Abstract
Pseudoaneurysm development is a relatively common complication encountered in patients with an arteriovenous access. Pseudoaneurysms frequently develop due to repeated cannulation at the same site (one-site-itis) during dialysis therapy. Because of their risk of rupture, pseudoaneurysms can create a major catastrophe including death. Both interventional and surgical approaches are available to successfully combat this complication. In this chapter, we present pathophysiology, clinical features, diagnosis, and management for patients with dialysis access-related pseudoaneurysms. Because multiple solutions are available, we also highlight the importance of multidisciplinary evaluation approach for pseudoaneurysms, including the assessment by a vascular surgeon.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pseudoaneurysm
- AV fistula bleeding
- Aneurysm rupture
- Dialysis access bleeding
- Complications of dialysis access
- Stent-graft
- Angioplasty
- Vessel rupture
- Hematoma
Introduction
No matter how one looks at hemodialysis, vascular access continues to be considered the lifeline of hemodialysis patients. An arteriovenous access has also been referred to the Achilles heel for hemodialysis patients. Arteriovenous accesses fall into three main categories. Arteriovenous fistula is created by connecting the end of a vein to the side of an artery. An arteriovenous graft is created by using a synthetic graft. Here the end of a vein is connected to the end of a synthetic graft. The other end of the graft is connected to the side of an artery. Finally, a permanent hemodialysis catheter is tunneled under the skin and is usually inserted into the internal jugular vein. Of the three options available, arteriovenous fistulae (AVF) and arteriovenous graft (AVG) remain the preferred forms of hemodialysis access to provide long-term hemodialysis therapy. It is important to mention that optimal functioning of these vascular accesses is essential for long-term hemodialysis and survival of a hemodialysis patient.
Both AVF and AVGs are at risk of developing multiple complications including vascular access stenosis, access thrombosis, infection, and the development of pseudoaneurysms due to multiple factors. These pseudoaneurysms can lead to access failure secondary to thrombosis, infection, and rupture. Pseudoaneurysm incidence rates have been reported to range from 2% to 10% [1, 2]. Because pseudoaneurysms can lead to access failure and the fact that sites for creation of an arteriovenous fistula are limited, it is important to have a heightened awareness of this complication. It is also important to have an understanding of when to diagnose and refer the patient to an interventionalist/surgeon for optimal treatment.
Pseudoaneurysm Development
The most common cause resulting in the development of pseudoaneurysms is the disruption in vascular wall of an arteriovenous graft. The most common cause of this disruption is repeated trauma due to repeated cannulation of an arteriovenous access at the same site. This situation is commonly referred to as one-site-itis. Due to repeated trauma coupled with sustained arterial pressure, blood dissects into the tissues around the damaged vessel and forms a perfused sac that communicates with the vascular lumen. In extreme cases, the only thing that separates blood from the external environment is the thin layer of skin over the arteriovenous access.
It is important to make a distinction between a pseudoaneurysm and a true aneurysm [1,2,3]. In contrast, pseudoaneurysms are formed due to the damage to vascular wall causing disruption of the vessel wall. On the other hand, true aneurysm is a dilatation due to weakness in the blood vessel that involves all the layers of a vessel without any damage.
Multiple factors may result in the development of pseudoaneurysms in an arteriovenous access [2]. These could develop from either multiple infiltrations during cannulation of an arteriovenous access or repeated trauma from needle puncture at the same site which causes wall damage and pseudoaneurysm formation. Nevertheless, from a mechanistic standpoint, certain elements need specific attention for pseudoaneurysms. Because venous system is directly connected to the arterial system, an arteriovenous access is a high-pressure system. Additionally, the development of vessel stenosis is predominantly located at the vein-graft anastomosis in an arteriovenous graft. High-pressure wave is transmitted from an artery directly into an arteriovenous graft through the artery-graft anastomosis. In the presence of a venous outflow tract stenosis (at vein-graft anastomosis), the pressure inside the graft will further increase [1]. If there had been repeated cannulation injury to the wall of the graft, the development of a pseudoaneurysm and its progression would be rapid due to the augmented pressure inside the graft due the outflow obstruction (i.e., vein-graft anastomotic stenosis). Venous outflow stenosis leading to increased intra-access pressure is a common cause of further development and enlargement of pseudoaneurysms [1]. It is worth noting that pseudoaneurysms are more commonly seen in patients with arteriovenous grafts than those with arteriovenous fistulas.
Clinical Features
Pseudoaneurysms present as visible swelling over an arteriovenous graft or an arteriovenous fistula. Frequently, the overlying skin is shiny and thin [2]. Many a times, there is a scab overlying the pseudoaneurysm. Depigmentation can also be seen in certain accesses [2]. The swelling can be a visible mass that is pulsating with each heartbeat. Patients often complain of a “knot” on their dialysis access. Many patients place a wrist band to hide the swelling, a practice that can limit the physical examination and inspection of a dangerous situation. Some patients demonstrated signs of inflammation (redness and warmth) over the aneurysm. Others show a scab at the site of needle cannulation. It is important to mention that pseudoaneurysms can rupture and cause a catastrophe and death. Therefore, prompt diagnosis and appropriate management are needed to optimally deal with pseudoaneurysms.
Diagnosis
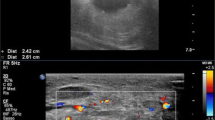
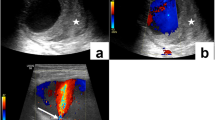
Physical examination is the cornerstone of the diagnosis of pseudoaneurysms [2]. A pulsatile mass overlying the access is diagnostic of pseudoaneurysm. Occluding the inflow drains the pulsatile mass with disappearance of pulsation [3]. Frequently, the neck of the swelling can be felt under the skin leading into the graft lumen. Both ultrasound and angiography can confirm the presence of an aneurysm but may not be needed to establish the diagnosis. There are features that can help nephrologists in assessing the severity of pseudoaneurysms. Thinning of the overlying skin, depigmentation, presence of a scab, and inflammation are all bothersome signs. A patient with these features should be promptly referred to an interventionalist/surgeon for further management. Delaying care of such patients can result in a catastrophe.
Management
As mentioned above, thinning of the overlying skin, depigmentation, presence of a scab, and inflammation regardless of the size of the pseudoaneurysm should prompt an evaluation by an interventionalist/surgeon. Similarly, prolonged bleeding after dialysis in the context of a pseudoaneurysm should also draw quick attention to the patient. Prolonged bleeding after dialysis indicates an outflow stenosis. Such a stenosis also raises the intra-access pressure and further deteriorates the pseudoaneurysm. Treatment of an outflow stenosis then becomes a part of the management of a pseudoaneurysm [1]. Treatment of an outflow stenosis is commonly performed by percutaneous balloon angioplasty with or without a stent. We recommend angioplasty alone (without a stent). This approach allows for a successful creation of a secondary fistula in the future when angioplasty fails or is needed frequently for a recurring stenosis. Angioplasty with a stent placed at the vein-graft anastomosis may jeopardize the creation of a secondary fistula and must be carefully considered. Creation of a secondary fistula fixes both, the recurring vein-graft stenosis and the pseudoaneurysm that carries a life-threating risk of a rupture [2, 3].
Endovascular stents have also been used for the treatment of pseudoaneurysm [1]. The placement of covered stents completely blocks of entry of blood into the aneurysm. However, these stents carry a risk of infection, and many pseudoaneurysms recur even after stent placement. One of the problems is that when these stents are placed inside a graft, the dialysis staff cannot tell their location. Many times, dialysis needles are inserted through the stent resulting in damage to the stent and recurrence of the aneurysm.
Surgical repair of the aneurysm can also be employed successfully. In fact, pseudoaneurysm patients should first be referred to a vascular surgeon in a multidisciplinary fashion. These specialists can guide the team on various options including angioplasty, stent for pseudoaneurysm, stent placement with angioplasty of the vein-graft anastomosis, or the creation of a secondary fistula.
Small aneurysm with no changes of the overlying skin should also be evaluated by the multidisciplinary team including the surgeon. Importantly, cannulation at the same site should be avoided. Once evaluated by the surgeon, they can then be followed up by observation and physical examination based upon the recommendation of a surgeon. During the follow-up period, a rapid enlargement or the development of skin changes mentioned above should prompt a quick surgical consultation.
References
McLennan G. Stent and stent-graft use in arteriovenous dialysis access. Semin Intervent Radio. 2016;33(1):10–4.
Inston N, Mistry H, Gilbert J, Kingsmore D, Raza Z, Tozzi M, Azizzadeh A, Jones R, Deane C, Wilkins J, Davidson I, Ross J, Gibbs P, Huang D, Valenti D. Aneurysms in vascular access: state of the art and future developments. J Vasc Access. 2017;18(6):464–72.
Mudoni A, Cornacchiari M, Gallieni M, Guastoni C, McGrogan D, Logias F, Ferramosca E, Mereghetti M, Inston N. Aneurysms and pseudoaneurysms in dialysis access. Clin Kidney J. 2015;8(4):363–7. https://doi.org/10.1093/ckj/sfv042. Epub 2015 Jun 10
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Asif, A., Ahmed, N., Agarwal, A.K. (2022). Approach to a Patient with Pseudoaneurysm. In: Yevzlin, A.S., Asif, A., Salman, L., Ramani, K., Qaqish, S.S., Vachharajani, T.J. (eds) Interventional Nephrology. Springer, Cham. https://doi.org/10.1007/978-3-030-81155-6_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-81155-6_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81154-9
Online ISBN: 978-3-030-81155-6
eBook Packages: MedicineMedicine (R0)