Abstract
Dental enamel, which is the most highly mineralized tissue in the human body, is critically important for the protection of the underlying tooth structures throughout a lifetime. When enamel is absent or poorly formed due to genetic defects, called amelogenesis imperfecta (AI), or through environmental or epigenetic effects such as fluorosis, or lost due to dental caries, the entire tooth structure may be compromised. Ameloblasts differentiate through secretory, transition, and maturation stages to generate the fully mineralized enamel matrix. Enamel matrix proteins produced during the secretory stage undergo limited hydrolyzed by matrix metalloproteinase 20 (MMP20) to initiate crystal growth. Then during maturation, the remaining matrix proteins are completely hydrolyzed by kallikrein 4 (KLK4). The protein fragments are endocytosed and this allows complete mineralization of the enamel space. Enamel mineralization is unique in that it requires pH cycling, in which the ameloblasts modulate the matrix pH between an acidic and neutral pH. In this chapter, Part 1 is an overview of enamel formation, and how pH cycling influences amelogenin hydrolysis by KLK4 and enamel mineralization. Part 2 describes how ion-pumps and transporters regulate the enamel matrix pH as mineralization occurs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tooth
- Enamel matrix
- Enamel maturation
- Ameloblasts
- Amelogenin
- MMP20
- KLK4
- pH cycling
- Enamel mineralization
- Ion transport
1 Part 1: Secretion of Matrix Proteins, Enamel Mineralization, and pH Regulation
1.1 Introduction
In the secretion stage of enamel formation, epithelial-derived ameloblasts secrete matrix proteins, to form a matrix with a cheese-like texture (Aoba and Moreno 1987; Smith and Nanci 1989). Enamel crystal formation is initiated in this matrix, and the crystals grow primarily in length and reach their full length at the end of the secretory stage. The secretory stage is followed by the transition stage, where the protein components in the enamel matrix dramatically decrease while the mineral components rapidly increase, resulting in a chalky appearing enamel matrix (Daculsi et al. 1984; Simmer et al. 2009). This transition leads to the maturation stage where the crystals continue to thicken and widen as proteins are simultaneously removed after degradation and replaced by hydroxyapatite (HAP) mineral. Following maturation, tooth enamel is a highly mineralized extracellular biomaterial, consisting of approximately 96–98% minerals and only 2–4% organic materials and water (He et al. 2011; Robinson 2014).
1.2 Secretory Stage Enamel
Enamel proteins are synthesized by the cells of the enamel organ, including ameloblasts. Ameloblasts elongate and form Tomes’ processes, through which enamel proteins are secreted in a prism-like structure, on top of the dentin matrix (Kallenbach 1973). The full thickness of the enamel matrix is established by matrix proteins that include amelogenins, ameloblastin, enamelin, tuftelin, and keratins (Robinson et al. 1998).
Amelogenins, which are transcribed from multiple alternatively spliced variants of the amelogenin gene, are the primary structural proteins, constituting 90–95% of total proteins in the secretory enamel protein matrix (Gibson 2011). Amelogenins play a central role in crystal growth and enamel thickness (Gibson et al. 2001; Robinson et al. 1998). The amelogenin knockout mouse has an enamel layer that is much thinner than that in the wild-type mouse, and the enamel crystals are disorganized, deformed, and disoriented (Gibson et al. 2001). The absence of ameloblastin (Ambn) results in a detachment of ameloblasts from the underlying matrix at the start of the secretory stage and generation of a thin enamel with an irregular prism pattern (Wazen et al. 2009). When enamelin is absent, ameloblasts are unable to adhere to the underlining enamel surface, and they prematurely undergo apoptosis (Hu et al. 2011). Mutations in keratin 75 are associated with an increased incidence of dental caries (Duverger et al. 2014).
The secretory matrix also contains proteinases, including matrix metalloproteinases (MMPs) (Bartlett 2013; Wöltgens et al. 1991), MMP2, MMP3, MMP9, MMP12, MMP13 and MMP20 (Bartlett 2013; DenBesten and Heffernan 1989; Goldberg et al. 2003; Llano et al. 1997; Wöltgens et al. 1991). MMP20 is the predominant enamel matrix proteinase in the secretory stage and cleaves enamel matrix proteins immediately after their secretion (Bartlett et al. 1998; Simmer and Hu 2002).
Mineral formed in the enamel matrix is initially amorphous calcium, which subsequently transforms to hydroxyapatite (HAP) (Beniash et al. 2009). The phosphorylated N terminal serine 16 on alternatively spliced amelogenins enhances the stabilization of amorphous calcium phosphate (ACP) mineral precursors and modulates the timing of conversion of ACP to apatite mineral (Shin et al. 2020). At the end of the secretory stage, long thin crystals of HAP extend the full length of the enamel matrix. The secretory stage ends as capillaries invaginate into the stellate reticulum layer of the enamel organ, overlying ameloblasts. Up to 50% of the ameloblasts undergo apoptosis during this transition so that there is no longer one ameloblast overlaying each enamel prism. The ameloblasts then further differentiate into maturation stage ameloblasts (Smith 1998).
1.3 Maturation Stage Enamel
Maturation ameloblasts cyclically modulate between ruffle-ended (RE) cells and smooth-ended (SE) cells (Fig. 11.1). Matrix proteins secreted at the maturation stage include amelotin (AMTN), odontogenic ameloblast-associated protein (ODAM) (Moffatt et al. 2008), and secretory calcium-binding phosphoprotein proline-glutamine rich 1 (SCPPPQ1). These proteins are thought to participate in structuring an extracellular matrix with the distinctive capacity of attaching epithelial cells to mineralized surfaces (Fouillen et al. 2017), and the formation of the final layer of aprismatic enamel (Abbarin et al. 2015).
Enamel formation and ameloblast modulation in the rodent incisor. In the secretory stage (a), ameloblasts secrete a protein-rich enamel matrix (yellow arrows) into which long thin crystals are formed. At the maturation stage (b) KLK4 is secreted (white arrow) and hydrolyzes matrix proteins. The hydrolyzed peptide fragments are endocytosed (red arrows) and mineral content (purple arrow) increases. In the maturation stage, ameloblasts become ruffle ended, facing an acidic enamel matrix (orange), and then cycle into smooth-ended ameloblasts, facing neutral enamel (light green). (c) A mouse incisor stained with pH indicator dye shows cycles of matrix acidification and neutralization in the maturation stage
Kallikrein 4 (KLK4), a serine proteinase previously named enamel matrix serine proteinase (EMSP1), first identified and purified from porcine enamel matrix by Simmer and colleagues (Simmer et al. 1998), is the predominant enamel matrix proteinase in the maturation stage (Simmer and Hu 2002). KLK4 hydrolyzes matrix proteins to create space for the mineralizing enamel crystals to expand in width.
1.4 Discovery of pH Cycling
Takano and coworkers first reported the visualization of ameloblast modulation on the enamel surface, by staining with the calcium-binding dye glyoxal bi (2-hydroxyanil) (GBHA). Enamel under SE stains red by GBHA, whereas enamel under RE stains a yellow/green color, or is unstained (Takano et al. 1982a) (see Fig.11.1). However, an unsolved question at that time was that Ca45 uptake is not correlated with RE and SE morphologies (Takano et al. 1982b).
Sasaki stained the enamel surface with a pH indicator dye and found that the enamel had alternating acidic and neutral pH. Furthermore, these pH cycles of acidic (5.8–6.0) and neutral (7.0–7.2) (see Fig. 11.1) were located at the same area stained by GBHA, indicating that enamel under RE ameloblasts was more acidic, and was more neutral under SE ameloblasts (Sasaki et al. 1991b) (see Figs. 11.1 and 11.2).
Staining of bovine tooth enamel surfaces. (a) A bovine developing tooth sagittally cut in half. The left half was stained with a pH Universal indicator mixture for 1–2 min. Alternate stripes of orange correspond to pH 5.5–6.0 and green to pH 7.0. The right half was stained with GBHA solution. Red stripes of staining correspond to the neutral bands of green staining with the pH indicator and unstained white zones to acidic orange zones. (b) Staining with the Universal indicator of a bovine enamel slice. Orange or green coloration occurs not only on the forming surface but also in depth. Color standards at different pH values are shown at the bottom (Sasaki et al. 1991b)
The correlation of enamel staining by GBHA and pH indicator dye later became clearer, as it was noted that GBHA stains poorly at acid pH. Therefore, ionic calcium staining by GBHA in the developing enamel matrix is also pH dependent. Calcein, another dye that stains ionic calcium, is also pH sensitive, and so has a similar property to that of GBHA, of only staining the neutral enamel matrix (Josephsen 1983; Picard et al. 2000).
Takagi et al. further characterized the profiles of the enamel matrix proteins collected from enamel either with acidic or neutral pH (Takagi et al. 1998). They discovered that the neutral enamel matrix contains relatively intact amelogenin and enamelin proteins, while the acid zones contain mostly lower molecular weight amelogenins and enamelin fragments. This suggests that amelogenin hydrolysis is more effective in the acidic conditions underlying RE ameloblasts.
We do not yet understand what drives the cyclic modulation of maturation ameloblasts. However, we do know that ion transporters localized to the ameloblast apical plasma membranes transport calcium into the enamel matrix to form HAP, while modulating matrix pH to direct this process. Bicarbonate ion transporters, including Ae2 and NBCe1, neutralize the acidified matrix. Matrix acidification is driven by the formation of HAP crystals which release protons into the matrix (Bronckers 2017; Smith 1998). Matrix acidification may also be controlled by proton secretion by V-ATPase translocated to the RE apical plasma membrane (Damkier et al. 2014; Toei et al. 2010). Modulations of ion transporter activities by either genetic defects, or environmental factors, such as excess fluoride, affect pH cycling and enamel matrix formation (Bronckers et al. 2015; DenBesten and Li 2011). Therefore, pH cycling, which is related to both matrix protein endocytosis and matrix mineralization is a unique mechanism that drives the formation of biomineralized enamel.
1.5 Amelogenin-Mediated Enamel Matrix Mineralization
The amelogenin protein structure is highly pH dependent. At pH 3.0 amelogenin is globally unstructured, while at pH 5.6, it forms elaborated structures with increased oligomerization and nanosphere formation. At pH 7.2 amelogenin assembles into highly organized structures, binding together to form branched chains (Beniash et al. 2012). In addition, at neutral pH, changes in electrolyte concentration dramatically affect protein adsorption onto the HAP surface (Shimabayashi et al. 1997).
The C terminus of amelogenin is a primary site for amelogenin binding to HAP. Mice lacking the 13 terminal amino acids have poorly formed enamel (Pugach et al. 2010). Glutamate-containing proteins interact strongly with Ca2+ ions on the surface of the HAP crystal, and acidic amino acids have been considered as major determinants of protein binding (Jaeger et al. 2005). It is therefore likely that the acid glutamates and aspartate (2 each) in the amelogenin C terminus may facilitate binding to the HAP surface.
When amelogenins assemble in a neutral aqueous environment, the hydrophilic C termini extend on the outside of the amelogenin nanostructures (Margolis et al. 2006). The exposed C terminal amino acids can bind to the apatite crystal surfaces, and also to positively charged protons and calcium in solution (Le et al. 2006; Ryu et al. 1998). Moreover, the positively charged groups of amelogenin (such as amine groups at the N-terminus and side chains) may also contribute to the apatite binding through their interactions with the negatively charged groups, such as phosphates, on the HAP surface.
The concentration of amelogenin influences HAP crystal growth. HAP crystal formation requires a supersaturated calcium phosphate environment (Eanes 1976; Eanes et al. 1965). In solution, amelogenins inhibit crystal growth in the presence of high concentrations of calcium and phosphate (Aoba et al. 1989; Iijima and Moradian-Oldak 2004). However, Beniash and co-workers observed that pre-assembled full-length amelogenin (amelogenin lacking the exon 4 transcript) increases the size of formed crystals at low concentrations of calcium and phosphate (1.5 and 2.5 mM) (Beniash et al. 2005). In the presence of calcium and phosphate concentrations similar to those measured in enamel fluid (0.5 and 2.5 mM, respectively); crystals do not grow well on an apatite surface (Aoba and Moreno 1987; Habelitz et al. 2005). However, with the addition of amelogenin at low concentration (0.4 mg/ml), a few nanospheres assemble with minimal crystals formation, and at higher concentrations of amelogenin (1.6 mg/ml) more nanospheres assemble and longer fibrous crystals are formed (Habelitz et al. 2004).
At these higher amelogenin concentrations, amelogenin nanospheres assemble as a monolayer on the crystal surface. It appears that this monolayer of amelogenin nanospheres functions differently from amelogenin monomers in solution (Aoba et al. 1989) or in an amelogenin gel (Iijima et al. 2002). The amelogenin monolayer nanostructure may create a highly charged local environment to attract calcium and phosphate ions to form a saturated niche on the crystal surface for crystal growth.
Additional multilayers of amelogenin nanospheres physically protect the fragile crystals, but they also inhibit further crystal growth. Therefore, the layer of amelogenin adsorbed on the crystal surface needs to be preferentially removed to release the space for crystal growth.
1.5.1 Disassembly of Amelogenin Bound on HAP Crystals
When bound to solid surfaces, protein conformations usually change (Hlady and Buijs 1996). Proteins, such as amelogenin, which can easily adsorb onto solid surfaces, have large changes in conformation upon binding, and less internal stability (Gray 2004). Tarasevich et al. found that at neutral pH, self-assembled amelogenin that was bound onto a single fluoroapatite (FAP) crystal, formed much smaller structures on the FAP surfaces, than the original nanospheres present in solution. Their studies provide strong evidence that amelogenin nanospheres undergo possible quaternary structural changes upon interacting with apatite surfaces (Tarasevich et al. 2009a, b). These adsorption-induced conformational changes of the amelogenin protein may further alter the subsequent interactions between amelogenin and proteinases.
Tao et al. further quantitatively analyzed the dynamics of this adsorption-induced amelogenin nanosphere disassembly. They reported that the amelogenin nanospheres disassembled onto the HAP surface to form oligomeric adsorbates (25-mer), the subunits of the larger nanosphere. They concluded that a surface-triggered disassembly mechanism actually reversed the process of oligomer nanosphere self-assembly (Tao et al. 2015). This supports the possibility that amelogenin–crystal interactions are involved in determining the extent to which oligomers adsorb, assemble, and disassemble.
Chen et al. used in situ atomic force microscopy (AFM) to show that on a positively charged surface, amelogenin first assembles as a relatively uniform population of decameric oligomers, and then becomes two main populations: higher-order assemblies of oligomers and amelogenin monomers. On negatively charged surfaces, amelogenin nanostructure disassembles into a film of monomers (Chen et al. 2011) (see Fig. 11.3). An acidic pH of the enamel matrix, resulted from HAP formation, may further disassemble amelogenin nanospheres (Beniash et al. 2012).
A proposed pathway of amelogenin self-assembly and structural dynamics in vivo. Intracellular amelogenin monomers (green) and hexamers (red) in ameloblast cells (I). After amelogenin proteins are secreted into the matrix, they assemble into nanospheres (II). A monolayer of amelogenin monomers forms after the nanospheres interact with a negatively charged hydrophilic surface (III). Decameric amelogenin oligomers form following the disassembly of nanospheres through interaction with positively charged surfaces (IV). Decameric oligomers exhibit unexpected structural dynamics on positively charged surfaces in situ and form a mixture of higher-order assemblies of oligomers and monomers (V) (Chen et al. 2011)
Therefore, a possible sequence for amelogenin and pH-mediated enamel matrix mineralization is as follows. Amelogenins bind to HAP crystals and then disassemble to form monolayers that attract calcium and phosphate ions to form a saturated niche on the crystal surface for crystal growth. As crystal growth is initiated, the matrix begins to acidify and the N-terminus of amelogenins, which contain more positively charged histidines, reduces its binding affinity to HAP crystals, while the hydrophilic amelogenin C termini containing more acidic amino acids, still hangs on the surface of crystals. This allows more access sites for proteinases, facilitating the digestion of bound amelogenin. As the matrix neutralizes, HAP synthesis begins, and as the crystals grow, the surface of newly formed crystals will contact another layer of proteins in the enamel matrix. This then initiates amelogenin disassembly again, and the cycle repeats itself during enamel maturation.
1.6 Enamel Matrix Proteinases, MMP-20, and KLK4
The transition of enamel matrix from protein-dominated content to more than 95% mineralized tissue requires hydrolysis of matrix proteins to allow continued crystal growth as enamel matures. MMP20 and KLK4 are two major proteinases involved in the hydrolysis of amelogenins and other enamel matrix proteins, to provide space for enamel crystals growth (Bartlett et al. 1998; Bartlett and Simmer 1999; Caterina et al. 1999; Fukae et al. 1998; Hu et al. 2002; Ryu et al. 2002).
MMP20, first reported by Bartlett and co-workers (Bartlett et al. 1998; Llano et al. 1997), belongs to the MMP superfamily and shares a similar structure with most other family members (Massova et al. 1998). Recombinant porcine, mouse, bovine, and human MMP20s have been expressed, purified, and activated in vitro and can hydrolyze both recombinant and native amelogenins (Li et al. 1999; Llano et al. 1997).
Amelogenin hydrolysis is initiated in secretory stage enamel at its C-terminal telopeptide. The most prevalent splice variants code the full length 25 kDa amelogenin that lacks exon 4 and the leucine-rich amelogenin polypeptide (LRAP) precursor. The 25 kDa amelogenin is cleaved into products of 20 kDa amelogenin and then a 5.0 kDa tyrosine-rich amelogenin polypeptide (TRAP). Similarly, the LRAP precursor is cleaved at the C terminus to form a 6.5 kDa LRAP (Fincham et al. 1981, 1989). Twenty kDa amelogenin, LRAP, and TRAP are the three major hydrolytic products in the secretory enamel protein matrix (Brookes et al. 1995).
KLK4 is upregulated during the transition stage. KLK4 rapidly cleaves amelogenins at multiple sites, accelerating amelogenin degradation during enamel maturation, to allow the final thickening of enamel crystals. The importance of KLK4 in the final stage of enamel mineralization is shown in the KLK4 knockout mouse, where the enamel layer is normal in thickness with an intact decussating structure but the crystals remain separated, resembling a pattern of uncooked angel hair spaghettis (Fig.11.4) (Bartlett and Simmer 1999; Ryu et al. 2002; Simmer et al. 2009).
Comparison of enamel from the wild-type and Klk4 null mice. (a, b) SEM at the same scale showing mandibular incisor enamel from the DEJ (bottom) to the surface (top) that has been fractured in the erupted portion by pressing on it with a knife. There is no observable difference in the overall thickness of the enamel layer between the wild-type and Klk4 null mice. (c, d) Higher magnification showing the decussating patterns of enamel rods just above the dentin enamel junction. (e, f) enamel rods in the wild-type mice have tightly packed crystallites that lose some aspect of their individuality. Enamel rods in the Klk4 null mice are composed of distinctly individual crystallites resembling angel hair spaghetti. Holes or vacancies in some rods give the impression that smaller bundles of crystallites broke at a slightly deeper level and slid out of the rod (Simmer et al. 2009)
1.6.1 Optimal Conditions for Proteinase Activity in the Enamel Matrix
In the secretory matrix, MMP-20 cleaves amelogenin at neutral pH (Fukae et al. 1998), whereas KLK4 has an optimal pH close to 6.1 (Lu et al. 2008). In vitro, optimal KLK4 activity against a peptide substrate occurs even lower, around pH 5, and at neutral pH, KLK4 activity was reduced by almost half (Fig. 11.5, unpublished data).
This acidic optimal pH for KLK4 suggests the importance of acidic conditions in the degradation of amelogenin and other enamel proteins during tooth enamel maturation (Sasaki et al. 1991a). In addition, MMP20 is reported to be able to activate KLK4 (Yamakoshi et al. 2013). Interestingly, some MMPs are reported to be activated by low pH, which plays an important role in caries formation (Amaral et al. 2018). Therefore, the sequential activation of KLK4 by MMP20 remaining in the maturation stage matrix may also contribute to the tooth enamel maturation.
The absorption of amelogenin onto hydroxyapatite also affects its hydrolysis. When amelogenin is absorbed onto hydroxyapatite it is readily hydrolyzed and removed by enamel matrix proteinases (88% for MMP20 and 98% for KLK4), at significantly higher rates as compared to hydrolysis of amelogenin in solution. There are also a greater number of cleavage sites as identified by LC-MALDI MS/MS, when amelogenin is hydrolyzed after absorption onto HAP as compared to those in solution (Zhu et al. 2014). These results suggest that the adsorption of amelogenin to HAP results in their preferential and selective degradation and removal from HAP by both MMP20 and KLK4. It may be that the disassembly of nanospheres into monomers at low pH and through amelogenin–crystal interactions results in more cleavage sites accessible to enzymes and lead to a higher rate of hydrolysis.
1.7 A Model for pH Cycling and KLK4-Regulated Enamel Maturation
How does pH cycling affect enamel matrix mineralization? A possible cyclic sequence of mineralization and protein removal is in Fig. 11.6.
Diagram showing a hypothetical sequence of the effect of pH-cycling on HAP crystal growth in maturing tooth enamel. When amelogenins (depicted as spheres) move from neutral (yellow) to acidic (orange) KLK4 activity is increased and amelogenins are hydrolyzed. When the matrix returns to neutral pH, HAP crystal growth (arrows) increases in width, and then this process repeats as the matrix acidifies, to inhibit HAP growth and increase amelogenin hydrolysis.
Adsorption of amelogenins onto the crystal surface results in conformational changes of the bound proteins, which then disassemble into oligomers or monomers on the surface of HAP crystals. These conformational changes may both increase local supersaturation of calcium and phosphate at the HAP surface, and expose more amelogenin cleavage sites to proteinases.
The hydrolysis and removal of amelogenins from the crystal surface open up the space surrounding the crystals, possibly with rapid precipitation of amorphous calcium phosphate (ACP), which then subsequently transforms to HAP. HAP formation releases protons contributing to matrix acidification, which then enhances amelogenin disassembly and KLK4 activity resulting in more protein hydrolysis. When the pH is regulated by ameloblasts to neutral, nascent crystals growing into this new space come into contact with the next layer of amelogenin nanospheres, and yet another cycle of the interaction-mediated preferential removal of bound amelogenin and crystal growth is initiated. The cycles of binding-growth-hydrolysis repeat until most of the matrix proteins are removed and the HAP crystals grow to fill up the entire enamel space to form the hardest tissue in our body.
2 Part 2: Regulations of Ion-Pumps, Transporters and Carbonic Anhydrases (CAs) Involved in Enamel Mineralization and pH Modulation
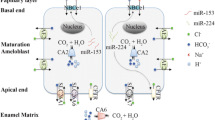
Maturation stage ameloblasts are responsible for the transport of 86% of minerals that are required for enamel matrix biomineralization (Smith 1998). As maturation stage ameloblasts cycle between smooth- and ruffle-ended ameloblasts, the expression and spatial cellular distribution of ion-transporters and carbonic anhydrases modulate to direct extracellular matrix pH cycling (Smith et al. 1996) while also transporting calcium and phosphate into the enamel matrix for crystal growth. Genes coding ion pumps (V-type-H+ATPase), transporters (CFTR, SLC26A, AE2, NHE1, NBCe1, NKCC1), and CAs (CA2 and CA6) are upregulated in ruffle-ended ameloblasts (Lacruz et al. 2011; Kim and Hong 2018). Reciprocal interactions between maturation ameloblasts and underlying enamel matrix contribute to the changes in gene expression between ruffle-ended and smooth-ended ameloblast in this unique process of enamel biomineralization.
2.1 Regulation of Matrix pH
2.1.1 Matrix Acidification
Cyclic acidification of the enamel matrix is associated with matrix mineralization (see Part 1). Matrix acidification occurs with the formation of hydroxyapatite crystals, which generate protons as the crystals form, and may also possibly be enhanced by extrusion of protons into the extracellular enamel matrix by V-H+ATPase and NHE1 (SLC(A1).
V-Type H+-ATPase subunits (Atp6v0d2, Atp6v1b2, Atp6v1c1 and Atp6v1e1) are highly upregulated in maturation stage ameloblasts. These subunits are involved in vesical acidification required for endocytosis of matrix proteins. The presence of subunits ATP6v1c1, ATP6v1e1, and Atp6v0a1 at the apical membrane of ruffle-ended maturation ameloblasts (Damkier et al. 2014; Josephsen et al. 2010; Sarkar et al. 2016), suggests an additional possibility, that these subunits of V-type H+-ATPase may export protons to assist the enamel matrix acidification. However, only a relatively small amount of bicarbonate is available for buffering the protons that are rapidly released as hydroxyapatite crystals form (Smith 1998). Thus, protons adding to the enamel matrix through V-type H+-ATPase may have only a limited role in further acidifying the enamel matrix.
NHE1, which is encoded by the SLC9A1 gene extrudes protons into the matrix as it mediates Na+ influx and H+ efflux. NHE1 as an acid extruder was identified in the lateral membrane during both the secretory and the maturation stages of ameloblasts (Josephsen et al. 2010). SLC9A1 is widely expressed with a preferential expression in the kidney, intestine, and liver, and NHE1 protein has a central role in regulating pH homeostasis, cell migration, and cell volume.
Calcium Transport
Acidification of the enamel matrix occurs when calcium and phosphate are transported into the matrix to form hydroxyapatite crystals. Maturation stage ameloblasts are responsible for the deposition of more than 70% of the calcium in enamel tissues (Smith 1998). This transport of calcium by the ameloblasts requires tight control of intracellular calcium concentrations ([Ca2+]) to avoid Ca2+ toxicity (Hubbard 2000).
Calcium transport into ameloblasts is mediated by store-operated calcium entry (SOCE) (Eckstein et al. 2017; Nurbaeva et al. 2017), ORAI, as well as by the cation channel TRPM7 (transient receptor potential cation channel subfamily M) (Faouzi et al. 2017; Nakano et al. 2016) and connexin 43 (Al-Ansari et al. 2018; Toth et al. 2010). The majority of Ca2+ is stored in the endoplasmic reticulum lumen, and the rest of excess Ca2+ binds to cytoplasmic calcium-binding proteins, including calbindin-D9k, -D28k, calmodulin (Berdal et al. 1993; Berdal et al. 1996; Hubbard 1995).
Calcium extrudes from ameloblasts into the enamel matrix through the basolateral and apical transmembrane Ca2+ pump, cotransporters/ion-exchangers, including Ca2+-ATPase (PMCA), potassium-independent Na+/Ca2+ (NCX) and potassium-dependent Na+/Ca2+ exchangers (NCKX) (Nurbaeva et al. 2017). Among the four types of PMCA subunits, PMCA-1 (ATP2B1) and PMCA-4 (ATP2B4) are the predominant isoforms of PMCA in human and mouse ameloblasts. Though the expression levels of these two isoforms remain comparable between secretory and maturation stage ameloblasts (Borke et al. 1993; Borke et al. 1995; Zaki et al. 1996), in maturation stage ameloblasts PMCA has been immunolocalized at the apical surface (Salama et al. 1987; Zaki et al. 1996).
As compared to PMCA, ion exchangers NCX and NCKX have a higher turnover rate of delivering calcium (Eisenmann et al. 1982), which is critical for providing a large amount of calcium for the rapid growth of hydroxyapatite crystals. The NCX isoform exchanges one Ca2+ outward for three Na+ inward (Yu and Choi 1997). Ncx1 and Ncx3 have been found to be equally expressed by secretory and maturation stage ameloblasts (Lacruz et al. 2012), suggesting that NCXs may act as housekeeping calcium transport in both secretory and maturation stage ameloblasts.
Whole transcriptome analysis shows that NCKX4 is the dominant isoform of the NCKX family expressed by ameloblasts, and is significantly upregulated in maturation ameloblasts as compared to secretory ameloblasts (Hu et al. 2012; Lacruz et al. 2012). NCKX4 can extrude one Ca2+ and one K+ in exchange for four Na+ inward (Lytton 2007). In humans, the NCKX4 gene has been identified as a causal gene of amelogenesis imperfecta (Jalloul et al. 2016; Parry et al. 2013; Smith et al. 2017).
Phosphate Transport
Hydroxyapatite has a calcium/phosphate ratio of 1.67, and therefore, also requires phosphate to be transported to the enamel matrix. A Na+-dependent Pi transporter such as NaPi-2b (SLC34A2), which is located in ruffle-ended ameloblasts, may operate in a coordinated way with NCKX4 to direct phosphate into the cells (Bronckers 2017; Bronckers et al. 2015).
2.1.2 Buffering to Neutralize Matrix pH
Secretory Stage
In the secretory stage enamel, where hydroxyapatite crystal formation is initiated to form long thin crystals, matrix acidification as a result of crystal formation, is likely buffered by the amelogenins in the secretory enamel matrix (Guo et al. 2015). Amelogenins are rich in histidine, which can absorb protons (Bansal et al. 2012; Simmer and Fincham 1995).
Maturation Stage
In the maturation stage, there are open junctions between smooth-ended ameloblasts, whereas ruffled-ended ameloblasts have tight apical junctions and open basal junctions (Skobe et al. 1985). The intercellular space between smooth-ended ameloblasts allows the free paracellular movement of fluid, ions, and small molecules, which might be able to correct the imbalance of pH immediately in the confined enamel space (Hanawa et al. 1990; Kawamoto and Shimizu 1997; McKee et al. 1986; Smith et al. 1987; Takano 1995; Takano et al. 1982b). This has been described as a “fluid flush” between smooth-ended ameloblasts, which brings in “base” and takes away “acid” to aid in the pH regulation activities.
In addition to this possibility, it is widely accepted that extrusion of bicarbonate by ameloblasts into the extracellular spaces buffers the intense acid loading during the rapid crystal growth in mineralizing enamel (Lacruz et al. 2010; Smith 1998; Varga et al. 2018; Yin and Paine 2017). Whole transcriptome analyses have shown that as compared to secretory ameloblasts, maturation ameloblasts have significantly upregulated genes involved in bicarbonate transport. These genes include: the chloride/bicarbonate channel CFTR (cystic fibrosis transmembrane conductance regulator); NBCe1 (sodium bicarbonate exchanger, solute carrier family 4 member 4); CA2 (carbonic anhydrase CAII) and CA6 (CAVI); AE2 (anion exchange protein 2) and the bicarbonate chloride exchangers SLC26A3/A4/A6/A7 (solute carrier 26, members A3, A4, A, and A7) (Bronckers 2017; Kim and Hong 2018; Lacruz et al. 2012; Simmer et al. 2014).
Ion Transporters
Ion transporters localized on apical ameloblast membranes include CFTR (Bronckers et al. 2010), SLC26 members (Yin et al. 2015), and CA (Bronckers 2017). CFTR likely contributes to bicarbonate transport in epithelial cells both directly by permeation through the channel, and indirectly by facilitating the function of Slc26 (Fong 2012). Using large-scale transcriptomic analysis, SLC26A4 has been shown as a target gene modulated by NaF and bisphenol in dental epithelia of rats (Jedeon et al. 2016b). To maintain bicarbonate supplies for extracellular transport, maturation-stage ameloblasts use carbonic anhydrases, CA2 and CA6 to catalyze the hydration of CO2. CA2 generates bicarbonate in the cytosol of ameloblasts (Josephsen et al. 2010; Lin et al. 1994; Toyosawa et al. 1996), and CA6, the secreted form of carbonic anhydrase, may generate bicarbonate in the enamel space (Smith et al. 2006).
Ion transporters localized on the basal ameloblast membranes include AE2, NBCe1, NHE1, and NKCC1. AE2 encoded by the SLC4A2 gene is a Cl−/HCO3− exchanger that exports bicarbonate into the extracellular space, in exchange for Cl− (Lyaruu et al. 2008; Paine et al. 2008). Bicarbonate can also be transported into ameloblasts by NBCe1, encoded by the SLC9A1 gene, which also mediates inward Na+ influx and H+ efflux (Josephsen et al. 2010; Lacruz et al. 2010; Paine et al. 2008). NKCC1, encoded by the SLC12A2 gene, mediates sodium and chloride transport and reabsorption.
pH Sensing Proteins
pH sensing proteins that may direct ameloblast regulation of matrix pH include the G-protein-coupled receptor 68 (GPR68). Defects in GPR68 are associated with amelogenesis imperfecta, suggesting a functional role of this pH sensor in ameloblast modulation of matrix pH cycling (Varga et al. 2018). GPR68 can also regulate steroid receptors, also present in maturation-stage ameloblasts (Houari et al. 2016). Steroid hormones and their receptors were reported to regulate CFTR, SLC26A6, NHE, and CA in several tissues (Gholami et al. 2013).
External Factors Regulating Ion Transport by Ameloblasts
Ameloblasts targeted by environmental toxicants and chemicals with endocrine-disrupting activities result in alterations in enamel formation (Babajko et al. 2017; Jedeon et al. 2013, 2016a, b) (see also Chap. 12). All of the transporters described in this chapter, with the possible exception of AE2, can be modulated by hormones or molecules with endocrine activity.
Hormonal changes that may affect enamel formation also include possible effects on the function of the HPA (hypothalamic–pituitary–adrenal) axis during enamel formation. Evidence for this possibility is the finding by Boyce et al., that children entering kindergarten who had increased salivary cortisol reactivity, had primary (deciduous) mandibular incisors with thinner and more hypomineralized enamel (Boyce et al. 2010). Cortisol reactivity in young children is associated with maternal prenatal stress (Luecken et al. 2013), and as the primary mandibular incisors are formed during the prenatal period of life, this suggests a possible link between alternations in HPA axis regulations and enamel formation.
2.2 Conclusions
Ameloblast Regulation of pH Cycling
Our understanding of how ameloblasts modulate and regulate pH cycling, as described in this chapter, has been advanced through immunolocalization of channels, transporters, and exchangers on ameloblast membranes, as well as in the use of cell culture models to investigate calcium transport and intracellular pH regulation (Bori et al. 2016; Bronckers 2017; Bronckers et al. 2015; Nurbaeva et al. 2015) (see Fig. 11.7).
However, much remains to be understood about the regulation of pH cycling by ameloblasts. As we further understand the mechanisms by which pH is regulated in enamel we will be better able to ensure optimal amelogenesis, enamel formation and mineralization.
References
Abbarin N, San Miguel S, Holcroft J, Iwasaki K, Ganss B (2015) The enamel protein amelotin is a promoter of hydroxyapatite mineralization. J Bone Miner Res 30:775–785. https://doi.org/10.1002/jbmr.2411
Al-Ansari S et al (2018) The importance of Connexin 43 in enamel development and mineralization. Front Physiol 9:750. https://doi.org/10.3389/fphys.2018.00750
Amaral SF, Scaffa PMC, Rodrigues RDS, Nesadal D, Marques MM, Nogueira FN, Sobral MAP (2018) Dynamic influence of pH on metalloproteinase activity in human coronal and radicular dentin. Caries Res 52:113–118. https://doi.org/10.1159/000479825
Aoba T, Moreno EC (1987) The enamel fluid in the early secretory stage of porcine amelogenesis: chemical composition and saturation with respect to enamel mineral. Calcif Tissue Int 41:86–94. https://doi.org/10.1007/BF02555250
Aoba T, Moreno EC, Kresak M, Tanabe T (1989) Possible roles of partial sequences at N- and C-termini of amelogenin in protein-enamel mineral interactions. J Dent Res 68:1331–1336. https://doi.org/10.1177/00220345890680090901
Babajko S, Jedeon K, Houari S, Loiodice S, Berdal A (2017) Disruption of steroid axis, a new paradigm for molar incisor hypomineralization (MIH). Front Physiol 8:343. https://doi.org/10.3389/fphys.2017.00343
Bansal AK, Shetty DC, Bindal R, Pathak A (2012) Amelogenin: a novel protein with diverse applications in genetic and molecular profiling. J Oral Maxillofac Pathol 16:395–399. https://doi.org/10.4103/0973-029X.102495
Bartlett JD (2013) Dental enamel development: proteinases and their enamel matrix substrates. ISRN dentistry 2013:684607. https://doi.org/10.1155/2013/684607
Bartlett JD, Simmer JP (1999) Proteinases in developing dental enamel. Crit Rev Oral Biol Med 10:425–441
Bartlett JD, Ryu OH, Xue J, Simmer JP, Margolis HC (1998) Enamelysin mRNA displays a developmental defined pattern of expression and encodes a protein which degrades amelogenin. Connect Tissue Res 39:101–109. https://doi.org/10.3109/03008209809023916
Beniash E, Simmer JP, Margolis HC (2005) The effect of recombinant mouse amelogenins on the formation and organization of hydroxyapatite crystals in vitro. J Struct Biol 149:182–190. https://doi.org/10.1016/j.jsb.2004.11.001
Beniash E, Metzler RA, Lam RS, Gilbert PU (2009) Transient amorphous calcium phosphate in forming enamel. J Struct Biol 166:133–143. https://doi.org/10.1016/j.jsb.2009.02.001
Beniash E, Simmer JP, Margolis HC (2012) Structural changes in amelogenin upon self-assembly and mineral interactions. J Dent Res 91:967–972. https://doi.org/10.1177/0022034512457371
Berdal A, Hotton D, Pike JW, Mathieu H, Dupret JM (1993) Cell- and stage-specific expression of vitamin D receptor and calbindin genes in rat incisor: regulation by 1,25-dihydroxyvitamin D3. Dev Biol 155:172–179. https://doi.org/10.1006/dbio.1993.1016
Berdal A, Hotton D, Saffar JL, Thomasset M, Nanci A (1996) Calbindin-D9k and calbindin-D28k expression in rat mineralized tissues in vivo. J Bone Miner Res 11:768–779. https://doi.org/10.1002/jbmr.5650110608
Bori E et al (2016) Evidence for bicarbonate secretion by ameloblasts in a novel cellular model. J Dent Res 95:588–596. https://doi.org/10.1177/0022034515625939
Borke JL, Zaki AE, Eisenmann DR, Ashrafi SH, Ashrafi SS, Penniston JT (1993) Expression of plasma membrane Ca++ pump epitopes parallels the progression of enamel and dentin mineralization in rat incisor. J Histochem Cytochem 41:175–181
Borke JL, Zaki A-M, Eisenmann DR, Mednieks MI (1995) Localization of plasma membrane Ca2+ pump mRNA and protein in human ameloblasts by in situ hybridization and immunohistochemistry. Connect Tissue Res 33:139–144
Boyce WT, Den Besten PK, Stamperdahl J, Zhan L, Jiang Y, Adler NE, Featherstone JD (2010) Social inequalities in childhood dental caries: the convergent roles of stress, bacteria and disadvantage. Soc Sci Med 71:1644–1652. https://doi.org/10.1016/j.socscimed.2010.07.045
Bronckers AL (2017) Ion transport by ameloblasts during amelogenesis. J Dent Res 96:243–253. https://doi.org/10.1177/0022034516681768
Bronckers A et al (2010) The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells. Bone 46:1188–1196. https://doi.org/10.1016/j.bone.2009.12.002
Bronckers AL, Lyaruu D, Jalali R, Medina JF, Zandieh-Doulabi B, DenBesten PK (2015) Ameloblast modulation and transport of Cl(−), Na(+), and K(+) during amelogenesis. J Dent Res 94:1740–1747. https://doi.org/10.1177/0022034515606900
Brookes SJ, Robinson C, Kirkham J, Bonass WA (1995) Biochemistry and molecular biology of amelogenin proteins of developing dental enamel. Arch Oral Biol 40:1–14. https://doi.org/10.1016/0003-9969(94)00135-X
Caterina J, Shi J, Krakora S, Bartlett JD, Engler JA, Kozak CA, Birkedal-Hansen H (1999) Isolation, characterization, and chromosomal location of the mouse enamelysin gene. Genomics 62:308–311. https://doi.org/10.1006/geno.1999.5990
Chen C-L, Bromley KM, Moradian-Oldak J, JJ DY (2011) In situ AFM study of amelogenin assembly and disassembly dynamics on charged surfaces provides insights on matrix protein self-assembly. J Am Chem Soc 133:17406–17413. https://doi.org/10.1021/ja206849c
Daculsi G, Kerebel B, Kerebel LM, Mitre D (1984) High-resolution study by transmission electron microscopy of a microhypoplasia of the human enamel surface. Arch Oral Biol 29:201–203. https://doi.org/10.1016/0003-9969(84)90055-4
Damkier HH, Josephsen K, Takano Y, Zahn D, Fejerskov O, Frische S (2014) Fluctuations in surface pH of maturing rat incisor enamel are a result of cycles of H(+)-secretion by ameloblasts and variations in enamel buffer characteristics. Bone 60:227–234. https://doi.org/10.1016/j.bone.2013.12.018
DenBesten PK, Heffernan LM (1989) Separation by polyacrylamide gel electrophoresis of multiple proteases in rat and bovine enamel. Arch Oral Biol 34:399–404. https://doi.org/10.1016/0003-9969(89)90117-9
DenBesten P, Li W (2011) Chronic fluoride toxicity: dental fluorosis. Monogr Oral Sci 22:81–96. https://doi.org/10.1159/000327028
Duverger O et al (2014) Hair keratin mutations in tooth enamel increase dental decay risk. J Clin Investig 124:5219–5224. https://doi.org/10.1172/JCI78272
Eanes ED (1976) The interaction of supersaturated calcium phosphate solutions with apatitic substrates. Calcif Tissue Res 20:75–89. https://doi.org/10.1007/BF02546399
Eanes ED, Gillessen IH, Posner AS (1965) Intermediate states in the precipitation of hydroxyapatite. Nature 208:365–367. https://doi.org/10.1038/208365a0
Eckstein M et al (2017) Store-operated Ca(2+) entry controls ameloblast cell function and enamel development. JCI Insight 2:e91166. https://doi.org/10.1172/jci.insight.91166
Eisenmann DR, Ashrafi S, Zaki AE (1982) Multi-method analysis of calcium localization in the secretory ameloblast. J Dent Res Spec No:1555-1562
Faouzi M, Kilch T, Horgen FD, Fleig A, Penner R (2017) The TRPM7 channel kinase regulates store-operated calcium entry. J Physiol 595:3165–3180. https://doi.org/10.1113/JP274006
Fincham AG, Belcourt AB, Termine JD, Butler WT, Cothran WC (1981) Dental enamel matrix: sequences of two amelogenin polypeptides. Biosci Rep 1:771–778. https://doi.org/10.1007/BF01114799
Fincham AG, Hu YY, Pavlova Z, Slavkin HC, Snead ML (1989) Human amelogenins: sequences of “TRAP” molecules. Calcif Tissue Int 45:243–250. https://doi.org/10.1007/bf02556044
Fong P (2012) CFTR-SLC26 transporter interactions in epithelia. Biophys Rev 4:107–116. https://doi.org/10.1007/s12551-012-0068-9
Fouillen A, Dos Santos NJ, Mary C, Castonguay JD, Moffatt P, Baron C, Nanci A (2017) Interactions of AMTN, ODAM and SCPPPQ1 proteins of a specialized basal lamina that attaches epithelial cells to tooth mineral. Sci Rep 7:46683. https://doi.org/10.1038/srep46683
Fukae M et al (1998) Enamelysin (matrix metalloproteinase-20): localization in the developing tooth and effects of pH and calcium on amelogenin hydrolysis. J Dent Res 77:1580–1588. https://doi.org/10.1177/00220345980770080501
Gholami K, Muniandy S, Salleh N (2013) In-vivo functional study on the involvement of CFTR, SLC26A6, NHE-1 and CA isoenzymes II and XII in uterine fluid pH, volume and electrolyte regulation in rats under different sex-steroid influence. Int J Med Sci 10:1121–1134. https://doi.org/10.7150/ijms.5918
Gibson CW (2011) The amelogenin proteins and enamel development in humans and mice. J Oral Biosci 53:248–256. https://doi.org/10.2330/joralbiosci.53.248
Gibson CW et al (2001) Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 276:31871–31875. https://doi.org/10.1074/jbc.M104624200
Goldberg M, Septier D, Bourd K, Hall R, George A, Goldberg H, Menashi S (2003) Immunohistochemical localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the forming rat incisor. Connect Tissue Res 44:143–153. https://doi.org/10.1080/03008200390223927
Gray JJ (2004) The interaction of proteins with solid surfaces. Curr Opin Struct Biol 14:110–115. https://doi.org/10.1016/j.sbi.2003.12.001
Guo J, Lyaruu DM, Takano Y, Gibson CW, DenBesten PK, Bronckers AL (2015) Amelogenins as potential buffers during secretory-stage amelogenesis. J Dent Res 94:412–420. https://doi.org/10.1177/0022034514564186
Habelitz S, Kullar A, Marshall SJ, DenBesten PK, Balooch M, Marshall GW, Li W (2004) Amelogenin-guided crystal growth on fluoroapatite glass-ceramics. J Dent Res 83:698–702. https://doi.org/10.1177/154405910408300908
Habelitz S, Denbesten PK, Marshall SJ, Marshall GW, Li W (2005) Amelogenin control over apatite crystal growth is affected by the pH and degree of ionic saturation. Orthod Craniofac Res 8:232–238. https://doi.org/10.1111/j.1601-6343.2005.00343.x
Hanawa M, Takano Y, Wakita M (1990) An autoradiographic study of calcium movement in the enamel organ of rat molar tooth germs. Arch Oral Biol 35:899–906
He B, Huang S, Zhang C, Jing J, Hao Y, Xiao L, Zhou X (2011) Mineral densities and elemental content in different layers of healthy human enamel with varying teeth age. Arch Oral Biol 56:997–1004. https://doi.org/10.1016/j.archoralbio.2011.02.015
Hlady VV, Buijs J (1996) Protein adsorption on solid surfaces. Curr Opin Biotechnol 7:72–77. https://doi.org/10.1016/s0958-1669(96)80098-x
Houari S, Loiodice S, Jedeon K, Berdal A, Babajko S (2016) Expression of steroid receptors in ameloblasts during amelogenesis in rat incisors. Front Physiol 7:503. https://doi.org/10.3389/fphys.2016.00503
Hu JC, Sun X, Zhang C, Liu S, Bartlett JD, Simmer JP (2002) Enamelysin and kallikrein-4 mRNA expression in developing mouse molars. Eur J Oral Sci 110:307–315. https://doi.org/10.1034/j.1600-0722.2002.21301.x
Hu JC, Lertlam R, Richardson AS, Smith CE, McKee MD, Simmer JP (2011) Cell proliferation and apoptosis in enamelin null mice. Eur J Oral Sci 119(Suppl 1):329–337. https://doi.org/10.1111/j.1600-0722.2011.00860.x
Hu P, Lacruz RS, Smith CE, Smith SM, Kurtz I, Paine ML (2012) Expression of the sodium/calcium/potassium exchanger, NCKX4, in ameloblasts. Cells Tissues Organs 196:501–509. https://doi.org/10.1159/000337493
Hubbard MJ (1995) Calbindin28kDa and calmodulin are hyperabundant in rat dental enamel cells. Identification of the protein phosphatase calcineurin as a principal calmodulin target and of a secretion-related role for calbindin28kDa. Eur J Biochem 230:68–79. https://doi.org/10.1111/j.1432-1033.1995.tb20535.x
Hubbard MJ (2000) Calcium transport across the dental enamel epithelium. Crit Rev Oral Biol Med 11:437–466. https://doi.org/10.1177/10454411000110040401
Iijima M, Moradian-Oldak J (2004) Interactions of amelogenins with octacalcium phosphate crystal faces are dose dependent. Calcif Tissue Int 74:522–531. https://doi.org/10.1007/s00223-002-0011-3
Iijima M, Moriwaki Y, Wen HB, Fincham AG, Moradian-Oldak J (2002) Elongated growth of octacalcium phosphate crystals in recombinant amelogenin gels under controlled ionic flow. J Dent Res 81:69–73. https://doi.org/10.1177/002203450208100115
Jaeger C, Groom NS, Bowe EA, Horner A, Davies ME, Murray RC, Duer MJ (2005) Investigation of the nature of the protein–mineral interface in bone by solid-state NMR. Chem Mater 17:3059–3061. https://doi.org/10.1021/cm050492k
Jalloul AH, Rogasevskaia TP, Szerencsei RT, Schnetkamp PP (2016) A functional study of mutations in K+-dependent Na+-Ca2+ exchangers associated with amelogenesis imperfecta and non-syndromic oculocutaneous albinism. J Biol Chem 291:13113–13123. https://doi.org/10.1074/jbc.M116.728824
Jedeon K et al (2013) Enamel defects reflect perinatal exposure to bisphenol A. Am J Pathol 183:108–118. https://doi.org/10.1016/j.ajpath.2013.04.004
Jedeon K, Berdal A, Babajko A (2016a) Impact of three endocrine disruptors, Bisphenol A, Genistein and Vinclozolin on female rat enamel. Bull Group Int Rech Sci Stomatol Odontol 53:e28
Jedeon K, Houari S, Loiodice S, Thuy TT, Le Normand M, Berdal A, Babajko S (2016b) Chronic exposure to bisphenol A exacerbates dental fluorosis in growing rats. J Bone Miner Res 31:1955–1966. https://doi.org/10.1002/jbmr.2879
Josephsen K (1983) Indirect visualization of ameloblast modulation in the rat incisor using calcium-binding compounds. Scand J Dent Res 91:76–78. https://doi.org/10.1111/j.1600-0722.1983.tb00780.x
Josephsen K, Takano Y, Frische S, Praetorius J, Nielsen S, Aoba T, Fejerskov O (2010) Ion transporters in secretory and cyclically modulating ameloblasts: a new hypothesis for cellular control of preeruptive enamel maturation. Am J Physiol Cell Physiol 299:C1299–C1307. https://doi.org/10.1152/ajpcell.00218.2010
Kallenbach E (1973) The fine structure of tomes’ process of rat incisor ameloblasts and its relationship to the elaboration of enamel. Tissue Cell 5:501–524. https://doi.org/10.1016/S0040-8166(73)80041-2
Kawamoto T, Shimizu M (1997) Pathway and speed of calcium movement from blood to mineralizing enamel. J Histochem Cytochem 45:213–230. https://doi.org/10.1177/002215549704500207
Kim HE, Hong JH (2018) The overview of channels, transporters, and calcium signaling molecules during amelogenesis. Arch Oral Biol 93:47–55. https://doi.org/10.1016/j.archoralbio.2018.05.014
Lacruz RS, Nanci A, Kurtz I, Wright JT, Paine ML (2010) Regulation of pH during amelogenesis. Calcif Tissue Int 86:91–103. https://doi.org/10.1007/s00223-009-9326-7
Lacruz RS, Smith CE, Chen Y-B, Hubbard MJ, Hacia JG, Paine ML (2011) Gene-expression analysis of early- and late-maturation-stage rat enamel organ. Eur J Oral Sci 119:149–157
Lacruz RS et al (2012) Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J Cell Physiol 227:2264–2275. https://doi.org/10.1002/jcp.22965
Le TQ, Gochin M, Featherstone JD, Li W, DenBesten PK (2006) Comparative calcium binding of leucine-rich amelogenin peptide and full-length amelogenin. Eur J Oral Sci 114(Suppl 1):320–326. https://doi.org/10.1111/j.1600-0722.2006.00313.x
Li W, Machule D, Gao C, DenBesten PK (1999) Activation of recombinant bovine matrix metalloproteinase-20 and its hydrolysis of two amelogenin oligopeptides. Eur J Oral Sci 107:352–359
Lin HM, Nakamura H, Noda T, Ozawa H (1994) Localization of H(+)-ATPase and carbonic anhydrase II in ameloblasts at maturation. Calcif Tissue Int 55:38–45
Llano E et al (1997) Identification and structural and functional characterization of human enamelysin (MMP-20). Biochemistry 36:15101–15108. https://doi.org/10.1021/bi972120y
Lu Y, Papagerakis P, Yamakoshi Y, Hu JCC, Bartlett JD, Simmer JP (2008) Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem 389:695–700. https://doi.org/10.1515/BC.2008.080
Luecken LJ, Lin B, Coburn SS, MacKinnon DP, Gonzales NA, Crnic KA (2013) Prenatal stress, partner support, and infant cortisol reactivity in low-income Mexican American families. Psychoneuroendocrinology 38:3092–3101. https://doi.org/10.1016/j.psyneuen.2013.09.006
Lyaruu DM et al (2008) The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol 27:119–127. https://doi.org/10.1016/j.matbio.2007.09.006
Lytton J (2007) Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406:365–382. https://doi.org/10.1042/BJ20070619
Margolis HC, Beniash E, Fowler CE (2006) Role of macromolecular assembly of enamel matrix proteins in enamel formation. J Dent Res 85:775–793. https://doi.org/10.1177/154405910608500902
Massova I, Kotra LP, Fridman R, Mobashery S (1998) Matrix metalloproteinases: structures, evolution, and diversification. FASEB J 12:1075–1095
McKee MD, Martineau-Doize B, Warshawsky H (1986) Penetration of various molecular-weight proteins into the enamel organ and enamel of the rat incisor. Arch Oral Biol 31:287–296. https://doi.org/10.1016/0003-9969(86)90042-7
Moffatt P, Smith CE, St-Arnaud R, Nanci A (2008) Characterization of Apin, a secreted protein highly expressed in tooth-associated epithelia. J Cell Biochem 103:941–956. https://doi.org/10.1002/jcb.21465
Nakano Y et al (2016) A critical role of TRPM7 as an ion channel protein in mediating the mineralization of the craniofacial hard tissues. Front Physiol 7:258. https://doi.org/10.3389/fphys.2016.00258
Nurbaeva MK et al (2015) Dental enamel cells express functional SOCE channels. Sci Rep 5:15803. https://doi.org/10.1038/srep15803
Nurbaeva MK, Eckstein M, Feske S, Lacruz RS (2017) Ca(2+) transport and signalling in enamel cells. J Physiol 595:3015–3039. https://doi.org/10.1113/JP272775
Paine ML et al (2008) Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res 87:391–395
Parry DA et al (2013) Identification of mutations in SLC24A4, encoding a potassium-dependent sodium/calcium exchanger, as a cause of amelogenesis imperfecta. Am J Hum Genet 92:307–312. https://doi.org/10.1016/j.ajhg.2013.01.003
Picard V, Govoni G, Jabado N, Gros P (2000) Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem 275:35738–35745. https://doi.org/10.1074/jbc.M005387200
Pugach MK et al (2010) The amelogenin C-terminus is required for enamel development. J Dent Res 89:165–169. https://doi.org/10.1177/0022034509358392
Robinson C (2014) Enamel maturation: a brief background with implications for some enamel dysplasias. Front Physiol 5:388. https://doi.org/10.3389/fphys.2014.00388
Robinson C, Brookes SJ, Shore RC, Kirkham J (1998) The developing enamel matrix: nature and function. Eur J Oral Sci 106(Suppl 1):282–291. https://doi.org/10.1111/j.1600-0722.1998.tb02188.x
Ryu OH, Hu CC, Simmer JP (1998) Biochemical characterization of recombinant mouse amelogenins: protein quantitation, proton absorption, and relative affinity for enamel crystals. Connect Tissue Res 38:207–214
Ryu O et al (2002) Porcine kallikrein-4 activation, glycosylation, activity, and expression in prokaryotic and eukaryotic hosts. Eur J Oral Sci 110:358–365
Salama AH, Zaki AE, Eisenmann DR (1987) Cytochemical localization of Ca2+-Mg2+ adenosine triphosphatase in rat incisor ameloblasts during enamel secretion and maturation. J Histochem Cytochem 35:471–482
Sarkar J, Wen X, Simanian EJ, Paine ML (2016) V-type ATPase proton pump expression during enamel formation. Matrix Biol 52-54:234–245. https://doi.org/10.1016/j.matbio.2015.11.004
Sasaki S, Takagi T, Suzuki M (1991a) Amelogenin degradation by an enzyme having acidic pH optimum and the presence of acidic zones in developing bovine enamel. In: Suga S, Nakahara H (eds) Mechanisms and phylogeny of mineralization in biological systems. Springer Japan, Tokyo, pp 79–81
Sasaki S, Takagi T, Suzuki M (1991b) Cyclical changes in pH in bovine developing enamel as sequential bands. Arch Oral Biol 36(3):227–231
Shimabayashi S, Uno T, Nakagaki M (1997) Formation of a surface complex between polymer and surfactant and its effect on the dispersion of solid particles. Colloids Surf A Physicochem Eng Aspects 123–124:283–295. https://doi.org/10.1016/S0927-7757(96)03820-4
Shin NY et al (2020) Amelogenin phosphorylation regulates tooth enamel formation by stabilizing a transient amorphous mineral precursor. J Biol Chem 295:1943–1959. https://doi.org/10.1074/jbc.RA119.010506
Simmer JP, Fincham AG (1995) Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med 6:84–108
Simmer JP, Hu JCC (2002) Expression, structure, and function of enamel proteinases. Connect Tissue Res 43:441–449. https://doi.org/10.1080/03008200290001159
Simmer JP et al (1998) Purification, characterization, and cloning of enamel matrix serine proteinase 1. J Dent Res 77:377–386. https://doi.org/10.1177/00220345980770020601
Simmer JP, Hu Y, Lertlam R, Yamakoshi Y, Hu JC (2009) Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J Biol Chem 284:19110–19121. https://doi.org/10.1074/jbc.M109.013623
Simmer JP, Richardson AS, Wang SK, Reid BM, Bai Y, Hu Y, Hu JC (2014) Ameloblast transcriptome changes from secretory to maturation stages. Connect Tissue Res 55(Suppl 1):29–32. https://doi.org/10.3109/03008207.2014.923862
Skobe Z, LaFrazia F, Prostak K (1985) Correlation of apical and lateral membrane modulations of maturation ameloblasts. J Dent Res 64:1055–1061. https://doi.org/10.1177/00220345850640080601
Smith CE (1998) Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128–161. https://doi.org/10.1177/10454411980090020101
Smith CE, Nanci A (1989) Secretory activity as a function of the development and maturation of ameloblasts. Connect Tissue Res 22:147–156
Smith CE, McKee MD, Nanci A (1987) Cyclic induction and rapid movement of sequential waves of new smooth-ended ameloblast modulation bands in rat incisors as visualized by polychrome fluorescent labeling and GBHA-staining of maturing enamel. Adv Dent Res 1:162–175
Smith CE, Issid M, Margolis HC, Moreno EC (1996) Developmental changes in the pH of enamel fluid and its effects on matrix-resident proteinases. Adv Dent Res 10:159–169
Smith CE, Nanci A, Moffatt P (2006) Evidence by signal peptide trap technology for the expression of carbonic anhydrase 6 in rat incisor enamel organs. Eur J Oral Sci 114:147–153. https://doi.org/10.1111/j.1600-0722.2006.00273.x
Smith CEL, Poulter JA, Antanaviciute A, Kirkham J, Brookes SJ, Inglehearn CF, Mighell AJ (2017) Amelogenesis imperfecta; genes, proteins, and pathways. Front Physiol 8:435. https://doi.org/10.3389/fphys.2017.00435
Takagi T, Ogasawara T, Tagami J, Akao M, Kuboki Y, Nagai N, LeGeros RZ (1998) pH and carbonate levels in developing enamel. Connect Tissue Res 38:181–187. discussion 201-185. https://doi.org/10.3109/03008209809017035
Takano Y (1995) Enamel mineralization and the role of ameloblasts in calcium transport. Connect Tissue Res 33:127–137
Takano Y, Crenshaw MA, Bawden JW, Hammarström L, Lindskog S (1982a) The visualization of the patterns of ameloblast modulation by the glyoxal bis(2-hydroxyanil) staining method. J Dent Res Spec No:1580-1587
Takano Y, Crenshaw MA, Reith EJ (1982b) Correlation of 45Ca incorporation with maturation ameloblast morphology in the rat incisor. Calcif Tissue Int 34:211–213. https://doi.org/10.1007/BF02411236
Tao J, Buchko GW, Shaw WJ, De Yoreo JJ, Tarasevich BJ (2015) Sequence-defined energetic shifts control the disassembly kinetics and microstructure of amelogenin adsorbed onto hydroxyapatite (100). Langmuir 31:10451–10460. https://doi.org/10.1021/acs.langmuir.5b02549
Tarasevich BJ, Lea S, Bernt W, Engelhard M, Shaw WJ (2009a) Adsorption of amelogenin onto self-assembled and fluoroapatite surfaces. J Phys Chem B 113:1833–1842. https://doi.org/10.1021/jp804548x
Tarasevich BJ, Lea S, Bernt W, Engelhard MH, Shaw WJ (2009b) Changes in the quaternary structure of amelogenin when adsorbed onto surfaces. Biopolymers 91:103–107. https://doi.org/10.1002/bip.21095
Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49:4715–4723. https://doi.org/10.1021/bi100397s
Toth K, Shao Q, Lorentz R, Laird DW (2010) Decreased levels of Cx43 gap junctions result in ameloblast dysregulation and enamel hypoplasia in Gja1Jrt/+ mice. J Cell Physiol 223:601–609. https://doi.org/10.1002/jcp.22046
Toyosawa S, Ogawa Y, Inagaki T, Ijuhin N (1996) Immunohistochemical localization of carbonic anhydrase isozyme II in rat incisor epithelial cells at various stages of amelogenesis. Cell Tissue Res 285:217–225
Varga G, DenBesten P, Racz R, Zsembery A (2018) Importance of bicarbonate transport in pH control during amelogenesis - need for functional studies. Oral Dis 24:879–890. https://doi.org/10.1111/odi.12738
Wazen RM, Moffatt P, Zalzal SF, Yamada Y, Nanci A (2009) A mouse model expressing a truncated form of ameloblastin exhibits dental and junctional epithelium defects. Matrix Biol 28:292–303. https://doi.org/10.1016/j.matbio.2009.04.004
Wöltgens JH, Lyaruu DM, Bervoets TJ (1991) Possible functions of alkaline phosphatase in dental mineralization: cadmium effects. J Biol Buccale 19:125–128
Yamakoshi Y, Simmer JP, Bartlett JD, Karakida T, Oida S (2013) MMP20 and KLK4 activation and inactivation interactions in vitro. Arch Oral Biol 58:1569–1577. https://doi.org/10.1016/j.archoralbio.2013.08.005
Yin K, Paine ML (2017) Bicarbonate transport during enamel maturation. Calcif Tissue Int 101:457–464. https://doi.org/10.1007/s00223-017-0311-2
Yin K et al (2015) SLC26A gene family participate in pH regulation during enamel maturation. PLoS One 10:e0144703. https://doi.org/10.1371/journal.pone.0144703
Yu SP, Choi DW (1997) Na(+)-Ca2+ exchange currents in cortical neurons: concomitant forward and reverse operation and effect of glutamate. Eur J Neurosci 9:1273–1281. https://doi.org/10.1111/j.1460-9568.1997.tb01482.x
Zaki AE, Hand AR, Mednieks MI, Eisenmann DR, Borke JL (1996) Quantitative immunocytochemistry of Ca(2+)-Mg2+ ATPase in ameloblasts associated with enamel secretion and maturation in the rat incisor. Adv Dent Res 10:245–251
Zhu L, Liu H, Witkowska HE, Huang Y, Tanimoto K, Li W (2014) Preferential and selective degradation and removal of amelogenin adsorbed on hydroxyapatites by MMP20 and KLK4 in vitro. Front Physiol 5:268. https://doi.org/10.3389/fphys.2014.00268
Acknowledgements
The work was supported by NIH/NIDCR fundings R01DE027971, R01DE025709 and R01DE027076.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Li, W., Zhang, Y., Babajko, S., Besten, P.D. (2021). Enamel Matrix Biomineralization: The Role of pH Cycling. In: Goldberg, M., Den Besten, P. (eds) Extracellular Matrix Biomineralization of Dental Tissue Structures. Biology of Extracellular Matrix, vol 10. Springer, Cham. https://doi.org/10.1007/978-3-030-76283-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-76283-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76282-7
Online ISBN: 978-3-030-76283-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)