Abstract
One of the modern trends in construction is the orientation towards biopositive building materials and technologies. In this regard, the use of plant raw materials for the production of building materials is of relevance. The aim of the research was to study the possibility of chemical modification of the ligno-carbohydrate complex of Heracleum sosnovsky to obtain biostable plant raw materials. The hypothesis was formulated, according to which a significant increase in the biostability of plant raw materials based on Heracleum can be achieved by modifying it with a four-coordination boron compound–monoethanolamine(N→B)-trihydroxyborate. This goal was achieved through laboratory studies of the sorption of the modifier, studying the microstructure of the samples and carrying out IR spectroscopy. As a result of the research, it was found that the degree of modification of Heracleum stems multiply depends on the modification temperature and drying temperature, the corresponding dependences have been established. It was found that under the action of the modifier, depolymerization of lignin and hemicellulose occurs, as well as the chemical interaction of the reagent with the substrate. Analysis of the microstructure indicates a uniform distribution of the modifier in the intercellular space. The novelty of the conducted research consists in the substantiation and experimental confirmation of the possibility of increasing the biostability of plant raw materials—Heracleum. The significance of the result lies in determining the optimal parameters of the modification process.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Heracleum sosnovsky was imported to the Russian Midlands from Georgia in the middle of twentieth century, later domesticated and throughout few decades has been grown for feeding livestock [1,2,3,4]. This was due to the biological specific features of the plant species, namely low maintenance, rapid growth and development of green matter, frost tolerance, high content of vitamins, proteins and microelement in the composition of cell walls [5,6,7,8]. However, the presence of furocoumarins in the plant green matter has exacerbated the quality of agricultural products. Moreover, there was discovered the potential hazard of dermatitis caused in the case of contact with Heracleum [9]. Thus, its cultivation was halted as early as the 1980s, at first in Europe, than on the territory of the Commonwealth Independent States. Nevertheless, throughout the period of its cultivation, this species has become wide spread in Eastern Europe, in particular on the territories of the Former Soviet Republics.
In the Central Part of Russia, the first specimen of wilding Heracleum sosnovsky was first detected in 1948 in Moscow Region. Over the subsequent years, Heracleum have been found, mostly, near the cultivation areas; at the very least in Moscow Region this species had not exhibited the tendency to diffusion into natural vegetation until the 1970s when Heracleum naturalizing became wide spread [1, 10]. Nowadays, the expansion of this invasive plant is becoming environmental catastrophe. If the green matter of Heracleum was used more actively in the national economy, this would facilitate its removal from agricultural ecosystem as well as invasive offloading. Presently, the scientists are searching the possible applications of Heracleum biomass in engineering, pulp-and-paper production, medicine and pharmaceutical industry [11,12,13,14]. Porous stem structure of Heracleum has quickened scientists’ interest in this material in the function of concrete admixture lowering its thermal conductivity [15]. Taking into account the current trend for the development of biopositive building technologies [16], the use of plant raw materials, in particular Heracleum, for the production of various building materials is of relevance and practical interest.
However, this plant raw material requires pre-treatment with antiseptics, because despite the high content of extractives in the composition of cell walls of Heracleum, there was observed the active growth of fungi on the stem surface when stored in contact with moist environment. The previous studies have shown that boracium-nitrogen compounds are rather effective, from the point of bio stability enhancement of plant raw materials used in construction. For example, the papers [17, 18] have demonstrated that application of four-coordination compounds of boracium for surface modification allows ensuring 100% bio stability of timber structures for the period of at least 20 years. It was suggested that application of four-coordination boracium compound–monoethanolamine(N→B)-trihydroxyborate for modifying cell walls of Heracleum stems will allow to enhance its biostability. Thus, the purpose of the present research was the study of possible chemical modification of ingo-carbohydrate complex of Heracleum sosnovsky with four-coordination boracium compound in order to increase the biostability of plant raw materials for the production of building materials. In order to realize the set objective, the following tasks were to be solved: determining optimal temperatures for modifying and drying of source raw material on the base of monoethanolamine(N→B)-trihydroxyborate; identifying the pattern of interaction of monoethanolamine(N→B)-trihydroxyborate with ingo-carbohydrate complex of Heracleum sosnovsky cell walls.
2 Methodology
As source raw materials of plant origin, there were used grinded stems of Heracleum sosnovsky gathered in September 2020 in the Pushkinsky district of Moscow Region. The stems were preliminary cleaned from the extraneous bodies and air-dried until they reach the constant weight. Then they were mechanically grinded to the particle size ranging 1–5 mm; the particles’ shape being irregular. Modifying was carried out by means of dipping into 50% aqua solution of monoethanolamine(N→B)-trihydroxyborate, pH = 9, for 3 h. Modifying temperature accounted for 25, 50, 75 and 100 ℃ correspondingly. The samples were further filtered off on the filter paper and dried until they reach the constant weight by two methods: (a) air-drying at room temperature and (b) in the drying cupboard at 105 ℃. In order to remove the excess of unreacted modifier and hydrolysis products, threefold extraction of modified and reference samples was done by distilled water at room temperature with further air-drying at room temperature until they reach a constant weight. Then, the surface layer of samples was investigated by the methods of infrared spectroscopy and electronic microscopy. The measurements were taken by the infrared Fourier spectrometer VERTEX 70v made by BRUKER (Germany) with the application of the auxiliary device FTIR (frustrated total internal reflection) GladyATR made by PIKE (USA) with diamond operating element. Spectra were obtained directly from finely grinded samples, non-treated additionally. The spectra taken by the method of frustrated total internal reflection (FTIR) were converted into absorption spectra by factoring in the relationship between penetration depth of infrared radiation into the sample and wave length, done by the OPUS application incorporated into the device software. Microphotographs of modified samples were received by means of the raster electron microscope JSM-6510LV JEOL.
3 Results and Discussion
The results of samples’ weighing before and after modifying at various temperatures as well as after extraction and drying are outlined in the Table 1.
From the data presented in the Table 1 it is apparent that light porous base has high sorption capacity, it absorbs from 1.4 g up to 3.18 g of modifier per 1 g of the base as a function of temperature. However, less modifier is bonded chemically. This was stated by means of samples weighing after extraction. Extraction enables to eliminate the excess of unreacted monoethanolamine(N→B)-trihydroxyborate from the composition of support pores. According to the table data, the level of modification has multiply increased as the temperature grows. Along with that, not only the temperature of modification has the meaning but the temperature of drying. The highest degree of modifier’s sorption is observed at the temperature of 100 ℃ and drying in the drying cupboard at 105 ℃. Under such conditions, there has been observed threefold exceeding of the modifier’s content in the base composition if compared with the process of modifying at room temperature. But also at room temperature of modifying and drying, the content of modifier in the base composition is noticeable—46%. This could ensure good security against bio corrosion and further researches will be aimed at the lowering of modifier’s concentration and the search of the optimal concentration.
Lingo-carbohydrate complex (LCC) of cell walls of Heracleum has the highest reaction capacity in relation to monoethanolamine(N→B)-trihydroxyborate if compared with LCC of timber [19]. This phenomenon could be explained by the following. Firstly, in the contact with modifier LCC of cell walls of Heracleum experiences lower steric hindrances if compared with LCC of timber due to less dense structure of tissues. Secondly, there are essential differences in the component ration in the LCC-composition. In the LCC-composition of timber, less chemically reactive cellulose dominates over chemically reactive lignin twofold, while in the LCC-composition of Heracleum the ration of cellulose and lignin is equal to 1:1 approx. Along with that, the attention should be paid on the negative mass increment of reference samples after extraction. This gives an evidence that hydrolysis of extractive substance from the initial substance composition takes place. Taking into account that modifying solution is characterized by alkaline medium (pH = 9), then during the modifying process, the percent of extractive substance outwashing must be significantly higher. Moreover, the hydrolysis process for low molecular polysaccharose (hemicellulose) and lignin in alkaline medium is inevitable. Nevertheless, the data of the Table 1 demonstrate that the mass loss is not observed (the last column of the table). This could be explained by the fact that significant mass increment arising due to modification overlaps by far the mass loss occurring during hydrolysis.
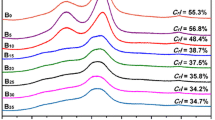
The Fig. 1 represents the fragments of infrared spectra in the area undergone significant changes during modification process. The Fig. 1 shows that all the spectra of modified samples 1.1.–1.8 are similar and have relatively insignificant differences. The variation from the reference sample spectrum (1.9) is much more prominent. The data presented at the Fig. 1 confirm that the most part of changes in the dipped base is observed at the curves peaks related to hemicellulose (1737, 1268, 1100, 1056 cm−1) and lignin (1601, 1268 cm−1). These changes are the result of lignin and hemicellulose depolymerization caused by a modifier, whereas cellulose has not been depolymerized that ensures secure fixation of modifier in the composite formulation [20].
Infrared spectrum of modified and references samples: 1.1 Tmod = 25 ℃, Tdrying = 25 ℃; 1.2 Tmod = 25 ℃, Tdrying = 105 ℃; 1.3 Tmod = 50 ℃, Tdrying = 25 ℃; 1.4 Tmod = 50 ℃, Tdrying = 105 ℃; 1.5 Tmod = 75 ℃ ℃, Tdrying = 25 ℃; 1.6 Tmod = 75 ℃, Tdrying = 105 ℃; 1.7 Tmod = 100 ℃, Tdrying = 25 ℃; 1.8 Tmod = 100 ℃, Tdrying = 105 ℃; 1.9 Reference sample (non-modified sample)
After modifying and drying the spectral band of 1735 cm−1 has almost disappeared, the spectral band of 1580 cm−1 is significantly reduced (has nearly disappeared).
This goes to prove chemical interaction between lignin hydroxyls and modifier’s molecules as well as partial hydrolysis of aromatic constituent of Heracleum LCC. The structure of the wide absorption band in the range of 1315–1470 cm−1 has also undergone a change. The changes prove that for all the samples, regardless the temperature of modifying and drying, chemical interaction of reactive chemical and the base takes place. Modified wide absorption band in the range of 1315–1470 cm−1 appeared after modification (Fig. 1) testifies the presence of the coordinate bond N→B in the composition of modified base [21]. Once can also observe the appearance of band in the range of 1630 cm−1, which corresponds to the effect of bonding NH2 in the spectra of the samples 1.1–1.8, Fig. 1 [21].
Microphotographs of modified and reference samples (magnification 500×) are presented in the Fig. 2. As the presented photographs show, modifier wraps around cell walls. At the microphotographs 2.1–2.3, even distribution of modifiers in the intercellular space is observed. In addition to this, the higher modification temperature, the more saturated near-wall layers by the modifier’s molecules.
This is explained by the fact that elevated temperature in alkaline medium leads to more constitutive hydrolysis of hemicellulose and lignin; and their place in the composite structure are taken by the molecules of monoethanolamine (N→B)-trihydroxyborate.
4 Conclusions
Thus, the obtained experimental data allow making the following conclusions. When modifying plant raw materials represented by grinded stems of Heracleum sosnovsky by the composition on the base of monoethanolamine(N→B)-trihydroxyborate, depolymerization of lignin and hemicellulose occurs, and the modifiers molecules replace them in the composition of lignin-carbohydrate complex of cell walls. This process is reinforced as the temperature of modifying and drying grows that leads to the increased content of boracium-nitrogen compound in the base composition. The process of dipping plant raw material in monoethanolamine(N→B)-trihydroxyborate is accompanied by chemo sorption. This is evidenced by the presence of modifier’s molecules confirmed by the data of infrared spectroscopy and microphotography, in the intercellular space after continuous water extraction. The novelty of the conducted research consists in the substantiation and experimental confirmation of the possibility of increasing the biostability of plant raw materials—Heracleum–by modifying with monoethanolamine(N→B)-trihydroxyborate, as well as establishing the mechanism of this process. When applied as preservatives of plant raw materials, it is recommended to carry out modifying and drying at room temperature as the high level of modification is reached at these conditions.
References
Luneva NN (2014) Zashchita i karantin rastenij [Plant protection and quarantine] 3:12–18
Mandenova IP (1970) Zametki po sistematiki i geografij rastenij [Notes on plant taxonomy and geography] 28:21–24
Nielsen C, Ravn HP, Nentwig W, Wade M (eds) (2005) The giant hogweed best practice manual. Guidelines for the management and control of an invasive weed in Europe. Forest and Landscape Denmark, Hoersholm
Kabysh TA (1985) Zashchita rastenij [Plant protection] 7:25–26
Geltman DV (2009) Rastitel’nye resursy [Plant resources] 3:68–75
Tkachenko KG (1989) Rastitel’nye resursy [Plant resources] 1:52–61
Tkachenko KG (2014) Vestnik udmurtskogo universiteta [Bulletin of the Udmurd University] 4:27–33
Cock M, Nentwig W, Ravn HP, Wade M (2007) Ecology and Management of Giant Hogweed (Heracleum Man-tegazziannum). CABI, Forest and Landscape Denmark
Mysnik EN (2013) Fitosanitarnaya optimizaciya agroekosistem [Phytosanitary optimization of agroecosystem] 2:301–302
Geltman DV, Buzunova IO, Konechnaya GYu (2009) Rastitel’nye resursy [Plant resources] 45:68–75
Vurasko AV, Simonova EI, Pervova IG, Minakova AR (2018) Vestnik Permskogo nacional’no-issledovatel’skogo politekhnicheskogo universi-teta. Prikladnaya ekologiya. Urbanistika. 2(30):21–32
Tabarov FS, Astakhov MV, Klimont AA, Kalashnik AT, Galimzyanov RR, Isaeva NV (2019) Rossijskie nanotekhnologii [Russian nanotechnology] 14:13–18
Ivanova TA, Getman VV, Koporulina EV (2019) Khimiya rastitel’nogo syr’ya [Chemistry of Plant Raw Materials] 2:311–319
Ivanova TA, Matveeva TN, Chanturia VA, Ivanova EN (2015) Fiziko-tekhnicheskie problemy razrabotki poleznyh iskopaemyh [Physical and technical problems of mining] 4:151–157
Musorina TA, Naumova EA, Shonina EV, Petrichenko MR, Kukolev MI (2019) Vestnik MGSU [MGSU Bulletin] 12(14):1555–1571
Chertes KL, Shestakov NI (2020) Moscow: MGSU [Monograph] 15(8):1135–1146
Koteneva IV (2011) Boron-nitrogen surface modifiers for protecting wood of building structures: monograph. MGSU, Moscow
Stepina IV, Sidorov VI, Klyachenkova OA (2014) Wood Res 584–586:1233–1236
Lesar B, Podlesnik B, Pohleven F, Humar M, Kralj P, Veber M (2008) J Membr Sci 53(3):17–26
Haider S, Park S-Y (2009) Carbohyd Polym 328:90–96
El Oudiani A et al (2017) Vestnik MGSU [MGSU Bulletin] 164:242–248
Acknowledgements
The team of authors would like to thank B.V. Lokshin [A.N.Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences (INEOS RAS)] and E.G. Leonova, T.B. Tumurova (CCU “Progress”, East Siberia State University of Technology and Management) for help in research.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Stepina, I., Sodomon, M., Semenov, V., Dorzhieva, E., Titova, I. (2022). Modifying Heracleum sosnowskyi Stems with Monoethanolamine(N→B)-trihydroxyborate for Manufacturing Biopositive Building Materials. In: Akimov, P., Vatin, N. (eds) Proceedings of FORM 2021. Lecture Notes in Civil Engineering, vol 170. Springer, Cham. https://doi.org/10.1007/978-3-030-79983-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-79983-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79982-3
Online ISBN: 978-3-030-79983-0
eBook Packages: EngineeringEngineering (R0)