Abstract
Drug delivery to the ocular diseases requires strategic approaches due to the existence of anatomical/static and physiological/dynamic barriers. Several ophthalmic conventional topical formulations are designed as solutions, suspensions, ointments, or emulsions to achieve an effective drug dose to the ocular tissues. In addition, various novel nanoformulation-based delivery systems have been explored through various routes of administration and showed promising results. In this book chapter, we briefly reviewed the routes of administration, ocular barriers with an emphasis on the topical route of drug administration, and the development of ocular suspension and nanosuspension formulations. The considerations in formulation development of suspension dosage forms are discussed to facilitate the development of safe, stable, and efficacious drug products. Moreover, the factors affecting the stability of suspensions/nanosuspensions and approaches to develop a stable product are summarized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ophthalmic formulations
- Suspension
- Nanosuspension
- Ocular barrier
- Topical delivery
- Stability
- Formulation development
- Excipients
Introduction
It is of paramount importance to understand the anatomy and physiology of human eye from both the front of the eye (anterior) and back of the eye (posterior) perspectives in order to develop effective ophthalmic products. The eye is a complicated organ and protected from external materials by impermeable epithelium, tear secretion, and ocular drainage pathways to clear any foreign object. The eye is comprised of connective, vascular, and neural tissues. The connective tissue consists of the transparent cornea connected to the sclera through the limbus. The vascular tissue is composed of the choroid and ciliary body connected by the iris in front of the globe. The retina constitutes the neural tissue, which transmits the electrical impulse to the brain through the optic nerve. Anatomically, the eye is subdivided into anterior and posterior segments. The anterior segment includes the cornea, pupil, iris, ciliary body, conjunctiva, lens, and aqueous humor, whereas the posterior segment consists of the sclera, choroid, and retina, surrounding the vitreous cavity filled with the vitreous humor. The aqueous humor provides nutrients for the lens and cornea and maintains the intraocular pressure (Janagam et al. 2017). The structure of the eye is schematically represented in Fig. 1 (reproduced with permission from (Barar et al. 2016)).
Schematic demonstration of the anatomy and the biological membranes and barriers of the eye. Panels (A, B, C, and D) represent the corneal epithelial barrier (CEB), the blood-aqueous barrier (BAB), the biostructures of the retina, and the blood-retinal barriers (BRB), both the inner endothelial and outer pigmented epithelial barriers. (Reproduced with permission from Barar et al. (2016))
Clinically, the anterior segment diseases are often treated by using solutions, suspensions, or ointments, however, the existence of anatomical/static (conjunctiva, cornea, sclera, blood aqueous, and retinal) and physiological/dynamic (choroid blood flow, efflux transporters, tear washing, nasolacrimal drainage) barriers limits the efficacy/bioavailability of these conventional dosage forms (Agrahari et al. 2017; Barar et al. 2016; Gaudana et al. 2010; Janagam et al. 2017). In addition to the eye’s intrinsic ability to exclude external molecules, the undesirable physicochemical properties, such as the low aqueous solubility of drugs, impose a significant challenge to ensure a high therapeutic efficacy. Furthermore, an ideal ocular formulation should be self-administered (for topically applied dosage forms) and nonirritating to ensure high patient compliance.
Routes of Ocular Drug Administration and the Associated Barriers to Consider for Developing Ophthalmic Products
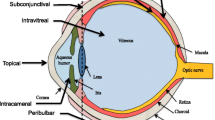
There are several routes of drug administration to the anterior segment of the eye: topical, intracameral, subconjunctival, and systemic (Janagam et al. 2017). The typical routes assessed for the posterior segment drug delivery are intraocular (suprachoroidal, intravitreal), topical, systemic, and periocular (subconjunctival, sub-Tenon, retrobulbar) (Agrahari et al. 2017; Peptu et al. 2015). These administration routes are briefly discussed below and schematically represented in Fig. 2. Depending on the route of administration, one or more ocular barriers need to be bypassed for drugs to reach the anterior or posterior segments.
Administration routes for delivering therapeutics to the anterior and posterior segments of the eye. (Adapted from Agrahari et al. (2017))
Intracameral administration is a local drug delivery method for direct injection into the anterior segment, avoiding the first-pass metabolism, cornea, conjunctiva, and blood-aqueous barriers (Janagam et al. 2017).
Subconjunctival administration administers drugs into the subconjunctival space around the outside of the sclera (Janagam et al. 2017). The drug then penetrates through the sclera and reaches to the anterior segment. It is a minimally invasive and local route avoiding the cornea and blood-aqueous barriers and the first-pass metabolism (Janagam et al. 2017).
Systemic administration can deliver drugs to both the anterior and posterior segments, but with low bioavailability due to the presence of the blood-aqueous barrier and blood-retinal barrier, respectively (Agrahari et al. 2017; Barar et al. 2016; Janagam et al. 2017). Because of the presence of the tight junctional complexes in the two layers comprising the blood-aqueous barrier, it restricts the penetration of drugs from the blood into the aqueous humor. Thus, high doses are required to achieve therapeutic drug levels in the aqueous humor, which can cause adverse effects. In addition, efflux transporters expressed on the apical and basolateral cell membranes of the human retinal pigment epithelium (RPE) limit drug permeation from the choroid to the retina after systemic administration (Barar et al. 2016; Janagam et al. 2017).
Intravitreal delivery has the potential to provide the highest intraocular bioavailability by circumventing several barriers of the posterior eye segment due to its proximity to the retina, choroid, and RPE (Rowe-Rendleman et al. 2014). However, intravitreal administration is invasive and painful, and repeated injections are associated with risks of hemorrhage, retinal detachment, increased intraocular pressure, cataract formation, bacterial endophthalmitis, and degeneration of photoreceptors (Falavarjani and Nguyen 2013).
Periocular delivery is an emerging, less-invasive route and utilizes the trans-scleral pathway to deliver drugs next to the choroid. However, drug losses via conjunctival, episcleral blood, and lymphatic flow are the limiting factors to periocular administration (Peptu et al. 2015; Raghava et al. 2004).
Suprachoroidal injection is one of the most suitable routes to reach the choroid and vitreous humor (Rai Udo et al. 2015; Hartman and Kompella 2018) since the suprachoroidal space lies internal to the sclera and provides a natural route for drugs injected across the sclera along the inner surface of the eye into the posterior segment.
Topical route of drug administration to the eye is the most convenient and self-administrable route for the anterior segment and provides high patient compliance and minimal side effects. Depending on the formulation and drug physiochemical characteristics, drugs can reach to the cornea, conjunctiva, sclera, aqueous humor, iris, ciliary body, vitreous humor, and retina sites after topical instillation (Janagam et al. 2017). However, precorneal factors and anatomical barriers adversely affect the bioavailability of topical formulations (Fig. 3) (reproduced with permission from (Janagam et al. 2017)). Precorneal factors include solution drainage, blinking, the tear film, tear turnover, and corneal/conjunctival barriers. Due to these factors, only ~1–7% of the topically administered drugs can reach to the aqueous humor (Janagam et al. 2017; Ghate and Edelhauser 2006). The tear film, composed of water, electrolytes, and various proteins, is the first obstacle for topically administered drugs and consists of three layers: an outermost lipid layer, a thicker aqueous middle layer, and an innermost mucin layer. Human tear volume is about 3.4–10.7 μl per eye (Scherz et al. 1974) with a turnover rate of 0.5–2.2 μl/min (Janagam et al. 2017; Worakul and Robinson 1997; Mishima et al. 1966). The tear film has a rapid restoration time of 2–3 min (Janagam et al. 2017; Worakul and Robinson 1997). Due to the fast turnover rate of the tear film, the topically administered doses are quickly washed away and drained into the nasolacrimal duct after instillation. Due to these factors, the contact time of topically administered formulations with the ocular membranes is low, and less than 5% of the applied dose permeates the eye and reaches the intraocular tissues (Gaudana et al. 2010; Ali et al. 2016).
Schematic representation of disposition of drug in the eye following topical administration. (Reproduced with permission from Janagam et al. (2017))
The cornea also limits the penetration of exogenous substances into the eye. It is composed of five layers: the epithelium, Bowman’s membrane, stroma, Descemet’s membrane, and endothelium (Janagam et al. 2017; Sridhar 2018). Each layer has a different polarity, and the epithelium, stroma, and endothelium layers form substantial barriers to drug penetration (Gaudana et al. 2010; Janagam et al. 2017). The corneal epithelium limits the permeation of hydrophilic molecules due to the hydrophobicity of the epithelium and the presence of tight junctional proteins between the corneal epithelial cells. The highly hydrated stroma poses a significant barrier for the penetration of lipophilic drugs. Most of the topical drugs permeate across the cornea to the aqueous humor, and from there, drugs distribute to the trabecular meshwork, iris, and ciliary body. However, the physical lenticular barrier, blood flow of the iris-ciliary body, and aqueous humor turnover limit drug distribution further to the vitreous and retina. Topically administered drugs can also be absorbed into the anterior segment through a non-corneal conjunctiva/sclera pathway (Ahmed et al. 1989; Ahmed and Patton 1985). The sclera has a large surface area and comparatively high permeability than the cornea. The trans-scleral permeation primarily depends on the size of the molecules rather than the lipophilicity. A schematic representation of the disposition of drug in the eye following various routes after ocular administration is provided in Fig. 4.
Pathways for distribution of drug to the eye following different delivery routes. (Adapted from Agrahari et al. (2017))
To improve the ocular bioavailability, various conventional (suspension, emulsion, ointments, aqueous gel) and novel drug delivery systems (nanosuspension, nanomicelle, nanoparticle, liposome, dendrimer, implant, microneedle, and in situ thermosensitive gel) are explored through various routes of administration and showed promising clinical/nonclinical results. An ophthalmic topical formulation could be designed as solution, suspension, ointment, or emulsion. Since the duration of drug action from eye drop solutions is relatively short, frequent drug administration is needed. Therefore, patient compliance is low, and thus, patient-friendly and long-acting topical delivery systems are needed. Several novel ocular drug delivery systems, as discussed by Barar et al. (2016), represent the recently developed products/devices for the treatment of anterior and posterior segment diseases. However, considering the scope of this book chapter, only the development of suspension and nanosuspension formulations for the anterior/posterior segment eye diseases is discussed.
Ophthalmic Suspension Formulation
Suspension dosage forms are dispersions of finely divided undissolved drug particles in an aqueous vehicle containing suitable suspending and dispersing agents. Suspension dosage forms offer distinct advantages in increasing the corneal contact time of drugs and thus provide a more sustained therapeutic action compared to solutions (Patel et al. 2013; Kaur and Kanwar 2002). These may also improve the stability, bioavailability, and efficacy of hydrophobic molecules. However, the formulation of an ophthalmic suspension is complex, challenging, and requires understanding of the properties of the dispersed phase and the dispersion medium.
Target Product Profile (TPP) and Desirable Attributes
Ophthalmic suspension formulations must fulfill the crucial requirements of safety, efficacy, stability, manufacturability, and bioavailability. In addition to these, special attention should be given to other formulation factors (components) that may affect ocular tolerability and safety. A typical ophthalmic product is sterile, nearly isotonic, contains preservatives, and is packaged into a suitable tamper-evident multidose dispensing system or form-fill-seal (FFS) package for unit dose. The ophthalmic suspension product development criteria below describe critical steps and necessary studies to develop the formulation with desired attributes, meeting pharmaceutical and regulatory requirements. In general, the desirable attributes of an ophthalmic suspension product are:
-
Safe, effective, and stable during the shelf life of the product
-
A particle size ≤10 μm in order to minimize the ocular irritation. Ideally, the particles (based on their shape, size, etc.) should not cause irritation to the eye
-
Physical attributes, such as particle size, size distribution, and formulation viscosity, should remain uniform throughout the shelf life of the product
-
The drug should not have a quick sedimentation rate and must suspend easily upon shaking without forming a cake
-
Resuspension should produce a homogeneous mix of drug particles to provide a reproducible content uniformity with each dose administered
-
The formulation viscosity must promote uniform flow from the container
-
Multidose product must meet regulatory criteria for preservative effectiveness
-
Must be sterile and endotoxin free for both anterior and posterior eye segment usage
The first step in rational product developmentis to construct the quality TPP that identifies quality attributes critical for product performance. The elements of a good TPP for an ophthalmic suspension formulation should consider:
-
Route of administration
-
Safety and efficacy
-
Target pH
-
Drug/formulation stability
-
Preservation for multidose products
-
Package type (bottle size, fill volume, types of plug)
-
Dosing frequency and dosing protocol (administration with or without shaking)
-
Ease of manufacturing process
-
Scalable and cGMP manufacturing capability
-
Shelf life and storage conditions
-
Sterility and endotoxin levels (<0.5 EU/mL)
-
Target population and distribution market
Key Considerations in the Development of Ophthalmic Suspension and Nanosuspension Formulations
In order to design an ophthalmic suspension product that addresses the above TPP and desirable attributes, a systematic approach is needed in identifying a prototype formulation during the product development phase. The important aspects when considering the development of dosage forms for ocular therapeutics are duration of therapy, intended targeted tissue, safety, and patient compliance. The first step in suspension product development, once the TPP and desired formulation profiles/attributes are identified, is establishing its physical and chemical attributes such as appearance, viscosity, osmolarity, resuspendability, and pH. Understanding of the interfacial, wetting, particle interaction, surface charge, aggregation, sedimentation, and rheological properties is required to formulate an effective and aesthetically good suspension. The choice of excipients and pH in formulation development should be based on the physiological comfort, product stability, and efficacy requirements. Accordingly, the formulation factors and processing parameters affecting physical and chemical stability should be considered. The critical factors that need to be considered during the formulation of ocular suspensions are discussed below.
Physical Properties of the Active Pharmaceutical Ingredient (API)
The critical issues in the development of a suspension formulation related to the physicochemical properties are non-homogeneity of the dosage form, settling, cake formation, aggregation of the suspended particles, and resuspendability issues. A continuous mixing is often required during the manufacturing and filling process to assure homogeneity of the dosage form. Considering these issues, one of the early preformulation activities in suspension formulation development is to evaluate the drug physicochemical properties, such as pKa, LogP, solubility in various solvents, dissolution rate, chemical stability of the solid and solution (pH-dependent) forms of the drug, polymorphism, melting point, density, particle size, hygroscopicity, surface area, and flow characteristics. The ionization constant is an important parameter in ocular absorption of drugs since it is predominantly the unionized form determines the extent of bioavailability, though both the ionizable and the unionized forms may diffuse across ocular membranes. Therefore, selecting the functional groups that maximize the unionized fraction at physiological pH without compromising solubility, stability, and potency, is important. The interfacial properties of the suspended drugs are also important, and the low interfacial tension particles are easily wetted by water and can be easily suspended. However, high interfacial tension particles are not easily wetted and need surfactants to increase the wettability of the particles by reducing the surface tension. Ideally, the drug should be insoluble in the continuous phase to develop a suspension dosage form; however, since many drugs are suitably soluble in the continuous phase, the problem is a consequence of storage temperature variations, which can lead to supersaturation and crystal growth (Ostwald ripening). This can be neutralized by the use of crystallization inhibitors such as povidone. Drug storage temperature, humidity, and packaging materials require evaluation as part of the formulation development process. Preformulation studies are important to carry out in this regard to characterize the drug substance. A list of such preformulation studies is summarized in Table 1.
Particle Size of the API
The particle size used in ocular suspensions is of primary importance due to its relationship with the ocular irritation and in formulating physically stable suspension. Drug particle size influences product appearance, settling rates, drug solubility, rate and extent of dissolution, in vivo absorption, resuspendability, and overall stability. In general, the drug particle size of <10 μm is recommended for ophthalmic suspension formulations to facilitate patient comfort, to minimize the damage to the cornea (Kaur and Kanwar 2002; Missel 2012), and to ensure that the suspension does not lead to irritation (foreign body sensation) of the sensitive ocular tissues. This is also important to ensure that uniform dosage is delivered to the eye since the drug solubility is favored by smaller particle size. However, other factors such as particle concentration, density, and shape may also contribute to the comfort threshold of the patients.
The processes to achieve the desired particle size distribution (e.g., grinding, air-jet micronization, wet milling with ceramic beads, spray drying, precipitation from supercritical fluid, and controlled crystallization) can affect the properties of the drug product. For example, comminution (grinding or milling) methods may generate heat that can cause polymorphic changes and the size of the drug particles, which can affect the dissolution and drug delivery features. The comminution of the particles results in the increase of the surface area and, hence, free surface energy, which makes the suspension thermodynamically unstable. In addition, the effect of proposed sterilization methods on the drug properties should be assessed. These preliminary evaluations indicate the optimal particle size of the API, size reduction and solubilization methods, and buffer pH range to provide a stable suspension formulation. The observations during the preformulation development are important and need to be considered in designing the scale-up manufacturing activities.
Drug particles may also exist in different crystalline forms (polymorphism) in a suspension dosage form. A change in crystal structure and particle size may occur during storage or manufacturing process, causing alterations in the solubility and bioavailability. Hence, the size distribution of particles and aggregates of drugs in a suspension formulation should be controlled in order to provide uniformity in the dosing and reproducible drug delivery characteristics. Thus, the potential for any changes in particle size due to Ostwald ripening or particle agglomeration needs to be evaluated. However, it is desirable to keep the particles below the recommended size of <10 μm (d90) for topical ocular administration (Kaur and Kanwar 2002; Missel 2012) if a prolonged drug delivery duration is desired to minimize potential irritation. For injectable formulation to the back of the eye, the optimum particle size is larger than the optimal size for topical administration of 10 μm (d90). The preferred size for injectable formulation is generally between 30 and 100 μm, and the preferred shape is rod-shaped particles (Thackaberry et al. 2017). The duration of drug action for suspension is particle size dependent. In addition to controlling the particle size, the drug crystal form selected should be thermodynamically the most stable form. Hence, performing the polymorphism form conversion studies under various processing, storage, and stability conditions should be a part of the preformulation activities. The most commonly used analytical techniques to characterize polymorphic conversion are X-ray powder diffraction (XRPD), differential scanning calorimetry (DSC), and microscopy-based methods.
While the ocular retention increases with an increase in the particle size, the rate of dissolution of the suspended drug increases with decreasing particle size. Thus, an optimum particle size has to be selected based on the therapeutic agent used. The compendial requirements of particle size specification in EP, JP, and USP are:
-
EP: Particles with diameter 20–50 μm should be 20 or less per 10 μg active ingredient. Particles with diameter 50–90 μm should be 2 or less per 10 μg active ingredient. Particles with diameter 90 μm or more should not be observed per 10 μg active ingredient.
-
JP: No particles >75 μm.
-
USP: Solid particles must be smaller than 5–10 μm to avoid ocular discomfort or irritation.
Role of Excipients
Ophthalmic suspension contains several inactive excipients, such as dispersing and wetting agents, suspending agents, buffering agents, tonicity agents, and preservatives. The selection of these materials is generally based on the route of administration, drug dose, drug physicochemical characteristics, excipient safety, and any possible adverse effects. Depending on their physicochemical properties, excipients such as surface-active agents can play several roles, ranging from wetting agents, stabilizers, solubilizers, preservatives (antimicrobial agents), to, potentially, corneal permeation enhancers (Ibrahim 2019). However, sometimes, the excipient use is limited by their potential toxicity, irritancy to the ocular tissues, and unwanted interactions with other excipients or drug. Hence, understanding the mechanisms of their different roles and the interactions with other formulation components can help determine their safe and effective concentration intended for ocular application. The amount of surfactant should be carefully evaluated, as excessive amounts can lead to eye irritation, foam formation during manufacturing and upon shaking the product, or affect the interactions with other excipients. A summary of various excipients and their recommended levels in ophthalmic suspension formulation is provided in Table 2.
Viscosity-Modifying (Enhancing) Agents
Viscosity of ocular topical (suspension) formulation is one of the most important factors. Increasing the formulation viscosity may reduce the drainage rate, prolong the precorneal residence time, enhance ocular absorption, and control the rate at which the drop flows out of the container, thus enhancing the ease of application. The viscosity of ocular formulations must be maintained to a certain level to avoid any blockage of the lacrimal ducts. The reported critical formulation viscosity threshold is 55 mPa/s, and no further increase in contact time between the dosage form and the eye occurs above this level (Jones 2016). The viscosities of commercially available products are frequently <30 mPa/s; otherwise, discomfort due to blurred vision and foreign body sensation occurs, resulting in a faster elimination due to reflex tears and blinks (Salzillo et al. 2016; Jones 2016). Polymers such as methylcellulose, polyvinyl alcohol, polyvinylpyrrolidone, polyethylene glycol, sodium carboxymethyl cellulose, and hydroxypropyl methylcellulose are common viscosity-enhancing agents of ocular formulations.
Wetting and Solubilizing Agents
Surface-active agents are predominantly employed in suspension to effectively disperse the drug during manufacture and product use and to enhance the physical stability of the dispersed particles. The wetting and solubilizing agents (to lower the contact angle between the solid surface and the wetting liquid and improve the solubility of poorly water-soluble drugs) that are generally used include Tweens (polysorbates), Spans (sorbitan monolaurate/monooleate/monopalmitate), and sodium lauryl sulfate. Nonionic surfactants are generally preferred because of their less toxicity compared to ionic surfactants.
Suspending Agents
Suspending agents prevent sedimentation by affecting the rheology of suspensions. These polymers adsorbed on the surface of the particle, creates a steric effect by preventing the individual particles from getting sufficiently close to each other so that they are prevented from getting to the primary minimum (DLVO theory, explained later in this chapter), and thus coagulate/aggregate and settle out as a deflocculated sediment that is difficult to redisperse. Since the driving force for the adsorption of these polymers would be a reduction in interfacial energy, the polymers that do adsorb onto the surface of the particles must be able to bridge the energy gap. Thus, polymers that are amphiphilic in nature (have both hydrophilic and lipophilic groups) (e.g., poloxamers) are required. In ophthalmic suspensions, methylcellulose, sodium carboxymethylcellulose, hydroxypropyl methylcellulose, and synthetic polymers such as carbomers, poloxamers, and polyvinyl alcohol are generally used as suspending agents.
pH Buffering Agents
The ocular formulation pH is an important determinants of the stability of the drugs and the drug absorption across the cornea. Ideally, the pH of the ocular suspension should be controlled at or around 7.4 (physiological pH of tear fluid) (Missel 2012) using the appropriate buffer system or vehicle while not causing any physical or chemical instability to the drugs. However, the outer surfaces of the eye can tolerate a wide pH range of 3.5–8.5 (Ammar et al. 2009), but the normal range to prevent corneal damage is 6.5–8.5. The drug pKa determines the ionization of the therapeutic agent at defined pH values. To be effectively absorbed, the drug must exhibit in the ionized and non-ionized forms. The non-ionized form is required to partition into and diffuse across the lipid-rich outer layer of the cornea (the epithelium), whereas the inner layer of the cornea (the stroma) is predominantly aqueous, and therefore, the ionized form of the drug is needed. The non-ionized drug then diffuses to the endothelium/aqueous humor interface where ionization and dissolution into the aqueous humor occur.
Tonicity Agents
An isotonic ophthalmic formulation is with the tonicity equal to that of a 0.9% NaCl solution (290 mOsm). However, the osmotic pressure of the aqueous intraocular fluid is slightly higher than that of normal tears (~305 mOsm) (Missel 2012). The external eye is much more tolerant of tonicity variations and usually can tolerate solutions equivalent to 0.5–1.8% NaCl (Missel 2012). However, tear fluid in some cases of dry eye keratoconjunctivitis sicca is reported to be hypertonic, and a hypotonic artificial-tear product is used to counteract this condition.
Clarifying Agents
Ophthalmic formulations must be free from foreign particles, which are generally accomplished by filtration (helps to achieve clarity of the product). Particles in ophthalmic formulations can cause damage to the eye by causing abrasions to the cornea or the eyelid membranes. Suitable clarifying agents such as polysorbate 20/80 and HPMC may be used in ocular formulations.
Preservatives
The addition of preservatives is required to prevent the growth of the microorganisms since the products can be contaminated with microorganisms during the therapeutic uses (for multiuse products) and manufacturing/filling processes. In general, an ideal preservative should be effective at low concentration against a wide spectrum of microorganisms, soluble in the formulation at the desired concentration, nontoxic, compatible with formulation and packaging components, not have any effect on the viscosity or formulation characteristics, and stable over a wide pH range and temperature conditions. The commonly used preservatives are cationic, surface active and ionizable, and as a result their performance can be affected by the pH, ionic strength, presence of ionized components, and the adsorption of the preservative to the surface of the suspended solid particles. Therefore, the compatibility of the preservatives with suspension vehicle, excipient, and drug needs to be assessed in advance with a suitable pH range, ionic strength, surfactant, and polymer, to significantly reduce the formulation development time. In addition to the compatibility, it is necessary to study the effect of other formulation excipients on preservative effectiveness as well as the physical stability. The efficacy of the preservatives must be assessed using the appropriate pharmacopoeial method. The concentration of the preservative should be optimized to provide adequate efficacy with minimal concentration-dependent toxicity. Other factors such as the loss of preservative to sorption in processing, adequate control of pH and temperature during processing, and the order of component addition can affect the preservative efficacy. The key preservatives of ophthalmic suspension products are briefly discussed below (Missel 2012; Kulshreshtha et al. 2010; Ibrahim 2019; Jones 2016).
Cationic preservatives: The common cationic preservatives are benzalkonium and benzethonium chlorides. Benzalkonium chloride is typically used at a concentration of 0.01% w/v in ocular suspensions (range between 0.002% and 0.02% w/v). However, the resistance of certain microorganisms to benzalkonium chloride (e.g., Pseudomonas aeruginosa) has been observed. Therefore, 0.1% w/v disodium edetate (disodium EDTA) is used to enhance the antimicrobial activity of benzalkonium chloride by chelating divalent cations in the outer membrane of the bacterial cell, thereby rendering the bacteria more permeable to the diffusion of the antimicrobial agent. Also, the antimicrobial properties of benzalkonium chloride decrease at pH < 5.0 (Jones 2016). Benzethonium chloride exhibits lower antimicrobial activity than benzalkonium chloride and commonly used within the concentration range of 0.01–0.02% w/v.
Esters of parahydroxybenzoates (parabens): Mixtures of methyl and propyl parabens, typically at a combined concentration of 0.2% w/w, are used in ophthalmic formulations. The concern regarding their ocular usage is the irritancy of the parabens, which limits their use in ophthalmic preparations.
Organic alcohols: Chlorobutanol and phenylethyl alcohol are the two primary agents in this category. Under alkaline conditions, hydrolysis of chlorobutanol occurs, releasing HCl, thus preferred to be used only for acidic ophthalmic preparations. Also, the formulations must be stored in glass containers since chlorobutanol is volatile and lost from solution if stored in polyolefin containers. Another issue with the use of chlorobutanol is its limited solubility (typically used at the level of 0.5% v/v and saturation solubility is 0.7% w/v at room temperature). Therefore, below room temperature, precipitation of the preservatives may occur. Phenylethyl alcohol has similar properties and issues as of chlorobutanol, such as poor solubility, volatility, and partitioning into plastic containers. The typical concentration of phenylethyl alcohol used in ophthalmic preparations is 0.25–0.50% v/v.
Organic mercurials: Phenylmercuric nitrate, phenylmercuric acetate, and thimerosal are compounds in this category. The phenylmercurics are reported to have deposited in the lens of the eye, and thimerosal has been associated with ocular sensitization, thus not the first options as preservative (Jones 2016).
Antioxidants: Antioxidants, such as sodium metabisulfite, sodium sulfite, and EDTA, are added to ocular suspensions to enhance the stability of drugs that are susceptible to oxidation or degradation by free radicals. However, the acceptance criteria for antioxidant content should be established based on the levels necessary to maintain the product’s stability throughout its proposed usage and shelf life.
Chelating agents: Most commonly used chelating agent in ophthalmic suspension is ethylenediaminetetraacetic acid (EDTA). With this mechanism, EDTA can also enhance stability of the active drug by sequestering the heavy metal ions and thus serves as an antioxidant for drugs that have their oxidation catalyzed by heavy metals. EDTA has multiple functions, as a buffer for free divalents and preventing their buildup in the cornea while also enhancing the antimicrobial action of other preservatives.
Preservative’s Safety and Efficacy Assessment in Ocular Formulation
Appropriate care should be taken on selecting preservatives at lowest possible but effective concentration because of the high sensitivity of ocular tissues. Quaternary ammonium compounds such as benzalkonium chloride are capable of destroying bacteria and mycoplasma by binding to their negatively charged cell membrane followed by dissociation and leakage of cellular contents. Unfortunately, this effect is also capable of causing injuries even to ocular epithelial cells, especially at high concentrations. However, not only the preservative but also several other factors interplay to determine formulation’s toxicity including the types and concentrations of the excipients, dosing frequency, and the residence time on the ocular surface. Additional formulation factors that can be adjusted to affect the preservative efficacy and toxicity at low concentrations are the storage temperature, processing parameters, and packaging components; thus, there is also a need of an appropriate optimization of these parameters. The ophthalmic suspension formulations must meet regulatory jurisdictional requirement of preservative effective test (PET) for multidose products at initial and throughout the product shelf life (Tables 3, 4, 5, and 6). Out of the regulatory criteria, the EPA PET criteria is the most astringent in the order of EPA > EPB > USP > JP.
Sterility
Ophthalmic suspensions must possess appropriate sterility with consideration given to preservation, osmolality, buffering capacity, viscosity, and packaging. Suspension products may pose challenges during manufacturing to achieve a sterile product since the possibilities of either degradation or morphological changes may occur during the sterilization process. Hence, the effect of sterilization methods (e.g., dry heat, autoclaving, ethylene oxide treatment, and gamma irradiation) on the drug and formulation properties should be assessed. The selection of sterilization procedure depends upon the nature of the dosage form, and a combination of methods can be used for ophthalmic products. Although it is preferable to sterilize ophthalmic formulations in their final container by autoclaving, this method may not be a suitable approach for thermally unstable drugs or formulations. As alternative aseptic manufacturing methods such as aseptic filtration, irradiation, or formulation of dosage form under aseptic conditions may also be applied.
The commonly used techniques in the formulation of a sterile suspension product are autoclaving (wet steam), dry heat, aseptic filtration, ethylene oxide, and irradiation. These all have their specific advantages and limitations; for example, the autoclaving and dry heat methods can only be used for thermostable products because of the high heat involved in these approaches. The aseptic filtration (generally through a 0.22 μm size filter) cannot be efficiently utilized for suspension products due to non-filterability of suspended particles especially of larger sized ones and of the higher viscosity products. The ethylene oxide method is advantageous for thermolabile molecules/products; however, the elimination of residual ethylene oxide from the product is time-consuming and challenging. The gamma radiation method can have degradative impact on the drug or excipients including the safety concern for human uses and therefore is not used much.
Container/Closure System
The container/closure characteristics of an ocular suspension product should be evaluated with a prototype formulation in order to demonstrate suitability of the final container/closure system. The tests to evaluate the protection for the formulation provided by the container/closure, the safety and compatibility of the container/closure, and the performance of the container/closure system are: light transmission, water vapor permeation, seal integrity/leakage, monitoring of extractable/leachable, evaluation of loss of excipients, and dosing uniformity. The most common container (bottle) is made of low-density polyethylene (LDPE), either natural/clear or opaque color, so it can be easily squeezed to deliver the required dose. In general, the round plug made of polyethylene and polypropylene closure is used in ophthalmic suspension products.
Nanosuspensions
As discussed earlier, ocular drug delivery is challenging, and the most conventional formulations are unable to efficiently deliver the drugs into the targeted areas due to the presence of several complex barriers and elimination mechanisms, which resulted in a significantly low bioavailability of the drugs. Nanotechnology became a common approach for pharmaceutical product development, including for suspension dosage form. Nanosuspensions are colloidal dispersion of submicron particles stabilized by polymers or surfactants. These systems are emerged as promising strategy for delivery of hydrophobic drugs in enhancing the retention time in precorneal tissues and improving the drug bioavailability due to the high solubility of nanosuspension formulations. Nanosuspensions can be solid or crystalline drug nanosuspensions (consist of crystalline nano-sized drug particles, stabilized with the help of surfactants or polymers) or polymer-coated drug nanosuspensions (drug is coated or encapsulated within a polymer matrix). Crystalline or solid nanosuspensions are preferred in terms of the minimal requirement for excipients, high drug loading, and ease of scale-up manufacturing. Nanosuspensions can be manufactured using the top-down techniques (using high-pressure homogenization, media milling, etc.) and bottom-up approaches (molecules are assembled to form nanoparticles using solvent-antisolvent method, emulsification solvent evaporation technique, lipid emulsion/microemulsion template, super critical fluid process, etc.) (Lai et al. 2015; Patravale et al. 2004; Rabinow 2004).
One of the primary reasons for a wide drug delivery application of nanosuspensions is their ability to provide formulations of poorly soluble drugs with higher dissolution rates because of their small particle size and thus high surface area. In general, the nanosuspension approach offers the following advantages in ocular drug delivery (Maharjan et al. 2019; Patravale et al. 2004; Rabinow 2004; Sutradhar et al. 2013; Yadollahi et al. 2015): (1) ease of application, (2) lesser eye irritation as smaller nano-sized particles are better tolerated than larger particles, (3) enhancement in the bioavailability of the drugs and thus reduction in the amount of dose, (4) increased precorneal residence time, and (5) enhancement of the physicochemical drug stability. Thus, nanosuspension is an effective and convenient approach in ocular drug delivery, offering maintained therapeutic drug concentration, reduced administration frequency, and increased patient compliance.
Manufacturing Process of Nanosuspension Formulations
Nanosuspensions can be manufactured by several processes broadly categorized as top-down, bottom-up, and combination of these two processes. The top-down approach consists of breaking the bigger particles into smaller ones using different milling techniques, such as media milling, high-pressure homogenization, and microfluidization. Though there is no use of toxic/harsh solvents and high drug loading can be achieved, these methods are high-energy processes with the generation of a lot of heat, and therefore, the processing of thermolabile materials is challenging. The bottom-up (precipitation) processes refer to the generation of small nano-sized particles from their molecular solutions using various approach such as solvent-antisolvent, supercritical fluid, emulsification-solvent evaporation, and spray drying. These can be carried out at ambient temperatures, and therefore, thermolabile molecules can be processed. A combination of precipitation and high-pressure homogenization methods (e.g., Nanoedge® technology) can also be applied. Several reviews described the methods of pharmaceutical nanosuspension production including their advantages and disadvantages.
Application of Nanosuspension Formulations in Ocular Drug Delivery
A number of studies have proved the efficacy of nanosuspension in improving ocular bioavailability of corticosteroids. Corticosteroids such as prednisone, dexamethasone, and hydrocortisone are the first choice for treatment of anterior segment inflammation, however, using these drugs in a large dose frequently may lead to cataracts, glaucoma, and optic nerve injury. Therefore, delivery of corticosteroid by nanosuspensions to improve its bioavailability is an attractive option. Kassem et al. found that the corticosteroids, such as hydrocortisone, prednisolone, and dexamethasone, coated by nanosuspensions resulted in an enhanced rate and extent of ophthalmic drug absorption, as well as a considerably higher intensity of drug action with extended duration of drug effect compared to solutions and microcrystalline suspensions (Kassem et al. 2007). In another study, Ali et al. used hydrocortisone nanosuspension for the treatment of inflammation disorders of the eye, and the results showed a better bioavailability of hydrocortisone in the form of nanosuspension (Ali et al. 2011). Nanosuspension can also deliver other drugs successfully. For instance, Abrego et al. prepared nanosuspensions and nanoparticles as ophthalmic delivery of pranoprofen (Abrego et al. 2014). The result showed that the release profiles of pranoprofen from the primary nanosuspensions and rehydrated nanoparticles (the primary nanosuspension was freeze-dried and rehydrated in water) were similar and exhibited a sustained drug delivery pattern. Another work showed the potential of nanosuspension in ocular drug delivery of ketotifen fumarate (Soltani et al. 2016). Nanosuspension has been able to localize the drug into the cornea ex vivo with an enhanced in vitro ocular drug delivery. The results from these studies concluded that nanosuspension could be an efficient ophthalmic drug delivery system. A list of approved nanosuspension or suspension products for the ocular diseases is provided in Table 7.
Manufacturing Consideration in Scale-Up Development of Ocular Suspension Dosage Form
Scale-up manufacturing of sterile ocular suspensions or nanosuspensions requires thorough understanding of the factors that influence their physicochemical stability and other critical attributes. For example, the drug particle morphology is a key factor in suspension product dissolution rate, resuspendability, and syringeability. The type and concentration of surfactants can impact resuspendability and chemical stability of the product. Additionally, the drug particle size reduction methods may impact the drug quality and should be evaluated in advance.
The scale-up manufacturing process development of suspension products should determine the operating conditions applicable to large-scale batches with no compromise of the quality in assuring the therapeutic effectiveness and stability of the product. The physical properties of the drug, such as particle size, polymorphism, and ionization characteristics, are key factors influencing the scale-up production of suspension dosage form. Specifications to ocular multidose suspension products should include particle size/size distribution of the drug, assay, degradation products (impurities), resuspendability, pH, viscosity, sterility, and preservative effectiveness test (PET, not required for unit dose suspension products). The particle size distribution is a very critical attribute and should be examined during each manufacturing step. Resuspendability of the product over the shelf life must also be assessed to assure in obtaining a precise dose after shaking of the suspension product bottle before use.
In suspensions, the insoluble drug is uniformly dispersed throughout the liquid phase with excipients using homogenization. However, suspensions are susceptible to changes in equipment speed, time, and processing temperature to produce desired dispersion of the drug. Depending on the types of homogenizing equipment as well as the processes, the results may vary in generating uniformly dispersed particles. Hence, the transition from lab-scale R&D batches produced using small-capacity equipment to large-scale homogenizer demands precise control of settings between various equipment models to generate desired results. In this regard, multiple small-scale batches are required to assure the success of large-scale manufacturing. The process validation may also require real-time sampling and in-process testing of the products relative to targeted specifications. Ophthalmic suspension products may also pose challenges during sterile manufacturing since the possibilities of either degradation or morphological changes may occur during the sterilization process. Hence, the effect of various sterilization methods on drug and formulation attributes should be assessed. If the suspension products cannot be manufactured by terminal sterilization methods due to stability issues; an alternative approach is aseptic manufacturing.
Stability Consideration of the Suspension and Nanosuspension Dosage Forms
In order to understand the role of excipients in ocular suspension and nanosuspension formulations, it is important to understand the stability and process by which these formulations are stabilized. Suspension dosage forms are kinetically stable but inherently thermodynamically unstable systems. When left undisturbed for a long time, the suspension particles aggregate, sediment, and finally cake, hence must redisperse readily to achieve dosage uniformity. A higher viscosity of dispersion medium offers the advantage of slower sedimentation of the particles; however, it may compromise spreadability for topical ophthalmic suspensions. Thus, the shear thinning is necessary so that the suspension is highly viscous with slow sedimentation during storage, i.e., when minimal shear is present but has low viscosity after agitation (high shear) to facilitate ease of pourability from the storage containers. In general, the properties and stability of the suspension are influenced by the physicochemical characteristics of the dispersed phase, the dispersion medium/vehicle, and their interactions when mixed. There are three important attributes for the stability of the suspension drug product: chemical stability, physical stability, and microbiological stability (preservative efficacy) (Missel 2012; Kulshreshtha et al. 2010).
Physical Stability
The common physical stability issues of suspension formulation include agglomeration, sedimentation/creaming, crystal growth, and change of crystallinity (polymorphism) (Wu et al. 2011). Ideally, the particles in physically stable suspension remain uniformly distributed throughout the dispersion. However, the large surface area of small particles creates high total surface energy, which is thermodynamically unfavorable. Thus, the system tends to decrease the surface area in order to minimize the free energy by formation of agglomerates. This may lead to flocculation or aggregation, dependent on the attractive and repulsive forces within the system. Agglomeration can cause rapid settling/creaming, crystal growth, and inconsistent dosing of the dosage form. The most common strategy to solve this is the use of stabilizers to reduce interfacial tension and prevent agglomeration to generate a stable nanosuspension formulation. The common stabilizers are phospholipids, polymers, surfactants (ionic and nonionic), or a combination of these materials.
Flocculated and deflocculated suspension: When the particles are held together in a loose open structure, the system is in the state of flocculation (Kulshreshtha et al. 2010). The loose aggregates have a larger size compared to the single particle and, thus, higher sedimentation rate. The loose structure of the rapidly settling flocs contains a significant amount of entrapped medium; thus, the final flocculation volume is relatively large, and the flocs can be easily redispersed by simple agitation, which is highly desirable to ensure uniform dosing. In deflocculated suspension, the particles settle as small individual particles, resulting in a slow sedimentation rate. This leads to a high-density sediment that may be difficult to redisperse as the energy barrier is much higher compared with a flocculated suspension (Kulshreshtha et al. 2010). A deflocculated suspension remains dispersed for a longer time; however, it leads to formation of a close-packed arrangement, resulting in cake formation in case of sedimentation. A comparison of deflocculated and flocculated suspension is provided in Table 8.
Role of particle size distribution: Particle size distribution (PSD) plays a key role in the physical stability of the suspension products. The rate of sedimentation, agglomeration, suspendability, and thus the bioavailability of APIs and rheological behavior of formulation are directly affected by the particle size. The particles, through random motion over time, aggregate because of the natural tendency to decrease the large specific surface area and excess surface energy. The frequency of particle-particle collision depends on PSD, particle concentration, dispersion medium viscosity, and temperature. Stokes’ law (Eq. 1). This indicates the important role of particle size, medium viscosity, and density differences between medium and dispersed phase on the particle sedimentation rate (Kulshreshtha et al. 2010).
Here, r is the radius of the particle/sphere, η is the viscosity of the liquid, v is the flow velocity, d1 is the density of the particle/sphere, d2 is the density of the liquid, and g is the gravitational constant.
The Stokes’ equation applies to dilute suspensions and assumes spherical and monodisperse particles, which may not be encountered in real systems. Equation 2 gives the changed sedimentation velocity (Alexander et al. 1990).
Here, v′ is the hindered sedimentation velocity, v is the sedimentation velocity from Eq. (1), ε is the porosity of the system, and n is the measure of hindering.
According to the Stokes’ law, reduction of particle size leads to a decrease in the rate of sedimentation of the suspended particles. However, reducing particle size beyond a certain limit may lead to formation of a compact cake upon sedimentation. Hence, there should be a balance between particle size distribution, viscosity of the continuous phase, and the difference in density between the dispersed and the continuous phases. The other approaches to alleviate sedimentation issues are by matching the drug particle density with the medium or by increasing the medium viscosity. Figure 5 (reproduced with permission from (Wu et al. 2011)) shows different sedimentation types (deflocculated, flocculated, and open floc based) that occur in suspension formulations.
Sedimentation in (a) deflocculated suspension, (b) flocculated suspension, and (c) open floc-based suspension. (Reproduced with permission from Wu et al. (2011))
The attraction and repulsion between the particles depend on the potential energy barrier between them and arise from the difference in the extent of repulsive forces in comparison with attractive electrostatic forces. Colloidal suspensions can be stabilized in both aqueous and nonaqueous medium through electrostatic repulsion and steric stabilization. Stability due to electrostatic repulsion can be explained by the classical Derjaguin-Landau-Verwey-Overbeek (DLVO) theory; according to which, there are two major forces acting on colloidal particles in a dispersion medium: electrostatic repulsive forces due to overlap of electrical double layers (EDL) and van der Waals attractive forces. The EDL arises because of the charge at the solid-liquid interface. To maintain electrical neutrality of the system, counter ions present in the media are attracted toward the surface to form a double layer of ions: a tightly bound first layer of ions, also known as the Stern layer; and a diffuse layer of ions, also called the Gouy or Gouy-Chapman layer (Fig. 6, reproduced with permission from (Wu et al. 2011)). The possible lowest electrolyte concentration should be used since as the ionic strength of the medium increases, the thickness of EDL decreases, and the force of repulsion becomes smaller due to screening of the surface charge.
Illustration of classical DLVO theory. Attractive forces are dominant at very small and large distances, leading to primary and secondary minimum, while repulsive forces are prevailing at intermediate distances and create net repulsion between the dispersed particles, thus preventing particle agglomeration. (Reproduced with permission from Wu et al. (2011))
In steric stabilization mechanism, the high concentrations of polymers added to the suspension or nanosuspensions get adsorbed onto the surfaces of newly formed particles of the hydrophobic drug with the hydrophobic parts of the polymer attached to the particle surface and the hydrophilic chains extending into the aqueous environment (Fig. 7, reproduced with permission from (Wu et al. 2011)). Due to steric effects, the long polymeric chains extended into the water prevent the two particles from coming very close to each other. Thus, the dispersion medium must be a good solvent for the adsorbed macromolecule to allow the polymer chains to extend into bulk solution. In practice, the most common steric stabilizers are block and graft copolymers, composed of two parts: one is insoluble in the dispersion medium and firmly anchors the stabilizing moiety, and the second is soluble in the dispersion medium, providing the steric repulsion. In comparison, electrostatic stabilization is more susceptible to the ionic strength of the dispersion medium, and the high concentrations of ions in the dispersion medium lead to the screening of the surface charge, which decreases the thickness of the diffuse double layer. The depleted double layer makes the dispersed particles susceptible to aggregation. On the other hand, the hydration of the polymers is more susceptible to temperature changes. Hence, sterically stabilized suspensions are more prone to destabilization by temperature fluctuations. Therefore, a combination of both ionic surfactants and a polymeric stabilizer reduces the self-repulsion between the ionic surfactants, facilitating close packing of the stabilizer molecule layer around the particle, a more efficient approach in preventing particle agglomeration.
Schematic summary of instability issues and general stabilization mechanisms of nanosuspension/suspension products. (Reproduced with permission from Wu et al. (2011))
Crystal growth: Crystal growth in colloidal suspensions is generally known as Ostwald ripening (Wu et al. 2011; Kulshreshtha et al. 2010), which is a process where large particles grow at the expense of smaller particles and subsequently leads to a shift in the particle size and size distribution to a higher range. According to Ostwald-Freundlich equation, small particles have higher saturation solubility than larger particles (Wu et al. 2011), creating a drug concentration gradient between them. Consequently, molecules diffuse from the higher concentration surrounding small particles to around larger particles with lower drug concentration, generating supersaturated solution around the large particles, leading to drug crystallization. This process leaves an unsaturated solution surrounding the small particles, causing dissolution of the drug molecules from the small particles into the bulk medium. A narrow particle size distribution can minimize the saturation solubility difference and drug concentration gradients within the medium and, thus, help to inhibit the occurrence of Ostwald ripening. Stabilizers being absorbed on the particles surface can reduce the interfacial tension between the solid particles and liquid medium, thus preventing the Ostwald ripening. Solubility, temperature, and mechanical agitation may also affect the Ostwald ripening process. A summary of instability issues and general stabilization mechanisms of nanosuspension and suspension products is schematically represented in Fig. 7 (reproduced with permission from (Wu et al. 2011)).
In summary, the formulation factors that can be adjusted to affect the physical stability of the formulation include (Kulshreshtha et al. 2010):
-
Flocculation/deflocculation: (a) Add charged surface-active polymer or surfactant, (b) add oppositely charged flocculation agent (to shield the surface charges of the particles and to reduce the zeta potential to zero, at which point flocculation is observed), (c) add nonionic surface-active polymer or surfactant, (d) adjust ionic strength of vehicle (high concentrations of ions in the dispersion medium lead to the screening of the surface charge, which decreases the thickness of the diffuse double layer and makes the dispersed particles susceptible to aggregation), and (e), depending on the drug pKa, adjust pH to modify the surface charge.
-
Sedimentation rate: (a) Increase the viscosity of the vehicle (polymer stabilizers adsorbed on the surface of the particle, create a steric effect by preventing the individual particles from getting sufficiently close to each other, and help in settling out as a deflocculated sediment that is difficult to redisperse) and (b) decrease the particle size of the drug (leads to a decrease in the rate of sedimentation of the suspended particles; however, reducing the particle size beyond a certain limit may lead to formation of a compact cake upon sedimentation).
-
Ostwald ripening and crystal growth: (a) Generation of narrow particle size distribution (a narrow particle size distribution can minimize the saturation solubility difference around large and small particles and drug concentration gradients within the medium and, thus, help to inhibit the occurrence of Ostwald ripening), (b) addition of stabilizers (stabilizers being absorbed on the particles surface can reduce the interfacial tension between the solid particles and liquid medium, thus preventing the Ostwald ripening), and (c) optimize the solubility, temperature, and mechanical agitation (all these can lead to supersaturation in solubility and crystal growth (Ostwald ripening)).
Chemical Stability
The chemical stability for suspension products is mostly drug specific since each drug molecule has specific functional groups that affect the stability. Several factors such as storage temperature and pH, chemical stability of entrapped drugs, as well as the type and molecular weight of the polymer used can lead to the chemical instability of suspension. There are primarily three frequently encountered chemical stability issues: hydrolysis, oxidation, and photodegradation (Kulshreshtha et al. 2010). The formulation parameters that can be adjusted to address these chemical stability issues are (Kulshreshtha et al. 2010):
-
Hydrolysis: (a) Reduce solubility of the drug in the vehicle, (b) adjust the pH to avoid acid or base catalysis, or (c) reduce the storage temperature.
-
Oxidation: (a) Add an antioxidant to the formulation, (b) remove oxygen from the manufacturing process and package, (c) use a more protective package, or (d) reduce the storage temperature.
-
Photodegradation: (a) Reduce the solubility of the drug in the vehicle (if photodegradation occurs to drug in solution), or (b) use a more protective package and/or storage condition.
In summary, the topical drug delivery to the ocular diseases requires strategic approaches due to the presence of several anatomical/static and physiological/dynamic barriers. Considering this, the development of conventional or nanoformulation-based delivery systems requires appropriate selection of the excipients and formulation development strategy to achieve an effective drug dose to the ocular tissues. In this book chapter, we emphasized on the topical route of drug administration and the development of ocular suspension and nanosuspension formulations. The considerations in the formulation development approaches summarized here may help in facilitating the development of safe, stable, and efficacious ocular drug products.
Abbreviations
- API:
-
Active pharmaceutical ingredient
- EDTA:
-
Ethylenediaminetetraacetic acid
- LDPE:
-
Low-density polyethylene
- PSD:
-
Particle size distribution
- RPE:
-
Retinal pigment epithelium
References
Abrego G, Alvarado HL, Egea MA, Gonzalez-Mira E, Calpena AC, Garcia ML. Design of nanosuspensions and freeze-dried PLGA nanoparticles as a novel approach for ophthalmic delivery of pranoprofen. J Pharm Sci. 2014;103(10):3153–64. https://doi.org/10.1002/jps.24101.
Agrahari V, Agrahari V, Mandal A, Pal D, Mitra AK. How are we improving the delivery to back of the eye? Advances and challenges of novel therapeutic approaches. Expert Opin Drug Deliv. 2017;14(10):1145–62. https://doi.org/10.1080/17425247.2017.1272569.
Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985;26(4):584–7.
Ahmed I, Francoeur ML, Thombre AG, Patton TF. The kinetics of timolol in the rabbit lens: implications for ocular drug delivery. Pharm Res. 1989;6(9):772–8.
Alexander KS, Azizi J, Dollimore D, Uppala V. Interpretation of the hindered settling of calcium carbonate suspensions in terms of permeability. J Pharm Sci. 1990;79(5):401–6. https://doi.org/10.1002/jps.2600790508.
Ali HSM, York P, Ali AMA, Blagden N. Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J Control Release. 2011;149(2):175–81. https://doi.org/10.1016/j.jconrel.2010.10.007.
Ali J, Fazil M, Qumbar M, Khan N, Ali A. Colloidal drug delivery system: amplify the ocular delivery. Drug Deliv. 2016;23(3):710–26. https://doi.org/10.3109/10717544.2014.923065.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech. 2009;10(3):808–19. https://doi.org/10.1208/s12249-009-9268-4.
Barar J, Aghanejad A, Fathi M, Omidi Y. Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts. 2016;6(1):49–67. https://doi.org/10.15171/bi.2016.07.
Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond). 2013;27(7):787–94. https://doi.org/10.1038/eye.2013.107.
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–60. https://doi.org/10.1208/s12248-010-9183-3.
Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3(2):275–87. https://doi.org/10.1517/17425247.3.2.275.
Hartman RR, Kompella UB. Intravitreal, subretinal, and Suprachoroidal injections: evolution of microneedles for drug delivery. J Ocul Pharmacol Ther. 2018;34(1–2):141–53. https://doi.org/10.1089/jop.2017.0121.
Ibrahim SS. The role of surface active agents in ophthalmic drug delivery: a comprehensive review. J Pharm Sci. 2019;108(6):1923–33. https://doi.org/10.1016/j.xphs.2019.01.016.
Janagam DR, Wu L, Lowe TL. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev. 2017;122:31–64. https://doi.org/10.1016/j.addr.2017.04.001.
Jones DS. FASTtrack: pharmaceutics - dosage form and design. Chicago: Pharmaceutical Press; 2016.
Kassem MA, Abdel Rahman AA, Ghorab MM, Ahmed MB, Khalil RM. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm. 2007;340(1–2):126–33. https://doi.org/10.1016/j.ijpharm.2007.03.011.
Kaur IP, Kanwar M. Ocular preparations: the formulation approach. Drug Dev Ind Pharm. 2002;28(5):473–93. https://doi.org/10.1081/DDC-120003445.
Kulshreshtha AK, Singh ON, Wall GM. Pharmaceutical suspensions, from formulation development to manufacturing. New York: Springer; 2010.
Lai F, Schlich M, Pireddu R, Corrias F, Maria Fadda A, Sinico C. Production of nanosuspensions as a tool to improve drug bioavailability: focus on topical delivery. Curr Pharm Des. 2015;21(42):6089–103.
Maharjan P, Cho KH, Maharjan A, Shin MC, Moon C, Min KA. Pharmaceutical challenges and perspectives in developing ophthalmic drug formulations. J Pharm Investig. 2019;49(2):215–28. https://doi.org/10.1007/s40005-018-0404-6.
Mishima S, Gasset A, Klyce SD Jr, Baum JL. Determination of tear volume and tear flow. Investig Ophthalmol. 1966;5(3):264–76.
Missel MC. Remington: essentials of pharmaceutics. Chicago: Pharmaceutical Press; 2012.
Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47–64. https://doi.org/10.5497/wjp.v2.i2.47.
Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56(7):827–40. https://doi.org/10.1211/0022357023691.
Peptu CA, Popa M, Savin C, Popa RF, Ochiuz L. Modern drug delivery systems for targeting the posterior segment of the eye. Curr Pharm Des. 2015;21(42):6055–69.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–96. https://doi.org/10.1038/nrd1494.
Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1(1):99–114. https://doi.org/10.1517/17425247.1.1.99.
Rai Udo J, Young SA, Thrimawithana TR, Abdelkader H, Alani AW, Pierscionek B, Alany RG. The suprachoroidal pathway: a new drug delivery route to the back of the eye. Drug Discov Today. 2015;20(4):491–5. https://doi.org/10.1016/j.drudis.2014.10.010.
Rowe-Rendleman CL, Durazo SA, Kompella UB, Rittenhouse KD, Di Polo A, Weiner AL, Grossniklaus HE, Naash MI, Lewin AS, Horsager A, Edelhauser HF. Drug and gene delivery to the back of the eye: from bench to bedside. Invest Ophthalmol Vis Sci. 2014;55(4):2714–30. https://doi.org/10.1167/iovs.13-13707.
Salzillo R, Schiraldi C, Corsuto L, D’Agostino A, Filosa R, De Rosa M, La Gatta A. Optimization of hyaluronan-based eye drop formulations. Carbohydr Polym. 2016;153:275–83. https://doi.org/10.1016/j.carbpol.2016.07.106.
Scherz W, Doane MG, Dohlman CH. Tear volume in normal eyes and keratoconjunctivitis sicca. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;192(2):141–50.
Soltani S, Zakeri-Milani P, Barzegar-Jalali M, Jelvehgari M. Comparison of different nanosuspensions as potential ophthalmic delivery Systems for ketotifen fumarate. Adv Pharm Bull. 2016;6(3):345–52. https://doi.org/10.15171/apb.2016.046.
Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66(2):190–4. https://doi.org/10.4103/ijo.IJO_646_17.
Sutradhar KB, Khatun S, Luna IP. Increasing possibilities of Nanosuspension. J Nanotechnol. 2013;2013:12. https://doi.org/10.1155/2013/346581.
Thackaberry EA, Farman C, Zhong F, Lorget F, Staflin K, Cercillieux A, Miller PE, Schuetz C, Chang D, Famili A, Daugherty AL, Rajagopal K, Bantseev V. Evaluation of the toxicity of intravitreally injected PLGA microspheres and rods in monkeys and rabbits: effects of depot size on inflammatory response. Invest Ophthalmol Vis Sci. 2017;58(10):4274–85. https://doi.org/10.1167/iovs.16-21334.
Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. Eur J Pharm Biopharm. 1997;44(1):71–83. https://doi.org/10.1016/S0939-6411(97)00064-7.
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–69. https://doi.org/10.1016/j.addr.2011.02.001.
Yadollahi R, Vasilev K, Simovic S. Nanosuspension technologies for delivery of poorly soluble drugs. J Nanomater. 2015;2015:13. https://doi.org/10.1155/2015/216375.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
Agrahari, V., Singh, O.N. (2021). Ocular Suspension and Nanosuspension Products: Formulation Development Considerations. In: Neervannan, S., Kompella, U.B. (eds) Ophthalmic Product Development. AAPS Advances in the Pharmaceutical Sciences Series, vol 37. Springer, Cham. https://doi.org/10.1007/978-3-030-76367-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-76367-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76366-4
Online ISBN: 978-3-030-76367-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)