Abstract
Non Celiac Wheat Sensitivity (NCWS) is a recently ‘rediscovered’ disorder that seems to be even more common than the principal gluten -related disorder, i.e. celiac disease, at least in adults. In recent years, the number of both patients and publications on NCWS have increased greatly. The clinical picture of NCWS is variable and usually includes irritable bowel—like gastrointestinal manifestations and minimal neurological complaints, such as foggy mind and headache. Treatment with the gluten -free diet may dramatically improve the quality of life of these patients. In view of the currently high rate of perceived gluten sensitivity and the possible placebo effect of any dietary intervention, the demonstration of a clear-cut relationship between gluten ingestion and symptoms appearance by means of the gluten/wheat challenge is a crucial step in the diagnostic algorithm of NCWS. Although validated biomarker(s) for the diagnosis of NCWS are not available yet, the so-called Salerno diagnostic criteria can help to recognize this disorder, optimize clinical care, avoid self-diagnosis, and advance the science of NCWS. The identification and validation of biomarkers will be instrumental to gain insights in NCWS pathogenesis, to investigate the epidemiology of this “new” disorder, and ultimately to improve the health and the quality of life of a large number of individuals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gluten-related disorders
- Wheat intolerance
- Celiac disease
- Gluten
- Gluten-free diet
- FODMAP
- Amylase/trypsin inhibitors
1 Introduction
Non-celiac gluten/wheat sensitivity (NCGS/NCWS) is characterized by irritable bowel syndrome (IBS)-like symptoms and extra-intestinal manifestations, occurring in a few hours or days after ingestion of gluten/wheat-containing food, improving rapidly with gluten/wheat withdrawal and relapsing soon after gluten/wheat challenge. Pre-requisite for suspecting NCGS/NCWS is the exclusion of both celiac disease (CD) and wheat allergy (WA) when the patient is still on a gluten-containing diet [1].
The terminology of this disorder is still a matter of debate. Although the first cases of NCGS were reported in the 1970s [2, 3], this entity has been characterized only recently (year 2010) by Sapone et al. [4] who described the clinical and pathophysiological features of NCGS. Since then, the number of papers reporting on NCGS has grown exponentially, as well as the number of non-celiac individuals treated with the gluten -free diet (GFD) because of a wide array of symptoms or conditions. However, in recent years it has become clear that wheat components other than gluten, particularly so-called Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols (FODMAPs) [5] and Amylase -Trypsin Inhibitors (ATIs) [6], may elicit symptoms of NCGS. Since it is often impossible to establish which wheat component/s is/are the disease trigger/s, the disorder here described is best defined as NCWS. The major limitation of NCWS terminology is the exclusion of other gluten-containing grains, such as rye and barley, that might trigger the disorder. Figure 1 shows an updated classification of NCWS and other gluten -related disorders.
2 Epidemiology of NCWS
Due to lack of a disease biomarker, the frequency of NCWS in the general population is still unclear. An early estimate from the Center For Celiac Research at the University of Maryland in Baltimore (US) suggested a NCWS prevalence of about 6% [7]. The limitation to this observation was that this is a tertiary centre seeing patients within a fee-paying system. Due to a selection bias, this may not accurately reflect international prevalence figures for NCWS. More recent data from the US National Health and Nutrition Examination Surveys (NHANES) indicate that the prevalence of NCWS increased significantly from 0.5% in 2009–10 to 1.0% in 2011–12 to 1.7% in 2013–14 in the general population [8].
NCWS has mostly been described in adults, particularly in females aged 30–50 years [9]; however, paediatric case series have also been reported [10, 11]. In a recent study conducted in Italy, Francavilla and coworkers investigated the prevalence of NCWS on a sample of 1114 children affected with functional gastrointestinal disorders (FGID) and found that 3.3% of them had “suspected” NCWS, but only 1.1% had the diagnosis confirmed by a gluten challenge [12]. Since FGID affect about 20% of children, the estimated prevalence of NCWS in the overall paediatric population is about 0.2–0.3%.
An emerging epidemiological issue is represented by self-reported gluten intolerance, i.e., people excluding gluten-containing food without a medical diagnosis of a specific gluten -related disorder. Many individuals perceive the GFD as healthy lifestyle practice, others erroneously believe that the GFD may help in losing weight or improving physical fitness. These “gluten avoiders” or lifestylers have nothing to do with true NCWS or any other gluten -related disorder, but are widely diffused in many countries, with a prevalence of 6.2–13% [13, 14].
In conclusion, current estimates indicate that the prevalence of NCWS is around 2% in the general population and 0.2–0.3% in children.
3 Clinical Picture
As noted previously, NCWS is characterized by symptoms that usually occur soon after wheat ingestion, disappear with wheat/gluten withdrawal, and relapse following wheat/gluten challenge within hours or days. Therefore, the latency between wheat ingestion/withdraw and the appearance/disappearance of symptoms is typically much shorter in NCWS (few hours/days) than in CD (weeks/months).
The ‘classical’ presentation of NCWS is a combination of irritable bowel syndrome (IBS)-like symptoms, including abdominal pain, bloating, bowel habit abnormalities (either diarrhoea or constipation), and minimal neurological manifestations such as ‘foggy mind’, headache and chronic fatigue. Other complaints may include joint and muscle pain, leg or arm numbness, dermatitis (eczema or skin rash), depression, gynaecologic problems (recurrent vaginitis and cystitis) and anaemia, and major neurological manifestations. When seen at a specialty clinic, many NCWS patients already report the causal relationship between the ingestion of gluten-containing food and worsening of symptoms. In children, NCWS usually manifests with IBS-like symptoms, such as abdominal pain and chronic diarrhoea, while the extraintestinal manifestations are less frequent [4, 10, 15, 16].
The prevalence of IBS worldwide is 10–20% [17, 18]. Approximately 50% of patients with gastrointestinal complaints seen in primary care have IBS-type symptoms [19]. Patients with IBS report a reduced quality of life and there is an associated economic and societal cost [20, 21]. Patients have always reported that food plays an important role in their IBS-type symptoms with estimates of up to 80% of patients having postprandial symptomology, and up to 40% reporting specific “food intolerances” [22,23,24]. Over the last 15 years there has been renewed interest in the concept of dietary interventions for FGID [25, 26]. IBS dietary research has focused on the role of two common components of the western diet, specifically FODMAPs and gluten in relation to the induction of IBS symptoms. To note, both these two components are found in large amounts in wheat that contains both gluten and fructans FODMAPs. Several randomized control trials have demonstrated the efficacy of the low FODMAP diet. There is overlap between NCWS and IBS-type symptoms [13, 15]. The fundamental difference between NCWS and IBS is that patients with NCWS self-report symptoms when consuming wheat and have identified or perceive wheat as the culprit. Conversely IBS patients do not report wheat as a specific stimulus for their symptoms. However, previously published literature has demonstrated that wheat is a commonly reported “food intolerance” when IBS patients are specifically questioned [22,23,24].
In recent years, several studies explored the relationship between the ingestion of gluten-containing food and the appearance of neurological and psychiatric disorders/symptoms, an issue that is analyzed in detail in the next two paragraphs.
4 Gluten Sensitivity and Autism
Research on the effect of diet on autistic spectrum disorder (ASD) has been increasing in recent decades. One of the most popular interventions for ASD is the gluten-free casein-free (GFCF) diet. Although an association between CD and autism has been anecdotally reported, the possible effect of the GFCF in children with autism is not due to underlying CD, but to an entirely different pathophysiological mechanism [27, 28]. It has been hypothesized that symptoms may be caused by opioid peptides formed from the incomplete breakdown of foods containing gluten and casein. Increased intestinal permeability, also referred to as the ‘leaky gut syndrome’, has been suspected in ASD to be part of the chain of events that allows these peptides to cross the intestinal membrane, enter the bloodstream, and cross the blood–brain barrier, affecting the endogenous opiate system and neurotransmission within the nervous system. The resulting excess of opioids is thought to lead to behaviours noted in ASD, and the removal of these substances from the diet could determine an improvement in autistic behaviours [29]. The leaky gut/autism connection has fuelled a strong debate within the scientific community, which is far from being settled.
One study reported a high percentage of increased intestinal permeability, [as established by the lactulose/mannitol (L/M) ratio], among patients with autism and their relatives compared with normal subjects. After starting the GFD, patients with autism had significantly lower intestinal permeability test values compared with those who were on an unrestricted diet and controls [30]. However, Robertson et al. [31] did not detect any changes in intestinal permeability in a small cohort of ASD children. In another small study, neither the L/M ratio nor behavioural scores were different between groups exposed to gluten/dairy or placebo [32]. The finding of IgG-class antibodies directed against food antigens is considered indirect evidence of increased intestinal permeability. Children with autism have significantly higher levels of IgG AGA (but not IgA) compared with healthy controls, particularly those with gastrointestinal symptoms [33]. Another study confirmed these findings and also reported an increase in antibodies directed to several other food allergens, including casein and whole milk [34].
A recent study reported that levels of serological markers of an impaired gut barrier (zonulin and intestinal fatty acid binding protein I-FABP) in children with ASD were similar to those found in healthy control. AGA IgG were found in 27.3% of ASD children while celiac specific antibodies (anti-TG2 IgA and anti-DPG IgG) were negative, but increased levels of antibodies against neural TG6 were found in 6.49% of children. According to this study, the mechanism of immune activation and gluten antibody response in ASD patients is probably not strictly connected with increased gut permeability [35].
Despite its popularity, the efficacy of the GFCF diet in improving autistic behaviour remains to be proven. A 2008 Cochrane review reported that only two small randomized controlled trials investigated the effect of the GFCF diet in children with ASD (n = 35). There were only three significant treatment effects in favour of the diet intervention: overall autistic traits, mean difference (MD) = −5.60; social isolation, MD = −3.20, and overall ability to communicate and interact, MD = 1.70. In addition, three outcomes were not different between the treatment and control group, while differences for ten outcomes could not be analyzed because data were skewed. The review concluded that the evidence for efficacy of these diets is poor, and large-scale good-quality randomized controlled trials are needed [36]. Similar conclusions were reached by a recently published systematic review on treatment of autistic children with the GFCF diet [37].
In a two-stage randomized controlled study of the GFCF diet in children with ASD, Whiteley et al. reported significant group improvements in core autistic and related behaviours after 8 and 12 months of diet. The results showed a less dramatic change between children having been on diet for 8 months and those on diet for 24 months, possibly reflective of a plateau effect [38]. Analyses indicated several factors to be potentially pertinent to a positive response to the dietary intervention in terms of symptom presentation. Age was found to be the strongest predictor of response, where those participants aged between 7 and 9 years seemed to derive most benefit from the dietary intervention [39]. The above data suggest that removing gluten from the diet may positively affect the clinical outcome in some children diagnosed with ASD, indicating that autism may be part of the spectrum of NCGS, at least in some cases.
On the other hand, a randomized controlled double-blind trial was performed on 74 children with ASD with severe maladaptive behavior and increased urinary intestinal fatty acids binding protein (I-FABP, i.e. a marker of enterocyte damage) to test the potential ‘toxicity’ of gluten/casein. Subjects on a regular diet were randomized to receive a gluten/casein or placebo supplement for 7 days. Administrating gluten/casein to children with ASD for 1 week did not increase maladaptive behavior, gastrointestinal symptom severity or urinary I-FABP excretion [40].
Another double-blind placebo-controlled study was conducted placing 14 children with autism on gluten-free/casein-free diet for 4–6 weeks. They did not find significant effects of the diet on autism symptoms or ASD-related behaviors [41].
Ghalichi et al. investigated the effect of the GFD on gastrointestinal symptoms and behavioral indices in children with ASD. The first group (40 children) was assigned to the GFD and reported a significant decrease both in gastrointestinal symptoms and behavioral disorders; the second group (40 children) on regular diet did not obtain significant results. The study concluded that some children with ASD can improve their stereotyped behaviors, communication and social interaction following a GFD [42].
In another study, a group of patients aged 3–8 years diagnosed with ASD on GFCF diet was compared with a patients on ketogenic diet and a control group (on regular diet). Both diet groups reported improvement in autistic manifestations [43].
In contrast, a randomized, controlled, single-blinded trial demonstrated that there were no differences between children with ASD after an 8-week GFD (n = 33) and those on a gluten-containing diet (n = 33) in autistic symptoms, maladaptive behaviors and intellectual abilities [44].
Gonzalez-Domenech et al. conducted a clinical trial to determine the influence of a GFCF diet not only on behavior disorders in patients with ASD, but also on urinary beta-casomorphin concentrations, which is a peptide with opioid activity resulting from an incomplete degradation of casein in the intestine. Each patient of the study consumed a GFCF diet for six months and a normal diet for another six months. GFCF diet did not induce a significant change in behavioral symptoms of autism and urinary beta-casomorphin concentrations [45].
A very recent systematic review and meta-analysis identified six RCTs investigating a GFCF diet compared to a regular diet in children aged 3 to 17 years with ASD. They reported no effect of a GFCF diet on clinician-reported autism core symptoms, parent reported functional level and behavioral difficulties. In addition, the study showed that a GFCF diet may trigger gastrointestinal adverse effects (RR 2.33). So, this study suggests caution in starting the GFCF diet in children with ASD [46].
A review published in 2021 [47] discussed the pathological mechanisms and the evidence on the use of a GFD in people with ASD. The result was that it is still unclear if the interaction between ASD and gluten is due to gluten specifically (opioid activity from improperly digested gluten, inflammation caused by oxidative stress or reactivity with anti-gluten antibodies) or it is a consequence of a generally raised autoimmune profile in ASD. In addition, authors concluded that there is not a proven benefit of the GFD in people with ASD (who do not have a clinical diagnosis of CD) according to the current literature.
In conclusion, further studies are needed to clarify the possible link between gluten ingestion and autism. Investigations are particularly required to identify possible phenotypes based on the best response and non-response to dietary modifications and to assess biological correlates before considering a dietary intervention.
5 NCGS and Other Psychiatric Disorders
An association between schizophrenia and CD was noted in reports spanning back to the 1960s [48]. In 1986, a double-blind gluten-free vs gluten-load controlled trial of 24 patients conducted by Vlissides et al. [49] showed changes in the symptom profile of schizophrenic patients in response to exclusion of gluten from the diet. On the other hand, a small blind study conducted by Potkin et al. [50] showed no differences in the clinical status of 8 schizophrenic patients on a 5-week gluten challenge in an inpatient setting, as measured by the Brief Psychiatric Rating Scale. A subsequent study by Storms et al. [51] tested 26 schizophrenic patients on a locked ward assigned to either a gluten-free or gluten-rich diet. No differences were found between the groups on their performance in a battery of psychological tests. A recent study using blood samples from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found that 5.5% of the subjects with schizophrenia had a high level of anti-tTG antibodies (compared to 1.1% in the healthy control sample) and 23.1% had AGA IgG positivity compared with 3.1% of controls. Interestingly enough, a large proportion of transglutaminase (tTG)-positive subjects were endomysial antibody (EMA) negative, questioning the possibility that their tTG positivity was related to CD. Indeed, only 2% of schizophrenic patients fulfilled the CD diagnostic criteria (both anti-tTG and EMA positive), questioning the role of CD in schizophrenia [52]. Additional studies revealed that most of the tTG-positive subjects were tTG-6 positive, suggesting that these antibodies are more a biomarker of neuroinflammation than CD [53]. This study indicated the existence of a specific immune response to gluten in some of these patients, probably related to NCGS. Other studies confirmed the high prevalence of AGA among people with schizophrenia [54]; however, the exact mechanism underlying the observed improvement of symptoms with the GFD in some patients has remained elusive.
In 2019 Kelly et al. [55] performed the first double-blind clinical trial of gluten-free versus gluten-containing diets in patients with schizophrenia who were positive for AGA IgG and negative for CD. They noted improvement on the Clinical Global Impressions scale, in negative symptoms and in gastrointestinal symptoms in participants on the gluten -free diet. This study suggests that a subgroup of patients with schizophrenia could benefit from a GFD.
Another recent study compared concentrations of markers in patients with a first episode or chronic schizophrenia. The prevalence of increased AGA antibody titres was significantly higher in patients than in controls. In particular, chronic patients had significantly higher concentrations of AGA IgA antibodies. In this study, elevated AGA antibodies titres increased the risk of developing schizophrenia about four to seven times [56].
Another psychiatric disease that has been hypothesized to be associated with NCGS is depression. In an Australian study, a group of 22 patients with irritable bowel syndrome who had CD excluded underwent a double-blind crossover study with a placebo versus oral gluten supplementation after a GFD. The results showed that gluten induced depression scale worsening when compared to placebo and without producing effects on other symptoms, for example anxiety [57]. Similar results were reached by an Italian study: among the extraintestinal symptoms, depression was more severe in subjects with NCGS when they received gluten instead of placebo [58]. Porcelli et al. evaluated the presence of antibodies associated with gluten related disorders in patients with mood disorders. They considered IgA/IgG anti-gliadin antibodies, IgA/IgG anti-deamidated gliadin peptide antibodies and IgA/IgG anti-transglutaminase antibodies in patients with bipolar disorders or depressive disorders and controls. A significant difference was found only for anti-tTG IgG antibodies, in particular each unit increase in the anti-tTG IgG antibodies corresponded to about 5% increased risk of having a mood disorder [59].
Finally, some case-reports suggest a possible relationship between gluten ingestion and visual and auditory hallucinations [60, 61].
In conclusion, according to the literature, some subgroups of patients with psychiatric disorders could benefit from a GFD. So, further studies are needed on how to identify those patients with the altered response to gluten, in order to start the diet treatment in this disease subgroup.
6 Small Intestinal Biopsy Findings in NCWS
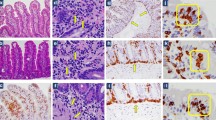
Unlike patients with active CD who show an increased number of intraepithelial lymphocytes (IELs) associated to a variable degree of villous atrophy and crypt hypertrophy (so called Marsh lesion grade 3a-3c) at the small intestinal biopsy, NCWS subjects show a normal to mildly inflamed mucosa (Marsh 0–1), sometimes with an isolated increase of duodenal IELs. In the Sapone’s pioneer paper, CD patients had increased numbers of CD3 + IELs (>50/100 enterocytes) compared to controls, while NCWS patients had a number of CD3 + IELs intermediate between CD patients and controls in the context of relatively conserved villus architecture. The numbers of TCR-γδ IELs were only elevated in CD subjects (>3.4/100 enterocytes), while in NCWS patients the numbers of γδ IELs were similar to those in controls [4].
In a recent review, Sergi and coworkers indicate the following hystological features as suggestive, but not specific, of NCWS: (a) a “nearly” standard number of T lymphocytes (<25 for 100 epithelial cells); (b) a peculiar disposition of T lymphocytes in a small “cluster” of 4 or 5 cells in the superficial epithelium; (c) the linear distribution of T lymphocytes in the deeper part of the lamina propria of the mucosa over the muscularis mucosae, and (d) an increased number of eosinophils in the lamina propria (>5 cells per high-power field, HPF) [62]. Interestingly, a relevant eosinophilic infiltration has been found in the rectal mucosa of patients with NCWS, which was more intense in the rectum than in the duodenum, suggesting that NCWS might involve inflammation of the entire intestinal tract [63].
Unpublished results indicate a higher mast cell density in NCWS in comparison to healthy controls and CD patients. This increased mast cell number in NCWS seems to be closely related with the presence of IBS-like symptoms such as bloating, abdominal pain, and impaired bowel function. Moreover, the close vicinity of mast cells and nerve fibres observed in these patients may have a role in the generation of symptoms, e.g., abdominal pain, via a neuroimmune mechanism [64].
7 Searching for Biomarker/s of NCWS
Given the non-specific findings at the small intestinal biopsy in subjects with NCWS, the search for a non-invasive biomarker of this condition is currently very active.
By investigating a large number of Intestinal cell damage and systemic immune activation markers (anti-tTG IgA, anti-deamidated gliadin IgG and IgA, AGA IgG, IgA and IgM, lipopolysaccharide-binding protein (LBP), soluble CD14 (sCD14), endotoxin-core antibodies IgG, IgA and IgM, anti-flagellin IgG, IgA and IgM, and fatty acid-binding protein 2 (FABP2) in individuals reporting NCWS, Uhde et al.found that Individuals with wheat sensitivity had significantly increased serum levels of soluble CD14 and LBP, as well as antibody reactivity to bacterial LPS and flagellin. Circulating levels of FABP2, a marker of intestinal epithelial cell damage, were significantly elevated in the affected individuals and correlated with the immune responses to microbial products. The principal component analysis showed that the “clouds” of NCWS, CD and control subjects were nicely separated on the graph, however none of these biomarkers alone showed enough sensitivity and specificity to be useful in clinical practice [65].
The most specific CD serological markers, such as IgA-class anti-tTG and EMA, are negative in NCWS patients by definition. However, IgG-class AGA directed against native gliadin (the first-generation AGA test) are found more frequently in these cases (about 50%) than in the general population. Therefore, the finding of isolated IgG-AGA positivity may be a clue to the diagnosis of NCGS. When initially positive, IgG AGA normalize more quickly in NCGS than in CD patients after starting treatment with the GFD [66]. Recently Uhde et al.showed that the AGA IgG antibody in NCWS is significantly different from CD in subclass distribution and in its relationship to intestinal cell damage. The observed increase in the gluten-reactive IgG2 and IgG4 subclasses and the correlation between the IgG4 subclass and FABP2 in NCGS may point to a protective response aimed at dampening the inflammatory effect of other antibodies and immune cells [67]. Again, due to poor sensitivity/specificity, IgG AGA determination may yield a clue but does not have a primary diagnostic role in clinical practice.
Zonulin is the protein prehaptoglobin 2, the only known human protein that can reversibly open the intestinal tight junctions. In CD, higher zonulin release correlates with increased epithelial permeability. Recently Barbaro et al. showed that serum zonulin levels were increased in patients with confirmed as well as self-reported NCWS over asymptomatic controls and patients with IBS-D. Zonulin was reduced with the elimination of wheat from the diets of participants with a genetic predisposition to CD. They developed a diagnostic algorithm based on zonulin serum levels, gender and abdominal symptoms and this provided a high performing diagnostic tool with an accuracy of 89.0% [68].
Finally, no association has so far been identified between NCWS and specific genetic markers. In NCWS subjects the prevalence of HLA-DQ2 and -DQ8, the genes that are strongly associated with CD, is comparable to that found in the general population (around 40%) and therefore has no significant positive or negative predictive value.
8 The “Salerno Diagnostic Criteria”
As anticipated in the introduction, the diagnosis of NCWS should be considered in patients with persistent intestinal and/or extraintestinal complaints showing a normal result of the CD and WA serological markers on a gluten-containing diet, usually reporting worsening of symptoms after eating gluten-rich food. Table 1 shows the major features of NCGS, CD, and WA that may help to differentiate these different gluten -related disorders. NCGS should not be an exclusion diagnosis only. Unfortunately, no biomarker is enough sensitive and specific of NCWS. Therefore, the diagnosis of NCWS is based on establishing a clear-cut cause-effect relationship between the ingestion of wheat/gluten and the appearance/disappearance of symptoms.
In the year 2014 a group of world experts on gluten -related disorders met in Salerno, Italy, to set up diagnostic criteria for NCGS. These criteria are known as the “Salerno diagnostic criteria” (SDC) [27]. It should preliminary be noted that these criteria can identify cases of NCGS, but do not necessarily fit for intolerances to other wheat components (e.g. FODMAP and ATIs).
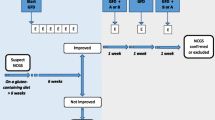
Patients suspected of suffering from a gluten -related disorder should preliminarily undergo a full clinical and laboratory evaluation to exclude CD and WA while still on a gluten-containing diet, according to a previously outlined diagnostic protocol [29]. At baseline the patient has to be on a normal gluten containing diet for at least six weeks. A self-administered instrument incorporating a modified version of the Gastrointestinal Symptom Rating Scale (GSRS) is filled in. The patient identifies one to three main symptoms that will be quantitatively assessed using a Numerical Rating Scale (NRS) with a score ranging from 1 (mild) to 10 (severe). At time 0 the patient is switched to the GFD for 6 weeks (Step 1). Responders are defined as patients who fulfil the response criteria (>30% reduction of one to three main symptoms or at least 1 symptom with no worsening of others) for at least 50% of the observation time.
The diagnosis of NCGS is excluded in subjects failing to show symptomatic improvement after six weeks of GFD. GFD-unresponsive patients should be investigated for other possible causes of IBS-like symptoms, e.g., intolerance to FODMAPs or small bowel bacterial overgrowth.
In view of the high rate of perceived gluten sensitivity and the possible placebo/nocebo effect of any dietary intervention, a double-blind, placebo-controlled (DBPC) gluten challenge is a crucial step in the diagnostic algorithm of NCGS (Step 2). The gluten challenge includes a 1-week challenge followed by a 1-week washout of strict GFD and by the crossover to the second 1-week challenge. The duration of the challenge period may occasionally be longer than 1 week in patients showing fluctuating symptoms, such as headache or neurobehavioral problems. During the challenge, the patient will identify and report one to three main symptoms. A variation of at least 30% between the gluten and the placebo challenge should be detected to discriminate a positive from a negative result. The suggested dose of gluten for the challenge is 8 g. Gluten and placebo preparations must be undistinguishable in look, texture, and taste as well as balanced in nutritional components.
9 NCWS/NCGS Remains a Difficult Diagnosis
As previously anticipated, many individuals start a GFD based on a self-diagnosis and/or without an expert medical advice. How many of these self-reported gluten intolerants are indeed affected by true NCWS/NCGS. During these last years several studies addressed this issue by performing so-called gluten re-challenge in subjects with a provisional diagnosis of NCGS/NCWS. By meta-analyzing these results, Lionetti et al. [69] found that there was a considerable heterogeneity related to different sample size, type, and amount of gluten administered, duration of challenge and different type of placebo. The overall pooled percentage of patients with a diagnosis of NCGS relapsing after a gluten challenge was only 30%, ranging between 7 and 77%. Surprisingly, the meta-analysis showed a not significant relative risk (RR) of relapse after gluten challenge as compared to placebo (RR = 0.4; 95% CI = −0.15–0.9; p = 0.16). On the other hand, the overall pooled percentage of patients with a diagnosis of NCGS relapsing after a gluten challenge performed according to the Salerno criteria was significantly higher as compared to the percentage of patients relapsing after placebo (40 vs. 24%; p = 0.003), with a significant RR of relapse after gluten challenge as compared to placebo (RR = 2.8; 95% CI = 1.5–5.5; p = 0.002). Authors attributed the low percentage of diagnosis confirmation to several factors, such as a strong “nocebo” effect of the challenge, clinical overlapping with IBS, resolution of NCWS over time, and methodological issues. A causal relationship between gluten and relapsing symptoms was observed in 40% of patients when the Salerno criteria were adopted. Therefore the “Salerno” algorithm is recommended for confirmation of NCWS diagnosis, until a valid biomarker will be available. The poor performance of re-challenge studies based on purified gluten is a novel argument in favour of a possible role of NCGS triggers different from gluten itself.
10 NCWS Pathophysiology: One, Two or Many Triggers?
The pathogenesis of NCWS is likely to be multifactorial, with the innate immune response playing a key role. The first studies on NCWS assumed that gluten was the only wheat component responsible of triggering this disorder, since symptoms disappeared with the GFD. Studies have shown that gliadin can cause an immediate and transient increase in gut permeability. This permeating effect is secondary to the binding of specific undigestible gliadin fragments to the CXCR3 chemokine receptor with subsequent release of zonulin, a modulator of intercellular tight junctions [70]. Several studies have identified an altered expression of innate immune components in response to gluten consumption in NCWS individuals, including mucosal Toll-like receptor 2 (TLR2), PBMC-derived interleukin-10 (IL-10), granulocyte-colony stimulating factor (GCSF), transforming growth factor-α (TGF-α), and the chemokine CXCL-10 [64].
In 2011, Biesiekierski et al. [71] reported that gluten caused gastrointestinal symptoms in non-CD IBS subjects investigated by a randomized, double-blind placebo-controlled (DBPC) trial. However, in a subsequent study, the same research group reached different conclusions based on the results of a different DBPC crossover trial on 37 patients with IBS/self-reported NCGS [16]. Patients were randomly assigned to a period of reduced low-fermentable, poorly absorbed, short-chain carbohydrates (FODMAPs) diet and then placed on either a gluten or whey protein challenge. In all participants, gastrointestinal complaints consistently improved during reduced FODMAP intake but significantly worsened to a similar degree when their diets included gluten or whey proteins. The FODMAP list includes fructans, galactans, fructose, and polyols that are contained in several foodstuffs, including wheat, vegetables, and milk derivatives. In the small and large intestine, the FODMAP molecules exert an osmotic effect and are also rapidly fermented by colonic microflora producing gas. The increase in fluid and gas distends the bowel, with consequent sensation of bloating and abdominal pain or discomfort, and diarrhoea. Data of this study raised the possibility that the positive effect of the GFD in patients with IBS is an unspecific consequence of reducing FODMAP intake, given that wheat is one of the possible sources of FODMAPs [72]. However, it should be noted that FODMAPs cannot be entirely and exclusively responsible for the symptoms reported by NCGS subjects, since these patients experience a resolution of symptoms while on the GFD despite continuing to ingest FODMAPs from other sources, like legumes.
ATIs are a family of at least 11 structurally similar, small and compact mono-, di- or tetrameric wheat proteins, which serve as protective proteins in wheat and other cereals by inhibiting enzymes (amylase and trypsin-like activities) of wheat and some parasites. In the developing grain, ATIs are deposited together with gluten proteins in the endosperm and become associated with the starch granules. Encoded mainly by the B and D genomes, ATIs are high in most modern hexaploid bread wheats, and low in spelt (old hexaploid), tetraploid (durum wheat, emmer) and diploid (einkorn) wheat species. They are also present in other gluten containing cereals such as barley and rye. Long known as major allergens in baker’s asthma, ATIs were identified as triggers of innate immune activation in intestinal myeloid cells via stimulation of Toll-like receptor 4 (TLR4). Notably, nutritional ATIs enhance intestinal inflammation in models of inflammatory bowel disease in mice, and immune activation is higher in the mesenteric lymph nodes than in the intestinal mucosa [73]. In intestinal tissues from control mice, ATIs induced an innate immune response by activation of Toll-like receptor 4 signalling to MD2 and CD14, causing barrier dysfunction in the absence of mucosal damage. Administration of ATIs to gluten-sensitized mice expressing HLA-DQ8 increased intestinal inflammation in response to gluten in the diet. Interestingly, ATIs are degraded by Lactobacillus, which reduced the inflammatory effects of ATIs [74]. However clinical data on the ability of ATIs to trigger symptoms of NCWS are still missing.
Finally, Carroccio et al. recently hypothesized that food antigens, including wheat proteins, initiate a Th2 response driving intestinal eosinophilia and suggested that NCWS is a form of non-IgE mediated food allergy [64]. They also reported that production of TNF-α by CD45+, CD3+, CD4+, and CD8+ cells and of IL-17 by CD4+ cells is higher in the rectal tissue of NCWS patients than in controls. Overall, these results suggest a significant role for an immune adaptive response in patients with NCWS [75].
In summary, these new studies seem to indicate that the pathophysiology of NCWS is much more complex than previously thought and may include different, non-mutually exclusive factors related to wheat consumption (gluten, FODMAPs and ATIs). The complex interplay between these dietary factors, the genetic background, the immune response and the intestinal microbiome drives the loss of tolerance to gluten/wheat and the development of different gluten/wheat-related disorders (Fig. 2).
11 Treatment
A strict gluten/wheat-free diet remains the only available treatment for NCWS. Since NCWS may be a transient condition, expert recommendation is to maintain the GFD for a given period, e.g. 12–24 months, and then test gluten tolerance again. Based on severity of symptoms, some gluten sensitive patients without CD may choose to follow a gluten -free diet indefinitely [70]. However, it is still unclear whether some basic principles of the “celiac” GFD, particularly the need of the complete exclusion of all wheat derivatives, holds for NCWS as well. For example it is theoretically possible that some NCWS patient may tolerate a small amount of gluten in their diet, particularly if clinical manifestations depend primarily on FODMAPs intolerance. Another interesting possibility is that a subgroup of NCWS might tolerate ancient wheat grains, e.g. einkorn, still containing gluten but much less of ATIs. Further studies are required to clarify these important treatment issues.
12 Conclusions
NCWS is a recently ‘rediscovered’ disorder that seems to be even more common than CD, at least in adults. In recent years, the number of both patients and publications on NCWS have increased greatly. The clinical picture of NCGS is variable and usually includes IBS-like gastrointestinal manifestations and minimal neurological complaints, such as foggy mind and headache. Treatment with the GFD may dramatically improve the quality of life of these patients, for which we have very little certainty and many knowledge ‘black holes’. In view of the currently high rate of perceived gluten sensitivity and the possible placebo effect of any dietary intervention, the demonstration of a clear-cut relationship between gluten ingestion and symptoms appearance by means of the DBPC gluten (or wheat) challenge is a crucial step in the diagnostic algorithm of NCWS. Although validated biomarker(s) for the diagnosis of NCWS are not available yet, the diagnostic criteria summarized in this paper can help to recognize this disorder, optimize clinical care, avoid self-diagnosis, and advance the science of NCWS. The identification and validation of biomarker(s) will be instrumental to gain insights in NCGS pathogenesis, to investigate the epidemiology of this “new” disorder, and ultimately to improve the health and the quality of life of a large number of individuals.
References
Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7(5):583–613. https://doi.org/10.1177/2050640619844125.
Ellis A, Linaker BD. Non-coeliac gluten sensitivity? Lancet. 1978;1(8078):1358–9. https://doi.org/10.1016/s0140-6736(78)92427-3.
Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology. 1981;81(1):192–4.
Sapone A, Lammers KM, Mazzarella G, Mikhailenko I, Carteni M, Casolaro V, et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol. 2010;152(1):75–80. https://doi.org/10.1159/000260087.
Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320–8 e1–3. https://doi.org/10.1053/j.gastro.2013.04.051.
Schuppan D, Zevallos V. Wheat amylase trypsin inhibitors as nutritional activators of innate immunity. Dig Dis. 2015;33(2):260–3. https://doi.org/10.1159/000371476.
Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. https://doi.org/10.1186/1741-7015-10-13.
Choung RS, Unalp-Arida A, Ruhl CE, Brantner TL, Everhart JE, Murray JA. Less hidden celiac disease but increased gluten avoidance without a diagnosis in the united states: findings from the national health and nutrition examination surveys from 2009 to 2014. Mayo Clin Proc. 2016.https://doi.org/10.1016/j.mayocp.2016.10.012
Catassi C. Gluten sensitivity. Ann Nutr Metab. 2015;67 Suppl 2:16–26.https://doi.org/10.1159/000440990
Francavilla R, Cristofori F, Castellaneta S, Polloni C, Albano V, Dellatte S, et al. Clinical, serologic, and histologic features of gluten sensitivity in children. J Pediatr. 2014;164(3):463–7 e1. https://doi.org/10.1016/j.jpeds.2013.10.007.
Feldman MF, Bird JA. Clinical, serologic, and histologic features of gluten sensitivity in children. Pediatrics. 2014;134(Suppl 3):S157–8. https://doi.org/10.1542/peds.2014-1817QQ.
Francavilla R, Cristofori F, Verzillo L, Gentile A, Castellaneta S, Polloni C, et al. Randomized double-blind placebo-controlled crossover trial for the diagnosis of non-celiac gluten sensitivity in children. Am J Gastroenterol. 2018;113(3):421–30. https://doi.org/10.1038/ajg.2017.483.
Aziz I, Lewis NR, Hadjivassiliou M, Winfield SN, Rugg N, Kelsall A, et al. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur J Gastroenterol Hepatol. 2014;26(1):33–9. https://doi.org/10.1097/01.meg.0000435546.87251.f7.
van Gils T, Nijeboer P, CE IJ, Sanders DS, Mulder CJ, Bouma G. Prevalence and characterization of self-reported gluten sensitivity in The Netherlands. Nutrients. 2016;8(11). https://doi.org/10.3390/nu8110714.
Volta U, Bardella MT, Calabro A, Troncone R, Corazza GR, Study Group for Non-Celiac Gluten S. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014;12:85. https://doi.org/10.1186/1741-7015-12-85.
Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O'Neill J, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903–11 e3. https://doi.org/10.1053/j.gastro.2013.01.049.
Dalrymple J, Bullock I. Diagnosis and management of irritable bowel syndrome in adults in primary care: summary of NICE guidance. BMJ. 2008;336(7643):556–8. https://doi.org/10.1136/bmj.39484.712616.AD.
Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–21 e4. https://doi.org/10.1016/j.cgh.2012.02.029.
Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. https://doi.org/10.2147/CLEP.S40245.
Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–56. https://doi.org/10.1053/gast.2002.32392.
Akehurst RL, Brazier JE, Mathers N, O’Keefe C, Kaltenthaler E, Morgan A, et al. Health-related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics. 2002;20(7):455–62. https://doi.org/10.2165/00019053-200220070-00003.
Morcos A, Dinan T, Quigley EM. Irritable bowel syndrome: role of food in pathogenesis and management. J Dig Dis. 2009;10(4):237–46. https://doi.org/10.1111/j.1751-2980.2009.00392.x.
Bohn L, Storsrud S, Tornblom H, Bengtsson U, Simren M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. https://doi.org/10.1038/ajg.2013.105.
McKenzie YA, Thompson J, Gulia P, Lomer MC. British Dietetic Association systematic review of systematic reviews and evidence-based practice guidelines for the use of probiotics in the management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet. 2016;29(5):576–92. https://doi.org/10.1111/jhn.12386.
Jones VA, McLaughlan P, Shorthouse M, Workman E, Hunter JO. Food intolerance: a major factor in the pathogenesis of irritable bowel syndrome. Lancet. 1982;2(8308):1115–7. https://doi.org/10.1016/s0140-6736(82)92782-9.
King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352(9135):1187–9. https://doi.org/10.1016/s0140-6736(98)02146-1.
Catassi C, Elli L, Bonaz B, Bouma G, Carroccio A, Castillejo G, et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients. 2015;7(6):4966–77. https://doi.org/10.3390/nu7064966.
Batista IC, Gandolfi L, Nobrega YK, Almeida RC, Almeida LM, Campos Junior D, et al. Autism spectrum disorder and celiac disease: no evidence for a link. Arq Neuropsiquiatr. 2012;70(1):28–33. https://doi.org/10.1590/s0004-282x2012000100007.
Marcason W. What is the current status of research concerning use of a gluten-free, casein-free diet for children diagnosed with autism? J Am Diet Assoc. 2009;109(3):572. https://doi.org/10.1016/j.jada.2009.01.013.
de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–24. https://doi.org/10.1097/MPG.0b013e3181dcc4a5.
Robertson MA, Sigalet DL, Holst JJ, Meddings JB, Wood J, Sharkey KA. Intestinal permeability and glucagon-like peptide-2 in children with autism: a controlled pilot study. J Autism Dev Disord. 2008;38(6):1066–71. https://doi.org/10.1007/s10803-007-0482-1.
Navarro F, Pearson DA, Fatheree N, Mansour R, Hashmi SS, Rhoads JM. Are “leaky gut” and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders? Nutr Neurosci. 2015;18(4):177–85. https://doi.org/10.1179/1476830514Y.0000000110.
Lau NM, Green PH, Taylor AK, Hellberg D, Ajamian M, Tan CZ, et al. Markers of celiac disease and gluten sensitivity in children with autism. PLoS One. 2013;8(6):e66155.https://doi.org/10.1371/journal.pone.0066155
de Magistris L, Picardi A, Siniscalco D, Riccio MP, Sapone A, Cariello R, et al. Antibodies against food antigens in patients with autistic spectrum disorders. Biomed Res Int. 2013;2013:729349.https://doi.org/10.1155/2013/729349
Jozefczuk J, Konopka E, Bierla JB, Trojanowska I, Sowinska A, Czarnecki R, et al. The occurrence of antibodies against gluten in children with autism spectrum disorders does not correlate with serological markers of impaired intestinal permeability. J Med Food. 2018;21(2):181–7. https://doi.org/10.1089/jmf.2017.0069.
Millward C, Ferriter M, Calver S, Connell-Jones G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008(2):CD003498. https://doi.org/10.1002/14651858.CD003498.pub3.
Mari-Bauset S, Zazpe I, Mari-Sanchis A, Llopis-Gonzalez A, Morales-Suarez-Varela M. Evidence of the gluten-free and casein-free diet in autism spectrum disorders: a systematic review. J Child Neurol. 2014;29(12):1718–27. https://doi.org/10.1177/0883073814531330.
Whiteley P, Haracopos D, Knivsberg AM, Reichelt KL, Parlar S, Jacobsen J, et al. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr Neurosci. 2010;13(2):87–100. https://doi.org/10.1179/147683010X12611460763922.
Pedersen L, Parlar S, Kvist K, Whiteley P, Shattock P. Data mining the ScanBrit study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders: behavioural and psychometric measures of dietary response. Nutr Neurosci. 2014;17(5):207–13. https://doi.org/10.1179/1476830513Y.0000000082.
Pusponegoro HD, Ismael S, Firmansyah A, Sastroasmoro S, Vandenplas Y. Gluten and casein supplementation does not increase symptoms in children with autism spectrum disorder. Acta Paediatr. 2015;104(11):e500–5. https://doi.org/10.1111/apa.13108.
Hyman SL, Stewart PA, Foley J, Cain U, Peck R, Morris DD, et al. The gluten-free/casein-free diet: a double-blind challenge trial in children with autism. J Autism Dev Disord. 2016;46(1):205–20. https://doi.org/10.1007/s10803-015-2564-9.
Ghalichi F, Ghaemmaghami J, Malek A, Ostadrahimi A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: a randomized clinical trial. World J Pediatr. 2016;12(4):436–42. https://doi.org/10.1007/s12519-016-0040-z.
El-Rashidy O, El-Baz F, El-Gendy Y, Khalaf R, Reda D, Saad K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metab Brain Dis. 2017;32(6):1935–41. https://doi.org/10.1007/s11011-017-0088-z.
Piwowarczyk A, Horvath A, Pisula E, Kawa R, Szajewska H. Gluten-free diet in children with autism spectrum disorders: a randomized, controlled, single-blinded trial. J Autism Dev Disord. 2020;50(2):482–90. https://doi.org/10.1007/s10803-019-04266-9.
Gonzalez-Domenech PJ, Diaz Atienza F, Garcia Pablos C, Fernandez Soto ML, Martinez-Ortega JM, Gutierrez-Rojas L. Influence of a combined gluten-free and casein-free diet on behavior disorders in children and adolescents diagnosed with autism spectrum disorder: a 12-month follow-up clinical trial. J Autism Dev Disord. 2020;50(3):935–48. https://doi.org/10.1007/s10803-019-04333-1.
Keller A, Rimestad ML, Friis Rohde J, Holm Petersen B, Bruun Korfitsen C, Tarp S, et al. The effect of a combined gluten- and casein-free diet on children and adolescents with autism spectrum disorders: a systematic review and meta-analysis. Nutrients. 2021;13(2). https://doi.org/10.3390/nu13020470.
Croall ID, Hoggard N, Hadjivassiliou M. Gluten and autism spectrum disorder. Nutrients. 2021;13(2). https://doi.org/10.3390/nu13020572.
Dohan FC. Cereals and schizophrenia data and hypothesis. Acta Psychiatr Scand. 1966;42(2):125–52. https://doi.org/10.1111/j.1600-0447.1966.tb01920.x.
Vlissides DN, Venulet A, Jenner FA. A double-blind gluten-free/gluten-load controlled trial in a secure ward population. Br J Psychiatry. 1986;148:447–52. https://doi.org/10.1192/bjp.148.4.447.
Potkin SG, Weinberger D, Kleinman J, Nasrallah H, Luchins D, Bigelow L, et al. Wheat gluten challenge in schizophrenic patients. Am J Psychiatry. 1981;138(9):1208–11. https://doi.org/10.1176/ajp.138.9.1208.
Storms LH, Clopton JM, Wright C. Effects of gluten on schizophrenics. Arch Gen Psychiatry. 1982;39(3):323–7. https://doi.org/10.1001/archpsyc.1982.04290030055010.
Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, et al. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011;37(1):94–100. https://doi.org/10.1093/schbul/sbp055.
Cascella NG, Santora D, Gregory P, Kelly DL, Fasano A, Eaton WW. Increased prevalence of transglutaminase 6 antibodies in sera from schizophrenia patients. Schizophr Bull. 2013;39(4):867–71. https://doi.org/10.1093/schbul/sbs064.
Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68(1):100–4. https://doi.org/10.1016/j.biopsych.2010.03.021.
Kelly DL, Demyanovich HK, Rodriguez KM, Cihakova D, Talor MV, McMahon RP, et al. Randomized controlled trial of a gluten-free diet in patients with schizophrenia positive for antigliadin antibodies (AGA IgG): a pilot feasibility study. J Psychiatry Neurosci. 2019;44(4):269–76.
Dzikowski M, Juchnowicz D, Dzikowska I, Rog J, Prochnicki M, Koziol M, et al. The differences between gluten sensitivity, intestinal biomarkers and immune biomarkers in patients with first-episode and chronic schizophrenia. J Clin Med. 2020;9(11). https://doi.org/10.3390/jcm9113707.
Peters SL, Biesiekierski JR, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment Pharmacol Ther. 2014;39(10):1104–12. https://doi.org/10.1111/apt.12730.
Di Sabatino A, Volta U, Salvatore C, Biancheri P, Caio G, De Giorgio R, et al. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: a randomized, double-blind, placebo-controlled, cross-over trial. Clin Gastroenterol Hepatol. 2015;13(9):1604–12 e3. https://doi.org/10.1016/j.cgh.2015.01.029.
Porcelli B, Verdino V, Ferretti F, Bizzaro N, Terzuoli L, Cinci F, et al. A study on the association of mood disorders and gluten-related diseases. Psychiatry Res. 2018;260:366–70. https://doi.org/10.1016/j.psychres.2017.12.008.
Genuis SJ, Lobo RA. Gluten sensitivity presenting as a neuropsychiatric disorder. Gastroenterol Res Pract. 2014;2014:293206.https://doi.org/10.1155/2014/293206
Lionetti E, Leonardi S, Franzonello C, Mancardi M, Ruggieri M, Catassi C. Gluten psychosis: confirmation of a new clinical entity. Nutrients. 2015;7(7):5532–9. https://doi.org/10.3390/nu7075235.
Sergi C, Villanacci V, Carroccio A. Non-celiac wheat sensitivity: rationality and irrationality of a gluten-free diet in individuals affected with non-celiac disease: a review. BMC Gastroenterol. 2021;21(1):5. https://doi.org/10.1186/s12876-020-01568-6.
Carroccio A, Giannone G, Mansueto P, Soresi M, La Blasca F, Fayer F, et al. Duodenal and rectal mucosa inflammation in patients with non-celiac wheat sensitivity. Clin Gastroenterol Hepatol. 2019;17(4):682–90 e3. https://doi.org/10.1016/j.cgh.2018.08.043.
Volta U, De Giorgio R, Caio G, Uhde M, Manfredini R, Alaedini A. Nonceliac wheat sensitivity: an immune-mediated condition with systemic manifestations. Gastroenterol Clin North Am. 2019;48(1):165–82. https://doi.org/10.1016/j.gtc.2018.09.012.
Uhde M, Ajamian M, Caio G, De Giorgio R, Indart A, Green PH, et al. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut. 2016;65(12):1930–7. https://doi.org/10.1136/gutjnl-2016-311964.
Caio G, Volta U, Tovoli F, De Giorgio R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 2014;14:26. https://doi.org/10.1186/1471-230X-14-26.
Uhde M, Caio G, De Giorgio R, Green PH, Volta U, Alaedini A. Subclass profile of IgG antibody response to gluten differentiates nonceliac gluten sensitivity from celiac disease. Gastroenterology. 2020;159(5):1965–7 e2. https://doi.org/10.1053/j.gastro.2020.07.032.
Barbaro MR, Cremon C, Morselli-Labate AM, Di Sabatino A, Giuffrida P, Corazza GR, et al. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity. Gut. 2020;69(11):1966–74. https://doi.org/10.1136/gutjnl-2019-319281.
Lionetti E, Pulvirenti A, Vallorani M, Catassi G, Verma AK, Gatti S, et al. Re-challenge studies in non-celiac gluten sensitivity: a systematic review and meta-analysis. Front Physiol. 2017;8:621. https://doi.org/10.3389/fphys.2017.00621.
Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. 2017;318(7):647–56. https://doi.org/10.1001/jama.2017.9730.
Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106(3):508–14; quiz 15. https://doi.org/10.1038/ajg.2010.487.
Catassi G, Lionetti E, Gatti S, Catassi C. The low FODMAP diet: many question marks for a catchy acronym. Nutrients. 2017;9(3). https://doi.org/10.3390/nu9030292.
Catassi C, Alaedini A, Bojarski C, Bonaz B, Bouma G, Carroccio A, et al. The overlapping area of Non-Celiac Gluten Sensitivity (NCGS) and wheat-sensitive Irritable Bowel Syndrome (IBS): an update. Nutrients. 2017;9(11). https://doi.org/10.3390/nu9111268.
Caminero A, McCarville JL, Zevallos VF, Pigrau M, Yu XB, Jury J, et al. Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology. 2019;156(8):2266–80. https://doi.org/10.1053/j.gastro.2019.02.028.
Mansueto P, Di Liberto D, Fayer F, Soresi M, Geraci G, Giannone AG, et al. TNF-alpha, IL-17, and IL-22 production in the rectal mucosa of nonceliac wheat sensitivity patients: role of adaptive immunity. Am J Physiol Gastrointest Liver Physiol. 2020;319(3):G281–8. https://doi.org/10.1152/ajpgi.00104.2020.
Funding
C. Catassi is a scientific consultant of Dr. Schär Food and Takeda. Other authors have no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Catassi, C., Guelzoni, G., Catassi, G.N. (2022). Non Celiac Wheat Sensitivity. In: Amil-Dias, J., Polanco, I. (eds) Advances in Celiac Disease . Springer, Cham. https://doi.org/10.1007/978-3-030-82401-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-82401-3_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82400-6
Online ISBN: 978-3-030-82401-3
eBook Packages: MedicineMedicine (R0)