematopoietic stem cell function is enhanced by dietary restriction. Exp Hematol 31:1097–1103
Chiba T, Tsuchiya T, Komatsu T, Mori R, Hayashi H, Shimokawa I (2010) Development of calorie restriction mimetics as therapeutics for obesity, diabetes, inflammatory and neurodegenerative diseases. Curr Genomics 11:562–567
Chung KW, Chung HY (2019) The effects of calorie restriction on autophagy: role on aging intervention. Nutrients 11:2923
Chung MM, Chen YL, Pei D, Cheng YC, Sun B, Nicol CJ, Yen CH, Chen HM, Liang YJ, Chiang MC (2015) The neuroprotective role of metformin in advanced glycation end product treated human neural stem cells is AMPK-dependent. Biochimica et Biophysica Acta (BBA) Molecular Basis of Disease 1852:720–731
Clevers H (2015) What is an adult stem cell? Science 350:1319–1320
Dadwal P, Mahmud N, Sinai L, Azimi A, Fatt M, Wondisford FE, Miller FD, Morshead CM (2015) Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Rep 5:166–173
Desai AK, Grossberg GT, Chibnall JT (2010) Healthy brain aging: a road map. Clin Geriatr Med 26:1–16
Dias IR, Santos CDS, Magalhães CODE, de Oliveira LRS, Peixoto MFD, de Sousa RAL, Cassilhas RC (2020) Does calorie restriction improve cognition? IBRO Rep 9:37–45
Ding H, Xu X, Qin X, Yang C, Feng Q (2016) Resveratrol promotes differentiation of mouse embryonic stem cells to cardiomyocytes. Cardiovasc Ther 34:283–289
Du G, Qiao Y, Zhuo Z, Zhou J, Li X, Liu Z, Li Y, Chen H (2020) Lipoic acid rejuvenates aged intestinal stem cells by preventing age-associated endosome reduction. EMBO Rep 21:e49583–e49583
Epel ES, Lithgow GJ (2014) Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol Ser A 69:S10–S16
Fabbiano S, Suárez-Zamorano N, Rigo D, Veyrat-Durebex C, Dokic AS, Didier JC, Trajkovski M (2016) Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab 24:434–446
Fatt M, Hsu K, He L, Wondisford F, Miller FD, Kaplan DR, Wang J (2015) Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Rep 5:988–995
Flanagan EW, Most J, Mey JT, Redman LM (2020) Calorie restriction and aging in humans. Annu Rev Nutr 40:105–133
Fontán-Lozano A, López-Lluch G, Delgado-García JM, Navas P, Carrión AM (2008) Molecular bases of caloric restriction regulation of neuronal synaptic plasticity. Mol Neurobiol 38:167–177
Forni MF, Peloggia J, Braga TT, Chinchilla JEO, Shinohara J, Navas CA, Camara NOS, Kowaltowski AJ (2017) Caloric restriction promotes structural and metabolic changes in the skin. Cell Rep 20:2678–2692
Franceschi C, Ostan R, Santoro A (2018) Nutrition and inflammation: are centenarians similar to individuals on calorie-restricted diets? Annu Rev Nutr 38:329–356
Fukada S, Ma Y, Uezumi A (2014) Adult stem cell and mesenchymal progenitor theories of aging. Front Cell Dev Biol 2:10
Gems D, Partridge L (2008) Stress-response hormesis and aging: “that which does not kill us makes us stronger.” Cell Metab 7:200–203
Gilbert EAB, Morshead CM (2019) Metformin activates neural stem and progenitor cells in the spinal cord and improves functional outcomes following injury. FASEB J 33:208.3
Glenny E, Liu J, Touvron M, Vance N, Magness S, Bulik C, Van LL, Carroll I (2020) Severe calorie restriction induces gut microbiota-dependent intestinal stem cell dysfunction. Curr Dev Nutr 4:1558–1558
Gonzalez MA, Bernad A (2012) Characteristics of adult stem cells. Adv Exp Med Biol 741:103–120
Goodell MA, Rando TA (2015) Stem cells and healthy aging. Science 350:1199–1204
Gorbunov N, Petrovski G, Gurusamy N, Ray D, Kim DH, Das DK (2012) Regeneration of infarcted myocardium with resveratrol-modified cardiac stem cells. J Cell Mol Med 16:174–184
Guerra GP, Rubin MA, Mello CF (2016) Modulation of learning and memory by natural polyamines. Pharmacol Res 112:99–118
Gurusamy N, Ray D, Lekli I, Das DK (2010) Red wine antioxidant resveratrol-modified cardiac stem cells regenerate infarcted myocardium. J Cell Mol Med 14:2235–2239
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395
He D, Wu H, Xiang J, Ruan X, Peng P, Ruan Y, Chen Y-G, Wang Y, Yu Q, Zhang H, Habib SL, de Pinho RA, Liu H, Li B (2020) Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat Commun 11:37
Heilbronn LK, Ravussin E (2003) Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 78:361–369
Hertel J, Friedrich N, Wittfeld K, Pietzner M, Budde K, van der Auwera S, Lohmann T, Teumer A, Völzke H, Nauck M, Grabe HJ (2016) Measuring biological age via metabonomics: the metabolic age score. J Proteome Res 15:400–410
Igarashi M, Guarente L (2016) mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 166:436–450
Igarashi M, Miura M, Williams E, Jaksch F, Kadowaki T, Yamauchi T, Guarente L (2019) NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell 18:12935
Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, Lane MA, Roth GS (2004) Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci 1019:412–423
Ingram DK, Roth GS (2015) Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev 20:46–62
Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, Decabo R (2006) Calorie restriction mimetics: an emerging research field. Aging Cell 5:97–108
Jackson JR, Ryan MJ, Alway SE (2011) Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol Ser A 66A:751–764
Jiang LL, Liu L (2020) Effect of metformin on stem cells: molecular mechanism and clinical prospect. World J Stem Cells 12:1455–1473
Jones DL, Rando TA (2011) Emerging models and paradigms for stem cell ageing. Nat Cell Biol 13:506–512
Kaptan Z, Akgün-Dar K, Kapucu A, Dedeakayoğulları H, Batu Ş, Üzüm G (2015) Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain Res 1618:194–204
Kawakami Y, Hambright WS, Takayama K, Mu X, Lu A, Cummins JH, Matsumoto T, Yurube T, Kuroda R, Kurosaka M, Fu FH, Robbins PD, Niedernhofer LJ, Huard J (2019) Rapamycin rescues age-related changes in muscle-derived stem/progenitor cells from progeroid mice. Mol Therapy Methods Clin Dev 14:64–76
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F (2014) Geroscience: linking aging to chronic disease. Cell 159:709–713
Kishi T, Hirooka Y, Nagayama T, Isegawa K, Katsuki M, Takesue K, Sunagawa K (2014) Calorie restriction improves cognitive decline via up-regulation of brain-derived neurotrophic factor. Int Heart J 56:14–168
Komatsu T, Park S, Hayashi H, Mori R, Yamaza H, Shimokawa I (2019) Mechanisms of calorie restriction: a review of genes required for the life-extending and tumor-inhibiting effects of calorie restriction. Nutrients 11:3068
Kornienko JS, Smirnova IS, Pugovkina NA, Ivanova JS, Shilina MA, Grinchuk TM, Shatrova AN, Aksenov ND, Zenin VV, Nikolsky NN, Lyublinskaya OG (2019) High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci Rep 9:1296
Kumar V, Pandey A, Jahan S, Shukla RK, Kumar D, Srivastava A, Singh S, Rajpurohit CS, Yadav S, Khanna VK, Pant AB (2016) Differential responses of Trans-Resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep 6:28142
Kusne Y, Goldberg EL, Parker SS, Hapak SM, Maskaykina IY, Chew WM, Limesand KH, Brooks HL, Price TJ, Sanai N, Nikolich-Zugich J, Ghosh S (2014) Contrasting effects of chronic, systemic treatment with mTOR inhibitors rapamycin and metformin on adult neural progenitors in mice. Age (dordr) 36:199–212
Lane MA, Ingram DK, Roth GS (1998) 2-Deoxy-D-glucose feeding in rats mimics physiologic effects of calorie restriction. J Anti Aging Med 1:327–337
Lazare S, Ausema A, Reijne AC, van Dijk G, van Os R, de Haan G (2017) Lifelong dietary intervention does not affect hematopoietic stem cell function. Exp Hematol 53:26–30
Lee NK, Maclean HE (2011) Polyamines, androgens, and skeletal muscle hypertrophy. J Cell Physiol 226:1453–1460
Li Z-H, Wang Y-L, Wang H-J, Wu J-H, Tan Y-Z (2020) Rapamycin-preactivated autophagy enhances survival and differentiation of mesenchymal stem cells after transplantation into infarcted myocardium. Stem Cell Rev Rep 16:344–356
Licausi F, Hartman NW (2018) Role of mTOR complexes in neurogenesis. Int J Mol Sci 19:1544
Ling L, Gu S, Cheng Y (2017) Resveratrol activates endogenous cardiac stem cells and improves myocardial regeneration following acute myocardial infarction. Mol Med Rep 15:1188–1194
Liu H, Zhang S, Zhao L, Zhang Y, Li Q, Chai X, Zhang Y (2016) Resveratrol enhances cardiomyocyte differentiation of human induced pluripotent stem cells through inhibiting canonical WNT signal pathway and enhancing serum response factor-miR-1 axis. Stem Cells Int 2016:2524092–2524092
Liu L, Rando TA (2011) Manifestations and mechanisms of stem cell aging. J Cell Biol 193:257–266
Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD (2016) Calorie restriction in overweight older adults: do benefits exceed potential risks? Exp Gerontol 86:4–13
Loeffler M, Roeder I (2002) Tissue stem cells: definition, plasticity, heterogeneity, self-organization and models–a conceptual approach. Cells Tissues Organs 171:8–26
López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G (2016) Metabolic control of longevity. Cell 166:802–821
Lu Q, Liu Y, Wang Y, Wang W, Yang Z, Li T, Tian Y, Chen P, Ma K, Jia Z, Zhou C (2017) Rapamycin efficiently promotes cardiac differentiation of mouse embryonic stem cells. Biosci Rep 37:BSR20160552
Luo Y, Li L, Zou P, Wang J, Shao L, Zhou D, Liu L (2014) Rapamycin enhances long-term hematopoietic reconstitution of ex vivo expanded mouse hematopoietic stem cells by inhibiting senescence. Transplantation 97:20–29
Ma S, Sun S, Geng L, Song M, Wang W, Ye Y, Ji Q, Zou Z, Wang S, He X, Li W, Esteban CR, Long X, Guo G, Chan P, Zhou Q, Belmonte JCI, Zhang W, Qu J, Liu G-H (2020) Caloric restriction reprograms the single-cell transcriptional landscape of rattus norvegicus Aging. Cell 180:984-1001.e22
Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G (2019) Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab 29:592–610
Madhyastha S, Sekhar S, Rao G (2013) Resveratrol improves postnatal hippocampal neurogenesis and brain derived neurotrophic factor in prenatally stressed rats. Int J Dev Neurosci 31:580–585
Maharajan N, Vijayakumar K, Jang C, Cho G-W (2020) Caloric restriction maintains stem cells through niche and regulates stem cell aging. J Mol Med 98:25–37
Mai V, Colbert LH, Berrigan D, Perkins SN, Pfeiffer R, Lavigne JA, Lanza E, Haines DC, Schatzkin A, Hursting SD (2003) Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in ApcMin mice through different mechanisms. Cancer Res 63:1752
Maiese K, Chong ZZ, Shang YC, Wang S (2013) mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med 19:51–60
Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen KM (2017) Metformin - a future therapy for neurodegenerative diseases : theme: drug discovery, development and delivery in alzheimer’s disease guest editor: davide brambilla. Pharm Res 34:2614–2627
Martel J, Chang S-H, Wu C-Y, Peng H-H, Hwang T-L, Ko Y-F, Young JD, Ojcius DM (2021) Recent advances in the field of caloric restriction mimetics and anti-aging molecules. Ageing Res Rev 66:101240
Matsui K, Ezoe S, Oritani K, Shibata M, Tokunaga M, Fujita N, Tanimura A, Sudo T, Tanaka H, McBurney MW, Matsumura I, Kanakura Y (2012) NAD-dependent histone deacetylase, SIRT1, plays essential roles in the maintenance of hematopoietic stem cells. Biochem Biophys Res Commun 418:811–817
Mattson MP (2008) Hormesis defined. Ageing Res Rev 7:1–7
Mazzoccoli G, Tevy MF, Borghesan M, Vergini MRD, Vinciguerra M (2014) Caloric restriction and aging stem cells: the stick and the carrot? Exp Gerontol 50:137–148
Mehmel M, Jovanović N, Spitz U (2020) Nicotinamide riboside-the current state of research and therapeutic uses. Nutrients 12:1616
Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R (2011) Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66:191–201
Mimeault M, Batra SK (2009) Aging of tissue-resident adult stem/progenitor cells and their pathological consequences. Panminerva Med 51:57–79
Moriya J, Chen R, Yamakawa J-I, Sasaki K, Ishigaki Y, Takahashi T (2011) Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull 34:354–359
Na HJ, Park JS, Pyo JH, Jeon HJ, Kim YS, Arking R, Yoo MA (2015) Metformin inhibits age-related centrosome amplification in Drosophila midgut stem cells through AKT/TOR pathway. Mech Ageing Dev 149:8–18
Na HJ, Pyo JH, Jeon HJ, Park JS, Chung HY, Yoo MA (2018) Deficiency of Atg6 impairs beneficial effect of metformin on intestinal stem cell aging in Drosophila. Biochem Biophys Res Commun 498:18–24
Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Hölter SM, Moreth K, Prehn C, Puk O, Rácz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Höfler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D (2013) Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest 123:3272–3291
Neumann B, Baror R, Zhao C, Segel M, Dietmann S, Rawji KS, Foerster S, McClain CR, Chalut K, van Wijngaarden P, Franklin RJM (2019) Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell stem cell 25:473-485 e8
Ould-Brahim F, Sarma SN, Syal C, Lu KJ, Seegobin M, Carter A, Jeffers MS, Doré C, Stanford WL, Corbett D, Wang J (2018) Metformin preconditioning of human induced pluripotent stem cell-derived neural stem cells promotes their engraftment and improves post-stroke regeneration and recovery. Stem Cells Dev 27:1085–1096
Paliouras GN, Hamilton LK, Aumont A, Joppé SE, Barnabé-Heider F, Fernandes KJL (2012) Mammalian target of rapamycin signaling is a key regulator of the transit-amplifying progenitor pool in the adult and aging forebrain. J Neurosci 32:15012
Parikh I, Guo J, Chuang K-H, Zhong Y, Rempe RG, Hoffman JD, Armstrong R, Bauer B, Hartz AMS, Lin A-L (2016) Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging 8:2814–2826
Park HR, Kong KH, Yu BP, Mattson MP, Lee J (2012) Resveratrol inhibits the proliferation of neural progenitor cells and hippocampal neurogenesis. J Biol Chem 287:42588–42600
Park J-H, Glass Z, Sayed K, Michurina TV, Lazutkin A, Mineyeva O, Velmeshev D, Ward WF, Richardson A, Enikolopov G (2013) Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur J Neurosci 37:1987–1993
Park JH, Lee NK, Lim HJ, Ji ST, Kim Y-J, Jang WB, Kim DY, Kang S, Yun J, Ha JS, Kim H, Lee D, Baek SH, Kwon S-M (2020) Pharmacological inhibition of mTOR attenuates replicative cell senescence and improves cellular function via regulating the STAT3-PIM1 axis in human cardiac progenitor cells. Exp Mol Med 52:615–628
Park SE, Chung HY, Park YJ (2017) Calorie Restriction Facilitates Aging-Related Muscle Loss in the Aged Mice. The FASEB Journal 31:1b306
Pavlidou T, Marinkovic M, Rosina M, Fuoco C, Vumbaca S, Gargioli C, Castagnoli L, Cesareni G (2019) Metformin delays satellite cell activation and maintains quiescence. Stem Cells Int 2019:5980465
Pavlidou T, Rosina M, Fuoco C, Gerini G, Gargioli C, Castagnoli L, Cesareni G (2017) Regulation of myoblast differentiation by metabolic perturbations induced by metformin. PLOS ONE 12:e0182475
Peña-Villalobos I, Casanova-Maldonado I, Lois P, Sabat P, Palma V (2019) Adaptive physiological and morphological adjustments mediated by intestinal stem cells in response to food availability in mice. Front Physiol 9:1821
Qiu XX, Liu Y, Zhang YF, Guan YN, Jia QQ, Wang C, Liang H, Li YQ, Yang HT, Qin YW, Huang S, Zhao XX, Jing Q (2017) Rapamycin and CHIR99021 coordinate robust cardiomyocyte differentiation from human pluripotent stem cells via reducing p53-dependent apoptosis. J Am Heart Assoc 6:e005295
Raman L, Kong X, Kernie SG (2013) Pharmacological inhibition of the mTOR pathway impairs hippocampal development in mice. Neurosci Lett 541:9–14
Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, Mackay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK (2012) Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med 4:144ra103
Rando TA (2006) Stem cells, ageing and the quest for immortality. Nature 441:1080–1086
Ratcliff M, Rees D, McGrady S, Buntwal L, Hornsby AKE, Bayliss J, Kent BA, Bussey T, Saksida L, Beynon AL, Howell OW, Morgan AH, Sun Y, Andrews ZB, Wells T, Davies JS (2019) Calorie restriction activates new adult born olfactory-bulb neurones in a ghrelin-dependent manner but acyl-ghrelin does not enhance subventricular zone neurogenesis. J Neuroendocrinol 31:e12755
Ravussin Y, Leduc CA, Watanabe K, Leibel RL (2012) Effects of ambient temperature on adaptive thermogenesis during maintenance of reduced body weight in mice. Am J Physiol Regul Integr Comp Physiol 303:R438–R448
Rimmelé P, Lofek-Czubek S, Ghaffari S (2014) Resveratrol increases the bone marrow hematopoietic stem and progenitor cell capacity. Am J Hematol 89:E235–E238
Romine J, Gao X, Xu X-M, So KF, Chen J (2015) The proliferation of amplifying neural progenitor cells is impaired in the aging brain and restored by the mTOR pathway activation. Neurobiol Aging 36:1716–1726
Ruddy RM, Adams KV, Morshead CM (2019) Age- and sex-dependent effects of metformin on neural precursor cells and cognitive recovery in a model of neonatal stroke. Sci Adv 5:eaax1912
Santoro A, Martucci M, Conte M, Capri M, Franceschi C, Salvioli S (2020) Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Res Reviews 64:101142
Sato S, Solanas G, Peixoto FO, Bee L, Symeonidi A, Schmidt MS, Brenner C, Masri S, Benitah SA, Sassone-Corsi P (2017) Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 170:664-677.e11
Schmuck EG, Mulligan JD, Saupe KW (2011) Caloric restriction attenuates the age-associated increase of adipose-derived stem cells but further reduces their proliferative capacity. Age (dordr) 33:107–118
Schüler SC, Gebert N, Ori A (2020) Stem cell aging: the upcoming era of proteins and metabolites. Mechanisms of Ageing and Development 190:111288
Schultz MB, Sinclair DA (2016) When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development 143:3
Sciarretta Sebastiano, Forte Maurizio, Castoldi Francesca, Frati Giacomo, Versaci Francesco, Sadoshima Junichi, Kroemer Guido, Maiuri Maria Chiara (2020) Caloric restriction mimetics for the treatment of cardiovascular diseases. Cardiovasc Res 117(6):1434–1449. https://doi.org/10.1093/cvr/cvaa297
Selman C, Sinclair A, Pedroni SMA, Irvine EE, Michie AM, Withers DJ (2016) Evidence that hematopoietic stem cell function is preserved during aging in long-lived S6K1 mutant mice. Oncotarget 7:29937–29943
Senesi P, Montesano A, Luzi L, Codella R, Benedini S, Terruzzi I (2016) Metformin treatment prevents sedentariness related damages in mice. J Diabetes Res 2016:8274689
Sharpless NE, Depinho RA (2007) How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8:703–713
Shen C, Cheng W, Yu P, Wang L, Zhou L, Zeng L, Yang Q (2016) Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol Med Rep 14:3646–3654
Sheng Y, Xia F, Chen L, Lv Y, Lv S, Yu J, Liu J, Ding G (2020) Differential responses of white adipose tissue and brown adipose tissue to calorie restriction during aging. J Gerontol Ser A 76:393–399
Shintani H, Shintani T, Ashida H, Sato M (2018) Calorie Restriction mimetics: upstream-type compounds for modulating glucose metabolism. Nutrients 10:1821
Signor C, Girardi BA, Wendel AL, Frühauf PKS, Pillat MM, Ulrich H, Mello CF, Rubin MA (2017) Spermidine improves the persistence of reconsolidated fear memory and neural differentiation in vitro: Involvement of BDNF. Neurobiol Learn Memory 140:82–91
Sigrist SJ, Carmona-Gutierrez D, Gupta VK, Bhukel A, Mertel S, Eisenberg T, Madeo F (2014) Spermidine-triggered autophagy ameliorates memory during aging. Autophagy 10:178–179
Smith JA, Daniel R (2012) Stem cells and aging: a chicken-or-the-egg issue? Aging Dis 3:260–268
Speakman JR (2013) Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. Annu Rev Nutr 33:289–317
Stockinger J, Maxwell N, Shapiro D, Decabo R, Valdez G (2018) Caloric restriction mimetics slow aging of neuromuscular synapses and muscle fibers. J Gerontol Ser A 73:21–28
Storlien L, Oakes ND, Kelley DE (2004) Metabolic flexibility. Proc Nutr Soc 63:363–368
Tang D, Tao S, Chen Z, Koliesnik IO, Calmes PG, Hoerr V, Han B, Gebert N, Zörnig M, Löffler B, Morita Y, Rudolph KL (2016) Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging. J Exp Med 213:535–553
Thornton KJ, Chapalamadugu KC, Doumit ME, Murdoch GK (2013) Polyamines enhance satellite cell activation and expression of myogenic regulatory factors. FASEB J 27:1146.7
Troca-Marín JA, Alves-Sampaio A, Montesinos ML (2011) An increase in basal BDNF provokes hyperactivation of the akt-mammalian target of rapamycin pathway and deregulation of local dendritic translation in a mouse model of down’s syndrome. J Neurosci 31:9445
van Cauwenberghe C, Vandendriessche C, Libert C, Vandenbroucke RE (2016) Caloric restriction: beneficial effects on brain aging and Alzheimer’s disease. Mamm Genome 27:300–319
Wang J, Gallagher D, Devito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD (2012) Metformin activates an atypical pkc-cbp pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11(1):23–35
Wilkinson AC, Yamazaki S (2018) The hematopoietic stem cell diet. Int J Hematol 107:634–641
Xie W-Q, Xiao W-F, Tang K, Wu Y-X, Hu P-W, Li Y-S, Duan Y, Lv S (2020) Caloric restriction: implications for sarcopenia and potential mechanisms. Aging 12:24441–24452
Xu T-T, Li H, Dai Z, Lau GK, Li B-Y, Zhu W-L, Liu X-Q, Liu H-F, Cai W-W, Huang S-Q, Wang Q, Zhang S-J (2020) Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 12:6401–6414
Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y, Fu L (2014) mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res 264:82–90
Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM (2012) mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486:490–495
Yousefi M, Nakauka-Ddamba A, Berry CT, Li N, Schoenberger J, Simeonov KP, Cedeno RJ, Yu Z, Lengner CJ (2018) Calorie restriction governs intestinal epithelial regeneration through cell-autonomous regulation of mTORC1 in reserve stem cells. Stem Cell Rep 10:703–711
Yousuf Y, Datu A, Barnes B, Amini-Nik S, Jeschke MG (2020) Metformin alleviates muscle wasting post-thermal injury by increasing Pax7-positive muscle progenitor cells. Stem Cell Res Ther 11:18
Zhang L, Gong H, Sun Q, Zhao R, Jia Y (2018) Spermidine-activated satellite cells are associated with hypoacetylation in ACVR2B and Smad3 binding to myogenic genes in mice. J Agric Food Chem 66:540–550
Zhang Q-S, Tang W, Deater M, Phan N, Marcogliese AN, Li H, Al-Dhalimy M, Major A, Olson S, Monnat RJ, Grompe M (2016) Metformin improves defective hematopoiesis and delays tumor formation in Fanconi anemia mice. Blood 128(24):2774–2784
Zhang QS, Marquez-Loza L, Eaton L, Duncan AW, Goldman DC, Anur P, Watanabe-Smith K, Rathbun RK, Fleming WH, Bagby GC, Grompe M (2010) Fancd2-/- mice have hematopoietic defects that can be partially corrected by resveratrol. Blood 116:5140–5148
Zheng B, Wang J, Tang L, Shi J, Zhu D (2017) mTORC1 and mTORC2 play different roles in regulating cardiomyocyte differentiation from embryonic stem cells. Int J Dev Biol 61:65–72
Acknowledgements
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP09058312) and the CRP grant of Nazarbayev University No. 091019CRP2113.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
All authors declare they have no conflict of interest.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Umbayev, B., Safarova, Y., Yermekova, A., Saliev, T. (2021). Calorie Restriction Mimetics and Adult Stem Cells. In: Rattan, S.I.S., Kaur, G. (eds) Nutrition, Food and Diet in Ageing and Longevity. Healthy Ageing and Longevity, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-030-83017-5_25
Download citation
DOI: https://doi.org/10.1007/978-3-030-83017-5_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83016-8
Online ISBN: 978-3-030-83017-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)