Abstract
Peripheral artery disease (PAD) is associated with a significantly high risk of cardiovascular morbidity and mortality in patients with chronic kidney disease (CKD). In addition, patients with CKD have higher risk of failure of treatment of PAD and lower limb amputations compared to general population. Therefore, close monitoring for signs and symptoms and timely treatment is extremely important in this population. Whereas asymptomatic patients are usually treated with risk factor control, the treatment of symptomatic patients depends on the severity and location of the disease. Patients with mild symptoms like intermittent claudication can be treated with a trial of antiplatelet agents. Those who do not respond or those with critical limb ischemia (CLI) need invasive treatment which could be either endovascular or surgical. Endovascular intervention is the mainstay of the treatment in most of the patients with CKD. Angioplasty of the lesions with or without stent placement can be done based on the severity and location of the lesion. It is important that patients with CKD get pre-exposure prophylaxis and minimum dose of iodinated contrast to minimize the risk of contrast-induced nephropathy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Peripheral artery disease
- Chronic kidney disease
- Intermittent claudication
- Critical limb ischemia
- Invasive angiography
- Angioplasty
- Stent placement
Introduction
Cardiovascular disease (CVD) is the cause of death in nearly half of end-stage renal disease (ESRD) patients [1, 2]. An individual with ESRD has a CVD mortality rate 15 times of that found in the general population. Previous studies have shown that peripheral arterial disease (PAD) is associated with a significantly elevated risk of cardiovascular disease morbidity and mortality and is generally regarded as CVD equivalent in terms of mortality risk [3,4,5]. The purpose of this chapter is to describe an approach to PAD treatment in CKD patients with a discussion of indications, outcomes, techniques, and precautions.
Even though PAD is more prevalent in CKD patients, very few trials on PAD treatment have looked at this population [6]. Furthermore, large observational cohort studies have shown that for the same degree of severity of PAD, fewer CKD patients underwent endovascular procedures than those without CKD [7, 8]. CKD patients, however, had higher incidence of amputations [8]. One possibility is the higher risk of treatment failure in this population as even with a successful intervention, these patients have higher risk of re-intervention and amputations in the future [9]. In the light of the above evidence, it is vital to have an accurate assessment of indications, risks, and benefits of treatment in these patients.
Indications
The pathophysiology of vascular disease in the CKD population differs from the nonrenal disease population. Traditional vascular disease comprises intimal disease with lipid-rich plaques producing focal stenoses and the potential for plaque rupture and subsequent thrombosis. In CKD, on the other hand, plaques are characterized by intense medial calcification, which tends toward chronic stenotic disease rather than acute plaque rupture [10, 11]. What is more, PAD appears to be more common in the CKD population than in the general population [12], with some studies suggesting that patients with moderate to severe CKD were at ninefold increased risk to have an ABI <0.9 [13]. In addition, lower extremity amputations resulting from PAD are more prevalent in the ESRD population than in the general population with PAD [14].
Given the prevalence of PAD in CKD, KDIGO guidelines published in 2012 recommend that adults with CKD be regularly examined for signs of peripheral artery disease and be considered for usual approaches to therapy [15]. However, the recommendation to screen CKD/ESRD patients for PAD who are asymptomatic is not supported by clinical data [16]. In patients with PAD who do not have classic claudication (asymptomatic patients), a reduced ABI is highly associated with cardiovascular events [17]. The purpose of screening asymptomatic patients in the general population is to attempt to modify their CVD risk by prescribing aspirin, lipid medications, diet, etc., if they are discovered to have PAD. For this reason, the ABI has become a routine measurement in the primary care practice of medicine [18]. In CKD and ESRD patients, however, the presence of CKD alone is an independent risk factor for CVD. Thus, by virtue of CKD alone, independent of PAD diagnosis, patients should be treated with an aggressive CVD risk reduction regimen. For this reason, screening of asymptomatic CKD patients for PAD is not recommended.
Likewise, treatment of PAD is only indicated if the CKD/ESRD patient exhibits symptoms and signs of claudication, rest pain, impaired ability to walk, or nonhealing lower extremity ulcerations. Claudication, the symptom classically associated with PAD, usually presents as reproducible muscle pain that occurs with activity and improves with rest. It results from a mismatch between oxygen supply to and demand of muscle group during exercise. Physical examination should focus on skin integrity (e.g., hair loss, presence of wounds or ulcers) and assessment of peripheral pulses with accurate documentation of all pulses at each visit. Diminished bilateral peripheral pulses, femoral bruits, and prolonged capillary refill are very specific for PAD [19]. If a CKD patient complains of the symptoms outlined above and if the suspected diagnosis is corroborated with noninvasive screening tests (ABI < 0.9), then treatment should be strongly considered.
Medical Treatment of PKD
The evidence for medical therapies that reduce symptoms and attenuate disease progression is strongest for antiplatelet therapies. There may be a modest benefit with clopidogrel over aspirin, as suggested by Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial which found a reduced cardiovascular risk in the clopidogrel-treated group [20]. Although the ACC/AHA guidelines recommend clopidogrel as an aspirin alternative, severe CKD was an exclusion criterion for enrollment in the CAPRIE trial, so the potential benefits of clopidogrel versus aspirin in our patients are unclear [21]. However, the TransAtlantic Inter-Society Consensus (TASC) guidelines recommend either aspirin or clopidogrel [22]. A recent meta-analysis which evaluated the effects of antiplatelet agents on maximal walking distance (MWD), one of the key parameters of symptom relief in the general PAD population [23], found that the overall pooled estimate was in favor of treatment but with a modest increase in MWD of 59 m.
Cilostazol and pentoxifylline are phosphodiesterase inhibitors that reduce platelet aggregation and act as mild vasodilator. Several studies have suggested that cilostazol can reduce claudication and increase walking times [24,25,26]. Studies with cilostazol showed a significant effect on walking distance at doses of 50 and 100 mg. MWD increased 36 m (95% CI: 30e41 m) with 50 mg, but almost twice that 70 m (95% CI: 47e93) with the 100-mg dose [23]. It is important to note that the use of cilostazol is contraindicated in patients with congestive heart failure, although there are no studies in this population. In addition, information in the package insert indicates that cilostazol has reduced clearance in severe renal impairment. Since this drug has not been studied in dialysis patients, caution is advised for use in individuals with a creatinine clearance <25 ml/min [13]. Pentoxifylline was similarly found to be of modest benefit on MWD [23]; clearance is reduced in renal failure, so dosages must be adjusted appropriately in those settings.
A comprehensive medical therapy algorithm based on the above observations is offered in Fig. 7.1.
Invasive Diagnostics of PKD
Severe forms of PAD often manifest as the clinical entity known as critical limb ischemia, which is defined by rest pain and ischemic skin lesions such as ulcers and gangrene. In the general population, revascularization is the optimal therapy for critical limb ischemia [25, 27]. Revascularization via percutaneous transluminal angioplasty (PTA) procedures is preferred to surgical revision in most cases. There are no randomized, controlled trial data regarding revascularization techniques in patients with CKD and dialysis patients, however. Not surprisingly, a retrospective analysis of patients who had CKD and underwent lower limb revascularization found lower rates of limb loss and mortality compared with ESRD [28]. What is more, mortality rates were found to be inversely correlated with kidney function [29]. Patients with ESRD often are not good candidates for PTA because of distal disease and vascular calcifications. Nevertheless, a retrospective analysis of hemodialysis patients saw lower mortality and higher limb salvage rates in those who underwent percutaneous revascularization compared with a surgical approach [30].
Once a patient is judged to be a likely candidate to benefit from intervention, an angiogram is scheduled. Contrast angiography remains the gold standard for diagnosis and the assessment of the severity of atherosclerotic PAD. The value of this diagnostic modality has been buoyed by the recently described association of nephrogenic systemic fibrosis (NSF) with magnetic resonance contrast agents required for MR angiography [31]. Angiography, further, allows evaluation of the abdominal aorta, renal arteries, and branch vessels, the presence of accessory renal arteries, as well as cortical blood flow and renal dimensions [32]. Moreover, pressure gradients across arterial lesions can be obtained to evaluate the hemodynamic significance of the said lesions if the angiographic or noninvasive testing data is equivocal. Digital subtraction angiography (DSA) has become available in many institutions, and, although its resolution is inferior to film, it permits the use of lower concentrations of iodinated contrast, as well as of alternative contrast agents such as CO2 [33].
The disadvantages of angiography include the use of iodinated contrast and ionizing radiation, relative cost, need for patient sedation and monitoring, and the potential occurrence of associated complications. The potential complications of arterial angiography include bleeding, infection, cholesterol embolization, and vascular injury. Patients with CKD not yet on dialysis, and even those on dialysis in whom residual renal function is an issue, may not be able to safely undergo conventional angiography. However, the use of various preparatory methods prior to angiography seems to diminish the risk of acute kidney injury in the setting of CKD [34]. Furthermore, contrast dose can be very strictly managed in these patients by a careful and deliberate approach to diagnostic evaluation in the CKD population.
Typically, an abdominal aortogram is performed with distal runoff, usually positioning a pigtail catheter at the lower edge of the first lumbar vertebra and power-injecting 40 ml of dye at 20 ml/s. The abdominal aortogram will provide information regarding the aorta itself, the position of the renal arteries, and the presence of iliac calcification. Runoff into the lower extremities must be done properly by moving the image intensifier or table such that the flow of contrast can be followed. This takes a certain degree of experience and can lead to repeated aortograms if not performed properly. In most instances, the aortogram provides adequate visualization of the peripheral arterial tree, but if optimal imaging or pressure gradient measurement are needed, selective catheterization becomes necessary. This can be achieved with a variety of different 4–6 F diagnostic catheters. Whatever catheter shape is used, the goal is to achieve selective cannulation of the artery in question without excessive catheter manipulation, as atheromas are often adjacent to areas of disease and distal embolization can occur [32].
Interventional Approach
Percutaneous intervention on PAD in CKD patients should be considered only if one of the following conditions is met:
-
1.
Symptomatic and refractory to medical therapy.
-
2.
Critical limb ischemia is present.
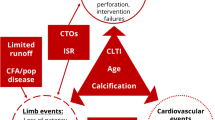
Medical therapy of the CKD patient with claudication is described above. If the CKD patient has been treated with medical therapy with no improvement in symptoms, then percutaneous revascularization may be considered (Fig. 7.1). Critical limb ischemia (CLI) is different from claudication per se and can be defined as limb pain that occurs at rest or impending limb loss that is caused by severe compromise of blood flow to the affected extremity. All CKD patients with rest pain, ulcers, or gangrene attributable to objectively proven arterial occlusive disease should be categorized as CLI. Unlike individuals with claudication, patients with CLI have resting perfusion that is inadequate to sustain viability in the tissue bed which frequently leads to amputation (Fig. 7.2). Therefore, CLI should be considered for percutaneous revascularization at the same time that medical therapy is initiated [21].
Claudication and CLI exist on a continuum, which makes it challenging to simplify this complex disease state into two distinct categories. For this reason, guidelines have been developed to subcategorize PAD into several distinct subtypes based on characteristics of lesion morphology [25]. These classifications are summarized in Tables 7.1 and 7.2 for supra- and infrainguinal disease, respectively. The symptoms that a given lesion is causing can also be divided into stages (Table 7.3) [35]. Based on classification of symptoms and lesion morphology, the interventional plan can then be determined based on the algorithms described in Figs. 7.3 and 7.4.
Precautions
Careful attention to contrast dye load is required, especially in patients at high risk for contrast nephropathy. As mentioned above, CO2 can be used as alternative contrast agents at least during some parts of the intervention. Adjuvant pharmacology before and after peripheral percutaneous intervention has not been systematically studied. Heparin to maintain an ACT of 250–300 s is frequently used as the anticoagulant of choice during interventional procedures; most interventionalists are quite familiar with its use, and it can be easily reversed with protamine. Patients are usually pretreated with aspirin which is continued indefinitely. The use of clopidogrel seems theoretically necessary following percutaneous intervention; however, there are no controlled studies exploring its use on CKD patients with PAD. However, if the CKD patient has symptoms of PAD justifying intervention, they should be treated with clopidogrel by virtue of that alone. Other possible intra-procedural anticoagulants, such as glycoprotein 2B3A receptor antagonists, and direct thrombin inhibitors, such as bivalirudin, have not been formally studied in peripheral interventions in CKD. PAD intervention requires close attention to several important details that are typically not a major concern in the venous system. When performing arterial intervention, one must not oversize the balloon or undersize the stent [36]. In addition, there is no reason to use a longer balloon than you need, and one should aim for 5-mm extension beyond the length of the lesion when selecting balloon length. The guidewire should be kept across the lesion at all times, even when retracting the balloon in what one feels is a successful intervention. One should seldom intervene before giving anticoagulation. One should not inflate an angioplasty balloon over nominal pressure in the arterial system, since balloon rupture can lead to acute limb ischemia in the arterial system. A partially inflated balloon should not be retracted, and it is best to intervene in the arterial system with a sheath that engages the lesion in question [32]. Most importantly, one must not allow air into the arterial system, especially when operating on the great vessels of the thoracic aorta. In most cases a manifold can be used to minimize the probability of air embolization.
Given the above precautions, PAD intervention should be performed only by skilled interventionalists who are specifically trained for PAD intervention. These practitioners should have a thorough knowledge of the medical/cognitive components of the decision making process as described above. To date, there is no entity that trains, certifies, or accredits nephrologists in this discipline. Furthermore, the procedures described above should only be performed if there is adequate monitoring and surgical backup to allow detection and treatment of the potential complications of PAD intervention, including but not limited to acute limb ischemia, arterial thrombosis, and arterial dissection.
Conclusion
PAD is a problem that affects CKD patients out of proportion to the general population and mirrors CVD outcomes very closely. Unlike the general population, PAD in CKD occurs due to medial calcification; as opposed to an intimal atherosclerotic process, PAD intervention should be performed in select symptomatic patients, as described by the guidelines, and CVD risk factor modification should occur in all CKD patients, regardless of the presence of PAD.
As a discipline, interventional nephrology has emerged out of a desire to create better outcomes for our patients and to “fix a problem.” The core values of our discipline have evolved out of this fundamental desire to meet an unmet clinical need, provide insight into a disease state specific to our patients, and offer clinical/academic excellence in doing so. We must endeavor to follow a similar path in our approach to PAD.
References
USRDS: United States renal data system annual data report, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney Urologic and Hematologic Diseases; 2000. p. 339–48.
Murabito JM, Evans JC, Larson MG, et al. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham study. Arch Intern Med. 2003;163(16):1939–42.
Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study: the cardiovascular health study group. Arterioscler Thromb Vasc Biol. 1999;19(3):538–45.
Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–6.
Kerr PG, Guérin AP. Arterial calcification and stiffness in chronic kidney disease. Clin Exp Pharmacol Physiol. 2007;34(7):683–7.
Wen Y, Potok OA, Wei X, et al. Exclusion of persons with kidney disease in trials of peripheral artery disease: a systemic review of randomized trials. Clin J Am Soc Nephrol. 2020;15(1):117–9.
Luders F, Bunzemeier H, Engelbertz C, et al. CKD and acute and long-term outcome of patients with peripheral artery disease and critical limb ischemia. Clin J Am Soc Nephrol. 2016;11:216–22.
O’Hare AM, Bertenthal D, Sidawy AN, et al. Renal insufficiency and use of revascularization among a national cohort of men with advanced lower extremity peripheral arterial disease. Clin J Am Soc Nephrol. 2006;1:297–304.
Patel VI, Mukhopadhyay S, Guest JM, et al. Impact of severe chronic kidney disease on outcomes of infrainguinal peripheral arterial intervention. J Vasc Surg. 2014;59:368–75.
Guérin AP, Pannier B, Marchais SJ, London GM. Cardiovascular disease in the dialysis population: prognostic significance of arterial disorders. Curr Opin Nephrol Hypertens. 2006;15(2):105–10.
Leskinen Y, Salenius JP, Lehtimäki T, Huhtala H, Saha H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis. 2002;40(3):472–9.
Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–43.
DeLoach SS, Mohler ER 3rd. Peripheral arterial disease: a guide for nephrologists. Clin J Am Soc Nephrol. 2007;2(4):839–46.
Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56(4):1524–33.
National Kidney Foundation. KDIGO 2012 Clinical practice guidelines for evaluation and management of chronic kidney disease. KI Suppl. 2013;3:100–1.
Inker LA, Astor BC, Chester F, et al. KDOQI US Commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–35.
Heald CL, Fowkes FG, Murray GD, Price JF, Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: systematic review. Atherosclerosis. 2006;189(1):61–9.
Steffen LM, Duprez DA, Boucher JL, Ershow AG, Hirsch AT. Management of peripheral arterial disease. Diabetes Spectrum. 2008;21(3)
McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32(6):1164–71.
Clagett GP, Sobel M, Jackson MR, Lip GY, Tangelder M, Verhaeghe R. Antithrombotic therapy in peripheral arterial occlusive disease. The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3 Suppl):609S–26.
Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines. Circulation. 2006;113(11):e463 654.
Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67.
Momsen AH, Jensen MB, Norager CB, Madsen MR, Vestersgaard-Andersen T, Lindholt JS. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg. 2009;38(4):463–74.
Beebe HG, Dawson DL, Cutler BS, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Arch Intern Med. 1999;159(17):2041–50.
Mohler ER 3rd, Beebe HG, Salles-Cuhna S, et al. Effects of cilostazol on resting ankle pressures and exercise-induced ischemia in patients with intermittent claudication. Vasc Med. 2001;6(3):151–6.
Reilly MP, Mohler ER 3rd. Cilostazol: treatment of intermittent claudication. Ann Pharmacother. 2001;35(1):48–56.
Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC working group. TransAtlantic Inter-Society Consensus. J Vasc Surg. 2000;31(1 Pt 2):S1–296.
O’Hare AM, Sidawy AN, Feinglass J, et al. Influence of renal insufficiency on limb loss and mortality after initial lower extremity surgical revascularization. J Vasc Surg. 2004;39(4):709–16.
O’Hare AM, Feinglass J, Sidawy AN, et al. Impact of renal insufficiency on short-term morbidity and mortality after lower extremity revascularization: data from the Department of Veterans Affairs’ National Surgical Quality Improvement Program. J Am Soc Nephrol. 2003;14(5):1287–95.
Jaar BG, Astor BC, Berns JS, Powe NR. Predictors of amputation and survival following lower extremity revascularization in hemodialysis patients. Kidney Int. 2004;65(2):613–20.
Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148–57.
Yevzlin AS, Schoenkerman AB, Gimelli G, Asif A. Arterial interventions in arteriovenous access and chronic kidney disease: a role for interventional nephrologists. Semin Dial. 2009;22(5):545–56.
Hawkins IF Jr, Wilcox CS, Kerns SR, Sabatelli FW. CO 2 digital angiography: a safer contrast agent for renal vascular imaging? Am J Kidney Dis. 1994;24(4):685–94.
Li JH, He NS. Prevention of iodinated contrast-induced nephropathy. Chin Med J. 2011;124(23):4079–82.
Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–38.
Asif A, Yevzlin AS. Arterial stent placement in arteriovenous dialysis access by interventional nephrologists. Semin Dial. 2009;22(5):557–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gardezi, A.I., Yevzlin, A.S. (2022). Overview of PAD Treatment in the CKD Population: Indications, Medical Strategies, and Endovascular Techniques. In: Yevzlin, A.S., Asif, A., Salman, L., Ramani, K., Qaqish, S.S., Vachharajani, T.J. (eds) Interventional Nephrology. Springer, Cham. https://doi.org/10.1007/978-3-030-81155-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-81155-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81154-9
Online ISBN: 978-3-030-81155-6
eBook Packages: MedicineMedicine (R0)