Abstract
Encephalitis is an inflammation of brain parenchyma that is usually associated with inflammation of the meninges or other structures of the central nervous system such as myelitis and optic neuritis. In the present chapter we discuss the most common etiologies of acute encephalitis in previously healthy children diagnostic evaluation and treatment with a neurosurgical perspective.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Inflammation of cerebral parenchyma (or Encephalitis) is a relatively rare disease associated with a high mortality rate if left untreated. The introduction of antiviral regimens against herpes simplex virus (HSV) such as acyclovir, the increase in the number of immunosuppressed patients, the implementation of widespread vaccination for diseases such as mumps have caused significant alterations in the epidemiological landscape and the management of patients with encephalitis [1, 2]. The risk for encephalitis development is probably higher in the pediatric age group, while different pathogens are observed among different age groups. Currently, new types of auto-immune encephalitis such as Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis have been recognized. In this chapter, we discuss epidemiological, clinical, imaging, and treatment features of pediatric encephalitis and the association of encephalitis with common neurosurgical conditions [3, 4].
2 Epidemiology
The epidemiology of encephalitis in the pediatric population is not well described due to a lack of prospective studies in the literature. An incidence ranging from 2–10/100.000 cases has been reported, while the incidence is probably higher in infants. The mean age of children with encephalitis is approximately 6 years. No significant sex predilection has been observed [5, 6]. The implementation of vaccination programs against viruses such as measles, rubella, polio, and mumps has eliminated the encephalitis associated with these pathogens, which consisted a significant cause in the past [7]. Despite that, these diseases should be suspected in unvaccinated children with symptoms of encephalitis. Moreover, the spread of Human Immunodeficiency Virus (HIV) and the use of immunosuppressive or chemotherapeutic regimens in patients receiving transplantation or in patients with cancer respectively have caused a significant increase in the number of immunosuppressive patients . These patients are at high risk of encephalitis from herpesviruses (HSV1–2, CMV, EBV, HHV 6–7) [8, 9] Epidemic outbreaks (local or national) of encephalitis are usually associated with infections from arboviruses or enteroviruses [10, 11]. The seasonal distribution of encephalitis is not sufficiently studied. However, some authors suggest the use of oseltamivir as a part of the initial empirical regimen in patients with encephalitis during periods of seasonal (winter months) flu outbreak [12].
3 Aetiology
The complete understanding of pediatric encephalitis etiology remains unclear, with viral agents being the responsible pathogen in approximately 60–80% of the cases. Table 47.1 summarizes the main causes of encephalitis in children and their treatment. The cause of encephalitis is not identified in more than one-third of cases of encephalitis despite extensive laboratory testing [5, 6]. Contrary to adult encephalitis where HSV-1 is the leading cause, a wider spectrum of pathogens is involved in children. Despite that, HSV-1 remains a remarkable cause and accounts for 5–15% of pediatric encephalitis cases. The leading pathogens of pediatric encephalitis are Varicella-Zoster Virus (VZV), respiratory viruses and enteroviruses, which account for 20% of encephalitis cases each. Other viruses associated with encephalitis are adenoviruses, Cytomegalovirus (CMV), Human herpesvirus (HHV) 6–7, Epstein–Barr virus (EBV), with immunosuppressed patients being at greater risk for developing encephalitis from these viruses [6]. Arboviruses such as West-Nile Virus (WNV) and Japanese Encephalitis Virus (JEV) are other causes of viral encephalitis and are associated with epidemic outbreaks [13, 14]. Measles, mumps, rubella, and chickenpox should be considered a cause of encephalitis in unvaccinated children.
Bacterial encephalitis accounts for 10–30% of pediatric encephalitis cases and may affect solely the cerebral parenchyma or occur as meningoencephalitis. Mycobacterium tuberculosis (M. tuberculosis), Mycoplasma pneumoniae (M. pneumoniae), and Listeria monocytogenes (L. monocytogenes) are the leading causes of bacterial encephalitis in children . Nonetheless, a wide spectrum of bacteria have been reported as a cause of encephalitis including Streptococcus pneumoniae (S. pneumoniae), Neisseria meningitidis (N. meningitidis), Borrelia burgdorferi (B. burgdorferi), Bartonella quintana (B. quintana) [6, 15]. An increased incidence of encephalitis associated with Chlamydia pneumoniae (C. pneumoniae) has been reported in recent years [16]. Fungi and parasites are known causes of encephalitis in immunosuppressed patients, but in pediatric series, very few reports of such cases exist. Common pathogens included in this category are Toxoplasma gondii (T. gondii) and Cryptococcus neoformans (C. neoformans) [17]. Naegleria fowleri encephalitis should be suspected when the patient has a current history of swimming in lakes and is associated with a high mortality rate [18].
Although infective encephalitis is the most common form of encephalitis, many autoimmune causes of encephalitis have also been identified [19]. ADEM is probably the most common form of non-infective encephalitis. It is a demyelinating disease that shares clinical and imaging features with Multiple Sclerosis (MS) and is a type of anti-MOG associated encephalomyelitis. ADEM typically occurs after a viral infection (e.g. VZV, EBV, CMV, Enteroviruses, COVID-19) or very rarely following vaccinations and bacterial infections (e.g. M. pneumoniae) [4, 20, 21]. Anti-NMDAR encephalitis is another typical encephalitis that occurs in children and may account for 30–50% of all autoimmune encephalitis cases. Auto-antibodies against the GluN1 subunit of NMDAR are detected and it has been associated with HSV-1 infection. Moreover, it is associated with the existence of ovarian teratoma in approximately 30–50% of the cases in women [19, 22, 23]. Other antibodies that are associated with encephalitis are anti- Gamma Amino Butyric Acid (GABA) Receptors, anti-glutamic acid decarboxylase (GAD) in limbic encephalitis, and voltage-gated potassium channel (VGKC) –protein complex antibodies. Despite that, 60% of autoimmune encephalitis are seronegative and different criteria have been proposed for their diagnosis [5, 19, 24, 25]. Rasmussen encephalitis is another possibly immune-mediated encephalitis associated with chronic seizures in children. This encephalitis in particular is of neurosurgical interest because the definite treatment is functional hemispherectomy [26].
4 Clinical Manifestation
Approximately 60% of patients with encephalitis usually have a prodrome period of flu or diarrheal syndrome . The prominent clinical feature of encephalitis is the occurrence of encephalopathy (altered consciousness or behavioral changes) together with fever. Focal deficits, hemiparesis, and seizures may typically be observed in patients with encephalitis [5, 6]. Meningism (headache, neck stiffness, photophobia) is suggestive of meninges involvement and thus of meningoencephalitis usually associated with bacterial infections. Clues that facilitate in the differential diagnosis from other Central Nervous System pathologies such as tumors include the onset of acute symptoms, the presentation of fever, a recent history of viral infection, or recent vaccination (in ADEM). Limbic encephalitis is usually immune-mediated or paraneoplastic and affects structures of the limbic system (e.g. temporal lobe, amygdala, hippocampus), and patients usually occur with loss of short-term memory, seizures, confusion, hallucination, or other psychiatric disturbances. Rasmussen encephalitis is an exceedingly rare type of encephalitis that typically affects one cerebral hemisphere of children and occurs with seizures and loss of functions of the affected hemisphere (e.g. hemiparesis, hemianopia, cognitive impairment) [26]. The onset of hydrocephalus in encephalitis without the involvement of meninges is very uncommon. In the majority of cases, the hydrocephalus concerns patients who have bacterial meningoencephalitis and is treated with an external or ventriculoperitoneal (VP) shunt [27].
5 Diagnosis
Encephalitis is generally defined as the onset of encephalopathy of at least one-day duration, as well as exclusion of other pathologies that could explain the patient’s clinical picture, with additionally two or more of the following criteria [28, 29]:
-
Fever
-
CSF pleocytosis (more than 4–5 white cells per mm3).
-
Seizures or neurological deficits that cannot be attributed to other conditions.
-
Imaging findings suggestive of encephalitis.
-
Electroencephalography (EEG) findings suggestive of encephalitis (slow waves-high amplitude).
Features from the patient’s history that may assist in the encephalitis diagnosis is a recent history of flu or gastroenteritis, bacterial infections associated with encephalitis, recent vaccinations , known local epidemic waves of viruses related to encephalitis (e.g. WNV, JEV), and the existence of any known immunodeficiency (e.g. HIV infection). However, as previously discussed, the cause of encephalitis will not be identified in approximately half of the patients.
CSF puncture is the most important initial test that should be performed in any patient with a suspicion of encephalitis. Nevertheless, it is important to exclude any possible signs of raised intracranial pressure (ICP) prior to the procedure due to the risk of brain herniation. Clinical features that raise a concern for elevated ICP is papilledema, seizures, and decreased level of consciousness. In these patients, a Commutated Tomography (CT) scan should be performed to evaluate ICP before the lumbar puncture. CSF findings that suggest encephalitis are pleocytosis (more than 5 cells per mm3) with most of them being lymphocytes. Glucose and protein levels are usually within the normal range in contrast to cases of bacterial meningitis where glucose levels are low and protein levels are increased [30]. Despite that, low glucose levels and increased protein levels are observed in bacterial, fungal, or protozoal encephalitis (or meningoencephalitis). Polymerase Chain Reaction (PCR) of CSF is performed in all cases with suspicion of encephalitis to detect HSV1–2, VZV, HIV, CMV, HHV 6–7, (Para) Influenza, and Enteroviruses in accordance with the patient’s history and the CSF is usually sent for bacterial and fungi cultures. It is important for the clinicians to note that the PCR analysis of CSF may be negative in the first 2 days of the infection. Thus, a single negative result does not exclude an infection . Despite that, the sensitivity of the test is very high after the second day even with the administration of an empirical antiviral regimen [6, 29]. Additionally, blood, throat, and nasopharyngeal samples are collected for culture, biochemical, serology, and PCR analysis for the common pathogens of encephalitis. Serology is commonly utilized to detect an increase in the title of IgM antibodies, or a new increase in the title of IgG antibodies which may facilitate the diagnosis. Stool samples may be collected to detect enteroviruses [5, 6].
Clinically, the differential diagnosis between autoimmune and infectious encephalitis is challenging and cannot be based on the patient’s clinical presentation. In both types of encephalitis, fever and a prodrome period of flu-like symptoms may be observed, but in autoimmune encephalitis, the fever is usually developed later in the progression of the disease. In autoimmune encephalitis, and especially in encephalitis associated with NMDAR, psychiatric symptoms and behavioral alteration are more prominent than in infective encephalitis, while in encephalitis associated with anti-GABA Receptors (GABA-R) seizures are usually the prominent feature. Auto-immune encephalitis is very rare in immunosuppressed individuals [31]. The typical serological workup for auto-immune encephalitis in children includes autoantibodies test for NMDAR, GABA-R, VGKC–protein complex, GAD, MOG (in ADEM), and Leucine-rich glioma inactivated 1 (LGI-1). Despite that, approximately 30–50% of autoimmune encephalitis will be seronegative and their diagnosis is mainly guided by the exclusion of any other known causes of acute encephalitis [19].
6 Imaging
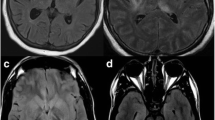
Although neuroimaging is typically not diagnostic of encephalitis, it is the most important examination to exclude other intracranial or other CNS pathologies. At the time of admission, a brain CT scan will be performed in the majority of patients to evaluate a possible contradiction for lumbar puncture (increased ICP or herniation) [30]. Additionally, in the initial CT, the presence of a hemorrhagic stroke or space-occupying lesions (e.g. abscesses, neoplasms) can be evaluated. Brain Magnetic Resonance Imaging (MRI) is the neuroimaging examination of choice to evaluate a patient with suspected encephalitis. Generally, encephalitis’ lesions display high signal in T-2 weighted images and restricted diffusion in Diffusion-Weighted Images (DWI). In HSV-1 the middle temporal lobes, insular cortexes, inferolateral frontal lobes, and limbic system structures are usually affected bilaterally, while HSV-2 affects diffusely the brain parenchyma. In children, extra limbic lesions are not uncommon. The main differential diagnoses that should be considered are low-grade gliomas, gliomatosis cerebri, and limbic encephalitis (unusual in children). In VZV, the cerebral cortex, the cerebellum, and basal ganglia are usually affected, while areas with hemorrhages may be observed [32].
With the exception of ADEM and probably limbic encephalitis, most types of autoimmune encephalitis have no specific imaging findings, and in many cases the initial brain MRI does not display any pathological feature. In ADEM the prominent feature is the presentation of tumefactive demyelinating lesions with high signal in T2-WI bilaterally in the brain and the spinal cord. These lesions display little to no mass effect even though many of them can be large in size [33]. After Gadolinium (Gd) administration, a ring enhancement of the lesion may be observed in T1-WI. Differential diagnoses for ADEM include MS (dissemination in space and time), Hurst disease, lymphomas, high-grade gliomas (Anaplastic astrocytoma and Glioblastoma). The absence of mass effect and the prominent involvement of the white matter are the key imaging features that assist in the differential diagnosis from brain neoplasms [34]. NMDAR associated encephalitis usually appears with no lesions in MRI in the initial imaging, and if present the findings are atypical and presented as areas with high signal in T2-WI [35].
7 Treatment
At the time of the admission, the pathogen of encephalitis is usually unknown and an empirical regimen is administrated against the pathogens of encephalitis associated with a high mortality rate (HSV 1–2) and against the common pathogens of bacterial meningitis (N. meningitides, S. pneumoniae, H. influenza), especially in cases where the patient presents symptoms of meningism. Infection from HSV-1 and HSV-2 is highly lethal with approximately 80% mortality rate if left untreated, and thus it is of crucial significance to cover the patients with an anti-viral regimen as early as possible [30].
Ideally, a lumbar puncture should be performed immediately after the clinical suspicion of encephalitis , but if the lumbar puncture is delayed more than 6 h a treatment regimen that includes acyclovir should be administrated. The anti-viral regimen of choice for HSV and VZV encephalitis is intravenous (iv) acyclovir. Oseltamivir may be co-administrated in the flu seasons. The UK protocol of pediatric viral encephalitis management suggests the use of iv acyclovir for 2 weeks (or 3 weeks in immunosuppressed), and evaluation of CSF with PCR at the end of treatment. If the virus is still detected in the CSF, acyclovir should be continued for one week . The circle of weekly acyclovir treatment is continued until HSV/VZV is not detected in CSF [30, 36]. If evidence of meningism is present, ceftriaxone is administrated to cover the bacterial causes of meningitis. Moreover, if the patient’s history set a suspicion of other causes of encephalitis such as recent pneumonia with M. pneumoniae, consumptions of contaminated products (eg. infection from L. monocytogenes), a recent tick (eg. Lyme disease) or tuberculosis, the empirical regimen should be modified with the addition of appropriate antibiotics for each pathogen [5, 29]. An anti-fungal and anti-protozoal regimen may be considered in patients with HIV infection if low glucose and high protein levels are present in CSF. A specific protocol for the treatment of auto-immune pediatric encephalitis in children has not been established. In the majority of the cases, corticosteroids are the first-line treatment. Alternative treatments include the use of intravenous immunoglobin and plasma exchange. In women with NMDAR encephalitis associated with a teratoma, removal of the teratoma can lead to a full recovery in approximately 60–70% of the patients [19].
Neurosurgical intervention is usually not required in patients with infectious encephalitis. A call for neurosurgical evaluation is required in cases of hydrocephalus development. In these cases, a VP or external CSF shunt may be required. Risk factors for the onset of hydrocephalus development include cases of mumps encephalitis, bacterial meningoencephalitis, signs and symptoms of meningism, and recurrent seizures (especially status epilepticus). An additional role of the neurosurgeon in cases of encephalitis used to be to obtain a brain biopsy in cases where the diagnosis cannot be established with the conventional laboratory and imaging methods. However, the introduction of PCR in clinical practice has eliminated the need for a brain biopsy in encephalitis diagnosis [5]. Finally, in the case of Rasmussen encephalitis which does not respond to conservative treatment with corticosteroids, plasmapheresis or iv immunoglobulin, a functional hemispherectomy may be needed to control the seizures and to improve patients’ quality of life [26, 35].
8 Prognosis
Despite the application of novel treatment protocols and the use of acyclovir, the mortality rate of infectious encephalitis remains high and ranges from 10–30%. The younger age of affected children and infection from HSV1 and HSV-2 are possibly the most significant risk factors for survival. Severe neurological sequelae such as seizures, truncal ataxia , hemiparesis, behavioral disorders, and quadriplegia may be observed in 10–20% of children [6]. In ADEM and encephalitis associated with NMDAR antibodies, the mortality rate is lower (5–15%). Nevertheless, the morbidity remains high, and moderate to severe neurological impairments are observed in approximately 20% of children after the treatment [37,38,39].
References
Granerod J, Crowcroft NS. The epidemiology of acute encephalitis. Neuropsychol Rehabil. 2007 Aug–Oct;17(4–5):406–28. https://doi.org/10.1080/09602010600989620.
Venkatesan A. Epidemiology and outcomes of acute encephalitis. Curr Opin Neurol. June 2015;28(3):277–82. https://doi.org/10.1097/WCO.0000000000000199.
Barbagallo M, Vitaliti G, Pavone P, Romano C, Lubrano R, Falsaperla R. Pediatric autoimmune encephalitis. J Pediatr Neurosci. 2017;12(2):130–4. https://doi.org/10.4103/jpn.JPN_185_16.
Lee YJ. Acute disseminated encephalomyelitis in children: differential diagnosis from multiple sclerosis on the basis of clinical course. Korean J Pediatr. 2011;54(6):234–40. https://doi.org/10.3345/kjp.2011.54.6.234.
Thompson C, Kneen R, Riordan A, Kelly D, Pollard AJ. Encephalitis in children. Arch Dis Child. 2012 Feb;97(2):150–61. https://doi.org/10.1136/archdischild-2011-300100.
Galanakis E, Tzoufi M, Katragkou A, Nakou I, Roilides E. A prospective multicenter study of childhood encephalitis in Greece. Pediatr Infect Dis J. 2009 Aug;28(8):740–2. https://doi.org/10.1097/INF.0b013e318199eff9.
Koskiniemi M, Vaheri A. Effect of measles, mumps, rubella vaccination on pattern of encephalitis in children. Lancet. 1989 Jan 7;1(8628):31–4. https://doi.org/10.1016/s0140-6736(89)91683-8.
Saylor D, Thakur K, Venkatesan A. Acute encephalitis in the immunocompromised individual. Curr Opin Infect Dis. 2015 Aug;28(4):330–6. https://doi.org/10.1097/QCO.0000000000000175.
Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS, UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–44. https://doi.org/10.1016/S1473-3099(10)70222-X. Erratum in: Lancet Infect Dis. 2011 Feb; 11(2): 79
Hollidge BS, González-Scarano F, Soldan SS. Arboviral encephalitides: transmission, emergence, and pathogenesis. J Neuroimmune Pharmacol. 2010;5(3):428–42. https://doi.org/10.1007/s11481-010-9234-7.
Jain S, Patel B, Bhatt GC. Enteroviral encephalitis in children: clinical features, pathophysiology, and treatment advances. Pathog Glob Health. 2014;108(5):216–22. https://doi.org/10.1179/2047773214Y.0000000145.
Mastrolia MV, Rubino C, Resti M, et al. Characteristics and outcome of influenza-associated encephalopathy/encephalitis among children in a tertiary pediatric hospital in Italy, 2017–2019. BMC Infect Dis. 2019;19:1012. https://doi.org/10.1186/s12879-019-4636-5.
Debiasi RL, Tyler KL. West Nile virus meningoencephalitis. Nat Clin Pract Neurol. 2006;2(5):264–75. https://doi.org/10.1038/ncpneuro0176.
Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in Southeast Asia. J Virol. 2003;77(5):3091–8. https://doi.org/10.1128/jvi.77.5.3091-3098.2003.
Elenga N, Roux A, Cuadro-Alvarez E, Martin E, Kallel H, Defo A. Etiology and prognosis of encephalitis in French Guianese children: a retrospective record-based study. J Infect Public Health. 2020 Jul;13(7):1051–3. https://doi.org/10.1016/j.jiph.2020.01.315.
Airas L, Kotilainen P, Vainionpää R. Encephalitis associated with Chlamydia pneumoniae. J Marttila Neurol. 2001;56(12):1778–9. https://doi.org/10.1212/WNL.56.12.1778.
Chang L, Lim BCW, Flaherty GT, Torresi J. Travel vaccination recommendations and infection risk in HIV-positive travellers. J Travel Med. 2019 Sep 2;26(6):taz034. https://doi.org/10.1093/jtm/taz034.
Grace E, Asbill S, Virga K. Naegleria fowleri: pathogenesis, diagnosis, and treatment options. Antimicrob Agents Chemother. 2015;59(11):6677–81. https://doi.org/10.1128/AAC.01293-15.
Dale RC, Gorman MP, Lim M. Autoimmune encephalitis in children: clinical phenomenology, therapeutics, and emerging challenges. Curr Opin Neurol. 2017 Jun;30(3):334–44. https://doi.org/10.1097/WCO.0000000000000443.
Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12(1):1–13. https://doi.org/10.3988/jcn.2016.12.1.1.
Tsiodras S, Kelesidis T, Kelesidis I, Voumbourakis K, Giamarellou H. Mycoplasma pneumoniae-associated myelitis: a comprehensive review. Eur J Neurol. 2006 Feb;13(2):112–24. https://doi.org/10.1111/j.1468-1331.2006.01174.x.
Sai Y, Zhang X, Feng M, Tang J, Liao H, Tan L. Clinical diagnosis and treatment of pediatric anti-N-methyl-D-aspartate receptor encephalitis: a single center retrospective study. Exp Ther Med. 2018;16(2):1442–8. https://doi.org/10.3892/etm.2018.6329.
Peery HE, Day GS, Doja A, Xia C, Fritzler MJ, Foster WG. Anti-NMDA receptor encephalitis in children: the disorder, its diagnosis, and treatment. Handb Clin Neurol. 2013;112:1229–33. https://doi.org/10.1016/B978-0-444-52910-7.00045-3.
Höftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81(17):1500–6. https://doi.org/10.1212/WNL.0b013e3182a9585f.
Misawa T, Mizusawa H. Anti-VGKC antibody-associated limbic encephalitis/Morvan syndrome. Brain Nerve. 2010;62(4):339–45. Japanese
Varadkar S, Bien CG, Kruse CA, et al. Rasmussen's encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13(2):195–205. https://doi.org/10.1016/S1474-4422(13)70260-6.
Kumar G, Kalita J, Misra UK. Raised intracranial pressure in acute viral encephalitis. Clin Neurol Neurosurg. 2009 Jun;111(5):399–406. https://doi.org/10.1016/j.clineuro.2009.03.004.
Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–44.
Ellul M, Solomon T. Acute encephalitis – diagnosis and management. Clin Med (Lond). 2018 Mar;18(2):155–9. https://doi.org/10.7861/clinmedicine.18-2-155.
Kirkham FJ. Guidelines for the management of encephalitis in children. Dev Med Child Neurol. 2013 Feb;55(2):107–10. https://doi.org/10.1111/j.1469-8749.2012.04410.x.
Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27(3):361–8. https://doi.org/10.1097/WCO.0000000000000087.
Jayaraman K, Rangasami R, Chandrasekharan A. Magnetic resonance imaging findings in viral encephalitis: a pictorial essay. J Neurosci Rural Pract. 2018;9:556–60.
Rossi A. Imaging of acute disseminated encephalomyelitis. Neuroimaging Clin N Am. 2008;18(1):149–61.; ix. https://doi.org/10.1016/j.nic.2007.12.007.
Mordekar SR, et al. Glioblastoma Multiforme incorrectly diagnosed as ADEM in children. J Pediatr Neurol. 2015;6(1):053–6.
Zhang T, Duan Y, Ye J, Xu W, Shu N, Wang C, Li K, Liu Y. Brain MRI characteristics of patients with anti-N-methyl-D-aspartate receptor encephalitis and their associations with 2-year clinical outcome. Am J Neuroradiol. 2018;39(5):824–9. https://doi.org/10.3174/ajnr.A5593.
Kneen R, Michael BD, Menson E, Mehta B, Easton A, Hemingway C, Klapper PE, Vincent A, Lim M, Carrol E, Solomon T, National Encephalitis Guidelines Development and Stakeholder Groups. Management of suspected viral encephalitis in children – Association of British Neurologists and British Paediatric Allergy, Immunology and Infection Group national guidelines. J Infect. 2012 May;64(5):449–77. https://doi.org/10.1016/j.jinf.2011.11.013.
Cole J, Evans E, Mwangi M, Mar S. Acute disseminated encephalomyelitis in children: an updated review based on current diagnostic criteria. Pediatr Neurol. 2019 Nov;100:26–34. https://doi.org/10.1016/j.pediatrneurol.2019.06.017.
Barry H, Byrne S, Barrett E, Murphy KC, Cotter DR. Anti-N-methyl-d-aspartate receptor encephalitis: review of clinical presentation, diagnosis and treatment. BJPsych Bull. 2015;39(1):19–23. https://doi.org/10.1192/pb.bp.113.045518.
Wang H, Xiao Z. Current Progress on assessing the prognosis for anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. Biomed Res Int. 2020;2020:7506590. Published 2020 Apr 14. https://doi.org/10.1155/2020/7506590.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lampros, M., Alexiou, G., Prodromou, N. (2022). Encephalitis. In: Alexiou, G., Prodromou, N. (eds) Pediatric Neurosurgery for Clinicians. Springer, Cham. https://doi.org/10.1007/978-3-030-80522-7_47
Download citation
DOI: https://doi.org/10.1007/978-3-030-80522-7_47
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80521-0
Online ISBN: 978-3-030-80522-7
eBook Packages: MedicineMedicine (R0)