Abstract
Environmental pollution is a serious issue nowadays due to urbanization and overpopulation. Pollutants such as heavy metals, fertilizers, pesticides, herbicides, industrial effluents and organic compounds are the major source of environmental pollution. Combination of these diverse compounds and its degradation in natural environment is very tough. To overcome this problem, nanotechnology plays a significant role in the environmental remediation. The application and performance of various nanomaterials in ecological remediation depends mainly on methodologies preferred for the elimination of target pollutants from wastewater. The superior properties like high surface area and efficiency of nanomaterials are key reason for the elimination of pollutants from contaminated water. Adsorption, absorption, chemical reaction, filtration and photocatalysis-mediated nanotechnology degrades highly contaminated sites effectively. The environmental remediation could be explored by several engineered nanomaterials such as carbon, graphene, chitosan, polymer supported, metal oxide, membranes, dendrimers and fibrous clay-based nanocomposites. Nanoremediation helps to diminish cost of overall cleaning up of large-scale contaminated sites compared to conventional method of remediation. Nano-based remediation reduces degradation time and has the potential of 100% pollutant remediation capacity. This chapter affords the knowledge on environmental pollutant remediation using nanomaterials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
21.1 Introduction
Water is the prime requirement for the endurance of animals and human beings. The earth is covered by 70% of water, and only 2.5% is clean water. Water is utilized for domestic purpose and numerous industrial activities being discharged enormous quantity of untreated wastewater into water bodies. Besides, farming activities consume a range of fertilizers, pesticides and insecticides which reach water bodies through run-off.
In general, the microorganisms, inorganic and organic materials, cause water contamination. The inorganic pollutants consist of metals like Pb, Hg, Cd, Cr, etc., and the organic pollutants consist of agricultural pesticides, pharmaceuticals, textile wastes such as dyes, personal care products, household wastes like detergents, phenolic compounds and halogenated and aromatic compounds. Discharge of these pollutants in the lakes, rivers and/or drinking water resources leads to serious environmental issues like water pollution, water scarcity and health risks (Saleh 2015). According to the World Health Organization (WHO), up to 12 million people are affected by consumption of polluted water every year.
In present decades, water treatment techniques have turn into a considerable attention worldwide in increasing fast growth of industries and ecological contamination (Geise et al. 2010; Sharma et al. 2019; Yahya et al. 2018). Removal of pollutants from water is most important for the developing countries and some of the techniques are employed for the water treatment. Among, various wastewater treatment technologies, nanotechnology acquire several advantages owing to its unique physicochemical properties. Oxidation, adsorption and degradation are the nano-techniques used for the removal of contaminants which persist in the polluted water. Chemical oxidation and advanced oxidation are efficient processes for the removal of organic contaminants in water. The oxidants used in the chemical oxidation process are ozone, chlorine, chlorite, hydrogen peroxide, etc. Fenton process is one of the advanced oxidation process (AOP) which degrades the organic contaminants from water by redox reactions, dehydrogenation and electrophilic addition. AOP does not eliminate the byproducts completely during the treatment which leads to health risk to humans (Feng et al. 2013; Celiz et al. 2009).
Adsorption is a critically significant method to remediate pollutants from the polluted water, and it is an economic method. The efficiency of the adsorption depends on the nature of adsorbate, adsorbent and condition of the operation. The mechanism of adsorption generally attributes to electrostatic, π–π and Van der Waals interactions. Activated carbon, carbon nanomaterials, metal organic frameworks, clays and zeolites are used as the adsorbent (Yu et al. 2016). This remediation mechanism can be applied in water treatment nanotechnology to eliminate a number of contaminants. Hence, this chapter highlights sources of wastewater, properties of various nanomaterials, mechanism of nanoremediation and efficiency of nanomaterials used for target pollutants in wastewater.
21.2 Sources of Wastewater
Organic contaminants in waster ecosystem cause serious issues in the environment. In addition to the organic contaminants, heavy metals in water also lead to the health issue to the living organism. The pharmaceutical products are also identified in surface and groundwater ecosystem. The personal care products and endocrine disrupting compounds are high volatile and high polar in nature. Due to this intractable and persistent nature in the environment, these compounds does not undergo the degradation process (Archer et al. 2017; Tijani et al. 2019; Snyder et al. 2003).
The world population is increasing day by day, and so the world faces the lack of food. Pesticides play a significant part in the production of crops and vegetables, and it includes fungicides, herbicides, insecticides, etc. To control the pest in farming, more than a million tons of pesticides have been prepared each year. Usage of pesticides in farming leads to fast transfer of pesticide residue to the water bodies and penetrated into food chain. In farming countries, water pollution due to pesticides are incredibly frequent, and it is hazardous to human beings. Consumption of water affected by pesticide causes organ damage, cancer, reproduction effects, nervous system damage and also birth defects. The famous pesticides are organophosphate, organochlorine, N-methyl carbamate, arsenic-containing fungicides, chlorophenoxy, nitrophenol, pentachlorophenol, rodenticides and fumigants (Milne 2018).
Organic dyes are the most common organic pollutants obtained from textile, plastic, construction, leather, food industries, cosmetics and paper industries. It was anticipated that above 15% of dyes (approximately 400 ton/day) were liberated into the water body during the dyeing process (Garcia et al. 2007; Vanhulle et al. 2008). These are synthetic organic compounds which have the mutagenic and carcinogenic effects (Tang et al. 2016). The azo dyes, for instance, methylene blue and rhodamine B, are commonly used in the recent decades. These organic dyes are nondegradable naturally (Chen et al. 2011; Mirzazadeh and Lashanizadegan 2018; Han et al. 2015). Methylene blue causes eye and skin irritation and also respiratory tract irritation upon contact during the consumption (Jain et al. 2007; Kumar and Kumaran 2005). In addition, usage of detergents has increased in our daily life, and it affects the water ecosystem. These detergents are classified as cationic detergents like quaternary ammonium cations; anionic detergents like linear alkylbenzene sulfonates; and non-ionic and zwitterionic detergents. The general industrial chemicals like aromatic compounds and bisphenol A cause many health issues during consumption (Sui et al. 2011).
The water contaminated with heavy metals shows high density and atomic weight as high as five times than pure water, and these metals naturally occur via different sources. Heavy metal sources including soils, rocks and volcanic explosion and other anthropogenic sources including mining actions contaminates the water bodies. Arsenic (As), chromium (Cr), lead (Pb), mercury (Hg), cadmium (Cd), copper (Cu) and nickel (Ni) are some of the common metals which cause heavy metal contamination in water. Amongst all the heavy metals, arsenic and chromium are the principal reasons of water contamination by both natural and anthropogenic sources. According to the World Health Organization (WHO), arsenic is classified as group 1 human carcinogenic substance. Consumption of arsenic-polluted water leads to various types of skin diseases such as hyperpigmentation, hyperkeratosis and cancers like kidney, skin, lung and bladder (Khare 2016; Singh et al. 2015; Halem et al. 2009).
21.3 Mechanism
Elimination of contaminants present in wastewater can be done by photocatalysis, and the catalyst used for the degradation process in photocatalysis is known as photocatalyst which alters rate of the reaction only. After the completion of degradation process, the catalyst can be recovered and reused. During the photocatalytic activity, the following reactions are taking place in the remediation of contaminants in the water body:
-
The contaminants transferred are adsorbed on the surface of the catalyst.
-
The photonic activation and decomposition of the adsorbed contaminants.
-
Desorption of the reaction product.
-
Reaction products are eliminated from the surface of the catalyst.
where OC organic contaminants.
The photocatalytic degradation of pollutants present in water can be carried out with the use of photons, electrons and catalyst (Fig. 21.1). The photocatalytic degradation of materials such as dyes, heavy metals, organic pollutants, microplastics, etc., engages the photons, catalyst and electrons. These were engaged the individual energy stage in the atom. Depending upon the atoms, every individual energy state was divided into several energy levels. The energy bands are formed depends on close energy state. Atom filled with electrons by donation of the energy and conductivity band was created. This action can be done by photocatalyst, and then the different factors were contributed in the important parts of photocatalytic activity. The electrons are energized to conductance band from valence band in the presence of sunlight. The assortment of the photogenerated n-type and p-type from nanoparticles prohibited beneath sunlight gives enhanced time of charge transporters and additional proficient redox properties (Nivetha et al. 2019).
21.4 Remediation
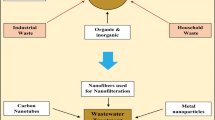
Nowadays, wastewater obtained from industrial sources are treated by different processes, and they have the capability to remediate the contaminants. There are numerous methods that have been adopted for the wastewater treatment such as phytoremediation , bioremediation , etc. But these methods have some disadvantages like inefficiency, regeneration, waste products, weak selectivity, etc. To overcome these demerits, alternate method must be needed. Hence, nanotechnologies play a pivotal role in wastewater treatment processes. Adsorption and degradation process is considered as the most significant process in the water purification. The degradation efficiency of contaminants relies on several factors like source of irradiation, adsorbent dosage, contact time, effect of pH, temperature and nature and type of the catalyst used in the wastewater remediation. Some of the important nano-based materials are depicted in Fig. 21.2 for wastewater remediation.
21.4.1 Magnetic Nanomaterials
The application of various nanomaterials and its degradation efficiency in pollutant remediation were depicted in Tables 21.1 and 21.2.
21.4.1.1 Zero-Valent Iron Nanoparticles
Zero-valent iron (ZVI) nanoparticles have been widely considered and applied for the wastewater treatment (Fig. 21.3). Owing to the ease of oxidation, high reactivity, non-hazardous nature, economic, plenty, synthesize easily, bioavailabilities and large surface area, it is used in wastewater treatment processes (Zhu and Chen 2019; Zhang 2003). The ZVI is used to eliminate the organic pollutants, polychlorinated biphenyl, organic chlorinated solvent and heavy metals present in wastewater. It is used as catalyst in the degradation and oxidation of organic pollutants transferred to hydrogen peroxide in presence of dissolved oxygen. From ZVI , two electrons are transported repeatedly to reduce the hydrogen peroxide into the water. In addition, the hydroxyl radicals are produced, and it has the ability to oxidize the organic pollutants during the permutation of hydrogen peroxide and Fe2+ (Fenton reaction). It has the standard redox potential value 0.440 V and shows that it can eliminate the halogenated compounds via reductive dehalogenation (Fu et al. 2014; He and Zhao 2007). It quickly removes hazardous non-aqueous phase liquids like halogenated partially volatile compound, halogenated volatile material and non-halogenated partially volatile materials to non-hazardous materials in polluted water.
In aqueous solution, zero-valent iron nanoparticles undergo oxidation readily either by oxygen or react with subsurface component which is occurring naturally leading to low reactivity and surface passivation. The reaction circumstances like pH, strength of ions, initial concentration of pollutant, method of synthesis, stabilizing agent used during the synthesis, medium for oxidation either air or water, and time play a crucial role in the degradation of organic contaminants. ZVI nanoparticles have some disadvantages as follows:
-
(i)
Passivation – Owing to the less active iron hydroxides formation on ZVI nanoparticles surface during the reaction
-
(ii)
Aggregation – Owing to the Van der Walls and magnetic force and also formation of less active small particles
-
(iii)
Poor retrievability
-
(iv)
Potential health and environmental risk – Because of bioaccumulation (Lu and Astruc 2020)
21.4.1.2 Iron Oxide
Iron oxide nanoparticles have existed in different structures like maghemite, hematite and magnetite (Fig. 21.3). These iron oxide nanoparticles acquire polymorphism which includes temperature-induced phase transition (Cornell and Schwertmann 2003). Owing to the strong magnetic property, porosity and precise surface area, iron nanoparticles displayed tremendous properties in adsorption process (Huang and Chen 2009; Nizamuddin et al. 2019).
Hematite (α-Fe2O3) is an n-type semiconductor, and it has the band gap value of 2.1–2.2 eV, and it has paramagnetic phase at Curie temperature (Tc = 682.85 °C). According to JCPDS file No. 33-0664, it has trigonal crystal structure which belongs to R-3c space group and unit cell parameter a = b = 4.9865 Å, c = 13.5016 Å (Rozenberg et al. 2002). Crystallinity, particle size, cation doping, exchange interactions and subparticle structure influence the magnetic properties of the hematite. Not only the size and shape of hematite but also the nature of the dopant plays a vital role to increase the adsorption properties and lead to the efficient catalyst in the wastewater treatment (Tadic et al. 2019). Owing to poor conductivity and low efficiency of separation, its photocatalytic activity is controlled (Zhang et al. 2017). During visible light irradiation, it absorbs approximately 43% of light which helps to degrade the effluent from the polluted water under light source (Santhosh et al. 2019). Kang et al. (2019) reported that hematite-based material can enhance the degradation efficiency of rhodamine B in the presence of photocatalyst. Chen et al. (2019) reported the admirable degradation kinetics of antibiotics such as ciprofloxacin, norfloxacin, sulfadiazine and tetracycline under solar light irradiation in the presence of AgBr/Ag3PO4@natural hematite as a photocatalyst. The rate constants of antibiotics are 0.16, 0.19, 0.34 and 0.10 min−1 for ciprofloxacin, norfloxacin, sulfadiazine and tetracycline, respectively.
Magnetite (Fe3O4) is nothing but a combination of ferrous and ferric ions, different to all other metal oxides. Magnetite divulges both p-type and n-type semiconductor with low band gap energy of 0.1 eV. According to JCPDS file No. 19-0629, it has a cubic inverse spinel structure which belongs to Fd3m space group (Okube et al. 2012). Owing to high surface energy, it is not stable in the aqueous environment. Surface functionalization generates the stability of the magnetite, and it increases the efficiency of elimination of pollutant. It can be used as adsorbent to remove the heavy metals from the polluted water compared with other adsorbents. Fan et al. (2019) tried to eliminate the lead from lead-containing solution using carboxymethyl-cellulose-immobilized magnetite nanoparticles, and it shows that the utmost adsorption capability of lead ion was attained at 152 mg/g.
Maghemite (γ-Fe2O3) has an analogous crystal arrangement to magnetite, and it has cubic structure. Because of high magnetization saturation, it is broadly employed as an appropriate adsorbent in wastewater treatment (Wu et al. 2015; Leone et al. 2018). It reveals ferromagnetic nature and more stable in aqueous environment. It is more superior adsorbent for heavy metal than magnetite due to small size and high specific surface area (Martinez-Boubeta and Simeonidis 2019). Rajput et al. (2017) reported the removal efficiency was found at 59.2 and 25 for lead (II) and copper (II), respectively, in the presence of maghemite.
21.4.1.3 Spinel Ferrites
Spinel ferrites have the general formula MFe2O4, where M represents the divalent metal ions with ionic radius ranging between 0.6 and 1 Å. Examples for divalent ions are Cu, Ni, Mg, Mn, Co, Zn, Cd, etc. (Ashour et al. 2014). These are magnetic semiconductors which are widely used in the field of water treatment. Depends on nature, sharing of cations and synthesis method, the properties and application of spinel ferrites varies (Fig. 21.3). The attractive band gap makes the spinel ferrite a more efficient catalyst in heavy metal removal and increases the ability of photodegradation. Owing to biocompatibility, magnetic behaviour and chemical stability, spinel ferrites and its composites are utilized in wastewater treatment. Aromatic nitro compounds are identified as common pollutant in agricultural and industrial wastewater owing to their stability and solubility in water which can be efficiently degraded by photocatalytic activity of spinel ferrites .
21.4.2 Transition Metal Oxide NPs
21.4.2.1 Titania
Titanium oxide (TiO2) is a semiconductor material which acts as photocatalyst under ultraviolet light. The electron present on the surface of substrates is transported to the conduction band. The various effluents present in the water can be degraded using titania because of its stability, abundance and less hazardous (Shakeel et al. 2016). Owing to the intrinsic properties like wide band gap (3.2 eV) and low quantum yield, the use of titania in water treatment was limited under visible light (Upadhyay et al. 2014; Qin et al. 2015). To induce the solar efficiency the nanomaterials are undergoing modification. During contact of catalyst with light sources , it produces electron and hole pairs. The electrons and hole pairs move around to the surface of the catalyst, and the redox reaction occurs for absorbing the pollutant (Fujishima et al. 2008; Di Paola et al. 2012). Transition metal or non-metal supporting is one of the tactics to decrease the band gap of titania which stimulates the photocatalyst under different light sources or direct sunlight radiation. Titania thin films and titania-decorated alumina showed high efficiency in the remediation of creatinine and methylene blue dye. Titania nanoparticles have the ability to degrade the highly hazardous materials such as antibiotic and chemotherapeutic doxorubicin present in water. Titania nanorods, nanobelts, nanowires, nanotubes, nanomembranes and nanofibers have been used to remediate the wastewater. Titania doped with other metals such as silver, iron, copper, zirconium, etc. improved electrical, catalytic and optical properties.
21.4.2.2 Copper Oxide
Copper oxide nanoparticles have been employed for the wastewater treatment owing to its incredible optical, superconductive, electrical, magnetic and thermal properties and also its low cost, low toxicity and abundance. The monovalent copper oxide nanoparticle is a p-type semiconductor with the narrow band gap of 2.0–2.5 eV. Copper oxide nanoparticles have the affinity to adsorb molecular oxygen to proliferate photogenerated electrons. Yadav et al. (2021) tried to remove the organic dyes such as Congo red (CR), methylene blue (MB), methyl orange (MO) and methyl red (MR) from the water using copper oxide nanoparticles. The increasing order of degradation efficiency was found to be MO > MB > CR > MR. Husein et al. (2019) synthesized copper nano-adsorbent for the removal of pharmaceutical pollutants from real wastewater samples. Ibuprofen, naproxen and diclofenac were identified as pollutants in real water samples. The removal capacities were calculated as 36.0, 33.9 and 33.9 mg/g for ibuprofen, naproxen and diclofenac. Dlamini et al. (2019) collected three different samples such as coal mine water, domestic wastewater and Mzingazi river water. Copper nanoparticle showed 85 and 76% removal efficiency of phosphate and sulphate from coal mine water, respectively. The removal efficiency was found at 80, 89, 63, 62 and 64% for phosphate, total nitrogen, nitrate, aluminium and sulphate , respectively, in domestic water sample. The removal efficiency was found at 92 and 52% for phosphate and total nitrogen in Mzingazi river water, respectively.
21.4.2.3 Zinc Oxide
Zinc oxide nanoparticles are a semiconductor material which acts as better photocatalyst in the removal of organic dyes. It is an n-type semiconductor with the band gap value of 3.37 eV. Owing to the non-hazardous nature, effective adsorption properties, good thermal, mechanical and chemical properties and zinc oxide nanoparticles can be employed for the elimination of organic and inorganic nanomaterials (Mustapha et al. 2020). It has the admirable UV and visible light adsorption and reflective properties, and it has high surface activity due to its large number of active adsorption sites. The wavelength and intensity of light source is more important, owing to the essential characteristic of the material in photocatalytic reaction. The catalyst used for the wastewater treatment not only eliminates the chemicals but also eliminates the microbial contaminants present in water. Photocatalytic inactivation of microbes was a tedious process, and the process differs with concentration, physiological state and kind of microbes. The morphology, nature and concentration of the catalyst influence the rate of microbial inactivation. Anusa et al. (2017) removed the heavy metals such as Cu(II), Pb(II) and Cd(II) using zinc oxide nanoparticles at different pH from simulated industrial wastewater. The removal efficiency of Cu(II) at pH = 2, 4, 6 and 8 was 99.15, 99.25, 100 and 100%. The removal efficiency of Pb(II) at pH = 2 was 63.61 and at pH = 4, 6 and 8 was 77.47%. The removal efficiency of Cd(II) at pH = 2, 4, 6 and 8 was 87.05, 96.50, 98.05 and 97.85%.
21.4.3 Carbon-Based Nanoparticles
21.4.3.1 Carbon Nanotubes (CNTs)
Currently, carbon-enriched materials such as carbon nanotubes, graphene oxides, activated carbon, carbon fibres and biochar are used as adsorbents in water purification. CNTs have π–π conjugative structure with hexagonal arrays, and every carbon atom has sp2 hybridization. It is hydrophobic in nature (Gupta et al. 2013). Carbon has the capability to form carbon to carbon long chains due to its binding ability in both straight and complex branching which facilitates double or triple bond formation and collection of atoms in different geometrical arrangements (Mubarak et al. 2014). Because of non-hazardous and high adsorption property, carbon-based nanomaterials have been broadly used for the elimination of heavy metals present in water. Electrostatic communication, ligand replacement, surface complex formation and adsorption–precipitation within metal ion and functional groups present in surface of carbon nanotubes (CNTs) are the steps followed in the mechanism of elimination of heavy metals in water. Due to the higher surface area to volume ratio of CNTs, the absorption property will be increased (Ruthiraan et al. 2015).
Multiwalled carbon nanotubes (MWCNTs) were synthesized by chemical vapour deposition, and the synthesized MWCNTs which has been found in between the width 60 and 70 nm range revealed the 100% efficiency in the removal of Cd(II) at pH = 10 and 12 with 0.5 mg/mL from 100 ppm metallic solution. The pH plays a vital role in the elimination of heavy metals. The heavy metals can be removed easily in the acidic condition. In the case of basic pH, the metals form precipitate because of the hydroxide formation. The MWCNTs shows the higher efficiency in the elimination of heavy metals in water, but its efficiency depends upon the pH of the reaction (Bhanjana et al. 2017).
Carbon microspheres can be utilized for the elimination of chromium, nickel and copper. Magnetic carbon nanomaterials have been employed for the elimination of heavy metals. MWCNTs-incorporated ZVI nanoparticles have been used in the removal of arsenic in the pH ranging between 6 and 7. During the oxidation in water, ZVI forms Fe2+ and Fe3+ hydroxides and leads to the formation of arsenic complexes, and these complexes can easily be eliminated from the water because the precipitation is taking place (Alijani and Shariatinia 2017).
21.4.3.2 Graphene Nanomaterials
Graphene have been obtained from graphite, and it acquires good electrical and mechanical properties and also it shows better thermal conductivity with honeycomb network structure (Aghigh et al. 2015; Chatterjee et al. 2015). It exists in various forms such as graphene oxide and reduced graphene oxide which are used for the removal of heavy metals (Gao et al. 2011). Graphene oxide (GO) is an oxidative product of graphene, and reduced graphene oxide (r-GO) is a reduction product of graphene oxide. GO have different oxygen functional group, while r-GO can be changed by functional groups, for instance, hydroxyl, amine and carboxylic acid group with more structural imperfection than graphene. To enhance the adsorption nature of GO , different kinds of functional groups are added to modify the GO. Due to the high surface area with fine chemical constancy, GO and r-GO are used for the wastewater remediation. The mechanism of heavy metal elimination from water using graphene-based nanomaterials depends on the electrostatic interactions and surface metal hydroxide. Based upon the surface area and surface charge, the interaction is taking place. Surface area is directly proportional to adsorption ability which is straightly with respect to particularly tunable morphology of the GO-supported nanomaterials. GO-supported metal oxides increase the ability of elimination of heavy metals in the water because of the increase of metal oxide’s electronegative charge over the GO (Ghorbani et al. 2020).
21.4.4 Nanomembranes
Filtration and membranes are extremely efficient methodologies for purification water and remediation of wastewater. The remediation includes elimination of heavy metals, inorganic ions, organic pollutants such as dyes, pesticides, pharmaceutical products, etc. and bio-based products like microorganisms (Zhang et al. 2018). Reverse osmosis is the process which is used to purify the water and desalination of seawater till now. In between ultrafiltration and reverse osmosis, there is a process called nano-filtration, using membranes that have been employed for the wastewater treatment and desalination of seawater. Nanomembrane filtration is a very efficient method for wastewater treatment . It can be divided into inorganic and organic membranes in which zeolite, silicon dioxide and 2D graphene-based nanomaterials are inorganic membranes and organic polymer-based nanomaterials belong to organic nanomembranes (Liu et al. 2014; Pedrosa et al. 2019; Huang et al. 2014). The organic polymer membranes consist of natural polymer such as chitosan, cellulose acetate and synthetic polymeric membrane such as polyacrylonitrile, polyurethane, polyamidoamine, polysulfone, polyethersulfone, polyvinyl alcohol and polyamide. The efficiency of membranes mainly depends on the structure and weight of the molecule, pore size and volume, polarity, hydrophilicity and hydrophobicity (Cyna et al. 2002; Ahmad et al. 2008; Bonne et al. 2000; Tepus et al. 2009).
21.5 Conclusion and Future Prospect
The universe is in need of advanced water treatment technologies to get freshwater for drinking and agricultural purposes. Nanotechnology mutiny will play a crucial part in resolving the difficulty of increasing demands of freshwater and disseminated water recycle. Nanomaterials are attractive material which is used for water treatment because of its fascinating physicochemical properties. Engineering nanomaterials like nanoadsorbents, photocatalysts, nanomembranes, etc. provide the prospective for new water treatment technologies, and it can be adapted to precise applications in the removal of pollutants from contaminated water. Owing to their distinctive properties such as high reaction rate, high surface area-to-volume ratio, increased surface associated behaviour (antimicrobial properties and catalysis), high conductivity and self-assembling property on substrate, nanomaterials show high efficiency in the removal of pollutants. Nanomaterials can act as catalyst to purify the water under ultraviolet light source and freely existing sun irradiation. Nanomaterials can be used to eliminate harmful organic pollutants, microplastics and microbes via catalysis using ultraviolet and solar irradiation. A nanomembrane (semi porous membranes) is used to convert hard water into soft water by blocking monovalent and bivalent ions present in water body. In future, nanotechnology plays a fascinating role in water treatment, water monitoring, etc. that can effectively stop an extensive assortment of contaminant present in water together with affordability and ease of operation. There is no debate that nanotechnologies play a vital role in the field of wastewater treatment because of its unique nature.
References
Abbas N, Shao GN, Imran SM, Haider MS, Kim HT (2016) Inexpensive synthesis of a high performance Fe3O4-SiO2-TiO2 photocatalyst: magnetic recovery and reuse. Front Chem Sci Eng 10:405–416
Aghigh A, Alizadeh V, Wong HY, Islam MS, Amin N, Zaman M (2015) Recent advances in utilization of graphene for filtration and desalination of water: a review. Desalination 365:389–397
Ahmad AL, Tan LS, Shukor SRA (2008) Dimethoate and atrazine retention from aqueous solution by nanofiltration membranes. J Hazard Mater 151:71–77
Ahmed MA, Bishay ST, Ahmed FM, El-Dek SI (2017) Effective Pb2+ removal from water using nanozerovalent iron stored 10 months. Appl Nanosci 7:407–416
Alijani H, Shariatinia Z (2017) Effective aqueous arsenic removal using zero valent iron doped MWCNT synthesized by in situ CVD method using natural α-Fe2O3 as a precursor. Chemosphere 171:502–511
Alp E, Esgin H, Kazmanli MK, Genç A (2019) Synergetic activity enhancement in 2D CuO-Fe2O3 nanocomposites for the photodegradation of rhodamine B. Ceram Int 45:9174–9178
Anusa R, Ravichandran C, Sivakumar EKT (2017) Removal of heavy metal ions from industrial waste water by nano-ZnO in presence of electrogenerated Fenton’s reagent. Int J ChemTech Res 10(7):501–508
Archer E, Petrie B, Hordern BK, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174:437–446
Ashour AH, Hemeda OM, Heiba ZK, Al-Zahrani SM (2014) Electrical and thermal behavior of PS/ferrite composite. J Magn Magn Mater 369:260–267
Ayazi Z, Khoshhesab ZM, Norouzi S (2016) Modeling and optimizing of adsorption removal of Reactive Blue 19 on the magnetite/graphene oxide nanocomposite via response surface methodology. Desalination Water Treat 57:25301–25316
Bagheri M, Najafabadi NR, Borna E (2020) Removal of reactive blue 203 dye photocatalytic using ZnO nanoparticles stabilized on functionalized MWCNTs. J King Saud Univ Sci 32(1):799–804
Bhanjana G, Dilbaghi N, Kim KH, Kumar S (2017) Carbon nanotubes as sorbent material for removal of cadmium. J Mol Liq 242:966–970
Bonne PAC, Beerendonk EF, Van der Hoek JP, Hofman JAMH (2000) Retention of herbicides and pesticides in relation to aging of RO membranes. Desalination 132:189–193
Cao J, Lai L, Lai B, Yao G, Chen X, Song L (2019) Degradation of tetracycline by peroxymonosulfate activated with zero valent iron: performance, intermediates, toxicity and mechanism. Chem Eng J 364:45–56
Celiz MD, Tso J, Aga DS (2009) Pharmaceutical metabolites in the environment: analytical challenges and ecological risks. Environ Toxicol Chem 28(12):2473–2484
Chatterjee SG, Chatterjee S, Ray AK, Chakraborty AK (2015) Graphene–metal oxide nanohybrids for toxic gas sensor: a review. Sens Actuators B Chem 221:1170–1181
Chen C, Liu J, Liu P, Yu B (2011) Investigation of photocatalytic degradation of methyl orange by using nano-sized ZnO catalysts. Adv Chem Eng Sci 1:9–14
Chen L, Yang S, Huang Y, Zhang B, Kang F, Ding D, Cai T (2019) Degradation of antibiotics in multi component systems with novel ternary AgBr/Ag3PO4@ natural hematite heterojunction photocatalyst under simulated solar light. J Hazard Mater 371:566–575
Clemonne J, Madadrang, Kim HY, Gao G, Wang N, Zhu J, Feng H, Gorring M, Kasner ML, Hou S (2012) Adsorption behavior of EDTA-graphene oxide for Pb(II) removal. ACS Appl Mater Interfaces 4:1186–1193
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses. John Wiley & Sons. ISBN: 3527302743
Cyna B, Chagneaub G, Bablon G, Tanghe N (2002) Two years of nanofiltration at the Méry-sur-Oise plant, France. Desalination 147:69–75
Danila V, Vasarevicius S, Valskys V (2018) Batch removal of Cd(II), Cu(II), Ni(II), and Pb(II) ions using stabilized zero-valent iron nanoparticles. Energy Procedia 147:214–219
Di Paola A, García-López E, Marcì G, Palmisano L (2012) A survey of photocatalytic materials for environmental remediation. J Hazard Mater 211–212:3–29
Dlamini NG, Basson AK, Pullabhotla VSR (2019) Optimization and application of bioflocculant passivated copper nanoparticles in the wastewater treatment. Int J Environ Res Public Health 16(12):2185
Dubey R, Bajpai J, Bajpai AK (2015) Green synthesis of graphene sand composite (GSC) as novel adsorbent for efficient removal of Cr (VI) ions from aqueous solution. J Water Process Eng 5:83–94
El-Dafrawy SM, Fawzy S, Hassan SM (2017) Preparation of modified nanoparticles of zinc oxide for removal of organic and inorganic pollutant. Trends Appl Sci Res 12:1–9
Fan H, Ma X, Zhou S, Huang J, Liu Y, Liu Y (2019) Highly efficient removal of heavy metal ions by carboxymethyl cellulose immobilized Fe3O4 nanoparticles prepared via high gravity technology. Carbohydr Polym 213:39–49
Feng L, Van Hullebusch ED, Rodrigo MA, Esposito G, Oturan MA (2013) Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes, a review. Chem Eng J 228:944–964
Franco P, Sacco O, De Marco I, Vaiano V (2019) Zinc oxide nanoparticles obtained by supercritical antisolvent precipitation for the photocatalytic degradation of crystal violet dye. Catalysts 9:346
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Fujishima A, Zhang XT, Tryk DA (2008) TiO2 photocatalysis and related surface phenomena. Surf Sci Rep 63:515–582
Gao B, Lim TM, Subagio DP, Lim TT (2010) Zr-doped TiO2 for enhanced photocatalytic degradation of bisphenol A. Appl Catal A 375:107–115
Gao W, Majumder M, Alemany LB, Narayanan TN, Ibarra MA, Pradhan BK, Ajayan PM (2011) Engineered graphite oxide materials for application in water purification. ACS Appl Mater Interfaces 3:1821–1826
Garcia JC, Oliveira U, Silva AEC, Oliveira CC, Nozaki J, de Souza NE (2007) Comparative study of the degradation of real textile effluents by photocatalytic reactions involving UV/TiO2/H2O2 and UV/Fe2+/H2O2 systems. J Hazard Mater 147:105–110
Geise GM, Lee HS, Miller DJ, Freeman BD, McGrath JE, Paul DR (2010) Water purification by membranes: the role of polymer science. J Polym Sci B Polym Phys 48(15):1685–1718
Ghorbani M, Seyedin O, Aghamohammadhassan M (2020) Adsorptive removal of lead (II) ion from water and wastewater media using carbon-based nanomaterials as unique sorbents: a review. J Environ Manag 254:109814
Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat M (2013) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv Colloid Interf Sci 193:24–34
Halem DV, Basker S, Amy G, Van Dijk J (2009) Arsenic in drinking water: a worldwide water quality concern for water supply companies. Drink Water Eng Sci 2:29–34
Hamdy A, Mostafa MK, Nasr M (2018) Zero-valent iron nanoparticles for methylene blue removal from aqueous solutions and textile wastewater treatment, with cost estimation. Water Sci Technol 78(2):367–378
Han PLJ, Zhu G, Hojamberdev M, Peng J, Zhang X, Lu Y, Ge B (2015) Rapid adsorption and photocatalytic activity for Rhodamine B and Cr(VI) by ultrathin BiOI nanosheets with high exposed {001} facets. New J Chem 39:1874–1882
He F, Zhao D (2007) Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ Sci Technol 41(17):6216–6221
Hinojosa-Reyes M, Camposeco-Solis R, Ruiz F, Rodríguez-González V, Moctezuma E (2019) Promotional effect of metal doping on nanostructured TiO2 during the photocatalytic degradation of 4-chlorophenol and naproxen sodium as pollutants. Mater Sci Semicond Process 100:130–139
Hu C, He M, Chen B, Hu B (2015) Simultaneous determination of polar and apolar compounds in environmental samples by a polyaniline/hydroxyl multi-walled carbon nanotubes composite-coated stir bar sorptive extraction coupled with high performance liquid chromatography. J Chromatogr A 1394:36–45
Huang SH, Chen DH (2009) Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J Hazard Mater 163(1):174–179
Huang HB, Ying YL, Peng XS (2014) Graphene oxide nanosheet: an emerging star material for novel separation membranes. J Mater Chem A 2:13772–13782
Husein DZ, Hassanien R, Al-Hakkani MF (2019) Green-synthesized copper nano-adsorbent for the removal of pharmaceutical pollutants from real wastewater samples. Heliyon 5:e02339
Ibrahim I, Athanasekou C, Manolis G, Kaltzoglou A, Nasikas NK, Katsaros F, Devlin E, Kontos AG, Falaras P (2019) Photocatalysis as an advanced reduction process (ARP): the reduction of 4-nitrophenol using titania nanotubes ferrite nanocomposites. J Hazard Mater 372:37–44
Jain R, Mathur M, Sikarwar S, Mittal A (2007) Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J Environ Manag 85(4):956–964
Kakavandi B, Takdastan A, Pourfadakari S, Ahmadmoazzam M, Jorfi S (2019) Heterogeneous catalytic degradation of organic compounds using nanoscale zero valent iron supported on kaolinite: mechanism, kinetic and feasibility studies. J Taiwan Inst Chem Eng 96:329–340
Kang MJ, Yu H, Lee W, Cha HG (2019) Efficient Fe2O3/Cg-C3N4 Z-scheme heterojunction photocatalyst prepared by facile one step carbonizing process. J Phys Chem Solids 130:93–99
Kassaee M, Motamedi E, Majdi M (2011) Magnetic Fe3O4-graphene oxide/polystyrene: fabrication and characterization of a promising nanocomposite. Chem Eng J 172:540–549
Kaur J, Kaur M (2019) Facile fabrication of ternary nanocomposite of MgFe2O4 – TiO2@ GO for synergistic adsorption and photocatalytic degradation studies. Ceram Int 45(7):8646–8659
Khalafi T, Buazar F, Ghanemi K (2019) Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci Rep 9:6866
Khare JSSS (2016) Heavy metal toxicity in the ecosystem and its impacts. Global J Eng Sci Soc Sci Stud 2. ISSN: 2394-3084
Kireeti KV, Chandrakanth G, Kadam MM, Jha N (2016) A sodium modified reduced graphene oxide-Fe3O4 nanocomposite for efficient lead (II) adsorption. RSC Adv 6:84825–84836
Kumar KV, Kumaran A (2005) A removal of methylene blue by mango seed kernel powder. Biochem Eng J 27(1):83–93
Le AT, Pung SY, Sreekantan S, Matsuda A (2019) Mechanisms of heavy metal ions removal by ZnO particles. Heliyon 5:e01440
Leone VO, Pereira MC, Aquino SF, Oliveira LCA, Correa S, Ramalho TC, Gurgel LVA, Silva AC (2018) Adsorption of diclofenac on a magnetic adsorbent based on maghemite: experimental and theoretical studies. New J Chem 42:437–449
Li YH, Ding J, Luan Z, Di Z, Zhu Y, Xu C, Wu D, Wei B (2003) Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 41(14):2787–2792
Liu T, Li B, Hao Y, Yao Z (2014) MoO3-nanowire membrane and Bi2Mo3O12/MoO3 nano-heterostructural photocatalyst for wastewater treatment. Chem Eng J 244:382–390
Lu F, Astruc D (2020) Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord Chem Rev 408:213180
Ma J, Yu F, Zhou L, Jin L, Yang M, Luan J, Tang Y, Fan H, Yuan Z, Chen J (2012) Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl Mater Interfaces 4(11):5749–5760
Mahdavi S, Jalali M, Afkhami A (2013) Heavy metals removal from aqueous solutions using TiO2, MgO, and Al2O3 nanoparticles. Chem Eng Commun 200:448–470
Malakootian M, Olama N, Malakootian M, Nasiri A (2019) Photocatalytic degradation of metronidazole from aquatic solution by TiO2-doped Fe3+ nano-photocatalyst. Int J Environ Sci Technol 16:4275–4284
Martinez-Boubeta C, Simeonidis K (2019) Chapter 20 – magnetic nanoparticles for water purification, in: S. Thomas, D. Pasquini, S.-.Y. Leu, D.A. Gopakumar (Eds.), Nanoscale Materials in Water Purification, Elsevier, pp. 521–552.
Milne GWA (ed) (2018) The Ashgate handbook of pesticides and agricultural chemicals. Routledge
Mirzazadeh H, Lashanizadegan M (2018) ZnO/CdO/reduced graphene oxide and its high catalytic performance towards degradation of the organic pollutants. J Serbian Chem Soc 83(2):221–236
Mubarak N, Sahu J, Abdullah E, Jayakumar N, Ganesan P (2014) Single stage production of carbon nanotubes using microwave technology. Diam Relat Mater 48:52–59
Mustapha S, Ndamitso MM, Abdulkareem AS, Tijani JO, Shuaib DT, Ajala AO, Mohammed AK (2020) Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: a review. Appl Water Sci 10:49
Nivetha A, Devi SM, Prabha I (2019) Fascinating physic-chemical properties and resourceful applications of selected cadmium nanomaterials. J Inorg Organomet Polym Mater 29:1423–1438
Nizamuddin S, Siddiqui MTH, Mubarak NM, Baloch HA, Abdullah EC, Mazari SA, Griffin GJ, Srinivasan MP, Tanksale A (2019) Chapter 17 – Iron oxide nanomaterials for the removal of heavy metals and dyes from wastewater. In: Thomas S, Pasquini D, Leu SY, Gopakumar DA (eds) Nanoscale materials in water purification. Elsevier, pp 447–472
Okube M, Yasue T, Sasaki S (2012) Residual density mapping and site selective determination of anomalous scattering factors to examine the origin of the Fe K pre-edge peak of magnetite. J Synchrotron Radiat 19:759–767
Pedrosa M, Drazic G, Tavares PB, Figueiredo JL, Silva AMT (2019) Metal-free graphene-based catalytic membrane for degradation of organic contaminants by persulfate activation. Chem Eng J 369:223–232
Peng X, Luan Z, Ding J, Di Z, Li Y, Tian B (2005) Ceria nanoparticles supported on carbon nanotubes for the removal of arsenate from water. Mater Lett 59:399–403
Pizarro C, Rubio MA, Escudey M, Albornoz MF, Muñoz D, Denardin J, Fabris JD (2015) Nanomagnetite-zeolite composites in the removal of arsenate from aqueous systems. J Braz Chem Soc 26:1887–1896
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339:2693–2700
Qin C, Li Z, Chen G, Zhao Y, Lin T (2015) Fabrication and visible-light photocatalytic behavior of perovskite praseodymium ferrite porous nanotubes. J Power Sources 285:178–184
Rajput S, Singh LP, Pittman CU Jr, Mohan D (2017) Lead (Pb2+) and copper (Cu2+) remediation from water using superparamagnetic maghemite (γ-Fe2O3) nanoparticles synthesized by Flame Spray Pyrolysis (FSP). J Colloid Interface Sci 492:176–190
Rozenberg GK, Dubrovinsky LS, Pasternak MP, Naaman O, Le Bihan T, Ahuja R (2002) High pressure structural studies of hematite Fe2O3. Phys Rev B 65:064112
Ruthiraan M, Mubarak NM, Thines RK, Abdullah EC, Sahu JN, Jayakumar NS, Ganesan P (2015) Comparative kinetic study of functionalized carbon nanotubes and magnetic biochar for removal of Cd2+ ions from wastewater. Korean J Chem Eng 32:446–457
Saleh TA (2015) Mercury sorption by silica/carbon nanotubes and silica/activated carbon: a comparison study. J Water Supply Res Technol AQUA 64(8):892
Santhosh C, Malathi A, Dhaneshvar E, Bhatnagar A, Grace AN, Madhavan J (2019) Chapter 16 – Iron oxide nanomaterials for water purification. In: Thomas S, Pasquini D, Leu SY, Gopakumar DA (eds) Nanoscale materials in water purification. Elsevier, pp 431–446
Shakeel M, Jabeen F, Shabbir S, Asghar MS, Khan MS, Chaudhry AS (2016) Toxicity of nano-titanium dioxide (TiO2-NP) through various routes of exposure: a review. Biol Trace Elem Res 172:1–36
Sharma S, Dutta V, Singh P, Raizada P, Sani AR, Bandegharaei AH, Thakur VK (2019) Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: a review. J Clean Prod 228:755–769
Sharaf El-Deen SEA, Zhang FS (2016) Immobilisation of TiO2-nanoparticles on sewage sludge and their adsorption for cadmium removal from aqueous solutions. J Exp Nanosci 11:239–58
Shin KY, Hong JY, Jang J (2011) Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles: isotherms and kinetic study. J Hazard Mater 190:36–44
Shokri M, Jodat A, Modirshahla N, Behnajady M (2013) Photocatalytic degradation of chloramphenicol in an aqueous suspension of silver-doped TiO2 nanoparticles. Environ Technol 34:1161–1166
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques a review. Ecotoxicol Environ Saf 112:247–270
Snyder SA, Westerhoff P, Yoon Y, Sedlak DL (2003) Pharmaceuticals, personal care products, and endocrine disruptors in water: implications for the water industry. Environ Eng Sci 20:449–469.
Sui Q, Huang J, Liu Y, Chang X, Ji G, Deng S, Xie T, Yu G (2011) Rapid removal of bisphenol A on highly ordered mesoporous carbon. J Environ Sci 23:177–182
Tadic M, Trpkov D, Kopanja L, Vojnovic S, Panjan M (2019) Hydrothermal synthesis of hematite (α-Fe2O3) nanoparticle forms: synthesis conditions, structure, particle shape analysis, cytotoxicity and magnetic properties. J Alloys Compd 792:599–609
Tang L, Wang J, Wang L, Jia C, Lv G, Liu N, Wu M (2016) Facile synthesis of silver bromide based nanomaterials and their efficient and rapid selective adsorption mechanisms toward anionic dyes. ACS Sustain Chem Eng 4:4617–4625
Tepus B, Simonic M, Petrinic I (2009) Comparison between nitrate and pesticide removal from ground water using adsorbents and NF and RO membranes. J Hazard Mater 170:1210–1217
Tian Z, Yang B, Cui G, Zhang L, Guo Y, Yan S (2014) Synthesis of poly(m-phenylenediamine)/iron oxide/acid oxidized multi-wall carbon nanotube for removal of hexavalent chromium. RSC Adv 5:2266–2275
Tijani JO, Fatoba OO, Babajide OO, Petrik LF, Zhu K, Chen C (2019) Chapter 6 – Application of nZVI and its composites into the treatment of toxic/radioactive metal ions. In: Chen C (ed) Interface science and technology. Elsevier, pp 281–330
Upadhyay RK, Soin N, Roy SS (2014) Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: a review. RSC Adv 4(8):3823–3851
Vanhulle S, Trovaslet M, Enaud E, Lucas M, Taghavi S, Van Der Lelie D, Van Aken B, Foret M, Onderwater RCA, Wesenberg D, Agathos SN, Schneider YJ, Corbisier AM (2008) Decolorization, cytotoxicity, and genotoxicity reduction during a combined ozonation/fungal treatment of dye-contaminated wastewater. Environ Sci Technol 42:584–589
Wen Y, Zhao Y, Guo M, Xu Y (2019) Synergetic effect of Fe2O3 and BiVO4 as photocatalyst nanocomposites for improved photo Fenton catalytic activity. J Mater Sci 54:8236–8246
Wu W, Wu Z, Yu T, Jiang C, Kim WS (2015) Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 16:023501
Xu Y, Liu Q, Xie M, Huang S, He M, Huang L, Xu H, Li H (2018) Synthesis of zinc ferrite/silver iodide composite with enhanced photocatalytic antibacterial and pollutant degradation ability. J Colloid Interface Sci 528:70–81
Yadav S, Chauhan M, Mathur D, Jain A, Malhotra P (2021) Sugarcane bagasse-facilitated benign synthesis of Cu2O nanoparticles and its role in photocatalytic degradation of toxic dyes: a trash to treasure approach. Environ Dev Sustain 23(2):2071–2091. https://doi.org/10.1007/s10668-020-00664-7
Yahya N, Aziz F, Jamaludin NA, Mutalib MA, Ismail AF, Salleh WNW, Jaafar J, Yusof N, Ludin NA (2018) A review of integrated photocatalyst adsorbents for wastewater treatment. J Environ Chem Eng 6(6):7411–7425
Yu F, Li Y, Han S, Ma J (2016) Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 153:365–385
Zha S, Cheng Y, Gao Y, Chen Z, Megharaj M, Naidu R (2014) Nanoscale zero valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem Eng J 255:141–148
Zhang WX (2003) Nanoscale iron nanoparticles for environmental remediation: an overview. J Nanopart Res 5:323–332
Zhang K, Liu Y, Deng J, Xie S, Lin H, Zhao X, Yang J, Han Z, Dai H (2017) Fe2O3/3DOM BiVO4: high performance photocatalysts for the visible light-driven degradation of 4-nitrophenol. Appl Catal B 202:569–579
Zhang Y, Wei S, Hu Y, Sun S (2018) Membrane technology in wastewater treatment enhanced by functional nanomaterials. J Clean Prod 197:339–348
Zhu K, Chen C (2019) Chapter 6: Application of nZVI and its composites into the treatment of toxic/radioactive metal ions, in: C. Chen (Ed.), Interface Science and Technology, Elsevier, pp. 281–330.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ramya, A., Dhevagi, P., Rakesh, S.S. (2022). Nanomaterials for Wastewater Remediation: Resolving Huge Problems with Tiny Particles. In: Krishnan, A., Ravindran, B., Balasubramanian, B., Swart, H.C., Panchu, S.J., Prasad, R. (eds) Emerging Nanomaterials for Advanced Technologies. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-80371-1_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-80371-1_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80370-4
Online ISBN: 978-3-030-80371-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)