Abstract
According to different sources of information the durability of magnesia binders, depending on raw material and technology of production, is equal to 20–100 MPa. It is considered that thanks to the presence of passive phase CaC03 in the caustic dolomite which doesn’t dissociate during the calcinations and which comprises 71% of the total mass of binder, the solidity of caustic dolomite must be lower than the durability of caustic magnesite. Judging by the quantitative correlation of active phrases consisting of MgO in caustic magnesite and caustic dolomite (100 and 29%), it is obvious that the durability of the first one must approximately be 3 times as much the durability of the second one. In fact, the correlation of durability is within the limits of 1:1.5 to 1:2. And what’s more some articles as well as our articles dealing with magnesia binders, show the possibility of receiving the caustic dolomite, or as we call it further, dolomite cement with the durability which can be correlated with the durability of caustic magnesite. Besides the durability limit was reached up to 110 MPa when the condition of calcination (temperature, duration, size of the grain of raw material) was optimized.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The role of alternative feedstocks for the production of building materials has been increasing in recent years. There are works, where the dolomite is used to get the natural cement and hydraulic lime [1,2,3,4] or as the filler for concrete [5,6,7]. The simplest way to use the dolomites in the binding substances consists in the preparation of mixed cements. The dolomite-based mixed cements are of wide range. There are complex additives both binary and many more [8,9,10,11]. The more updated way to get the dolomite-based binding components consists in the production of magnesia cement [12], which particularly facilitates the mitigation of carbon footprint [13, 14]. The magnesia cements are usually obtained by burning magnesium-containing carbonate rocks such as magnesites and dolomites. They are tempered by the water solutions of magnesium chloride. The magnesium phosphate cement is a kind of magnesia cements [15,16,17]. The magnesium phosphate cements feature the superior technical characteristics but require the higher burning temperature in comparison with the magnesia cement. The main trends in the magnesia cements enhancement are focused on the production of magnesia cement mixtures [18] as well as inclusion of crystallization centers [19] and other additives [20]. In our opinion, it is necessary to firstly upgrade the process of burning to get the maximal amount of active MgO. The reason why the durability rises is in the fact that the active phase (MgO) of dolomite cement and caustic magnesite after the calcinations of raw material is different in quality. It is obvious that these differences are formed during the process of calcination and continue during the hydration and hardening. The establishment of such differences and their utilization will give the opportunity to utilize magnesite binding dolomites instead of magnesite more soundly. The replacement of magnesite by dolomite will allow to lower the cost of binder by reducing the cost of raw material and to use local natural resources.
Research aims. Considering the finite reserves of limestone and magnesite, the raw materials base for the production of binding substances is rapidly reducing. The problem may be solved by use of dolomites to get the magnesia cement. The latter, firstly codenamed as Sorel’s cement or caustic magnesite, caustic dolomite, has been used in the course of building to one extent or another for 150 years. The name «dolomite cement» was also stuck for the caustic dolomite. It focuses on assessing the calcination regime of dolomite for binder strength. The object of research is dolomite cement; the subject of research is calcination processes and internal structure of binder.
2 Materials and Methods
To establish the qualitative difference of active phase (MgO) in magnesite binders depending on the parameters of calcination the authors carried out the analysis of the products of calcination by using different types of magnesite and dolomite without admixtures. The chemical structure of magnesite and dolomite samples are shown in Table 1. To avoid the influence of secondary factors on calcination, the samples were selected with approximately identical composition of admixtures.
Calcination was held under the same specific power consumption for dissociation of 1 unit of raw material taking into account the value of endothermic effects of magnesite and dolomite dissociation. The temperature of calcination was 650–750 °C relatively. The materials of fractions 5–7 mm were calcinated within time interval of 0.5–3.5 h at constant ventilation of space under binding.
To establish physical and mechanical indexes of dolomite cement and caustic magnesite tests on durability as well as methods of qualitative chemical and X-ray phase analysis were used. The research took place immediately after the calcinations. When fully expanded, 260 MPa versus 160 MPa, respectively.
3 Results and Discussion
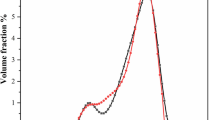
The kinetics of the process of magnesite and dolomite dissociation where the presence of magnesium oxide and of the product under calcination at the moment of taking information is shown in Tables 2–3 and Fig. 1–2.
The analysis of the results demonstrated shows that within 3 h practically complete dissociation of magnesite and dolomite takes place. It is established both by X-ray phase and chemical analyses. However, at the primary stage the dissociation of magnesite takes place with much higher speed than that of dolomite.
After 2 h of dissociation (Table 2, Fig. 1) practically all magnesite dissociates and later can’t be displayed by X-rays. At maximum durability of caustic magnesite, the dissociation of magnesite is 100%. The quantity of the active phase of MgO formed as a result of dissociation of magnesite and defined by X-ray analysis, practically coincides with the quantity of active phase defined by chemical analysis (curves 1 and 2, Fig. 1).
Moreover, the amount of magnesite that did not decompose during the burning process (curve 3, Fig. 1), practically corresponds to the amount of formed MgO, determined by the same analysis (amount 100%). According to graph (Fig. 2) and data from Table 3, dolomite decomposition is slower during the initial period. By the end of the second hour of RFA burning, the presence of up to 20% of undecomposed dolomite is still noticeable (to simplify – the amount of calcite formed during the burning process was not taken into account).
When comparing the dolomite composition burned products content at different stages of burning (curves 1 and 2, Fig. 2), based on chemical and X-ray phase analysis, we can see, that during the initial period of burning, chemical analysis shows more magnesium oxide than X-ray diffraction analysis (for example, 42% and 24%, respectively, with a burning duration of 1 h). Moreover, the amount of undecomposed dolomite during burning (curve 3, Fig. 2), according to XRF, does not correspond to the amount of formed MgO, determined by the same analysis (the amount is less than a 100%). And, at the same time, it corresponds to the amount of MgO, determined by chemical analysis (100% sum).
At first, this fact was taken for an error in chemical analysis, but the results were similar after the repeated test analysis.
Tests on durability of dolomite cement at various stages of calcination were held and the factors of durability and quantity of MgO were compared. The comparison was made with graphical correlations (taken earlier and are not demonstrated in the present document) of durability of dolomite cement on the quantity of MgO in a wide range of factors. The result shows that durability indexes of dolomite cement correspond to quantity of MgO, defined by chemical analysis.
When the results were analyzed, the authors came to a conclusion that unlike chemical analysis which shows both the quantity of crystal and amorphous MgO in dolomite cement, X-ray-phase analysis on defractional maxima of MgO fixes only the quantity of crystal MgO. The other amorphous part of MgO, during the qualitative X-ray phase analysis is not taken into account. That’s why according to X-ray phase analysis the substance of residual dolomite and newly-formed crystal MgO in calcinated sample is equal to less than 100%. The area of the largest divergence of curves 1 and 2 in the graph in Fig. 2 is defined as X-ray-amorphous area of MgO.
Thus, the results of the analyses show the qualitative and quantitative differences between active phases MgO of caustic magnesite and dolomite cement. Since the conditions of calcination are equal thus the reasons of high durability of dolomite cement in comparison with the durability of caustic magnesite should be found in the peculiarities of active phase of MgO of both binders. The actuality of this supposition is proved in the works dedicated to the study of reactionary ability of hard substances.
Additional Xray graphic research and the analysis of the rate of crystallinity of MgO (Table 4) proved that what was said above is right. In fact, MgO which is formed at thermal dissociation of dolomite has lower rate of crystallinity, than MgO, formed at thermal dissociation of magnesite.
The results of comparison of the sizes of crystals of magnesium oxide prove the abovementioned (Table 4). The average size of crystals of active phase of MgO of caustic dolomite, measured on half the wideness of its defractional maxima is 15 to 42 nm depending on the time of calcinations since the same index for caustic magnesite is 22 to 50 nm.
Consequently, at similar duration of calcination, the forming phase of MgO in dolomite cement is more active during the hardening, if compared with the phase of caustic magnesite; the smaller size of its crystal, is the proof to it. We can exclude the fact that one of the reasons of this effect is the presence of non-dissociated carbonate phase in the form of CaCO3 in dolomite cement, which can prevent the formation and the grow of crystals of MgO owing to epitaxional and endotaxial phenomena.
4 Conclusions
Thus, the active phase of dolomite cement presented as magnesium oxide, owing to above mentioned reasons is more dispersed, has highly abnormal surface power and, relatively, highly abnormal reactional ability, and thus magnesium oxide of dolomite cement at other equal conditions of calcination of raw material is more active than magnesium oxide of caustic magnesite. And as a binder substance dolomite cement is more effective than caustic magnesite.
References
Shelikhov, N., Rakhimov, R., Sagdiev, R.: Inorganic Mater. 52, 1172–1178 (2016). https://doi.org/10.1134/S0020168516110169
Shelikhov, N., Sagdiev, R.: IOP Conf. Series: Mater. Sci. Eng. 890 (2020). https://doi.org/10.1088/1757-899X/890/1/012097
Rakhimova, N.R., Rakhimov, R.Z., Morozov, V.P., Eskin, A.A.: Periodica Polytechnica Civil Eng. 65(1), 204–214 (2021). https://doi.org/10.3311/PPci.14084
Ermilova, E., Kamalova, Z., Ravil, R.: IOP Conf. Series: Mater. Sci. Eng. 890(1) (2020). https://doi.org/10.1088/1757-899X/890/1/012087
Sychugov, S., Tokarev, Y., Plekhanova, T., Mikhailova, O., Pudov, I., Faizullin, R., Gaifullin, A., Sagdiev, R.: Proc. Eng. 982–990 (2017). https://doi.org/10.1016/j.proeng.2017.02.124
Rakhimov, R.Z., Rakhimova, N.R., Gaifullin, A.R., Bikmukhametov, A.R., Morozov, V.P.: News KSUAE 3(49), 172–180 (2019)
Shelikhov, N., Sagdiev, R., Timirgaliev, M.: News KSUAE 4(50), 394–400 (2019)
Hailong, Y.: Cement Concr. Res. 139 (2021). https://doi.org/10.1016/j.cemconres.2020.106267
Xu, J., Lu, D., Zhang, S., Xu, Z., Hooton, R.D.: Constr. Build. Mater. 270 (2021). https://doi.org/10.1016/j.conbuildmat.2020.121375
Shaaban, M.: Heliyon 7(2021). https://doi.org/10.1016/j.heliyon.2021.e06311
Rakhimova, N.R.: Geosyst. Eng. 23(5), 287–298 (2020). https://doi.org/10.1080/12269328.2020.1713911
Baghrichea, M., Achourb, S., Baghrichec, O.: Case Stud. Constr. Mater. 13 (2020). https://doi.org/10.1016/j.cscm.2020.e00386
Chena, L., Wangab, L., Tsanga, D.C.W., Mechtcherineb, V., Poona, C.S.: J. Clean. Prod. 258 (2020). https://doi.org/10.1016/j.jclepro.2020.120997
Hamdy Abdel-Gawwad, A., Soltan Hassan, H., El-Kattan, I.M.: J. Clean. Prod. 28 (2019). https://doi.org/10.1016/j.jclepro.2019.119875
Yu, J., Qian, J., Wang, F., Li, Z., Jia, X.: Cement Concr. Res. 138 (2020). https://doi.org/10.1016/j.cemconres.2020.106235
Yu, J., Qian, J., Wang, F., Qina, J., Daia, X., Youb, C., Jia, X.: Constr. Build. Mater. 253 (2020). https://doi.org/10.1016/j.conbuildmat.2020.119147
Peng, L., Chen, B.: Constr. Build. Mater. 281 (2021). shttps://doi.org/10.1016/j.conbuildmat.2021.122609
Liu, Z., Wang, S., Huang, J., Wei, Z., Guan, B., Fang, J.: Constr. Build. Mater. 85, 247–255 (2015). https://doi.org/10.1016/j.conbuildmat.2015.01.056
Wu, C., Chen, C., Zhang, H., Tan, Y., Yu, H.: Constr. Build. Mater. 172, 597–607 (2018). https://doi.org/10.1016/j.conbuildmat.2018.04.005
Guan, Y., Hu, Z., Zhang, Z., Chang, J., Bi, W., Cheeseman, C.R., Zhang, T.: Cement Concr. Res. 143 (2021). https://doi.org/10.1016/j.cemconres.2021.106387
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Shelikhov, N., Sagdiev, R. (2021). Carbonate Phase in the Formation of Binding Substance in Dolomite Cement. In: Vatin, N. (eds) Proceedings of STCCE 2021. STCCE 2021. Lecture Notes in Civil Engineering, vol 169. Springer, Cham. https://doi.org/10.1007/978-3-030-80103-8_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-80103-8_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80102-1
Online ISBN: 978-3-030-80103-8
eBook Packages: EngineeringEngineering (R0)