Abstract
Globally, cardiovascular disease is a significant threat responsible for the higher death rate in the current scenario. Myocardial infarction causes ischemic injury to irreversibly damages cardiomyocytes, making them non-functional and leading to heart failure due to the lack of regeneration capacity. Clinically, organ transplantation and autologous cell-based therapies are potentially used to replace and restore damaged and unhealthy tissues. Nevertheless, the deficit of donor cells and lack of cell potency to differentiate into cardiac cells is another major problem for repairing damaged tissue. Thus, identifying the mechanism of cell differentiation, proliferation, and specification into the cardiac cells is a crucial step. This process can be accelerated by utilizing stem cells like pluripotent embryonic stem (ES) cells, which have the potency to differentiate into different cell lines and infinite probability to act as a source of cardiovascular cells under the influence of unspecified regulatory elements. This pluripotent ES differentiates into cardiomyocytes through the complex cellular pathways, controlled under gene regulation, expression, specific signaling molecules, and physiological parameters. To understand the diverse molecular machinery and regulatory pathway of stem cell differentiation is one of the difficult conundrums for our researcher. Although, to date, many innovations have been made to resolve this uncertainty to the formation of novel cures such as induced pluripotent stem cells (iPSCs). The chapter reviews the concepts of stem cell differentiation into cardiomyocytes through various in vitro cell culture and in vivo therapies at the pre-clinical to clinical level to evaluate the therapeutic application for the regeneration of the bio-functional heart.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Differentiation of Stem Cells into Cardiomyocytes Lineage: In Vitro Cell Culture
Introduction to Pluripotent Embryonic Cells
The stem cells that have unlimited capability to undergo self-renewal and the ability to undergo differentiation for the formation of ectoderm, endoderm, and mesoderm are called Pluripotent embryonic stem cells. These stem cells can arise into various cells that can form a complete human body (Ameen et al., 2008). The pluripotent stem cell-derived, in vitro, differentiated cardiomyocytes retain the functional properties of the pluripotent stem cell and own the phenotype of cardiac cells that are more stabilized and reproducible on both clinical and physiological aspects. Further, these pluripotent stem cell-derived, in vitro differentiated cardiomyocytes are the important models for in dissection of molecular events involved in cardiogenesis. These pluripotent stem cell-derived cardiomyocytes may serve as significant in vitro tools for developing and generating safe drugs in the pharmaceutical industry. The previous studies have reported the numerous methodologies in the formation of functional cardiomyocytes through the differentiation of human Embryonic Stem cells (hESCs) and induced pluripotent stem (iPS) cell technology (Zhang et al., 2009; Zwi et al., 2009; Haase et al., 2009).
There is a significant contribution of pluripotent stem cell-derived, in vitro differentiated cardiomyocytes from the future health perspective. Therefore, it is a critical requirement to validate standardized assays of such cells in the in vitro models to ensure the safety potential and efficacy of new drugs in the pharmaceutical industry (Vidarsson et al., 2010; Bram et al., 2009). However, there are impediments to generate the pluripotent stem cell-derived, in vitro differentiated cardiomyocytes cellular preparations that may cause cancer risk. The successful transplantation may reduce the chances of immune rejection (Braam et al., 2009). Furthermore, the advancement of research in the field of stem cell technology has generated the possibilities for the treatment of damaged and degenerated cardiac tissue through the repairing of damaged myocardium using stem cell-derived cardiomyocytes in preclinical studies in translational medicine (Nelson et al., 2009; van Laake, et al., 2007).
Development of Cardio Myocytes from Pluripotent Stem Cells
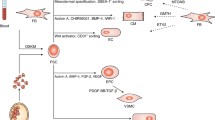
There are efficient methods that induce differentiation in cardiomyocytes to generate the qualitative and quantitative homogenous populations of cardiomyocytes for futuristic cell-based therapeutic applications. Perhaps, these futuristic cell therapies will urge well-structured methodologies and protocols for repetitive results fulfilling the regulatory requirements. The process of cardiogenesis is an extraordinarily vigorous and well-coordinated process that involves sequential expression of signaling molecules such as signal transduction molecules and transcription factors. The process of early differentiation of early mesoderm through cardiac mesoderm and committed cardiac progenitors to functional beating cardiomyocytes with the expression of markers is explained in Fig. 5.1.
There are four major steps in the generation of cardio myocytes from pluripotent stem cells:
-
1.
Mesoderm formation which requires T/Brachyury, Mesp1 and Nkx2.5, Tbx5/20, Gata4, Mef2c, and Hand1/2 transcription factors
-
2.
Pattern formation of mesoderm toward cardiogenic mesoderm
-
3.
Formation of cardiac mesoderm
-
4.
Maturation of early cardio myocytes.
The transcription factors, signaling pathways, growth factors, and microRNAs involved in developing specialized cardiac subtypes and differentiation process during the cardiogenesis are summarized in Table 5.1 and Fig. 5.1.
Steps of Pluripotent Stem Cells Differentiation into Cardiomyocytes
-
1.
Formation of embryoid body and spontaneous cardiomyocyte differentiation- The embryoid body consists of derivatives of the three germ layers (ectoderm, endoderm, and mesoderm), which develop spontaneously. These populations of cells are the mixed cells with functional properties of cardiomyocytes. These cardiomyocytes are the first cell types induced from pluripotent stem cells in embryoid bodies, which induce the stimulation of expression of markers for mesodermal and early cardiac cell lineages through the process of cell to cell communication (Tran et al., 2009).
-
2.
Co-culture of Pluripotent Stem Cells and cardio inductive Cell- The widely used for cardiomyocyte in vitro differentiation approach of co-culture involves the crucial function of anterior endoderm in the cardiac induction of adjacent mesodermal structures (Synnergren et al., 2008; Cao et al., 2008a, b; Xu et al., 2009). In this method, co-culture of the visceral endoderm-like cell line (END-2), a derivative of mouse P19 embryonic carcinoma (EC) cells and pluripotent stem cells which forms the beating clusters of cells that also demonstrate the characteristics of cardiomyocytes (Passier, 2008).
-
3.
Guided cardiomyocyte differentiation with Specific Factors-In this method, signaling pathways that are responsible for the regulation of cardiogenesis are mimicked in the form of cell culture. To this system, growth factors such as FGFs, BMPs, and Wnts are supplemented that has the capability to induce mesoderm or endoderm development in pluripotent stem cells (Xu et al., 2006; Rust et al., 2009; Kolossov et al., 2005).
-
4.
Cardiac progenitor cells-The three major cardiac cell lineages may arise from a common multipotent cardiovascular progenitor cell population that has the capability to display the specific expression of markers (Bu et al., 2009; Kattman et al., 2006; Moretti et al., 2006).
Methods in Cardiomyocyte Differentiation
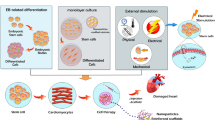
The methods in cardiomyocyte differentiation from pluripotent stem cells are explained in Fig. 5.2.
Concept and Methods of In Vitro Differentiation of ESCs and iPSCs Cells into Cardiomyocyte In Vitro Differentiation of ESCs
Embryonic stem cells (ESCs) or ES cells act as the best source for genetic manipulation through cultivation in vitro in the form of 3D aggregates, which are called embryoid bodies. ES cells can differentiate into derivatives of all three primary germ layers, including cardiomyocytes.
Several parameters specifically influence ES cells’ differentiation potency to form cardiomyocytes in culture (Wobus et al., 2002), as summarized in Fig. 5.3.
-
(1)
The initial number of cells in the EBs.
-
(2)
Supplements in media, FBS, growth factors
-
(3)
ES cell lines, and
-
(4)
The time of EB plating..
Differentiation of iPSCs into Human Cardio Myocytes
Induced pluripotent stem cells iPSCs are derived from patients who had different cardiac diseases or disorders, such as congenital heart disease, and become essential tools for studying the mechanisms underlying the disease pathogenesis and for the development of new treatment opportunities (Itzhaki et al., 2011; Moretti et al., 2010). However, on the basis of the methodology using embryonic stem cells, human iPSCs are capable of differentiating into beating cardiomyocytes through exposure to a variety of stimuli (Shiba et al., 2009; Yoshida & Yamanaka, 2011) (Table 5.2). ESCs, methods for producing embryoid bodies using iPSCs, have been successfully differentiated into cardiomyocytes (Zhang et al., 2009).
Methods for Differentiation of iPSCs and ESCs into Cardiomyocytes
Figure 5.3 and Table 5.2 summarizes the differentiation approaches currently used for cardiomyocyte differentiation from human embryonic stem cells, and human-induced pluripotent stem cells involve different growth factors as well as signaling molecules to form cardiomyocytes.
Future Trends of iPSCs in Stem Cell Research with Regard to Cardio Myocytes
-
Although iPSCs are good candidates for drug screening and disease modeling applications, they are not without limitations. The following reasons make them unfavorable promising entities.
-
The late-onset nature of many diseases likely shows a failure in reiterating the disease development accurately.
-
There is no assurance of result without in vivo studies as complex cellular interactions in human metabolic processes often cannot be recapitulated in these in vitro culture systems.
-
A large panel of patient-derived iPSCs needs to be evaluated to consider when the wide range of immune responses may elicit when applied to a larger heterogeneous human population. It requires clinical trials of a specific drug (Grskovic et al., 2011).
-
For these reasons, and despite their considerable promise, patient-specific iPSC models failed to substitute animal models. The persistent issue with maturity and accurate recapitulation of onset disease phenotypes has led to an explosion of bioengineering strategies to improve engineered cardiac tissue development in vitro. Such techniques seek to impersonate multiple aspects of the cardiac micro environmental niche.
Applications of In-Vitro Culture in Cardiogenesis
Cardiac transplantation is currently the treatment of choice for end-stage heart failure; however, the number of available donor organs limits this treatment to a minority of patients. In-vitro Cell-based therapies have recently emerged as an innovative approach for the treatment of degenerative heart diseases. Significant challenges remain to be overcome before this therapy can be practiced in clinics. Other critical problems include inefficient differentiation, tumorigenicity, immunogenicity, as well as complicated ethical issues surrounding the isolation of cells from in vitro fertilized human embryos (Blin et al., 2010). Human ES cells obviously represent a potentially valuable and renewable source of cells that could be used for transplantation therapy. Human ES cell lines are immortal and pluripotent, and consequently, derivatives of these cell lines can theoretically be used to treat a wide range of devastating diseases whose underlying pathology involves cell degeneration, death, or acute injury. Significant challenges to the therapeutic application are the sustenance against the host system immune rejection. The development of large stem cell banks that represent a wide array of histocompatibility backgrounds is a suggested trial to overcome immune rejection challenge.
Differentiation of Stem Cells into Cardiomyocyte Lineage: In Vivo Transplantation in Animal Models
Introduction to Embryonic Cells Involved in In-Vivo Development of Cardiomyocyte Lineage
In invertebrates, the heart is the first functional organ developed after the gastrulation phase of embryogenesis. The intercalating anterior mesodermal cell between the ectoderm and endoderm germ layer forms the primary mid-streak act as progenitor cardiomyocytes. These cardiac progenitors’ cells specifically differentiate into cardiomyocyte lineages to form heart muscle cells (Aguilar-Sanchez et al., 2018). Besides that, the endocardia cells form the endothelial cell lining, and vascular smooth muscle forms the vascular system. The cardiomyocytes have a low division rate, and their division rate subsequently reduces or stops until the postnatal time reached. This results in a less or restricted number of proliferated cells, but the heart grows with enlarged cell size to perform more activity (Senyo et al., 2013; Ali et al., 2014). Therefore, in the case of adults, the cardiomyocytes’ self-renewal or proliferation rate is significantly much lesser than the endothelial and mesenchymal cell; as a result heart injury or damage cannot be cured selfheal themselves (Kajstura et al., 2010). The turnover rate of the human cardiomyocytes is very poor; only the cardiac progenitor cells present in the heart possess the self-renewing capacity and have multipotent stem cell that could differentiate into cardiac cell lineages (Bang et al., 2016; Bergmann et al., 2009). In a developing embryo body, the early mesodermal cell differentiated to form cardiovascular progenitors, and these progenitors potentially differentiate into cardiac cells, i.e., cardiomyocytes, smooth muscle cells, and endothelial cells, respectively (Brade et al., 2013). These cardiac progenitor matures and results in the separated chambered heart formation. Thus, embryonic stem (ES) cells could be utilized as sources for the repair and regeneration of cardiac incision or injury.
-
Embryo stem cell → Ectoderm, Endoderm, Mesoderm
-
Mesoderm → Cardiovascular progenitor → Smooth muscle cells, Endothelial cells, Cardiac progenitors
-
Cardiac progenitors → Mature cardiomyocytes (MC)
-
Mature cardiomyocytes (MC), Smooth muscle cells, endothelial cells → Bio-functional heart.
-
5.
These progenitors, embryonic pluripotent stem cells differentiated into differentiated cell lineages, make a complex cardiac system. Other than it different cell lines such as adult stem cells, cardiac cells, vascular endothelial cells, mesenchymal cells act as the progenitor to cardiac tissue / whole organ generation (Brade et al., 2013; Wysoczynski & Bolli, 2020). However, during the post-natal period, the differentiation or capacity of cardiac cells to regenerate the tissue or replace the damaged one reduces; stem cells are the only opportunity.
-
6.
In cardiac damage, grafting or whole organ transplantation is the only clinical treatment to save a life. In clinical studies, it was reported that patients own stem cells (multipotent cell or bone-marrow) used for repair of damaged tissue, but due to lack of potency they are less effective and have a short life-span (Keller, 2005). ES cells have pluripotency, differentiate into any cell lineage, and make a better opportunity for clinical studies and tissue regeneration purposes. ES cells derived from the inner blastomeres of a developing embryo, subsequently cultured, maintained into cardiomyocytes lineages and function of different factors of signal transduction pathways involved in ES cell differentiation (Guo et al., 2016). It was reported that the ES cell obtained from the mouse demonstrated pluripotency and regenerated in all types of tissue cell under in vivo conditions (Morey et al., 2015). The potential of ES cells to differentiate and form cardiac lineage, can be identified on the basis of their physiological properties, such as the contracting nature of cardiomyocytes (Bartosh et al., 2008). The development of cardiomyocyte lineage form ES cells results in the formation of hematopoietic and vascular systems as same as the in vivo development (Arabadjiev et al., 2014). The applicability of ES cell differentiated cardiomyocytes used for the transplantation and to cure the cardiovascular problems. Therefore, ES cells have vast potential in the area of therapeutics and tissue regeneration, such as to treat cardiovascular diseases and restore cardiac function.
Function of Different Factors of Signal Transduction Pathways Involved in ES Cell Differentiation
ES cells are isolated from the embryonic inner cell mass self-renewal and pluripotent, but they need specific signaling to differentiate and proliferate into specific cell lineage. Besides that, to improve the cell functioning and survivability of ES cells derived cardiomyocyte, there is a need for extrinsic and intrinsic factors. (Arabadjiev et al., 2014). The progenitor cell lineage commitment and differentiation into the cardiomyocyte require the specific intrinsic factors such as zinc finger transcription factor GATA4, Nkx2.5 for the activation of myocardial differentiation genes and plays a role in the activation of many myocardial differentiation genes (Arabadjiev et al., 2014; Chen et al., 2008). The specific differentiation of genes expressed at different myocardial differentiation stages is listed in the figure below (Table 5.3).
Thus, in 2002, Yang and co-worker designed VEGF incorporated cardiomyocytes to treat myocardial infarction in mice model (Yang et al., 2002). The transplanted VEGF-expressing cells result in neo-vascularization and regain functioning of damaged hearts compared to non-VEGF-expressed transplanted cells. Besides the intrinsic factors, extrinsic factors i.e., growth factors, and chemicals induced the signaling and gene expression for the ES cells differentiation into the myocardial lineages (Bartosh, 2008; Gude et al., 2018; Pal et al., 2012). In cardiomyogenesis, these factors (Table 5.4) play an essential role in heart functioning and be a potential therapeutic agent used for cardiomyocyte transplantation.
Evaluation of Efficacy of Embryonic Cells in Functional and Anatomical Cardiac Repair in Animal Model
The embryonic and cardiac progenitor cells in the heart have self-renewing capability; thus, they will differentiate into the neo myocardial cell lineages to repair the cardiac incision or injury. It was reported that the mesenchymal cells used for the treatment of myocardial infarction have paracrine effects but lack the differentiation property into the cardiomyocytes (Brade et al., 2013). Besides that, the adult cardiac cell lineages in vivo differentiation result in enhancement of sarcomere organization and high beating rate (Bang et al., 2016). Hence, embryonic cells must be the focused cell line for cardiac repair.
In 1996, the first in vivo study in mice model having dystrophy to demonstrate the role of ES cell-derived cardiomyocytes for expressing α-cardiac MHC and improve the survivability (Klug et al., 1996). The survivability rate of in vivo transplanted ES-cell-derived cardiac cells up to 32 weeks and cured the myocardial infraction (Min et al., 2003). In the twentieth century, in vivo studies revealed the human ES cells (hESC) differentiation into cardiomyocytes for cardiac injury and diseases (Nir et al., 2003; Wysoczynski & Bolli, 2020). They observed both mouse ES and hES cells have the same in vivo maturation rate, but the cardiomyocytes derived from hES cells lack complete maturation and conduction.
After that, it was shown that the clinical applicability of hES cell-derived cardiomyocytes for the treatment of bradycardia in pig model by making as biological pace-makers’ for xenogeneic transplantation (Kehat et al., 2004; Shiba et al., 2012). The regenerated, repaired heart showed the spontaneous rhythmic contradiction of the transplanted cells. In the animal model study, in mouse treated with stem cell injection, results in regeneration of ventricular ejection fractions. However, in another study, the myocardial infraction was repaired with the engraftment of the matrix associated with the allogeneic stem cells (Cheraghi et al., 2016).
The ES cells differentiated into the cardiomyocyte lineages is mimic the in vivo development of the cardiomyocyte. The histopathological analysis studies reveal that the ES differentiated cardiac cell lineages require the incorporation of another signaling factor such as VEGF for the regeneration of functional cardiac tissue with better vascularization (Cao et al., 2008a, b).
However, it was found that the injected mature differentiated cardiomyocyte has a lesser therapeutic effect as compared to the immature ES cell lineage (Cheraghi et al., 2016; Nir et al., 2003). Thus, the identification, selection, and utilization of ES cells at specific cell dividing stage still query that has been focused by the researcher at pre-clinical for the evaluation of ES cell treatment.
Evaluation of Embryonic Cell Therapy for the Repair and Regeneration of Cardiac Tissue at the Clinical Level
ES cells is the most focused and novel source for the treatment of any kind of tissue injury, to repair, regenerate the neo-tissue or organ, and to cure different diseases or deformities such as cancer, cardiovascular, neural damages, etc. (Cheraghi et al., 2016). However, the in vivo success rate of the ES cell therapy demonstrates the limited functional potential and requires standard cell therapy parameters (Terashvili & Bosnjak, 2019). Thus, for the application of ES cell cardiac tissue repair and regeneration, there are some merits and demerits that we have to focus on the better outstanding. The challenges for the clinical application of ES cells are the identification of optimized cell lineages, therapy duration, chances of host rejection, the susceptibility of evocation of immune response, optimization of dosages, and enhancement of cell signaling for cardiac repair infarction (Wysoczynski & Bolli, 2020). Thus, there is a need to develop the methodologies, protocols for the enhancement and propagation of injected stem cells in the host body of myocardial regeneration.
The isolation of human ES cell (hESC) was focused by the researcher, as well as their applicability as a therapeutic agent has been done. The application of donor allogeneic embryonic cells for the treatment of cardiac injury is mocktail of cell population involves the MSCs, CPCs, pluripotent cells. At the clinical level, the ES cell therapy for the repairing of myocardial infraction results in an improved healing rate with better cardiac efficiency. The maturation rate of hES cells is not the same as the mES cells. Therefore, it shows more effectiveness as compared to the other cell lineages (Hoshino et al., 2007; Terashvili & Bosnjak, 2019). However, the standard dosage and treatment parameter for the cardiomyocyte is still a question.
Cardiac stem cell therapies may also result in inflammation and graft rejection by the host body; thus, clinical cell therapy has no significant outcome (Tang et al., 2018). The selection of the specific immature cell lineages and to provide a micro-environment to differentiate them into specific cell lineage under in vivo conditions is an ongoing issue to be resolved by the researcher. The success rate of the ES cell lines for cardiac repair has been going on, and research focused on the cell lineages associated with matrix or grafts to overcome the problem of graft rejection, fibrosis, and bio-functional tissue regeneration (Cheraghi et al., 2016). In situ injection of the bio-engineered hydrogel enriched with ECM protein and seeded with cells results in enhanced cardiac differentiation at in vitro level as well as repair of myocardial injury (Bai et al., 2019). Matrix associated cell grafts open the new era for embryonic cell-based therapies to study their synergistic effect for the complete neo-tissue regeneration. At the worldwide level, scientists and medical experts work together to cure millions of patients suffering from myocardial infarctions, congenital disabilities and improve the survival rate.
References
Aguilar-Sanchez, C., Michael, M., & Pennings, S. (2018). Cardiac stem cells in the postnatal heart: Lessons from development. Stem Cells International.
Ali, S. R., Hippenmeyer, S., Saadat, L. V., Luo, L., Weissman, I. L., & Ardehali, R. (2014, June 17). Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proceedings of the National Academy of Sciences, 111(24), 8850–8855.
Ameen, C., Strehl, R., Bjorquist, P., Lindahl, A., Hyllner, J., & Sartipy, P. (2008). Human embryonic stem cells: current technologies and emerging industrial applications. Critical Reviews in Oncology/Hematology, 65(1), 54–80.
Arabadjiev, A., Petkova, R., Chakarov, S., Pankov, R., & Zhelev, N. (2014). We heart cultured hearts. A comparative review of methodologies for targeted differentiation and maintenance of cardiomyocytes derived from pluripotent and multipotent stem cells. BioDiscovery, 14, p. e8962.
Bai, R., Tian, L., Li, Y., Zhang, J., Wei, Y., Jin, Z., Liu, Z., & Liu, H. (2019). Combining ECM hydrogels of cardiac bioactivity with stem cells of high cardiomyogenic potential for myocardial repair. Stem Cells International.
Bang, O. Y., Kim, E. H., Cha, J. M., & Moon, G. J. (2016). Adult stem cell therapy for stroke: Challenges and progress. Journal of Stroke, 18(3), 256.
Bartosh, T. J. (2008). Molecular regulation of cardiac stem cell growth and differentiation by extrinsic factors and novel intracellular signaling pathways. University of North Texas Health Science Center at Fort Worth.
Bergmann, O., Bhardwaj, R. D., Bernard, S., Zdunek, S., Barnabé-Heider, F., Walsh, S., Zupicich, J., Alkass, K., Buchholz, B. A., Druid, H., & Jovinge, S. (2009). Evidence for cardiomyocyte renewal in humans. Science, 324(5923), 98–102.
Blin, G., Nury, D., Stefanovic, S., Neri, T., Guillevic, O., Brinon, B., & Bellamy, V., et al. (2010). A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. The Journal of Clinical Investigation, 120(4), 1125–1139.
Braam, S. R., Passier, R., & Mummery, C. L. (2009). Cardiomyocytes from human pluripotent stem cells in regenerative medicine and drug discovery. Trends in Pharmacological Sciences, 30(10), 536–545.
Brade, T., Pane, L. S., Moretti, A., Chien, K. R., & Laugwitz, K. L. (2013). Embryonic heart progenitors and cardiogenesis. Cold Spring Harbor Perspectives in Medicine, 3(10), a013847.
Bu, L., Jiang, X., Martin-Puig, S., et al. (2009). Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature, 460(7251), 113–117.
Burridge, P. W., Keller, G., Gold, J. D., & Wu, J. C. (2012). Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell, 10(1), 16–28. https://doi.org/10.1016/j.stem.2011.12.013.
Callis, T. E., Deng, Z., Chen, J. F., & Wang, D. A. Z. (2008). Muscling through the microRNA world. Experimental Biology and Medicine, 233(2), 131–138.
Cao, F., Wagner, R. A., Wilson, K. D., et al. (2008). Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One, 3(10), Article ID e3474.
Cao, F., Wagner, R. A., Wilson, K. D., Xie, X., Fu, J. D., Drukker, M., Lee, A., Li, R. A., Gambhir, S. S., Weissman, I. L., & Robbins, R. C. (2008). Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PloS One, 3(10), e3474.
Chen, K., Wu, L., & Wang, Z. Z. (2008). Extrinsic regulation of cardiomyocyte differentiation of embryonic stem cells. Journal of Cellular Biochemistry, 104(1), 119–128.
Cheraghi, M., Namdari, M., Eatemadi, A., & Negahdarib, B. (2016). Recent advances in cardiac regeneration: Stem cell, biomaterial and growth factors. Biomedicine & Pharmacotherapy, 87(2017).
Elliott, D. A., Braam, S. R., Koutsis, K., Ng, E. S., Jenny, R., Lagerqvist, E. L., & Biben, C., et al. (2011). NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nature Methods, 8(12), 1037–1040.
Grskovic, M., Javaherian, A., Strulovici, B., & Daley, G. Q. (2011). Induced pluripotent stem cells–opportunities for disease modelling and drug discovery. Nature Reviews. Drug Discovery, 10, 915–929. [PubMed: 22076509].
Gude, N. A., Broughton, K. M., Firouzi, F., & Sussman, M. A. (2018). Cardiac ageing: Extrinsic and intrinsic factors in cellular renewal and senescence. Nature Reviews Cardiology, 15(9), 523–542.
Guo, G., von Meyenn, F., Santos, F., Chen, Y., Reik, W., Bertone, P., Smith, A., & Nichols, J. (2016). Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Reports, 6(4), 437–446.
Haase, A., Olmer, R., Schwanke, K., et al. (2009). Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell, 5(4), 434–441.
Hiroi, Y., Kudoh, S., Monzen, K., et al. (2001). Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nature Genetics, 28(3), 276–280.
Hoshino, K., Ly, H. Q., Frangioni, J. V., & Hajjar, R. J. (2007). In vivo tracking in cardiac stem cell-based therapy. Progress in Cardiovascular Diseases, 49(6), 414–420.
Hudson, J., Titmarsh, D., Hidalgo, A., Wolvetang, E., & Cooper-White, J. (2012). Primitive cardiac cells from human embryonic stem cells. Stem Cells and Development, 21(9), 1513–1523.
Itzhaki, I., Maizels, L., Huber, I., et al. (2011). Modelling the long QT syndrome with induced pluripotent stem cells. Nature, 471(7337), 225–229.
Ivey, K. N., Muth, A., Arnold, J., et al. (2008). MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell, 2(3), 219–229.
Kajstura, J., Urbanek, K., Perl, S., Hosoda, T., Zheng, H., Ogórek, B., Ferreira-Martins, J., Goichberg, P., Rondon-Clavo, C., Sanada, F., & D’Amario, D. (2010). Cardiomyogenesis in the adult human heart.
Kattman, S. J., Huber, T. L., & Keller, G. (2006). Multipotent Flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Developmental Cell, 11(5), 723–732.
Kattman, S. J., Witty, A. D., Gagliardi, M., Dubois, N. C., Niapour, M., Hotta, A., Ellis, J., & Keller, G. (2011). Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell, 8(2), 228–240.
Kehat, I., Khimovich, L., Caspi, O., Gepstein, A., Shofti, R., Arbel, G., Huber, I., Satin, J., Itskovitz-Eldor, J., & Gepstein, L. (2004). Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nature Biotechnology, 22(10), 1282–1289.
Keller, G. (2005). Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes & Development, 19(10), 1129–1155.
Klug, M. G., Soonpaa, M. H., Koh, G. Y., & Field, L. J. (1996). Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. The Journal of Clinical Investigation, 98(1), 216–224.
Kolossov, E., Lu, Z., Drobinskaya, I., et al. (2005). Identification and characterization of embryonic stem cell-derived pacemaker and atrial cardiomyocytes. FASEB Journal, 19(6), 577–579.
Laflamme, M. A., Chen, K. Y., Naumova, A. V., Muskheli, V., Fugate, J. A., Dupras, S. K., & Reinecke, H., et al. (2007). Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology, 25(9), 1015–1024.
Marvin, M. J., Di Rocco, G., Gardiner, A., Bush, S. M., & Lassar, A. B. (2001). Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes and Development, 15(3), 316–327.
Mima, T., Ueno, H., Fischman, D. A., Williams, L. T., & Mikawa, T. (1995). Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proceedings of the National Academy of Sciences of the United States of America, 92(2), 467–471.
Min, J. Y., Yang, Y., Sullivan, M. F., Ke, Q., Converso, K. L., Chen, Y., Morgan, J. P., & Xiao, Y. F. (2003). Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. The Journal of Thoracic and Cardiovascular Surgery, 125(2), 361–369.
Moretti, A., Caron, L., Nakano, A., et al. (2006). Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell, 127(6), 1151–1165.
Moretti, A., Bellin, M., Welling, A., et al. (2010). Patient-specific induced pluripotent stem-cell models for long-QT syndrome. New England Journal of Medicine, 363(15), 1397–1409.
Morey, L., Santanach, A., & Di Croce, L. (2015). Pluripotency and epigenetic factors in mouse embryonic stem cell fate regulation. Molecular and Cellular Biology, 35(16), 2716–2728.
Nelson, T. J., Martinez-Fernandez, A., Yamada, S., PerezTerzic, C., Ikeda, Y., & Terzic, A. (2009). Repair of acute myocardial infarction with induced pluripotent stem cells induced by human stemness factors. Circulation, 120(5), 408–416.
Nir, S. G., David, R., Zaruba, M., Franz, W. M., & Itskovitz-Eldor, J. (2003). Human embryonic stem cells for cardiovascular repair. Cardiovascular Research, 58(2), 313–323.
Pal, R., Mamidi, M. K., Das, A. K., & Bhonde, R. (2012). Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation potential of human embryonic stem cells. Archives of Toxicology, 86(4), 651–661.
Pasquinelli, A. E., Hunter, S., & Bracht, J. (2005). MicroRNAs: A developing story. Current Opinion in Genetics and Development, 15(2), 200–205.
Passier, R., Ward-Van Oostwaard, D., Snapper, J., et al. (2005). Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells, 23(6), 772–780.
Peterkin, T., Gibson, A., & Patient, R. (2003). GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation. EMBO Journal, 22(16), 4260–4273.
Plageman, T. F., Jr., & Yutzey, K. E. (2004). Differential expression and function of Tbx5 and Tbx20 in cardiac development. Journal of Biological Chemistry, 279(18), 19026–19034.
Riley, L. A.-C., & Cross, J. C. (1998). The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nature Genetics, 18(3), 271–275.
Rust, W., Balakrishnan, T., & Zweigerdt, R. (2009). Cardiomyocyte enrichment from human embryonic stem cell cultures by selection of ALCAM surface expression. Regenerative Medicine, 4(2), 225–237.
Senyo, S. E., Steinhauser, M. L., Pizzimenti, C. L., Yang, V. K., Cai, L., Wang, M., Wu, T. D., Guerquin-Kern, J. L., Lechene, C. P., & Lee, R. T. (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature, 493(7432), 433–436.
Shiba, Y., Hauch, K. D., & Laflamme, M. A. (2009). Cardiac applications for human pluripotent stem cells. Current Pharmaceutical Design, 15, 2791–2806. [PubMed: 19689350].
Shiba, Y., Fernandes, S., Zhu, W. Z., Filice, D., Muskheli, V., Kim, J., Palpant, N. J., Gantz, J., Moyes, K. W., Reinecke, H., & Van Biber, B. (2012). Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature, 489(7415), 322–325.
Synnergren, J., Akesson, K., Dahlenborg, K., et al. (2008). Molecular ˚ signature of cardiomyocyte clusters derived from human embryonic stem cells. Stem Cells, 26(7), 1831–1840.
Tang, J. N., Cores, J., Huang, K., Cui, X. L., Luo, L., Zhang, J. Y., Li, T. S., Qian, L., & Cheng, K. (2018). Concise review: Is cardiac cell therapy dead? embarrassing trial outcomes and new directions for the future. Stem Cells Translational Medicine, 7(4), 354–359.
Terashvili, M., & Bosnjak, Z. J. (2019). Stem cell therapies in cardiovascular disease. Journal of Cardiothoracic and Vascular Anesthesia, 33(1), 209–222.
Tran, T. H., Wang, X., Browne, C., et al. (2009). Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells, 27(8), 1869–1878.
Uosaki, H., Fukushima, H., Takeuchi, A., Matsuoka, S., Nakatsuji, N., Yamanaka, S., & Yamashita, J. K. (2011). Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PloS One, 6(8), e23657.
van Laake, L. W., Passier, R., Monshouwer-Kloots, J., et al. (2007). Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Research, 1(1), 9–24.
van Rooij, E., Liu, N., & Olson, E. N. (2008). MicroRNAs flex their muscles. Trends in Genetics, 24(4), 159–166.
Vidarsson, H., Hyllner, J., & Sartipy, P. (2010). Differentiation of human embryonic stem cells to cardiomyocytes for in vitro and in vivo applications. Stem Cell Reviews and Reports, 6(1), 108–120.
Wang, Z., Luo, X., Lu, Y., & Yang, B. (2008). miRNAs at the heart of the matter. Journal of Molecular Medicine, 86(7), 771–783.
Watt, A. J., Battle, M. A., Li, J., & Duncan, S. A. (2004). GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 101(34), 12573–12578.
Willems, E., Spiering, S., Davidovics, H., Lanier, M., Xia, Z., Dawson, M., Cashman, J., & Mercola, M. (2011). Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circulation Research, 109(4), 360–364.
Winnier, G., Blessing, M., Labosky, P. A., & Hogan, B. L. M. (1995). Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes and Development, 9(17), 2105–2116.
Wobus, A. M., Guan, K., Yang, H.-T., & Boheler, K. R. (2002). Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods in Molecular Biology, 185, 127–156.
Wysoczynski, M., & Bolli, R. (2020). A realistic appraisal of the use of embryonic stem cell-based therapies for cardiac repair. European Heart Journal, 41(25), 2397–2404.
Xu, C., Police, S., Hassanipour, M., & Gold, J. D. (2006). Cardiac bodies: A novel culture method for enrichment of cardiomyocytes derived from human embryonic stem cells. Stem Cells and Development, 15(5), 631–639.
Xu, X. Q., Soo, S. Y., Sun, W., & Zweigerdt, R. (2009). Global expression profile of highly enriched cardiomyocytes derived from human embryonic stem cells. Stem Cells, 27(9), 2163–2174.
Yang, Y., Min, J. Y., Rana, J. S., Ke, Q., Cai, J., Chen, Y., Morgan, J. P., & Xiao, Y. F. (2002). VEGF enhances functional improvement of postinfarcted hearts by transplantation of ESC-differentiated cells. Journal of Applied Physiology, 93(3), 1140–1151.
Yoshida, Y., & Yamanaka, S. (2011). iPS cells: A source of cardiac regeneration. Journal of Molecular and Cellular Cardiology, 50, 327–332. [PubMed: 21040726].
Zhang, H., & Bradley, A. (1996). Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development, 122(10), 2977–2986.
Zhang, J., Wilson, G. F., Soerens, A. G., Koonce, C. H., Yu, J., Palecek, S. P., et al. (2009). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research, 104, e30–e41. [PubMed: 19213953].
Zhao, Y., & Srivastava, D. (2007). A developmental view of microRNA function. Trends in Biochemical Sciences, 32(4), 189–197.
Zwi, L., Caspi, O., Arbel, G., et al. (2009). Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation, 120(15), 1513–1523.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Malik, S., Dhasmana, A. (2021). Differentiation of Stem Cells into Cardiomyocyte Lineage: In Vitro Cell Culture, In Vivo Transplantation in Animal Models. In: Khan, F.A. (eds) Advances in Application of Stem Cells: From Bench to Clinics. Stem Cell Biology and Regenerative Medicine, vol 69. Humana, Cham. https://doi.org/10.1007/978-3-030-78101-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-78101-9_5
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-78100-2
Online ISBN: 978-3-030-78101-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)