Abstract
Brain-connectivity refers to a pattern of functional or effective connectivity of distinct modules of human brain due to interactions between them. In this paper, the authors have attempted to conduct a brain connectivity based analysis to study the brain circuitry in subjects from their electroencephalographic (EEG) data, while they are engaged in playing a horror video game. The main motive of our work is to understand the differences in the effective connectivity among phasmophobic and non-phasmophobic subjects. In the present analysis, we propose a modified version of the causality test, named as Convergent Cross Mapping (CCM) to perform the analysis. The proposed CCM improves the performance of the standard CCM with an added feature of finding the possible direction of causation in terms of conditional entropy or maximum information transfer among the brain signal-sources. Experimental results and statistical analysis show that the proposed method shows superior efficacy in estimating the directed brain-connectivity as compared to the very well-known classical Granger Causality, classical CCM and other off-the-shelf brain-connectivity algorithms.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Phasmophobia is a psychological condition where people experience irrational fear of supernatural things. Many people undergo fear of supernatural things from a young age. The onset age of any specific phobia depends on the type of phobia, any traumatic situation in the past or genetic tendency of the person [8]. For some people, mild development of phobia at their young age might disappear as they move into adolescence, but for others the fears and the anxieties persist and the severity of phobia increases gradually. It may even worsen into a chronic and potentially lead to several other forms of severe psychological disorders like trauma, intense anxiety, panic attacks, debilitating enough for a diagnosis. In [26], the authors demonstrate overall clinical and psychological case study of six patients having phobia of supernaturals. According to the study, the patients were diagnosed with persistent fear of supernatural things, intense anxiety, panic attacks, developing other types of phobia like nyctophobia (fear of darkness) or autophobia (fear of being alone), insomnia, dementia, and mild epilepsy. Although there exist plenty of literature [12, 14, 27, 36], which involve research outcomes of psychologists, who investigated different types of phobia and attempted to provide solutions for the diagnosis and prognosis of such phobias, knowledge based on the analysis of brain connectivity measures in specific phobia is still limited. The present paper fills the void by aiming at investigating the brain dynamics of phasmophobic and non-phasmophobic subjects with respect to the functional connectivity of the brain activation region while playing a horror video game.
In the past few years, a considerable amount of works [9, 19, 22] have been done focusing on functional neuro-imaging in specific phobia, which has revealed new perspectives into the neuro-biological mechanism underlying the fear response when exposed to a phobic stimulus. According to the existing literature [2, 3], neural analysis underpinning specific phobia are mostly based on animal subtype, particularly snake or spider phobia subjects. These studies show evidence of hyper-activation in the brain region involved in fear evaluation structures, including the amygdala, dorsal anterior cingulate cortex (ACC), thalamus and insula. The comparative study [15, 20] based on neural response analysis reveals that there exist two subtypes of specific phobia, named as animal subtype and blood-injection-injury (BII) sub-type. Elevated BOLD responses are observed in insula, thalamus, cingulate gyrus, supplementary motor area, superior frontal gyrus, parietal cortex, superior temporal gyrus and the cerebellum in case of snake phobia (animal subtype) whereas in dental phobia (BII subtype) only prefrontal and orbitofrontal cortex activates. The literature, [6, 10] enhances the understanding of neural connectivity mechanisms of social phobia and panic disorder by analysing brain activation regions through emotional face perception. Functional connectivity analysis of patients suffering from agoraphobia (fear of situation/places) [18] shows decrease in inferior frontal gyrus activity at advanced stage with depression and panic disorder: altered prefrontal cortex connectivity and intensifying connectivity in fear producing regions, low connectivity in two brain networks in patients with sub-clinical agoraphobia [16].
Additionally, there exists literature [2, 3, 6, 41], which includes fMRI and PET studies investigating functional connectivity of the whole brain for phobic patients. The fMRI studies reveal that there is a significant increase in cortical thickness in the insula region, and increase or decrease in cortical thickness in ACC region of phobic patients. It is also found that the volume depletion in the amygdala may occur for the patients with animal phobia, which can in turn cause severe vomiting, diarrhea, kidney failure, etc. The PET study in [1] presents the neural correlates of phobic patients which shows that para-hippocampal memory processes are influenced by amygdala-related arousal.
However, there exist only a handful of studies assessing functional connectivity of specific phobia using functional Near Infrared Spectroscopy (fNIRS) and electroencephalography (EEG). The study [11] using fNIRS shows that the activation of left inferior frontal gyrus (IFG) decreases over time in response to the semantic stimuli during phobia-relevant emotional Stroop paradigm. The analysis [33] of in-situ cortical blood oxygenation using fNIRS of arachnophobic (extreme fear of spiders) patient shows promising results for the application of the technique in the field of psychotherapy. In [33], the authors identified the severity of acrophobia using EEG. The literature [32] utilizes both the fNIRS and EEG devices to study the neural correlates for arachnophobic patients. Although there exist numerous research works based on various types of phobia but understanding the brain functional connectivity changes in phasmophobic patient is a new research era.
The novelty of the present study lies in understanding the difference in brain functional connectivity of phasmophobic and non-phasmophobic subjects from their EEG signals by applying a modified version of Convergent cross mapping (CCM) technique [35]. CCM technique has the ability to overcome the limitations provided by Granger Causality [13, 17]. CCM is a statistical test of detecting the causal relationships among the time-series data (here, brain signals) of a uni-directionally connected chaotic system (here, brain) and thus provides a new era of research in brain-connectivity analysis.
Our approach is to experimentally determine the information transfer among brain regions, participating in a specific cognitive task (here, playing horror game) and to detect the causal dependence of the time-varying signals collected from multiple brain lobes. The source signals can be acquired by means of a brain-rhythm capturing device. EEG consists of several number of metal electrodes which can be mounted over the scalp of the subject to capture the source signals. Determining functional brain connectivity from the EEG data is a challenging problem as they have wide variations within and across experimental sessions. Although there exist a few interesting approaches like transfer entropy [39], Probabilistic Relative Correlation Adjacency Matrix (PRCAM) [17], several forms of Granger Causality analysis [13, 17, 25, 31], none of these shows promising results in determining the causal relationships between the pairs of active EEG electrodes [4].
CCM can fortunately provide the solutions to the drawbacks experienced by the existing techniques and thus successfully has been utilized to understand the causal dependence of the time-series data [5, 21, 24]. Thereby, we undertake an extended version of classical CCM to test inter-connectivity between pairs of brain modules. In this paper, the possible causal connectivity from brain region X to Y is checked by the following 2 steps. First, the acquired EEG time-series signals, acquired from the brain regions X and Y are checked for the causality by employing the classical CCM technique and then the direction of causation between the observation variables (here, EEG signals) has been inferred by calculating the amount of information transfer among them using conditional entropy of probability distributions of the residuals of CCM output.
The paper is divided into 4 sections. In Sect. 2, the principle of classical CCM and its extension in brain-connectivity analysis is introduced. Section 3 provides a framework for the experimental protocol design along with results and interpretations. Performance analysis and statistical validation are also undertaken in this section. Conclusions are listed in Sect. 4.
2 Principles and Methodologies
This section provides a brief outline about the existing classical CCM algorithm and the proposed extension to determine the brain-connectivity from the acquired EEG signals for a given cognitive task.
2.1 Classical CCM
CCM [35] is a causality-detection algorithm, which can be effectively utilized to obtain the causal relationship between two time-series variables, say X and Y. The principle, that CCM uses, includes reconstruction of system states from X and Y using specific values of a few pre-determined parameters and then to quantify the relationship values between them using a nearest neighbor algorithm.
The mathematical basis of CCM algorithm are constituted using the concept of Takens’ Theorem [37], which is one of the very well-known time-delay embedding theorems. In [37], Taken states that “an attractor manifold can be reconstructed from a set of observation variables of a dynamical system”. Let, X(t) and Y(t) be the two discrete time-varying signals of a dynamical system (here, brain as shown in Fig. 1), that are sharing a common attractor manifold, denoted by M. Following the Taken’s principle, one can easily reconstruct the D-dimensional shadow manifolds, denoted by \({M_{X}}\) and \({M_{Y}} \)for the temporal signals X(t) and Y(t) respectively, with the time delay \(\tau \) between the successive steps. Numerically, one can represent the time-points on the shadow manifold \({M_{X}}\) as: \({\underline{x}(t)} = <{X(t), X(t-\tau ), X(t-2\tau ), ..., X(t-(D-1)\tau )}>\), where D is the embedding dimension. The optimal values of the parameters D and \(\tau \) can be obtained using Simplex Projection technique [36]. It is followed from the Taken’s theorem that, the time-points on the shadow manifold \({M_{X}}\) have one-to-one correspondence to that of the original manifold M [35]. In an alternate way, it can be said that \({M_{X}}\) is diffeomorphic reconstruction of M, as shown in Fig. 2. The other shadow manifold \({M_{Y}}\), which is also diffeomorphic to M can also be easily constructed in similar manner.
CCM actually aims at discovering the degree of correspondence between the local neighbors of \({M_{X}}\) and \({M_{Y}}\) [35], i.e., how well the local neighbors of \({M_{X}}\) correspond to \({M_{Y}}\), as shown in Fig. 2. Therefore, the next step is to find D + 1 nearest neighbors by a suitable nearest-neighbor algorithm. Now let, \({t_{1}}, ..., {t_{D+1}}\) be the time-indices of the D+1 nearest neighbors of \({\underline{x}(t)}\) on \({M_{X}}\). These time-indices can be utilized to construct the cross mapping of \({\hat{Y}(t)}\) using Eq. (1).
where, i belongs to 1 to D+1 and \({w_{i}}\)s are the weights corresponding to the distance between \({\underline{x}(t)}\) and each of the D+1 nearest neighbors.
The weights can be estimated by
where, j = 1, ..., D+1 and \(u_{i}\) is given by
In (3), \(R(\underline{x}(t) , \underline{x}(t_{i}))\) denotes the Euclidean distance between \(\underline{x}(t)\) and \(\underline{x}(t_{i})\). Similarly, \({\hat{X}(t)}|{M_{Y}}\) can be obtained. Next, Pearson’s Correlation Coefficient [29], \(\rho \) (rho), between the estimated (\({\hat{Y}(t)}|{M_{X}}\)) and observed values of Y(t) can be computed using the following transformation.
2.2 Estimating the Direction of Causation Using Conditional Entropy
In the classical CCM algorithm, the causation is unidirectional, i.e., either \({X}\rightarrow {Y}\) or \({Y}\rightarrow {X}\). The implication relation \({X}\rightarrow {Y}\) denotes that textitX can be estimated from Y, but the converse is not hold. To find the direction of causation one can compare the correlation coefficient values \(\rho _ {x, y}\), obtained between X and \(\hat{X}|M_{Y}\), and \(\rho _ {y, x}\), obtained between Y and \(\hat{Y}|M_{X}\). If \(\rho _ {x, y}\) is greater than \(\rho _ {y, x}\), then one may conclude that \(\textit{X}\rightarrow \textit{Y}\). However, this is a contradiction of Granger’s Intuitive Scheme [35].
One approach to find the direction of causation is to compare the probability distribution of the euclidean distance between manifold \(M_X\) and manifold \(M_{\hat{X}}|M_{Y}\) and the same between manifold \(M_Y\) and manifold \(M_{\hat{Y}}|M_{X}\). Let, \(R_x\) be the euclidean distance between \(M_X\) and \(M_{\hat{X}}|M_{Y}\), and \(R_y\) be the euclidean distance between \(M_Y\) and \(M_{\hat{Y}}|M_{X}\). It is observed from Fig. 3 that the probability distributions of \(R_x\) and \(R_y\) has negligible difference. A suitable way to enhance the inference of the direction of causality is to determine the amount of information transfer among the probability distributions of \(R_x\) and \(R_y\) [30] by computing Conditional Entropy of and combine them with the corresponding correlation coefficients \(\rho _ {x, y}\) and \(\rho _ {y, x}\).
Let \(P(R_x)\) and \(P(R_y)\) be the probability distribution function of \(R_x\) and \(R_y\). The Conditional entropy of \(R_y\) for given \(R_x\) is determined as
The entropy of \(R_y\) conditioned on \(R_x\) actually measures the uncertainty in \(R_y\) given \(R_x\). Similarly, \(H(R_x|R_y)\) is computed. Then the mutual dependence measures between the two variables, denoted by \(C_{X|Y}\) and \(C_{Y|X}\) are derived by
These are the measures of Information Quality Ratio (IQR), which quantifies the amount of information transferred from one variable to the other against the total uncertainty. The direction of the causality is then determined based on the higher IQR values. Thus, for all possible combinations of m number of EEG electrode-pairs, we obtain \(m^2\) IQR values, which are represented in a matrix form. We name the matrix as W\(_{ECM}\) which has the dimension \(m\times m\). The entire experimental workflow is illustrated in Fig. 4.
2.3 Classification Using Kernelized Support Vector Machine
To understand the performance of the proposed brain-connectivity algorithm, the outputs obtained from the proposed CCM are treated as features and fed to a classifier. To classify the two classes: phasmophobic and non-phasmophobics from the EEG responses of the subjects, W\(_{ECM}\) are first reshaped to form \((1 \times m^{2}\)) vector and then used as features to train and test a kernelized Support Vector Machine (KSVM) [7] classifier with polynomial kernel function.
3 Experiments and Results
This section describes the experimental results obtained using the principles introduced in Sect. 2 for analysing functional connectivity in phasmophobic and non-phasmophobic subjects.
3.1 Experimental Setup
The experiment has been performed in the Artificial Intelligence Laboratory of Jadavpur University, Kolkata, India. A 14-channel EMOTIV EPOC+ EEG device is used to capture the electrical response of the brain. 24 subjects: 13 phasmophobics and 11 non-phasmophobics, in the age group 15–27 years participated in the said experiment. Subjects are asked to play a horror video game, namely Amnesia: The Dark Descent and during that time, EEG signals were extracted from them. To obtain data with minimum possible noise, the subjects had to relax and concentrate on the screen before the experiment could begin and the experiment was conducted in a dark and quiet room. The game was played on a computer. The 14 electrodes used to acquire EEG data are AF3, F7, F3, FC5, T7, P7, O1, O2, P8, T8, FC6, F4, F8, AF4. Additionally, P3 and P4 electrodes are used as reference electrodes to collect the signals properly. The electrode placement diagram is shown in Fig. 5.
The EMOTIV EPOC+ EEG device has built-in characteristics, which assists us in properly fitting the headset by directing that which EEG channel reflects bad quality signal (indicated by red light) and which one reflects good quality signal (indicated by green light). Here, the term ‘good quality’ emphasizes the signal with less noise and the term ‘bad’ indicates the channel with more noise. To obtain the good quality signal to the maximum extent, subjects are asked to close their eyes for 20 s in a rest position and then to open it, and then again to close their eyes for 10 s. In this manner, we were able to locate and fix the faulty electrodes by tracking the EEG channels. We didn’t start the experiment until every EEG channel was properly fit to the scalp of the subjects and was in good working order. To capture a wide range of variations in the brain responses, the experiment was conducted over 3 days for each subject. Each day, the subjects were asked to play the horror computer game for 10 min. After each trial session, the subjects were asked to rate their level of scariness in a scale of 1–10. Based on this rating we further divided the dataset into 2 clusters to create 2 different categories:
-
1.
rating 0–5 – Non-Phasmophobic
-
2.
rating 6–10 – Phasmophobic
3.2 Data Preprocessing
The EEG signals, thus obtained, are then passed through various signal pre-processing steps to remove the noise or artifacts. The sampling frequency of the recording was set 128 Hz. Sample length of each EEG data is 76,800. The built-in filter is present in the EMOTIV EEG headset device, which is the 5th order sinc filter. The frequency bandwidth of 0.1–40 Hz is set to record the EEG signals in a particular window and this is the first step of preprocessing of the raw signal. EEG signals are contaminated with various forms of artifacts. Three most common forms of artifacts that need special mention include 1) step artifacts, 2) spike artifacts, and 3) physiological artifacts [23]. The step artifacts come into play, when there is a change in the surrounding environment. The step artifact can be removed by minimizing the variation of the external light and instrumental noise. The spike/motion artifacts are related to decoupling between the electrodes and their assigned positions due to head or muscle movement. They result in abrupt changes in the amplitude of the received signals. For example, a sudden change in the ambient light intensity results in a spike-like noise. Another important artifact is physiological artifact, which may include the artifacts due to eye-blinking [34], respiration, heart-beat, blood pressure fluctuations and Mayer wave [40]. To remove most of the EEG artifacts, the raw EEG signal is passed through an Elliptical type bandpass filter of order 10 and frequency band of 0.1–40 Hz.
3.3 Active Brain Region Selection Usings LORETA
In our study, the selection of activated brain regions of the subjects playing horror video games is done using sLORETA [28] software. The experiment is carried out by analyzing and estimating the electrical activity of the intra-cortical distribution from the EEG signal acquired during gameplay using sLORETA software. From the sLORETA solutions, it is found that the prefrontal, frontal, temporal and occipital regions have the greater activation for the phasmophobics as compared to the non-phasmophobics, as indicated in Fig. 6.
3.4 Effective Connectivity Estimation by CCM Algorithm
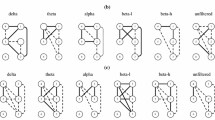
To get the effective functional connectivity between the active brain lobes, first of all we have checked for the causality between the electrodes by CCM method. To do so, the shadow manifold of each electrode is reconstructed by computing the optimum embedding dimension (D) and time delay (\(\tau \)) by simplex projection. For each electrode the computed value of D and \(\tau \) are 2 and 1 respectively. Hence from the reconstructed manifold we check for the cross map skill for every possible combination of electrode pair. Next to find the direction of causation, we have employed the conditional entropy based CCM approach described in Sect. 2. The directed brain-connectivity obtained by the proposed algorithm is depicted in Fig. 7 (a)-(f) for phasmophobic and non-phasmophobic subjects. Among 11 non-phasmophobics (denoted as NP1, NP2,. . . NP11) and 13 phasmophobics (denoted as P1, P2, . . . , P13), the brain-connectivity of only 3 subjects from each group is given in Fig. 7. It is observed from the figure that the phasmophobics have strong connectivity between left frontal and right temporal region, whereas the connections become weaker for non-phasmophobics.
3.5 Statistical Analysis Using One-Way ANOVA Test
We performed ANOVA tests to i) evaluate and determine the effect of stimuli in different brain regions and ii) to test the efficacy of the proposed connectivity algorithm. For the former case, we performed one way ANOVA test for each electrode individually which shows difference in impact of fear stimulus in the brain activation region between phasmophobic and non-phasmophobic subjects. As mentioned above, 13 subjects are phasmophobic and 11 are non- phasmophobic. We compared mean value of all the subjects from two groups (phasmophobic and non-phasmophobic) for each electrode of EEG data using F-distribution, initially considering null hypothesis i.e., two means are equal. The one-way ANOVA test shows statistically significant results, i.e., the probability (p-value) is less than the specific significant level (\(p < 0.05\)), which rejects the null hypothesis. The box-plot of the one-way ANOVA test is depicted in Fig. 8a for each of the occipital, frontal and temporal regions. Here, means of the high activation region are unequal between two groups: phasmophobic and non-phasmophobic. Brain Regions: (Occipital Lobe: O2 (\(p = 0.0009\)), Frontal region: F4 (\(p = 0.02\)), F7 (\(p = 0.007\)), F8 (p = 0.006), FC5 (\(p = 0.007\)), FC6 (\(p = 0.004\)), AF3(\(p = 0.002\))) and Temporal region: T7 (\(p = 0.005\)), T8 (\(p = 0.04\)).
Next, to test the differences in effective connectivity among phasmophobic and non-phasmophobic, obtained by the proposed CCM, again we utilize oneway ANOVA test. The significance level is set at \(p < 0.05\). The result is depicted in Fig. 8b, which emphasizes that there is increased connectivity for phasmophobic as compared to non-phasmophobic.
3.6 Relative Performance Analysis of the Proposed CCM
The proposed CCM algorithm for brain-connectivity analysis is compared here with 5 well-known techniques of functional connectivity analysis. They are Cross-correlation technique [38], probabilistic relative correlation adjacency matrix (PRCAM) [17], Granger Causality [13], standard CCM [4] and Transfer entropy based analysis [39]. The relative performance of the proposed algorithm with the existing algorithms, has been evaluated on the basis of three performance metrics of classifier: classifier accuracy, sensitivity and specificity. The results of the study are given in Table 1. It is apparent from the table that the proposed technique outperforms the existing techniques in all the cases.
4 Conclusion
The paper introduces a novel approach for brain-connectivity analysis for phasmophobic and non-phasmophobic subjects using conditional entropy based CCM technique in EEG-based Brain Computer Interfacing paradigm. The method is advantageous for its inherent potential to detect directional causality in brain-connectivity, which can not be carried out by traditional CCM or Granger causality techniques. The proposed approach has successfully been employed to determine brain-connectivity of phasmophobics and non-phasmophobics. Experiments undertaken reveal that phasmophobics possess brain-connectivity with weaker strength between left frontal and right temporal regions as compared to the non-phasmophobics. Thus the proposed method can easily segregate phasmophobics from their non-phasmophobic counterparts. This technique is useful for diagnosis of the subjects with phobia of supernatural at its early stage, which can in turn, will suggest an early treatment to reduce associated disorders like sleeplessness, anxiety, etc.
References
Åhs, F., et al.: Arousal modulation of memory and amygdala-parahippocampal connectivity: a pet-psychophysiology study in specific phobia. Psychophysiology 48(11), 1463–1469 (2011)
Åhs, F., et al.: Disentangling the web of fear: amygdala reactivity and functional connectivity in spider and snake phobia. Psychiatry Res. Neuroimaging 172(2), 103–108 (2009)
Britton, J.C., Gold, A.L., Deckersbach, T., Rauch, S.L.: Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depression Anxiety 26(9), 796–805 (2009)
Chowdhury, A., Dewan, D., Ghosh, L., Konar, A., Nagar, A.K.: Brain connectivity analysis in color perception problem using convergent cross mapping technique. In: Nagar, A.K., Deep, K., Bansal, J.C., Das, K.N. (eds.) Soft Computing for Problem Solving 2019. AISC, vol. 1138, pp. 287–299. Springer, Singapore (2020). https://doi.org/10.1007/978-981-15-3290-0_22
Clark, A.T., et al.: Spatial convergent cross mapping to detect causal relationships from short time series. Ecology 96(5), 1174–1181 (2015)
Danti, S., Ricciardi, E., Gentili, C., Gobbini, M.I., Pietrini, P., Guazzelli, M.: Is social phobia a “mis-communication’’ disorder? Brain functional connectivity during face perception differs between patients with social phobia and healthy control subjects. Front. Syst. Neurosci. 4, 152 (2010)
Das, S., Halder, A., Bhowmik, P., Chakraborty, A., Konar, A., Nagar, A.: Voice and facial expression based classification of emotion using linear support vector machine. In: 2009 Second International Conference on Developments in eSystems Engineering, pp. 377–384. IEEE (2009)
De Vries, Y.A., et al.: Childhood generalized specific phobia as an early marker of internalizing psychopathology across the lifespan: results from the world mental health surveys. BMC Med. 17(1), 1–11 (2019)
Del Casale, A.: Functional neuroimaging in specific phobia. Psychiatry Res. Neuroimaging 202(3), 181–197 (2012)
Demenescu, L., et al.: Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder. J. Psychiatric Res. 47(8), 1024–1031 (2013)
Deppermann, S., et al.: Functional co-activation within the prefrontal cortex supports the maintenance of behavioural performance in fear-relevant situations before an itbs modulated virtual reality challenge in participants with spider phobia. Behav. Brain Res. 307, 208–217 (2016)
Eaton, W.W., Bienvenu, O.J., Miloyan, B.: Specific phobias. Lancet Psychiatry 5(8), 678–686 (2018)
Granger, C.W.: Investigating causal relations by econometric models and cross-spectral methods. Econometrica J. Econometric Soc. 37, 424–438 (1969)
Grös, D.F., Antony, M.M.: The assessment and treatment of specific phobias: a review. Curr. Psychiatry Rep. 8(4), 298–303 (2006)
Hilbert, K., Evens, R., Maslowski, N.I., Wittchen, H.U., Lueken, U.: Neurostructural correlates of two subtypes of specific phobia: a voxel-based morphometry study. Psychiatry Res. Neuroimaging 231(2), 168–175 (2015)
Indovina, I., Conti, A., Lacquaniti, F., Staab, J.P., Passamonti, L., Toschi, N.: Lower functional connectivity in vestibular-limbic networks in individuals with subclinical agoraphobia. Front. Neurol. 10, 874 (2019)
Kar, R., Konar, A., Chakraborty, A., Nagar, A.K.: Detection of signaling pathways in human brain during arousal of specific emotion. In: 2014 International Joint Conference on Neural Networks (IJCNN), pp. 3950–3957. IEEE (2014)
Kunas, S.L., et al.: The impact of depressive comorbidity on neural plasticity following cognitive-behavioral therapy in panic disorder with agoraphobia. J. Affect. Disord. 245, 451–460 (2019)
Lange, I., et al.: Functional neuroimaging of associative learning and generalization in specific phobia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 89, 275–285 (2019)
Lueken, U., Kruschwitz, J.D., Muehlhan, M., Siegert, J., Hoyer, J., Wittchen, H.U.: How specific is specific phobia? Different neural response patterns in two subtypes of specific phobia. NeuroImage 56(1), 363–372 (2011)
Luo, C., Zheng, X., Zeng, D.: Causal inference in social media using convergent cross mapping. In: 2014 IEEE Joint Intelligence and Security Informatics Conference, pp. 260–263. IEEE (2014)
Linares, I.M., Chags, M.H.N., Machado-de Sousa, J.P., Crippa, J.A.S., Hallak, J.E.C.: Neuroimaging correlates of pharmacological and psychological treatments for specific phobia. CNS Neurol. Disord. Drug Targets (Formerly Curr. Drug Targets-CNS Neurol. Disord.) 13(6), 1021–1025 (2014)
Maulsby, R.L.: Some guidelines for assessment of spikes and sharp waves in EEG tracings. Am. J. EEG Technol. 11(1), 3–16 (1971)
McCracken, J.M., Weigel, R.S.: Convergent cross-mapping and pairwise asymmetric inference. Phys. Rev. E 90(6), 062903 (2014)
Nolte, G., Bai, O., Wheaton, L., Mari, Z., Vorbach, S., Hallett, M.: Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 115(10), 2292–2307 (2004)
de Oliveira-Souza, R.: Phobia of the supernatural: a distinct but poorly recognized specific phobia with an adverse impact on daily living. Front. Psychiatry 9, 590 (2018)
Pachana, N.A., Woodward, R.M., Byrne, G.J.: Treatment of specific phobia in older adults. Clin. Interv. Aging 2(3), 469 (2007)
Pascual-Marqui, R.D., et al.: Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 24(Suppl D), 5–12 (2002)
Pearson, K.: Vii. Note on regression and inheritance in the case of two parents. Proc. R. Soc. London 58(347–352), 240–242 (1895)
Pukenas, K.: An algorithm based on the convergent cross mapping method for the detection of causality in uni-directionally connected chaotic systems. Math. Models Eng. 4(3), 145–150 (2018)
Rathee, D., Cecotti, H., Prasad, G.: Estimation of effective fronto-parietal connectivity during motor imagery using partial granger causality analysis. In: 2016 International Joint Conference on Neural Networks (IJCNN), pp. 2055–2062. IEEE (2016)
Rosenbaum, D., et al.: Neuronal correlates of spider phobia in a combined fNIRS-EEG study. Sci. Rep. 10(1), 1–14 (2020)
Rosenbaum, D., et al.: Cortical oxygenation during exposure therapy-in situ fNIRS measurements in arachnophobia. NeuroImage Clin. 26, 102219 (2020)
Shoker, L., Sanei, S., Latif, M.A.: Removal of eye blinking artifacts from EEG incorporating a new constrained BSS algorithm. In: Processing Workshop Proceedings, 2004 Sensor Array and Multichannel Signal, pp. 177–181. IEEE (2004)
Sugihara, G., et al.: Detecting causality in complex ecosystems. Science 338(6106), 496–500 (2012)
Sugihara, G., May, R.M.: Nonlinear forecasting as a way of distinguishing chaos from measurement error in time series. Nature 344(6268), 734–741 (1990)
Takens, F.: Detecting strange attractors in turbulence. In: Rand, D., Young, L.-S. (eds.) Dynamical Systems and Turbulence, Warwick 1980. LNM, vol. 898, pp. 366–381. Springer, Heidelberg (1981). https://doi.org/10.1007/BFb0091924
Toyama, K., Kimura, M., Tanaka, K.: Cross-correlation analysis of interneuronal connectivity in cat visual cortex. J. Neurophysiol. 46(2), 191–201 (1981)
Vicente, R., Wibral, M., Lindner, M., Pipa, G.: Transfer entropy–a model-free measure of effective connectivity for the neurosciences. J. Comput. Neurosci. 30(1), 45–67 (2011)
Winkler, I., Haufe, S., Tangermann, M.: Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behav. Brain Functions 7(1), 1–15 (2011)
Zilverstand, A., Sorger, B., Sarkheil, P., Goebel, R.: fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front. Behav. Neurosci. 9, 148 (2015)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this paper
Cite this paper
Karmakar, S., Dewan, D., Ghosh, L., Chowdhury, A., Konar, A., Nagar, A.K. (2021). Brain-Connectivity Analysis to Differentiate Phasmophobic and Non-phasmophobic: An EEG Study. In: Papież, B.W., Yaqub, M., Jiao, J., Namburete, A.I.L., Noble, J.A. (eds) Medical Image Understanding and Analysis. MIUA 2021. Lecture Notes in Computer Science(), vol 12722. Springer, Cham. https://doi.org/10.1007/978-3-030-80432-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-80432-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80431-2
Online ISBN: 978-3-030-80432-9
eBook Packages: Computer ScienceComputer Science (R0)