Abstract
Limbic encephalitis (LE) is most commonly caused by either infectious or autoimmune phenomena. In a minority of cases, LE is paraneoplastic and arises from activation of the immune system by tumoral antibodies. Common causes of paraneoplastic autoimmune encephalitis include small-cell carcinoma of the lung, testicular germ cell tumor, thymic tumors, breast cancer, ovarian tumors (e.g., ovarian carcinoma and ovarian teratoma), hematological malignancies (e.g., Hodgkin lymphoma), gastrointestinal malignancies, and neuroblastoma. Causes of non-paraneoplastic autoimmune encephalitis include voltage-gated potassium channel (VGKC) antibody encephalitis, anti-N-methyl-D-aspartic acid (NMDA) receptor encephalitis, and systemic autoimmune conditions, such as systemic lupus erythematous [1–4].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Limbic Encephalitis
Limbic encephalitis (LE) is most commonly caused by either infectious or autoimmune phenomena. In a minority of cases, LE is paraneoplastic and arises from activation of the immune system by tumoral antibodies. Common causes of paraneoplastic autoimmune encephalitis include small-cell carcinoma of the lung, testicular germ cell tumor, thymic tumors, breast cancer, ovarian tumors (e.g., ovarian carcinoma and ovarian teratoma), hematological malignancies (e.g., Hodgkin lymphoma), gastrointestinal malignancies, and neuroblastoma. Causes of non-paraneoplastic autoimmune encephalitis include voltage-gated potassium channel (VGKC) antibody encephalitis, anti-N-methyl-D-aspartic acid (NMDA) receptor encephalitis, and systemic autoimmune conditions, such as systemic lupus erythematous [1,2,3,4].
FDG-PET has been shown to facilitate the visualization of changes (both hypo- and hypermetabolism) in glucose metabolism in the mesial temporal structures as well as extra-limbic regions and hence support the diagnosis of limbic encephalitis. Of note, the PET findings are oftentimes dependent upon the timing of the study and other factors including phase of encephalitis, i.e., relapsing course [5]. Metabolic information derived from [18F]-FDG-PET has been particularly helpful in patients with negative structural imaging. To date, studies have mostly focused either on morphological analysis of MRI or metabolic analysis of PET data alone for diagnosis of LE, but not on the diagnostic value of integrated [18F]-FDG-PET/MRI. A number of studies indicate that PET findings in the limbic system and extra-limbic regions are better associated with clinical symptoms and active disease status compared to structural MRI findings [6].
Deuschl and colleagues have done the largest investigation to date assessing the diagnostic value of integrated 18F-FDG-PET/MRI in the diagnostic workup of 20 patients with suspected LE [7]. Based on integrated PET/MRI, 19/20 patients were found to have morphologic and/or metabolic changes indicative of LE, whereas sole MRI enabled correct identification in 16/20 patients. Three patients with negative MRI showed metabolic changes in the limbic system or extra-limbic regions on FDG-PET, shifting the diagnosis from (negative) MRI to positive for LE by integrated PET/MRI. Furthermore, diagnostic confidence on a five-point Likert scale reached higher values for hybrid PET/MRI (2.7) when compared to sole MRI (2.4).
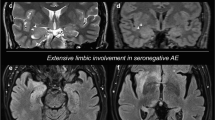
Diagnosis of autoimmune encephalitis has increased in parallel with the detection of new autoantibodies. Baumgartner and colleagues reported on the brain MRI, FDG-PET, and autoantibodies type of 18 patients with autoimmune limbic encephalitis. They found a significant association between PET findings and autoantibody type. Specifically, all patients with autoantibodies against intracellular antigens demonstrated mesiotemporal findings on FDG-PET (Fig. 52.1) [8], while only 2/9 patients with autoantibodies against surface antigens displayed mesiotemporal hypermetabolism. A similar association was noted using MRI only; however, this did not reach statistical significance.
FLAIR MRI (first row) depicts bilateral mesiotemporal signal hyperintensities, while FDG-PET (second row) shows extensive bilateral mesiotemporal hypermetabolism in addition to hypometabolism in the association cortices. Anti-Hu antibodies were detected in the patient’s serum. (Reproduced with permission from Baumgartner et al. [8])
Most of the FDG-PET studies of LE patients describe mesiotemporal hypermetabolism [9,10,11,12]. This is often accompanied by hypometabolism in the association cortex, relative metabolic sparing of primary cortices, cerebellum and striatum. However, several recent reports describe distinct, extra-mesiotemporal imaging findings in areas including the cerebellum, occipital or frontal regions, striatum and thalamus, or completely normal FDG-PET scans. In a retrospective study, Probasco and colleagues found marked medial occipital hypometabolism in 6 out of 8 patients with anti-NMDA receptor encephalitis and suggested that this imaging pattern may serve as an early biomarker for discriminating anti-NMDA receptor encephalitis from other autoimmune encephalitis etiologies [13].
NMDA receptor antibody encephalitis is a highly characteristic syndrome evolving in five stages, namely, the prodromal phase, psychotic phase, unresponsive phase, hyperkinetic phase, and gradual recovery phase, but only two clear stages have been supported with experimental data [14]. Temporal analysis of the onset of neurological features suggested progression through two main stages. The time of onset of the early features, characterized by neuropsychiatric symptoms and seizures preceded by a median of 10–20 days, the onset of movement disorders, reduction in consciousness and dysautonomia. This disorder often has a relapsing course; therefore, once patients overcome the hyperkinetic phase, gradual improvement happens in months and full recovery in 3 or more years. Good clinical outcomes, as defined by reductions in modified Rankin scores, correlate with decreased NMDA receptor antibody levels that are associated with early (<40 days) administration of immunotherapies in non-paraneoplastic patients. NMDA receptor antibodies in serum and CSF are considered to be highly specific for this condition [15]. In 45% of patients, the brain MRI is normal [15].
Diagnosis remains challenging due to its nonspecific clinical presentation with loss of short-term memory, mental status changes, or psychiatric symptoms commonly seen. Identifying the culprit autoantibodies in the cerebrospinal fluid is an important criterion for diagnosing LE. In the majority of cases, MRI may be unrevealing, especially early in the course of the disease, or demonstrate subtle increased T2/FLAIR signal abnormalities and/or volume loss most commonly affecting the amygdala, hippocampus, and/ or insula, typically with bilateral involvement [2]. Basal ganglia can also be involved which is an important feature in differentiating limbic encephalitis from HSV encephalitis, wherein the latter characteristically spares the basal ganglia [2].
Figure 52.2 demonstrates abnormal FDG distribution pattern with gradient of relative anterior hypermetabolism to posterior hypometabolism in a 20-year-old female who presented with a history of insomnia and changes in mental status. She was agitated, confused with visual hallucinations and combative behavior. She developed partial complex seizure with impairment of awareness and automatisms of lip smacking. During the first 2 weeks, she had become increasingly drowsy and confused; her level of consciousness was severely impaired, with no response to painful stimuli; and orofacial-limb dyskinesias appeared. Cardiac arrhythmia, characterized by alternating repeated episodes of tachycardia and bradycardia, was also observed.
FDG-PET cortical surface maps demonstrate abnormal FDG distribution pattern with gradient of relative anterior hypermetabolism to posterior hypometabolism and specifically decreased tracer uptake in the posterior parietal and temporal and occipital lobes bilaterally. There is moderate hypometabolism in the cerebellum and sensorimotor cortex which may represent sequelae of neurotropic medications. There is moderate to severe hypometabolism in the anteromedial temporal lobes bilaterally, which also may reflect treatment effects. There is preserved normal brain metabolism in the basal ganglia. There is mild nonspecific hypometabolism in the bilateral thalamus (Case courtesy of Dr. Ana M Franceschi, Northwell Health, NY, USA)
In addition to the diagnostic value of FDG-PET in patients with autoimmune encephalitis, the modality has also been used in evaluation of treatment response. Trevino-Peinado and colleagues reported on a 70-year-old woman with autoimmune encephalitis presenting with subacute cognitive impairment [16]. Initial MRI demonstrated only mild leukoraiosis; however, FDG-PET depicted diffuse cerebral hypometabolism. A 2-month follow-up FDG-PET following the administration of immunotherapy showed normalization of metabolic findings, with concurrent improvement in the patient’s clinical status.
Sarcoidosis
Sarcoidosis is a multisystem disorder characterized by the presence of noncaseating granulomas. The disease typically affects young and middle-aged adults and is more commonly noted in the African American population, generally presenting with pulmonary symptoms, although other organs/systems, including the skin, eyes, muscles, heart, and brain, can be affected initially or at later stages of the disease. The natural course of the disease in individual patients is variable. The signs and symptoms are nonspecific, posing a challenge for early and accurate diagnosis. Assessment of disease using conventional imaging methods is difficult and unreliable. However, accurate determination of disease activity is essential for timely administration of treatment to control the disease and relieve symptoms.
Neurologic complications occur in approximately 5% of patients with sarcoidosis. Neurosarcoidosis is a diagnostic dilemma in patients with known sarcoidosis who develop neurologic complaints and in patients presenting de novo with a constellation of findings consistent with CNS involvement. Approximately 50% of patients with eventual diagnosis of neurosarcoidosis present with neurologic difficulties at initial diagnosis. One-third of those with neurosarcoidosis will develop more than one neurologic manifestation of the disease.
The preferred imaging modality for the diagnosis of central nervous system sarcoidosis is contrast-enhanced MRI. Meningeal or parenchymal enhancement suggests active inflammation with disruption of the blood-brain barrier. If the diagnosis remains in doubt, meningeal, brain, or spinal cord biopsy is occasionally indicated. Performing a biopsy to establish the diagnosis, rather than initiating empiric therapy, should be considered if no evidence of systemic disease is present. In general, FDG-PET may be helpful for detecting clinically silent sites and directing biopsy for definitive diagnosis.
Sammarra et al. reported a case of a 52-year-old man that presented with history of focal seizures, progressive cognitive decline, and motor impairment. Brain 3T MRI demonstrated leukoencephalopathy with multifocal nodular lesions which were hyperintense on T2-weighted/fluid-attenuated inversion recovery (FLAIR) images involving the basal ganglia, periventricular, and deep white matter (Fig. 52.3) [17]. The interpretation of this pattern was unclear, and the differential diagnosis included inflammatory, infective, or neoplastic disorders. To better understand the nature of these abnormalities, single-voxel 1H-magnetic resonance spectroscopy (1H-MRS), contrast-enhanced computed tomography (CT) scan, and 18F-FDG-PET/3T-MRI were performed. The parenchymal lesions exhibited slight N-acetyl-aspartate/creatine reduction without abnormal lipid and lactate peaks on 1H-MRS, enhancement following contrast administration on CT and corresponding hypermetabolism on 18F-FDG-PET, suggesting a diagnosis of neurosarcoidosis in the proper clinical setting. Therefore, a whole-body 18F-FDG-PET/CT was performed in order to identify subclinical systemic sarcoidosis, and a hypermetabolic nodule in the left lung upper lobe was found.
Structural , spectroscopic, and metabolic findings of nodular lesions in the brain. Axial fluid-attenuated inversion recovery (FLAIR) (a) and T2-weighted (b) 3T-magnetic resonance imaging (MRI) images demonstrating several parenchymal nodular lesions and leukoencephalopathy. Single-voxel 1H-magnetic resonance spectroscopy (1H-MRS) of the basal ganglia lesions (c) and relative spectrum (d) showing N-acetyl-aspartate/creatine (NAA/Cr) reduction, without abnormal lactate or lipids peaks (NAA/Cr ratio was 0.873). Axial contrast-enhanced computed tomography (CT) (e) and fluorine-18-fluorodeoxyglucose positron-emission tomography (18F-FDG-PET)/3T-MRI (f) images demonstrating enhancement with hypermetabolism of all nodular lesions. (Reproduced with permission from Sammarra et al. [17])
Systemic Lupus Erythematosus (SLE)
CNS involvement has been found in 30–75% of all systemic lupus erythematosus (SLE) patients; however, clinical diagnosis of CNS lupus remains difficult, given lack of reliable and sensitive markers of CNS disease activity.
FDG-PET as a functional imaging technique has been compared with CT and MRI in the diagnosis of CNS-SLE. CT and MRI typically show only gross abnormalities including edema or small infarcts. In contrast, FDG-PET demonstrates decreased cerebral blood flow and glucose uptake during active focal and diffuse CNS lupus. Furthermore, there is a strong positive correlation between FDG-PET and neurologic findings, rendering PET a very sensitive method for detecting CNS involvement in SLE [18].
References
Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR. 2017;38(6):1070–8.
Oyanguren B, Sánchez V, et al. Limbic encephalitis: a clinical-radiological comparison between herpetic and autoimmune etiologies. Eur J Neurol. 2013;20(12):1566–70.
Tüzün E, Dalmau J. Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–71.
Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–94.
Padma S, Sundaram PS. PET/CT in the evaluation of anti-NMDA-receptor encephalitis: what we need to know as a NM physician. Ind J Nucl Med. 2011;26:99–101.
Morbelli S, Arbizu J, et al. The need of standardization and of large clinical studies in an emerging indication of [18F] FDG PET: the autoimmune encephalitis. Eur J Nucl Med Mol Imaging. 2017;44(3):353–7.
Deuschl C, Ruber T, et al. 18F-FDG-PET/MRI in the diagnostic work-up of limbic encephalitis. PLoS One. 2020;15(1):e0227906.
Baumgartner A, Rauer S, et al. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260:2744–53.
Chatzikonstantinou A, Szabo K, Ottomeyer C, et al. Successive affection of bilateral temporomesial structures in a case of non-paraneoplastic limbic encephalitis demonstrated by serial MRI and FDG-PET. J Neurol. 2009;256:1753–5.
Troester F, Weske G, Schlaudraff E, et al. Image of the month. FDG-PET in paraneoplastic limbic encephalitis. Eur J Nucl Med Mol Imaging. 2009;36:539.
Ances BM, Vitaliani R, et al. Treatment responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77.
Kassubek J, Juengling FD, et al. Limbic encephalitis investigated by 18 FDG-PET and 3D MRI. J Neuroimaging. 2001;11:55–9.
Probasco JC, Solnes L, Nalluri A, Cohen J, Jones KM, Zan E, Javadi MS, Venkatesan A. Decreased occipital lobe metabolism by FDG-PET/CT: an anti-NMDA receptor encephalitis biomarker. Neurol Neuroimmunol Neuroinflamm. 2017;5(1):e413.
Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–67.
Dalmau J, Tüzün E, Wu H, et al. Paraneoplastic Anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2006;61:25–36.
Trevino-Peinado C, Arbizu J, et al. Monitoring the effect of immunotherapy in autoimmune limbic encephalitis using FDG PET. Clin Nucl Med. 2015;40:e441–3.
Sammarra I, Barbagallo G, et al. (2019) Value of multimodal imaging approach to diagnosis of neurosarcoidosis. Brain Sci 9(10): 243.
Weiner S, Otte A, et al. Diagnosis and monitoring of central nervous system involvement in systemic lupus erythematosus: value of F-18 fluorodeoxyglucose PET. Ann Rheum Dis. 2000;59(5):377–85.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ghaderi Niri, S., Raghavan, P., Wintermark, M. (2022). Inflammatory Disease. In: Franceschi, A.M., Franceschi, D. (eds) Hybrid PET/MR Neuroimaging. Springer, Cham. https://doi.org/10.1007/978-3-030-82367-2_52
Download citation
DOI: https://doi.org/10.1007/978-3-030-82367-2_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82366-5
Online ISBN: 978-3-030-82367-2
eBook Packages: MedicineMedicine (R0)