Abstract
Nanotechnology has shown enhanced potential in multidisciplinary fields and has been an emerging area of research for decades. Cancer theranostics, a technology with a combined diagnostic and therapeutic impact, provides efficient drug delivery with enhanced targeting along with safe and efficient chemotherapy. Safety and toxicity are critical problems that need to be addressed for the successful clinical application of nanobiomaterials in cancer theranostics. The factors impacting the safety of nanobiomaterials include shape, size, surface charge, physiological fluids to which they are subjected, biocompatibility, dosage-related parameters, and exposure time. Zebrafish, a promising gold standard model for in vitro evaluation of toxicity of nanopharmaceuticals, can also be used for assessment of nanobiomaterials intended for the environment, health, and safety. Safe-by-design is an innovative approach proposed by scientists for managing the safety aspects of nanobiomaterials. It involves the use of various strategies like doping, coating, grafting, loading, optimizing size and shape, managing surface charge, reducing persistence and interaction, and passivating the defective site for safe production, development, and use of nanobiomaterials. It also helps in risk management to resolve uncertainty, indeterminacy, and design liability. The use of green nanobiomaterials is an emerging field which addresses safety toward the environment as well as human health. This chapter provides an overview of nanobiomaterials used in cancer theranostics, concept of safe-by-design strategy, toxicological studies, risk management, as well as emerging trends in the field of theranostics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cancer is the second leading cause of human death worldwide, with approximately 7.5 million deaths reported till date in 2020 (Cancer Statistics – Worldometer, n.d.. Globally, about one in six deaths is due to cancer (https://www.who.int/westernpacific/health-topics/cancer). As per the WHO, there might be approximately around 13 million deaths from cancer in 2030 (WHO | Key statistics, 2020). Cancer is one of the fatal diseases for which scientists have been battling for decades (Anand et al., 2020). The traditional therapy of cancer involves chemotherapy, radiotherapy, and surgery (Shukla et al., 2019). Chemotherapy kills both the normal and the healthy cells, also attributing to multidrug resistance (Brannon-peppas & Blanchette, 2004). Radiotherapy is appropriate for localized cancer, but it also lacks specificity, leading to toxicity and damage to neighboring cells (Lungu et al., 2019).
Cancer theranostics have combined action of diagnosis as well as a therapeutic effect (Gobbo et al., 2015). Nanotheranostics is a rapidly evolving area for tracking the delivery and release of drugs and therapeutic evaluation at the same time and efficacy by a single nanoscale carrier. Nanoparticles are modified to integrate different bioconjugated moieties for accurate detection and therapy (Gobbo et al., 2015; Indoria et al., 2020). The use of nanobiomaterials overcomes the drawback associated with the traditional methods. These materials are nontoxic, biocompatible, and biodegradable and allow controlled and sustained release of anticancer drugs. The major attractions include ease of size and charge manipulation, decrease in adverse effects, site specificity, and flexibility of route of administration (Pandurangan et al., 2016). Nanobiomaterials should interact with cancer cells without disturbing normal biological functions. In cancer therapy, they are useful in molecular imaging, early detection of cancer, bioinformatics, and targeting of cancer cells (Mody et al., 2016). These materials exhibit different properties in bulk form compared to when they are nanosized. The safety of the nanobiomaterials lies in the difference in this property (Tekade et al., 2018). This chapter addresses the thin line that exists between safety and toxicity of nanobiomaterials and will cover various nanobiomaterials used in cancer theranostics, their safety, evaluation parameters, and regulatory perspectives.
Nanobiomaterials Used in Cancer Theranostics
There are various nanobiomaterials used for cancer theranostics. For better understanding, they have been categorized into metal-based nanobiomaterials, polymeric nanobiomaterials, carbon-based nanobiomaterials, nanomaterials derived from natural origin, and protein-based nanomaterials. The metal-based nanomaterials include gold nanoparticles, silver nanoparticles, iron oxide nanoparticles, silica, selenium, titanium dioxide, and zinc oxide-based nanobiomaterials (Mody et al., 2016). Applications like bioimaging, phototherapy, gene delivery, drug delivery, and use as a biosensor are possible using the optical, surface, chemical, and electrical properties of gold nanoparticles. Gold nanoparticles can absorb near- infrared light and transfer light energy to localized surface plasmon resonance for bioimaging or as a photothermal agent in cancer theranostics (Jiang et al., 2012). The whole-body scan is possible using this optical property. Since epidermal growth factors express themselves in several cancers, one can conjugate gold nanoparticles with endothelial growth factor receptor
antibodies. Using these conjugates for imaging is possible with the help of a scanning confocal microscope (Guo et al., 2017). The oxidative stress induction and cytotoxic effects of gold nanoparticles make it a potential candidate for cancer therapy (Saravanan et al., 2019). Gold nanoparticles can serve as a suitable marker for increasing intratumor localization of anticancer drugs (Rejinold et al., 2015). The inherent optical property of silver nanoparticles makes silver interact with the wavelength of light (400nm) (Seeta et al., 2019). Compared to other nanobiomaterials, silver nanoparticles show better light absorption and resolution (Mihail et al., 2016). Silver alone or its amalgam with other metals can be used for medical imaging (Seeta et al., 2019). The nanosized silver nanoparticles can enter the tumor efficiently by passive or active targeting. They affect the mitochondria-producing reactive oxygen species, leading to oxidative stress and apoptosis of cancer cells. At 435 nm, it shows maximum cytotoxicity and anticancer activity due to activation of the apoptotic gene (Aydin et al., 2019). The capacity of iron oxide nanoparticles to bind with antibodies, chemotherapeutic agents, and nucleic acid also makes it a suitable candidate for cancer theranostics (Santhosh & Ulrih, 2013). Iron oxide nanoparticles (IONPs) are suitable as MRI contrast agents. These nanoparticles can bind covalently or noncovalently with the overexpressed cancer biomarker (Zhu et al., 2016). They can also navigate through tumor margin, metastasis, and inflammatory areas and can find the location of angiogenesis, making it possible to visualize and predict the stage of cancer. IONP labeling of cells can help to determine the spread of immune-competent cells in the tumor (Singh & Sahoo, 2013) and for biosensing. The study by Liu et al. depicted that they are sensitive and precise in detecting biomarkers at higher spatial and temporal resolution. Using the phenomenon of magnetic fluid hyperthermia, one can apply external heat to IONPs to target the cancer cells. It will convert magnetic energy to heat energy and will selectively kill the cancer cells (Liu et al., 2013). Research has increased in the development of mesoporous silica nanoparticles (MSNs) due to their unique properties like high surface area, flexibility in size, and ability to modify functional groups. Imaging and drug delivery are feasible using these properties. A study by Bobo et al. showed a synergistic effect of using a photosensitizer, porphyrin, and a drug, camptothecin, in lecithin-targeted mesoporous silica nanoparticles (Gary-Bobo et al., 2011). Cheng et al. developed polydopamine poly(ethylene)glycol-folic acid-modified MSNs for delivering doxorubicin for the treatment of cervical cancer. This novel system has higher antitumor activity in vivo and is a promising carrier (Cheng et al., 2017). Selenium is one of the extensively used chemotherapeutic agents. It is due to the ability to regulate cell cycle, inhibit migration of tumorcells, stimulate apoptosis, and invade them in vitro. Selenium can enhance anticancer effect of photodynamic therapy, modulate growth-stimulating hormone systems, and decrease selenium-binding protein expression for personalized therapy for people suffering from hepatocellular carcinoma (Sanmartin et al., 2012). The investigation of difference in disulfide, thioether, diselenide, carbon, and selenoether bonds depicts that selenoether and diselenide bonds produce more reactive oxygen species (ROS) and enhance cytotoxicity of paclitaxel-citronella prodrug conjugate (Sun et al., 2019). Titanium dioxide can stop the growth of tumors and bring improvement in cancer therapy. Nanoformulations developed from titanium oxide affect the proliferation of cells by blocking cell cycle. It exhibits an increased cytotoxicity effect and potential dose-dependent effect on cell proliferation and leads to cell death. It also leads to decrease in ATP level, inhibition of apoptosis, and necrotic cell death (Raja et al., 2020). Sonodynamic therapy involves the use of titanium dioxide and zinc oxide. Using a conjugation of antibodies with titanium oxide and zinc oxide helps in targeting specific receptors. It also decreases the adverse effects associated with daunorubicin and doxorubicin (Çeşmeli & BirayAvci, 2019). A study by Bai et al. depicted that the use of 20 nm-sized zinc oxide nanoparticles induced considerable cytotoxicity in human ovarian cancer cells by induction of ROS, which affects cells by apoptosis, autophagy, and mitochondrial malfunction. Photodynamic therapy using zinc oxide produces ROS, leading to significant cytotoxicity in cancer cells, thereby increasing the selectivity and decreasing the adverse effects. It gets localized in tumor cells, and by focusing light on that region, selective and specific therapeutic action is achievable (Ancona et al., 2018).
Polymeric nanobiomaterials include poly(lactide-co-glycolic acid), polycaprolactone, chitosan, polylactide acid, polyethylene glycol-poly(lactic acid-co-glycolic acid) (PEG-PLGA), etc. PEGylated form of PLGA nanoparticles (NPs) increases the therapeutic index of paclitaxel (Prabhu et al., 2015). The hyaluronic acid-based paclitaxel nano-lipid carrier gives a high therapeutic index and site specificity (Shukla et al., 2019). Chemotherapeutic drugs like paclitaxel, doxorubicin, docetaxel, camptothecin, and 5- fluorouracil are delivered using chitosan as a carrier. Conjugating chitosan with quantum dots, gadolinium, supermagnetic iron oxides, etc. is useful for imaging (Fernandez-Fernandez et al., 2011). PLGA has been used to encapsulate vincristine sulfate, dexamethasone, paclitaxel, doxorubicin, and cisplatin. It has completed its journey from benchside to bedside (Fernandez-Fernandez et al., 2011). Doxorubicin chitosan-polyalkyl cyanoacrylate nanoparticles show an increased therapeutic effect on cancer cells expressing folate receptors (Kumar et al., 2019). Polycaprolactone (PCL) can enhance the oral efficacy of the cytotoxic drugs – doxorubicin and paclitaxel. A PEGylated derivative of PCL was used as a nanoparticulate implant to be administered postsurgery. Polycaprolactone-hydrazine linkage releases the drug in acidic media with significant toxicity on cancer cells (Kumar et al., 2019). The cisplatin PEGylated polyglutamic acid micelles exhibited longer circulation time and accumulation in Lewis lung carcinoma cells. The treatment gave promising tumor regression, 20 times higher accumulation, and no weight loss in animal models (Prabhu et al., 2015).
Carbon nanomaterials consist of carbon nanotubes, carbon nanohorns, and fullerenes. Its graphene and carbon cage-like structure allows functional modification, imparts stability, and increases its drug-carrying capacity. Its unique property makes it flexible for incorporating both hydrophilic and hydrophobic drugs. Fullerenes being smaller in size can easily penetrate intracellularly (Yamashita et al., 2012). Drugs like paclitaxel and doxorubicin conjugated with fullerenes are efficient cancer theranostic. It is used as self-labeled probes designed for imaging, tracking drug delivery, mitigating adverse effects associated with chemotherapeutic drugs, and increasing selectivity toward cancer cells. The functional derivatives are capable of downregulating various angiogenic factors and suppress metastasis (Chen et al., 2012). Carbon nanotubes have the potential to penetrate the cell membrane and diffuse through lipid bilayer without killing normal cells. As per reports, it can selectively kill cancer cells using NIR light heating effect (Veerapandian et al., 2009), showing its utility in the field of thermal ablation and imaging. Gadolinium atoms are inserted inside carbon nanotubes for MRI and modified drug delivery (Mody et al., 2016). Carbon nanotubes take advantage of folic acid overexpression in cancer cells and get selectively bound to the surface of folic acid (Eskandari et al., 2014).
The natural origin-based nanobiomaterials include starch nanoparticles, alginate nanoparticles, pullulan nanoparticles, heparin-based nanoparticles, and silk fibroin (Mody et al., 2016). In the past decade, scientists have been attracted to natural polysaccharide like starch as a carrier. Due to the virtue of several hydroxyl groups, it can easily attach hydrophobic side chains and facilitate substitution, which enables the dissolution of the final product. It is biocompatible, biodegradable, nontoxic, non-immunogenic, renewable, economic, and biocompatible with drugs. Docetaxel is a potent chemotherapeutic drug. To tackle the low aqueous solubility, researchers have encapsulated this drug in starch nanoparticles (Dandekar et al., 2012). In the experiment conducted by Li H et al., the group
observed synergism in suppressing human lung cancer cells by simultaneously delivering doxorubicin and siRNA via folate-biotin-quaternized starch nanoparticles. The nanoparticles exhibited high cytotoxicity and inhibited proliferation in A549 cells and had specificity and potential in treating lung cancer (Li et al., 2019). Alginate is sensitive to acidic environment of the tumor vasculature. The polymer is being envisaged for its application in the design of smart cancer theranostics. Alginate-based magnetic nanogel by Peng N et al. caused rapid endocytosis and release of doxorubicin in the presence of a magnetic field. Also, it exhibited a desirable effect as an MRI and contrast agent (Peng et al., 2018). Pei M et al. discovered that in the acidic microenvironment of cancer, real-time noninvasive locating of cancer cells was possible (Pei et al., 2017). Pullulan nanoparticles have shown to prolong blood circulation time along with better stability and active tumor targeting. Pullulan-based doxorubicin nanoparticles developed by Li H et al. showed low cardiotoxicity. The group concluded that the developed pullulan-based doxorubicin nanoparticles not only improved the therapeutic efficacy but also eradicated chemoresistance and exhibited synergism effect compared to single-drug therapy (Li et al., 2015). Hua et al. loaded adriamycin-O-urocanyl pullulan nanoparticles to overcome drug resistance in cancer cells. There was higher cellular uptake of adriamycin due to avoidance of export by P-glycoprotein. This helped in reversing drug resistance in cancer cells (Guo et al., 2014). According to studies, heparin is a good anti-metastatic agent. It is due to heparinase, which inhibits metastasis or binding to growth factors or binding to platelets to expose circulating tumor cells to natural killer cells. The study performed by Sun H et al. displayed that doxorubicin-heparin had anti-metastatic activity and synergism (Sun et al., 2018). Yang and coworkers observed that heparin could overcome problems like low solubility, low selectivity, and improper release of drugs (Yang et al., 2017). Silk fibroin is obtained from silkworm. The biocompatibility, biodegradability, mechanical strength, and flexibility make it a suitable candidate for sustained release of drug. In a study performed by Montalban et al., curcumin silk fibroin nanoparticles showed cytotoxicity in two different cell lines (Hep3B cells and Kelly cells) without decreasing viability in normal cells (Montalban et al., 2018). Since most of the anticancer drugs have poor aqueous solubility, a carrier like silk fibroin can increase bioavailability. Application of doxorubicin-silk fibroin film in residual tumor bed, after removal of tumor, has shown to prevent regrowth of tumor (Jastrzebska et al., 2015). Cisplatin-loaded silk fibroin nanoparticles could internalize in the A549 cell line and could exhibit significant inhibition (Qu et al., 2014).
Albumin is protein-based commonly used as nanobiomaterial. Abraxane is the marketed formulation of albumin-based paclitaxel formulation used in the treatment of breast cancer. It has high penetration and selective anticancer activity with minimum harm to normal cells. Being flexible, it exhibits the EPR effect (Shukla et al., 2019). In a study performed on albumin nanoparticles, it was observed that there was an increase in cytotoxicity when tested in MCF-7 and A549 cells along with prolonged distribution in tumor, leading to slower tumor growth and increase in mice survival (Pandurangan et al., 2016). Concentration-dependent cytotoxicity is seen in MCF-7 cells by paclitaxel albumin nanoparticles. It gives optimal therapeutic efficacy with minimal side effects (Lomis et al., 2016). The combination of nano-albumin-bound paclitaxel along with gemcitabine for treating pancreatic cancer showed good antitumor activity and doubled the rate of survival. Nanoparticles also exhibited synergistic effect due to modulation of cytidine deaminase (Fanciullino et al., 2013).
Safety Aspects
Safety of Nanobiomaterials
It is of utmost importance for a formulation to be safe when administered to a patient. It is necessary to understand a thin line between safety and toxicity of these nanobiomaterials. Safety aspects of these nanobiomaterials are described in the following text.
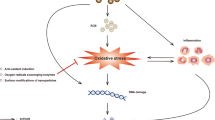
The bulk form of gold is inert, biocompatible, and nontoxic; however, with size reduction to prepare nanoparticles, safety is compromised (Fratoddi et al., 2015). The cytotoxicity of gold nanoparticles depends on the shape and concentration. A study by Steckiewicz et al. illustrated that star-shaped gold nanoparticles were the most cytotoxic, whereas spherical forms were the least cytotoxic. The larger the size, the lesser is the cytotoxicity (Steckiewicz et al., 2019). There is no significant toxicity of tumor necrosis factor observed with colloidal gold delivery (Powell et al., 2010). The toxicity also depends on the surface charge. Serious and five times severe toxicity is observed in cationic compared to anionic nanoparticles. It is due to the interaction between negatively charged cell membranes and positively charged cationic, which leads to internalization and disruption of membranes (Jiang et al., 2012). Silver nanoparticles are safe and effective for cancer treatment. There is a thin line between the safety and adverse effects of silver nanoparticles, which can be governed by monitoring the physicochemical properties (Mihail et al., 2016). The size of the silver nanoparticles influences the cell viability, reactive oxygen species generation, and lactate dehydrogenase action. Akter et al. demonstrated that the generation of reactive oxygen species in the macrophage cell lines was more by nanoparticles of size 15 nm and less by nanoparticles of size 55 nm (Akter et al., 2018). As per findings, toxicity was due to the changes in biological media. It disrupted the mitochondrial respiratory chain, thereby increasing the ROS level. It also interfered with the production of ATP, causing DNA damage. It had also inhibited cell proliferation by activating signaling pathways (Singh et al., 2017). Safety is maintained if we ensure the controlled release of silver ions. An in vivo study using zebrafish described size-dependent toxicity, with 100% mortality by the end of 120 hours. Toxicity can be decreased by sulfidation of silver nanoparticles, thereby reducing the release of silver ions. Another approach was to coat the nanoparticles with organic (citrates, proteins, polymers, etc.) or inorganic (carbonate, chloride, sulfide, etc.) capping agents. This coating stabilizes the nanoparticles, manages the surface chemistry, gives a proper shape, and reduces the amount of silver ions (Akter et al., 2018). In our body, iron oxide nanoparticles convert into elemental iron species for hemoglobin production. IONPs are safe up to a concentration of 200 μg/ml. A high dose of IONPs leads to ROS generation, affecting the normal functioning of the cell and cell apoptosis (Thomas et al., 2013). ROS generated in higher amounts leads to cell damage, DNA disruption, alteration in gene transcription, and protein alteration. The surface charge should be neutral. The cationic surface charge may lead to hemolysis and aggregation of platelets (Liu et al., 2013). Coating the surface will mask the oxidative sites, rendering the nanoparticles less reactive. For coating, various organic polymers like chitosan, poly(ethylene)glycol (PEG), dextran, and organic surfactants like sodium oleate, dodecyl amine, and inorganic metals can be used (Singh & Sahoo, 2013; Zhu et al., 2016). IONPs of 10–100 nm have promising pharmacokinetics (Zhu et al., 2016). Santhosh and Ulrich’s study concluded that PEG-coated IONPs showed no cytotoxicity, and cytotoxicity was due to uncoated IONPs (Santhosh & Ulrih, 2013).While evaluating dextran-coated IONPs, there was a decrease in proliferation; and cell death was observed. It was due to the breaking of the dextran coat that exposes cells to iron oxide aggregates (Singh et al., 2010). These nanoparticles may get detached from the surface of tumor due to cell division or leakage, so it is advisable to study the clearance pathway in the initial phase to develop efficient targeted systems (Liu et al., 2013). The fluorescent mesoporous nanoparticles were compatible at therapeutic doses, and it reduces the associated toxicity of chemotherapeutic agents along with excellent tumor suppression (Lu et al., 2010). Selenium in large quantity leads to toxicity. Some chemical forms have reported genotoxicity, but there is insufficient reported data to claim it a carcinogen. At times, selenosis occurs; hence, long-term use is not recommended. The toxic level leads to hair loss, damage, or removal of nails and skin lesions and affects the nervous system (Brozmanová et al., 2010).
Titanium dioxide exhibits noticeable toxicity like genotoxicity and cytotoxicity in humans. The large surface area and redox activity also contribute to the toxicity. The intraperitoneal injection of titanium dioxide in mice results in acute toxicity like tremor, lethargy, loss of appetite, and passive behavior. It showed more toxic effect on the kidney compared to the liver (Chen et al., 2009; Jinyuan et al., 2009). Zinc oxide can dissolve in the extracellular region, which increases the intracellular level of zinc oxide, leading to toxicity. Toxicity can also be due to uptake of zinc oxide by cells, followed by its dissolution. The systemic exposure of zinc oxide leads to neurological effects (Pandurangan & Kim, 2015).
In a study on polymeric nanobiomaterials by Jesus S. et al., the group concluded that oral toxicity associated with chitosan nanoparticles is ruled out, confirming compatibility of nanoparticles with the blood components. Dose-dependent toxicity is seen on intravenous (iv) injection. In various studies, there is a proportionate increase in ROS associated with chitosan nanoparticles. The generation of ROS is less in the nonlethal concentration (1%) of chitosan. Further, it is reported that PLGA NPs did not exhibit toxicity on oral or intravenous (iv) administration. Only one study reports the toxicity of daunorubicin-PEG-PLL-PLGA nanoparticles (Jesus et al., 2019). The toxicity evaluation of cationic and anionic polyamidoamine in zebrafish demonstrated that cationic form caused cardiovascular dysfunction and decreased survival rate, whereas anionic form did not show such toxicity (Jia et al., 2019). As per findings, bovine serum albumin decreased cytotoxicity associated with PLGA (Razavi & Khandan, 2017). Literature also reports that there is toxicity associated with polycaprolactone (PCL) on intravenous (iv) and intraperitoneal (ip) administration (Garcia et al., 2014). Administering paclitaxel-tamoxifen in polyethylene oxide-polycaprolactone via the iv route had a significant anticancer activity with minimal toxicity (Prabhu et al., 2015).
The macrophage cells are inefficient in completely engulfing the long fibers of carbon nanotubes. It leads to the production of ROS and inflammatory response. Contents of metal impurity in carbon nanotubes (CNTs) also determine the carcinogenicity. Apart from this, particle length and width also have an impact on safety. CNTs having a large diameter or tangled ones are comparatively less toxic. The fabrication should be such that it is biocompatible, biodegradable, and water-soluble; otherwise, it will lead to chronic toxicity (Yamashita et al., 2012). If CNTs are present as aggregates, it becomes difficult for macrophages to recognize them, leading to potential systemic toxicity. Doping the surface can increase or decrease toxicity. The acid-oxidized CNTs induce more toxicity, whereas nitrogen doping decreases toxicity (Narei et al., 2018). Injecting multi-walled CNTs in zebrafish leads to long-term reproductive toxicity and a higher death rate. It may be due to metal catalyst residues that is not removed during purification (Jia et al., 2019). Using purification techniques like sonication in different media, treatment with hydrochloric acid, ion-exchange chromatography, etc. can tackle this problem (Eatemadi et al., 2014).
The blank starch nanoparticles are safe, and there is no significant effect on cell viability, even at a dose of 2 mg/ml (Dandekar et al., 2012). Yu et al. performed chemo-photothermal therapy to eradicate tumors using hydroxyethyl starch-based nanoparticle systems. It was biodegradable and biocompatible and had efficient and safe in vivo performance (Yu et al., 2019). Zhao K et al. also found hydroxyethyl starch nanoparticles safe compared to the free doxorubicin. The conjugate had lower organ toxicity; hence, long-term administration is possible (Zhao et al., 2017). Saralkar P and Dash A prepared curcumin-resveratrol alginate nanoparticles for evaluating the effect on prostate cancer cell line DU145. The blank nanoparticles were found to be safe as they did not cause hemolysis and showed cytotoxic effect on cancer cells (Saralkar & Dash, 2017). Alginate is biocompatible, biodegradable, nontoxic, and hemocompatible (Bhunchu & Rojsitthisak, 2014). On investigating pullulan, it did not show any alteration in liver and kidney tissues. Even the orally administered dose did not show significant signs of toxicity. There was no change in clinical findings even after 14 days of repeat toxicity study (Raychudhuri et al., 2020). The folate-conjugated pullulan acetate nanoparticles for cervical cancer did not exhibit mortality in the control as well as the experimental group. There is a minor change in vital organs, along with inflammation in experimental groups (Tang et al., 2015). The long-term use of heparin leads to thrombocytopenia, osteoporosis, and bleeding in women. Also, it triggers the immune system, forms abnormal clots, and leads to myocardial infarction, stroke, and ischemia. Therefore, monitoring of patients is required (Hwang & Lee, 2016).The doxorubicin-silk fibroin hydrogel is safe and efficacious compared to iv doxorubicin in the treatment of breast cancer. It is efficient in reducing tumor growth and metastasis (Jastrzebska et al., 2015).
Albumin selectively accumulates in the tumor as the tumor cells require it to meet their increasing need of amino acids and energy (Li et al., 2020). Albumin is biodegradable, biocompatible, and non-immunogenic and has specificity for glycoprotein 60 receptor present in cancer cells. It allows the delivery of various anticancer drugs without inducing an immune response (Lomis et al., 2016). Drugs bound to albumin are likely taken up by cancer cells compared to normal cells rendering it safe. Usually, cationic polymer-based nanoparticles exhibit incompatibility and cause hemolysis and cytotoxicity. But, the bovine serum albumin nanoparticles do not damage RBC, cell line, or endothelial cells in vitro (Taguchi et al., 2013).
Importance of Dose of Nanobiomaterials
It is the dose that decides whether the outcome will exhibit a therapeutic effect or toxicity. It is necessary to determine a practically feasible dose from pharmacokinetic and pharmacodynamic studies. It is important to observe the effects of a high dose of nanobiomaterial as well as the toxicity due to long-term exposure. Also, determine appropriate dosage form, route of administration, dosing frequency, and exposure time, and establish safety protocols. There is a rare possibility of developing a neurodegenerative disorder, asthma, etc. due to exposure to high dose of nanomaterial. It necessitates the dose calculation of nanomaterial along with active pharmaceutical ingredients (Tekade et al., 2018). To explain the importance, we would like to quote certain examples. Using 10/20/50 nm-ranged gold nanoparticles can lead to liver damage. However, a single dose of gold nanoparticles did not lead to liver toxicity. Based on the observations of the same study, it was proposed that administering gold nanoparticles dose of 2.5 mg/kg after every 48 hours for 21 days did not cause any liver or brain toxicity (Pastoris Muller et al., 2017). In another example, the biotin-modified pullulan nanoparticle did not show apparent acute toxicity up to 200 mg/kg (Tang et al., 2015). The intraperitoneal injection of 1.5 g/kg of mesoporous silica nanoparticles leads to distress or death in mouse. It is attributed to high doses and size of nanoparticle (Lu et al., 2010). The use of selenium in low dose helps in cancer prevention, reduces inflammation, and regulates blood pressure, but intake of 300–700 μg/day leads to toxicity (Sanmartin et al., 2012).
Safe-by-Design Strategy for Developing Safer Nanotherapeutics
On seeing the adverse effects associated with nanomaterials, there is a need for efforts to minimize the risk during the development stage. Scientists have proposed a safe-by-design (SbD) strategy for developing safer nanotherapeutics (Yan et al., 2019). It is an innovative approach that stands on the pillars of safe materials, safe production, and safe use for maximizing safety while maintaining the efficacy of the final product (Schmutz et al., 2020).
The word “design” in safety-by-design does focus not only on the properties of nanomaterials that we modify but also on the entire process, the materials, as well as the final product. The implementation starts with defining the workflow of the project along with a schedule of evaluating the collected data of nanomaterial property, defining prerequisites, characterizing process, and product safety profile (Kraegeloh et al., 2018).
The product developed should be safe and meet all the regulatory requirements. Being a new concept, it is not a part of the ICH, FDA, or EMA guidelines (Schmutz et al., 2020). It follows REACH and OECD guidelines. REACH stands for Registration, Evaluation, Authorization, and Restriction of chemical substances. It emphasizes risk management by following three principles: evaluating the effect, assessing the exposure, and characterizing the risk. Effect evaluation involves the collection of data that affect toxicity like size, distribution of size, shape, surface area, aggregation, stability, surface property, and reactivity. As per reports, smaller particle size showed more toxicity due to greater uptake by cells. The positive charge exhibited more toxicity due to an increase in interaction with negative charge present on the biological membrane. Also, high ionic dissolution and rod-shaped nanomaterials caused damage to the cells. Exposure assessment identifies all the likely sources of exposure in the manufacturing process. Finally, risk characterization involves adapting a strategy for testing and managing the risk (Zielińska et al., 2020). The Organisation for Economic Co-operation and Development (OECD) has published a guideline on the quantitative structure-activity relationship for the environment, health, and safety (Schmutz et al., 2020). The OECD is used to develop nano-QSAR for developing a relationship between physicochemical properties of nanobiomaterials and observed desirable and undesirable effects. However, this technique requires more quantitative data of structure and chemical properties to develop a robust technique to co-relate the structure with the response (Yan et al., 2019).
Various strategies under safe-by-design can establish safety in products. It involves coating, doping, grafting, loading, optimizing size/shape, managing surface charge, reducing persistence, reducing interaction, and passivating defect site (Torres Andón & Fadeel, 2014; Yahaya & Zain, 2017; Yan et al., 2019; Reijnders, 2020). Coating involves encapsulating toxic material inside a biocompatible carrier to decrease the potential side effects (Yan et al., 2019). Coating the inorganic nanomaterial with polymers or silica decreases undesirable contact with biologics, while coating the rare-earth oxide with phosphate reduces the impact of damage caused by phosphonates due to phosphate stripping. The coating of carbon nanotubes with poloxamer reduced lung fibrosis (Reijnders, 2020). The coated gold nanoboxes were capable of developing personalized nanosystems for treating lung cancer (Movia et al., 2014). Doping involves the addition of a small amount of foreign atoms to modify the electrical, optical, or magnetic properties. It leads to change in energy near the surface, causing charge separation which will interfere ROS generation and oxidative stress (Yan et al., 2019). As per literature, doping the copper oxide nanoparticles with 1–10% iron reduced cytotoxicity, rendering it safe to the environment (Naatz et al., 2017). Doping nano-silica with iron and titanium minimizes inhalation hazards (Reijnders, 2020). Grafting is the covalent attachment of targeting ligands to nanomaterials. There are two modes of grafting – “grafting-to” and “grafting-from.” Grafting-to approach attaches reactive species to functionalized surface of nanomaterial, whereas grafting-from involves embedding nanomaterial inside a matrix (Yan et al., 2019). Grafting the carbon with small organic molecules reduced cytotoxicity. Loading is like grafting, but here it involves non-covalent bond formation. It helped in improving drug delivery and imaging (Reijnders, 2020). Next comes the optimization of properties. The cellular uptake and its distribution in the tissues depend upon the size of nanomaterial. The higher the uptake by the cells, the higher is the toxicity observed (Torres Andón & Fadeel, 2014). Studies have reported that the size of nanomaterials used in cancer should be in the range of 2–200 nm for having suitable half-life and accumulation in tumor via EPR effect (Yan et al., 2019). The small particle size in the range of 1–100 nm had a hazardous profile compared to bulk or large particle size formulation (Dekkers et al., 2020). The shape of nanomaterial will also determine the toxicity. The macrophages can efficiently engulf ellipsoidal-shaped particles compared to spherical nanoparticles. Nanoformulations that are in the form of needle or multi-walled nanotubes resist uptake of macrophages and cause damage to cellular membranes (Torres Andón & Fadeel, 2014). The shape of nanomaterial influences the stability, surface adsorption, transport, and absorption in the body (Zielińska et al., 2020). The surface charge of the nanocarrier is also responsible for the interaction with biological membranes. If the nanocarriers have net positive charge, it interacts with the negative charge of the cell surface. It increases the rate of internalization and associated toxicity of positively charged nanocarriers compared to negatively charged nanocarriers (Torres Andón & Fadeel, 2014). All the above discussed points were strategies to promote safety; in the following sections, we will discuss what parameters can be controlled or reduced to enhance safety of nanobiomaterials. Avoiding or reducing the use of toxic elements eventually decreases toxicity (Yahaya & Zain, 2017). Attempts to modify the oxidative state to alleviate reactivity of nanocarriers are required. One can reduce the release of toxic material from the matrix by optimizing Van-der Waals, coordination and ionic and covalent bonding present between matrix and nanoparticle with the aid of stabilizer or compatibilizers. There is a need to reduce the persistence of such materials or develop strategies to control their end life (Yahaya & Zain, 2017). Sometimes, the presence of defects like steps, kinks, corners, and edges have atoms possessing weak bonds that can lead to change in electronic structure and reactivity. Passivation of such defects during the development stage inhibits such toxic reactions without hampering desired activity of nanomaterials. It is possible by simple coating, for example, zinc oxide, and iron oxide nanoparticles can be coated with silica shells to shield the reactive site on the surface. Such coatings impart stability and biocompatibility, thereby maintaining functionality (Yan et al., 2019).
Safe-by-design (SbD) also helps in risk assessment, addressing the uncertainty, indeterminacy, and responsibility toward the design. This uncertainty is managed by identifying the risk and defining the consequences if the risk occurs without ignoring the facts. Sometimes, the risk is due to scenario uncertainty; to resolve this, apply a safety factor that makes it several times safer than expectations. Another approach is substituting all the dangerous parameters with less dangerous ones. However, one must understand that it is not possible to control all the hazards, but addressing only known parameters develops more uncertainty. Ruling out the indeterminacy will make the design more adaptable. The operators should have adequate expertise and skills to improve safety. There should be constant self-improvement within workers to remove indeterminacy and exposure to unknown hazards. Lastly, there should be a sense of responsibility, to not only safeguard themselves but also protect the end user (Van De Poel & Robaey, 2017).
“NaNoREG” and “Prosafe” are European projects for guiding the industry for SbD of manufactured nanomaterial. It is a combination of the innovative management process, risk assessment, environment, health, and safety assessment with regulatory affairs and data management. It consists of four elements: innovative projects, safety dossier, safety profile, and SbD protocols. The objective is to transfer precautionary measures to practical actions. It involves the use of all the precautionary measures to eradicate uncertainty and associated risks that may hinder the product’s entry to the market (Kraegeloh et al., 2018). NaNoREG has introduced an innovative approach for effective communication between regulators, researchers, decision-makers, and industry. It has developed SbD, on the stage-gate model, where the entire project is divided into various stages from the proposal of idea to its entry in the market and contains a gate between every stage and where decisions regarding cancellation, modification, or its entry into the next stage are taken (Micheletti et al., 2017). Another project named GoNanoBioMatSbD was developed from the SbD approach to deal with polymeric nanocarriers. It involves design of the material, evaluation, human health and environment risk, manufacturing and control, storage, and transport. The initial stage includes all the set of questions such as type of drug, its application, dose, and design of nanocarrier, which is an extensive literature search. It is followed by screening the model for toxicity with the aid of QSAR modelling. It is necessary to evaluate human risk at the initial stage with the help of literature search and toxicity modeling. The material design stage compares all the nanobiomaterials and attempts to maintain a proper balance between safety, efficacy, and budget. Then, it characterizes the polymer properties for optimizing the batch. It is tested for all types of toxicity, like immunotoxicity, carcinogenicity, and mutagenicity, and the endpoints are noted. All the environment risks are listed, and after comparing all the nanobiomaterials, at least one nanobiomaterial is selected by the end of this stage. It is followed by manufacturing and control steps. It is also necessary to apply the good manufacturing practices (GMPs) and define critical quality attributes (CQAs) and critical process parameters (CPPs), scale-up, storage, and transportation measures (Schmutz et al., 2020).
Gold Standard for Safety Assessment
The use of zebrafish is considered a gold standard for safety assessment (Jia et al., 2019). The evaluation is done on zebrafish from the environment, health, and safety perspective of nanomaterial, which helps in risk assessment and framing guidelines on safety, precautionary measures, control, and strategy development to improve characteristics of nanobiomaterial and decrease the associated toxicity (Chakraborty et al., 2016). Zebrafish is gaining importance due to similarities with humans. Compared to rodents’ models, it has more sensitivity toward toxins, and it develops a toxicity mechanism quickly. It is preferred due to small size, ease of handling, and less requirement of chemicals to determine toxicity (Kim et al., 2019). It is widely used in bioimaging to characterize the toxicity profile of nanomaterials. An experiment depicted that silver nanoparticles in the size range of 30–72 nm were able to diffuse in zebrafish embryos, leading to potential toxicity. To evaluate the cytotoxicity of gold nanoparticles, use the zebrafish model. The 20-day exposure of 16 and 55 μg/g dry weight of gold nanoparticles caused change in oxidative stress, neurotransmission, and mitochondrial metabolism. The evaluation of cytotoxicity of carbon nanotube was assessed using this model, which showed bioaccumulation of 16 L/kg wet weight of fish and biochemical alterations (Chakraborty et al., 2016). There was a disturbance in the behavior and development of zebrafish exposed to cadmium tellurium quantum dots. Titanium dioxide leads to neurotoxicity when it is in the form of nanoparticles compared to bulk titanium dioxide. The metal oxides interfered with hatching of zebrafish. Thus, to assess the safety of nanobiomaterials, the zebrafish response is evaluated (Haque & Ward, 2018).
Toxicology Study
The most common toxicological studies of nanomaterials involve analysis of physical and chemical parameters and in vitro, in silico, and in vivo evaluations. In vivo and in vitro toxicological studies are mostly carried out in animal models or cell models (Pandey & Mishra, 2019).
In Vitro and in Vivo Toxicology Study in Vitro Assessment of Nanomaterial Toxicity
The in vitro toxicology studies of nanobiomaterial involve the test for cytotoxicity, genotoxicity, apoptosis, and markers of oxidative stress. The benefits of in vitro study include reduced animal testing, faster analysis, and lower costs, and it is currently required to produce and confirm in vitro assays to determine nanomaterial toxicity. For evaluating the cytotoxicity of biomaterials, use multiple assays like cell membrane integrity, functionalization assay, and cell proliferation assay (Pandey & Mishra, 2019; Stone et al., 2009).
Table 13.1 gives a brief description of all assays for in vitro toxicology study.
In Vivo Toxicology
In vitro characterization used to estimate the nanotoxicity of nanomaterials is not sufficient to ensure complete human safety (Tekade et al., 2018). In vivo toxicology study is also commonly conducted on animal models like mice and rats. Zebrafish (Danio rerio) is also a popular model and has several distinct benefits in toxicological testing over its mammalian counterparts (Jia et al., 2019). The biodistribution, clearance, hematology, serum chemistry, and histopathology are among the evaluation techniques for in vitro toxicity. Biodistribution studies investigate the path of localization nanoparticles to the tissue or organ. Nanoparticles are detected in the killed or live animals through radiolabels. One can perform the clearance studies of nanoparticles to analyze the excretion and metabolism of nanoparticles at different intervals after exposure (Kumar & Sharma, 2017). The examination of alteration in serum chemistry and cell type following exposure of nanoparticles is another technique for in vivo toxicity evaluation. Studies have been conducted to evaluate the histopathology of the cell, tissue, or organ after exposure to determine the toxicity effect induced by nanoparticles (Lei et al., 2008). Histopathology examination determines nanoparticle accumulation in tissues such as the lungs, eyes, brain, liver, kidneys, heart, and spleen (Baker et al., 2008).
Biocompatibility Study
Biocompatibility is related to the capacity of a biomaterial to carry out its specific medical therapy role without having any unintended effect on the patient or effect of the patient on the therapy (WEBSTER et al., 2013). It determines the incompatibility with the biological system. The compatibility with blood is an important attribute to claim safety. Nanomaterial incompatibility with blood can result in protein complexes and complement activation of the system by forming a clot. Different nanomaterials have been documented to induce a hemolytic impact through different mechanisms, such as oxidative damage to the membrane, changes in osmotic stability, enzymatic modifications, and alterations in the physical properties of blood (Pandey & Mishra, 2019). Different assays, such as bleeding time, clotting time, prothrombin time, thrombin time, and activated partial thromboplastin time, are useful for analyzing the influence of nanomaterial on extrinsic and intrinsic pathways of blood (Tekade et al., 2018).
Risk Assessment of Nanomaterials
Risk assessment involves identifying the potential of risk, usually by giving a score or ranking. The main aim of risk assessment is to provide details that will be useful in evaluating substitutes (Hegde et al., 2015). To choose any nanomaterial, the human health risk assessment must be correlated with exposure to hazard assessment (Jesus et al., 2019). Exposure assessment is an estimation of the concentrations or doses that the human population experiences through the environment or environmental compartments (Hegde et al., 2015). Human exposure to nanomaterials is possible via numerous routes at various phases of nanomaterial synthesis (Sligo et al., 2018).The different exposure routes include respiratory, oral, ocular, dermal, and parenteral route (injectable and implantable). We will further discuss the most prominent exposure routes and characteristics of NMs (Sharma et al., 2016). The respiratory system is the most popular route of exposure for ENM in the occupational environment. The particle size in the respiratory system has a significant effect on their distribution and lung aggregation (Kreyling et al., 2009; Pietroiusti et al., 2018). In the alveolar area, particulate size around 20 nm has the largest percentage of rate of deposition, and a size less than 55 nm will reach the alveoli more successfully compared to particle size 200 nm or larger. Nanomaterials with a positive charge show higher interaction with the negative charge of mucus, thus avoiding fast mucociliary clearance (Jesus et al., 2019). The skin is the largest organ in the human body and hence has a potential role in dermal exposure to ENMs. Estimates of potential dermal exposure to generate ENMs have been recorded in the workplace. However, there is no convincing evidence for the entry of ENM into systemic circulation by intact or even injured or inflamed skin. However, dermal penetration may lead to nanoparticles penetrating the skin’s superficial layers, the dermis, causing a local inflammatory reaction (Gulson et al., 2010). For customers, the gastrointestinal path is theoretically important. However, at least in contrast to the pulmonary path, oral exposure was lower in staff. It is notable that a large proportion of nanoparticles inhaled are cleared into the oral cavity by the mucociliary escalator cells and then ingested into the gastrointestinal tract (Pietroiusti et al., 2018). The absorption depends on several variables, such as the form of nanoparticles, and essential physicochemical characteristics: particle size, dispersibility, and charge (Patricia, 2015). The particles having a diameter of about <50 nm and <500 nm cross epithelial barriers through paracellular and endocytosis. The nanomaterial having a positive charge has more affinity toward the intestinal mucus; hence, retention is more, and absorption is less. Neutrally charged nanomaterials diffuse more efficiently through the mucus layers s(Jesus et al., 2019). Hazard assessment is an evaluation of the nature and severity of biological effects (typically in toxicology studies). The hazard assessment concept is the same for nanomaterials as for other substances (Kuempel et al., 2012). Hazard evaluation of nanomaterial is done by using various toxicology tests and assays. Determination of hazard by using experimental testing helps to identify the properties of a chemical or substance and its potential that leads to harmful health effects of human, terrestrial, or aquatic organisms (Hegde et al., 2015).
Risk Management of Nanomaterials
Risk management for nanomaterials is assumed as naturalistic (Murashov, 2015). Risk management pays attention mostly to choosing and implementing the most appropriate risk monitoring step. It is widely seen that conventional structure and devices for risk management systems do not cover all problems related to developing, handling, and use of engineering nanomaterial. Hence, it is required to develop a new approach to become more responsive to the nano-specific problem (Marchant et al., 2008). There are a variety of technical documents and recommendations released by international organizations and standard-setting bodies that advise on risk management problems and control measures associated with ENMs. According to the risk management strategy, all possible hazards and exposures are determined, tested, and evaluated (Oksel, 2017). The most effective hazard control strategy is based on (1) limiting, substituting, and modifying the nanomaterials, (2) the engineering process to minimize or eliminate exposure to the nanomaterials, (3) implementing administrative controls that limit the quantity or duration of exposure to the nanomaterials, and (4) providing for use of PPE (NIOSH, 2012). The Control of Substances Hazardous to Health (COSHH) regulation, which requires employers to properly control the occupational exposure to all chemicals used in the workplace concentrates on preventing or reducing exposure to hazardous substances by controlling equipment, procedures, and worker behavior, demonstrating the clear importance given to management controls (e.g., supervision and training to reduce exposure; Oksel, 2017).
Regulatory Aspects
Legal Requirements
Cancer theranostics is one of the emerging field of cancer treatment and has multiple functions, such as diagnostic and therapeutic functions, targeted and regulated release of medicinal agents, and effectiveness of therapy (Svenson, 2013). Regulatory approval of pharmaceutical drug products for human use, particularly those that are biological products in which a nanomaterial in the finished products is present, needs extensive toxicology and safety studies. The same is applicable for any newly developed cancer theranostics. This can be a challenging job, as size, shape, composition, surface properties, loading of drug, dosage, route of administration, biodistribution, and pharmacokinetics are all variables that can influence the toxicity profiles (Cole et al., 2011).
Nanotechnology is used in a wide variety of products governed by the FDA, such as human drugs and biologics. Products containing nanobiomaterials have quality characteristics which differ from those products that do not contain nanobiomaterials and therefore require analysis. The guidance document and review processes provided by the FDA addresses issues such as public health impact, safety, effectiveness, or the regulatory status of pharmaceutical products containing nanomaterials on case-by-case basis; and the guidance provided should be used as supplementary with other documents (‘Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology’, 2011; FDA/CDER/"Yeaton, 2017).
It includes characterization of the nanomaterial, understanding of the expected use and application of the nanomaterial, and how nanomaterial attributes contribute to product quality, safety, and efficacy; it is also an effective structure for assessing potential risks associated with nanomaterial-containing drug products. All drugs including finished drug products and drugs that are subject to OTC monograph regulations should be manufactured under current good manufacturing practice (cGMPs) as mentioned in the following sections: 501(a)(2)(B) of the Food, Drug, and Cosmetic Act (FD&C Act); 21 CFR parts 210, 211, and 212; and the regulations in 21 CFR parts 600–680. Building a knowledge base to better understand potential threats to product safety, identification, strength, consistency, and purity characteristics during the manufacture of nanomaterial-containing drug products is important for robust control strategies and successful process validation protocols to be put in place (FDA/CDER/"Yeaton, 2017; Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011).
The current FDA guidance on determining new excipient safety is applicable when a standard excipient is intentionally transformed into a nanoscale material. An appropriate safety assessment is required when existing safety data does not completely demonstrate the safety of nanomaterials with regard to exposure period, exposure level, and route of administration. In case, a typical excipient has been deliberately modified to be a nanomaterial or inserted into a nanomaterial; it is important to research the effect on safety and exposure of the materials (Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology, 2011).
For Nonclinical Studies
All current guidelines from the International Council for Harmonisation (ICH) on nonclinical safety studies of the drug product as well as the components are generally applicable to nanomaterial-containing drug products. In terms of safety, newly developed drug products containing nanomaterials should be carefully analyzed:
-
(a)
absorption, distribution, metabolism, and excretion (ADME) – Nanomaterial drug products including excipients as drug carriers’ biological fate and possible safety impacts in addition to active ingredient are required to be studied. Radiolabelled or fluorescence of nanomaterial will help in biodistribution studies of materials.
-
(b)
Risk assessment for routes of administration – While evaluating safety of a drug product containing nanomaterials, the following route-specific issues should be addressed and may require special evaluation in addition to the nonclinical studies usually performed in support of drug product production.
-
(c)
Testing of representative nanomaterial – Before conducting toxicity studies, the nanomaterial to which the human is exposed should be known; and different factors, media, and in vitro and in vivo solvents that affect the aggregation and surface properties of the drug should be understood. Adequate validated method of analysis should be employed to examine the test articles used in nonclinical studies. In general, such nonclinical evaluations, normally carried out to support the manufacture of any drug product, will be sufficient to evaluate nanomaterial-containing drug products when the clinical content is tested in nonclinical studies.
-
(d)
Bridging toxicology (a drug product not containing nanomaterials to a drug product containing nanomaterials) – When an existing approved drug product is changed to nanomaterial ADME and a bridging toxicology analysis may be adequate and necessary to allow reliance on prior nonclinical expertise, provided that other regulatory requirements are met. Consideration should be given to the effect of the transition on the drug ADME and the possible effects of the transition on toxicity (FDA/CDER/"Yeaton, 2017; Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011).
For Clinical Studies
Nanomaterial-containing drug products should be manufactured according to all policies and guidelines that apply to the NDA, ANDA, IND, BLA, clinical efficacy, and safety studies (Narang et al., 2018).
505(b)(2) Submissions – For the NDA (New Drug Application) submitted under section 505(b)(1) and approved by 505(c) section (Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011)
505(j) Submissions – Approval for generic product referencing a nanomaterial drug product can be applied by submitting an ANDA under section 505(j)of FD&C Act (Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011).
351(k) Submissions – For the development of a biological reference product containing nanomaterials, existing guidelines on biosimilars should be followed. As part of product development, the contribution of the nanomaterial to product potency, safety, and purity should be assessed. Sponsors are encouraged to approach the FDA early on to develop nanomaterial-containing biosimilars (Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011).
Bioanalytical Methods – After the administration of products containing nanomaterials, the clinically important elements, i.e., the parent drug and major active metabolites, should be calculated in the required biological matrices. It is recommended to use verified, relevant, and highly sensitive methods for examination of free and nanomaterial-associated drugs (Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011).
In vitro Tests – The following parameters such as biocompatibility, plasma protein binding, stability, in vitro clearance, and metabolism should be carried out with human biomaterials (Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011).
Immunogenicity – Applications for general guidelines for risk reduction associated with adverse immune responses shall address the FDA Guidance on the Evaluation of Industry Immunogenicity for Therapeutic Protein Products and the ICH Guideline S8 Immunotoxicity Research for Human Pharmaceuticals on sample approaches. Assessments of the probability of immunogenicity of biological products having a nanomaterial nonbiological component should consider the adjuvant properties of the component. Accordingly, the biological products that contain a nanomaterial component may have different immunogenic properties, which are important to be assessed (Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011).
Environmental Impact Considerations
The National Environmental Policy Act (NEPA) requires federal agencies to determine the environmental effects and to ensure that environmental analysis is made known to the concerned and affected public. Applicants must submit an environmental assessment (EA) or claim categorical exclusion [21 CFR 25.15(a)]. The information registered in EA will be reviewed by the CDER (Center for Drug Evaluation and Research) or CBER(Center for Biologics Evaluation and Research) to decide if it is reliable, or the proposed action may have a substantial effect on the quality of the human environment. They encourage industries to notify them in early development phase of their plan to either demand a categorical exclusion or apply for an EA in order to assist them in decision-making and late-cycle information requests (Guidance for Industry Considering Whether an FDA- Regulated Product Involves the Application of Nanotechnology, 2011; Narang et al., 2018).
Global Regulatory Strategy
Organisation for Economic Cooperation and Development (OECD)
Within its chemical safety framework, the Organisation for Economic Cooperation and Development (OECD) initiated a strategic initiative in 2006 which provides a global forum to discuss and support responsible development of nanomaterials processed, in particular their safety assessment and risk assessment. They established Working Party on Manufactured Nanomaterials (WPMN). It facilitates global collaboration on aspects of the human health and environmental protection of manufactured nanomaterials and focuses on the production of suitable methodologies and techniques to ensure the safe use of nanotechnology (Rauscher et al., 2017).
They are divided into six groups, namely, environment, health, and safety research strategy on manufactured nanomaterials, development of research database nanomaterials, safety testing of a set or representative manufactured, cooperation on voluntary schemes and prevention, manufactured nanomaterial test guidelines, and cooperation on risk assessments and exposure measurement (Park & Yeo, 2016).
Data obtained in accordance with the guidelines are covered by the Mutual Acceptance of Data (MAD) agreement of the OECD for the assessment of chemicals. MAD is a critical component for international harmonization of chemical safety approaches by regulatory acceptance of these test guidelines. Therefore, MAD also includes data on nanomaterials collected following OECD test guidelines that are specific to nanomaterials (Rauscher et al., 2017).
International Organization for Standardization (ISO)
ISO was established in June 2005 and is composed of 33 member countries and 15 observing countries. The ISO/TC 229 conducted ISO standardization of nanotechnologies based on four working groups (WGs): terminology and nomenclature (WG 1), measurement and characterization (WG 2), health safety and environmental aspects of nanotechnologies (WG 3), and material specifications (WG 4). It plays a significant role in developing basic framework for global nanotechnologies for risk assessment, risk control, and standardization (Park & Yeo, 2016).
Emerging Green Nanomaterial Approach for Cancer Theranostics
Researchers are moving toward safer and environment friendly approaches, which are not only safe for the environment but also for health. Many advances had been made in research toward a greener approach, where natural plant extracts, sources, and microorganisms are used for the synthesis of biogenic nanoparticles. The manufacturing of nanoparticles can be carried out using three methods: physical, chemical, and biological (green) methods. The conventional methods, physical and chemical methods, have certain limitations such as high energy consumption, low production rate, use of toxic and hazardous chemicals, instability, huge production cost, and environmental and health hazards (Si et al., 2020). The alternative methods are biological methods based on green nanotechnology (Saravanan et al., 2019). The advantages of green nanotechnology over conventional are low cost production, less energy consumption, use of renewable sources, resolving sustainability problems of climate change, reduction in use of toxic chemicals, simplicity of handling, and biodegradable yet recyclable products (Barry, 2019; Si et al., 2020). In the last few years, metal nanoparticles such as gold, silver, platinum, palladium, selenium, zinc, and copper have proven to be of great interest in the field of cancer theranostics.
Plants Used as a Natural Source for Green Approach
The phytochemicals extracted from the plant source are useful in combating cancer. Phytochemicals present in the medicinal plants had shown cytotoxic effects against cancer cells. Though phytonanotechnology has high potential in synthesizing biogenic nanoparticles, the exact mechanism of the phytosynthesis is yet to be understood (Saravanan et al., 2019). Plant parts such as roots, leaves, and barks are collected, washed, and cut into small pieces for extraction under sterile conditions. The extract is purified by filtration and centrifugation. Extract, metal salt, and water are incubated for the growth of nanoparticles. The natural compounds used are starch, glucose, chitosan, sucrose, and calcium alginate as reducing and/or capping agents. Nontoxic, biodegradable polymers such as polyethylene glycol(PEG) and carboxymethyl cellulose(CMC). Nanoparticles synthesized are generally spherical in shape (Noruzi, 2015). Reduction and agitation methods, choice of suitable types of protecting agents, and concentration; synthesizing conditions such as pH, concentration of reductive biomass, temperature, and time; and use of alternative energy sources such as ultrasound and UV light are some of the factors responsible for the sizes and shapes of nanoparticles (Barry, 2019).
Microorganisms Used as a Natural Source for Green Approach
Various microorganisms such as yeast, fungi, bacteria, and algae are studied and used for synthesis of biogenic nanoparticles. One of the drawbacks of using microorganisms is that they require long incubation period for reduction, whereas the plant-based synthesis is quick. The use of microorganisms also has biosafety issues, where they are resolved in green synthesis using plant extracts (Ovais et al., 2016).
There are two approaches of synthesis, i.e., intracellular and extracellular. The advantage of extracellular process over intracellular process is that it is devoid of downstream processing steps. Downstream processing steps in an intracellular process are the recovery steps. It includes sonication, centrifugation, and washing steps for purification of nanoparticles. There are some important factors that play a crucial role in synthesis. Some important factors play a crucial role in synthesis, including metal-resistant agents, proteins, peptides, enzymes, reducing cofactors, and organic materials that play a role of a reducing agent (Soni et al., 2019).
For nanoparticles synthesis, the widely used bacterial species include Actinobacteria sp., Escherichia coli, Klebsiella pneumonia, Lactobacillus spp., Bacillus cereus, Corynebacterium sp., and Pseudomonas sp. (Soni et al., 2019).
In recent years, the studies have confirmed the biocompatibility and effectiveness of green nanoparticles and can be used as theranostic agent for cancer. The anticancer potential of phytosynthesized metallic NPs has grown over the past decade, with relatively little research on their genotoxicity, pharmacokinetics, pharmacodynamics, and safety profiles alone or in combination with others. The studies conducted on in vivo models elicit potential value of green nanoparticles, and future research would allow us to conclude more on the anticancer activity of green nanoparticles (Saravanan et al., 2019).
Conclusion and Future Aspects
The use of nanobiomaterial in cancer theranostic is an innovative approach toward cancer diagnosis and therapeutics. This dual-purpose technique aims at interacting with the cells such that there is no interaction with the normal cells, thereby promoting selectivity and sensitivity toward cancer cells and reducing the duration of cancer treatment. There are various nanobiomaterials used in cancer cells, briefly divided as metal-based, polymer-based, derived from natural origin, carbon-based, and protein-based nanobiomaterials. These nanobiomaterials show promising results in cancer therapy if used properly. Scientists need to optimize the physicochemical properties and the dose of these nanomaterials to achieve maximum safety and minimal toxicity. Though toxicity cannot be eradicated, efforts can be made to make the formulation safe for end users. Researchers have adopted the use of the “safe-by-design” strategy that not only focuses on the physicochemical properties of these materials but also considers the entire process, material, and final product. The REACH guideline emphasizes evaluating the effect, assessing the exposure, and characterizing the risk, whereas the OECD guidelines suggest using QSAR modelling to establish a relationship between physicochemical properties and observed effects. Various techniques under SbD include coating, grafting, loading, doping, optimizing size/shape/charge, reducing persistence, and passivating defects. NaNoREG has developed SbD on the stage-gate model, whereas GoNanoBioMatSbD was developed for dealing with polymeric NBM. Both were concerned about the product right from the initial stage to its entry into the market.
The in vitro evaluation of toxicology involves cell membrane integrity assay, trypan blue dye exclusion assay, lactate dehydrogenase assay, metabolic activity assay, MTS assay, MTT assay, XTT assay, cell proliferation assay, and assay for genotoxicity. In vivo evaluation involves various animal models, but zebrafish is preferable due to its peculiar characteristics. Biocompatibility studies are performed to evaluate if any incompatibility exists with the biological system. The various routes by which exposure to nanobiomaterials occurs involve pulmonary, dermal, oral, ocular, and parenteral root. Optimizing the size, shape, and surface charge minimizes the exposure and associated exposure. As per the Control of Substances Hazardous to Health (COSHH) regulation, the employers should properly control the occupational exposure to all chemicals used in the workplace. They should concentrate on the prevention and/or reduction of exposure to hazardous substances by controlling worker behavior, equipment, and procedures.
As per the FDA, characterization of the nanomaterial, understanding of the expected use and application of the nanomaterial, and how nanomaterial characteristics contribute to product safety, efficacy, and quality are an effective structure for assessing potential risks associated with nanomaterial-containing drug products. The current FDA guidance on determining new excipient safety applies where a typical excipient is intentionally transformed into a nanomaterial. An appropriate safety assessment should be ensured when existing safety data regarding exposure level, duration of exposure, and route of administration do not completely demonstrate the safety of the nanomaterials. New products containing nanobiomaterials should be tested for ADME and risk considerations for the route of administration, testing nanobiomaterial for in vivo, in vitro, vehicle, media, and surface properties, thereby bridging regulations of conventional dosage forms and nanoformulations. They should follow clinical trials similar to the recommendations for the IND, NDA, ANDA, and BLA. The FDA also requires applicants to submit an environmental assessment or some similar document. The OECD facilitates international collaboration on aspects of the human health and environmental protection of processed nanomaterials and focuses on the production of suitable methods and techniques to ensure the safe use of nanotechnology. ISO also plays a significant role in developing a basic framework for global nanotechnologies for risk assessment, risk control, and standardization.
Advances in research are being achieved by moving toward the use of green technology. The advantages of green nanotechnology over conventional are low cost production, less energy consumption, use of renewable sources, resolving sustainability problems of climate change, reduction in the use of toxic chemicals, simplicity of handling, and biodegradable yet recyclable products. The in vivo studies of green nanoparticles elicit its potential for developing safe nanotherapeutics in the future.
References
Adan, A., & Baran, Y. (2016). Cell proliferation and cytotoxicity assays. Current Pharmaceutical Biotechnology, 17, 1213–1221. https://doi.org/10.2174/13892010176661608081605
Akter, M., et al. (2018). A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. Journal of Advanced Research, 9, 1–16. https://doi.org/10.1016/j.jare.2017.10.008
Anand, P., et al. (2020). Expert review cancer is a preventable disease that requires major lifestyle changes. Pharmacy Research, 25(9), 2097–2116. https://doi.org/10.1007/s11095-008-9661-9
Ancona, A., et al. (2018). Lipid-coated zinc oxide nanoparticles as innovative ROS- generators for photodynamic therapy in cancer cells. Nanomaterials, 8(3), 143. https://doi.org/10.3390/nano8030143
Arne Biesiekierski, Y. L., Yin Xiao, Cuie Wen. (2018). Assessing the biocompatibility of biomaterial.
Aslantürk, Ö. S., & Aslantürk, S. (2018). In vitro cytotoxicity and cell viability assays: Disadvantages in vitro cytotoxicity and viability assays: Principles, advantages, and disadvantages, 1–18. https://doi.org/10.5772/intechopen.71923
Aydin, A. C., Yesilot, S., & Aydin, C. (2019). Silver nanoparticles; A new hope in cancer therapy? East Journal of Medicine, 24(1), 111–116. https://doi.org/10.5505/ejm.2019.66487
Baker GL, Gupta A, Clark ML, Valenzuela BR, Staska LM, Harbo SJ, Pierce JT, Dill JA. Inhalation toxicity and lung toxicokinetics of C60 fullerene nanoparticles and microparticles. Toxicol Sci. 2008 Jan;101(1):122–31. https://doi.org/10.1093/toxsci/kfm243. Epub 2007 Sep 17. PMID: 17878152.
Barry S. Biogenic synthesis of gold nanoparticles using red and green pear fruit extracts. http://hdl.handle.net/11394/7166
Bhunchu, S., & Rojsitthisak, P. (2014). Biopolymeric alginate-chitosan nanoparticles as drug delivery carriers for cancer therapy. Die Pharmazie, 69(8), 563–570.
Brannon-peppas, L., & Blanchette, J. O. (2004). Nanoparticle and targeted systems for cancer therapy. Advanced Drug Delivery Reviews, 56, 1649–1659. https://doi.org/10.1016/j.addr.2004.02.014
Brozmanová, J., et al. (2010). Selenium: A double- edged sword for defence & offence in cancer. Archives of Toxicology, 84(12), 919–938. https://doi.org/10.1007/s00204-010-0595-8
Cancer Statistics - Worldometer (n.d.). Available at: https://www.worldometers.info/cancer/. Accessed: 29 Nov 2020.
Çeşmeli, S., & BirayAvci, C. (2019). Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. Journal of Drug Targeting, 27(7), 762–766. https://doi.org/10.1080/1061186X.2018.1527338
Chakraborty, C., et al. (2016). Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. Journal of Nanobiotechnology, 14(1), 1–13. https://doi.org/10.1186/s12951-016-0217-6
Chen, J., et al. (2009). In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. Journal of Applied Toxicology: JAT, 29(4), 330–337. https://doi.org/10.1002/jat.1414
Chen, Z., Mao, R., & Liu, Y. (2012). Fullerenes for cancer diagnosis and therapy: Preparation, biological and clinical perspectives. Current Drug Metabolism, 13, 1035–1045.
Cheng, W., et al. (2017). A pH-sensitive delivery vehicle based on folic acid-conjugated polydopamine-modified mesoporous silica nanoparticles for targeted cancer therapy. Applied Materials and Interfaces, 9, 18462–18473.
Cole, A. J., Yang, V. C., & David, A. E. (2011). Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends in Biotechnology, 29(7), 323–332. https://doi.org/10.1016/j.tibtech.2011.03.001
Costa, S., & Paulo Teixeira, J. (2014). Comet assay. In Encyclopedia of toxicology (Vol. 1, 3rd ed., pp. 1020–1023). Academic Press. https://doi.org/10.1016/B978-0-12-386454-3.01072-1
Crouch, S. P. M., et al. (1993). The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. Journal of Immunological Methods, 160(1), 81–88. https://doi.org/10.1016/0022-1759(93)90011-U
Dandekar, P., et al. (2012). A hydrophobic starch polymer for nanoparticle-mediated delivery of docetaxel a. Macromolecular Bioscience, 12(2), 184–194. https://doi.org/10.1002/mabi.201100244
Dekkers, S., et al. (2020). Safe-by-design part I: Proposal for nanospecific human health safety aspects needed along the innovation process. Nano, 18, 100227. https://doi.org/10.1016/j.impact.2020.100227
Dusinska, M., et al. (2012). Critical evaluation of toxicity tests. In Adverse effects of engineered nanomaterials (pp. 63–83). Academic Press. https://doi.org/10.1016/B978-0-12-386940-1.00004-0
Eatemadi, A., et al. (2014). Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Research Letters, 9(1), 393. https://doi.org/10.1186/1556-276X-9-393
Eskandari, M., et al. (2014). Polymer-functionalized carbon nanotubes in cancer therapy: A review. Iranian Polymer Journal (English Edition), 23(5), 387–403. https://doi.org/10.1007/s13726-014-0228-9
Fanciullino, R., Ciccolini, J., & Milano, G. (2013). Challenges, expectations and limits for nanoparticles-based therapeutics in cancer: A focus on nano-albumin-bound drugs. Critical Reviews in Oncology/Hematology, 88(3), 504–513. https://doi.org/10.1016/j.critrevonc.2013.06.010
FDA/CDER/"Yeaton, A. (2017). Drug products, including biological products, that contain nanomaterials – guidance for industry, p. 29.
Fernandez-Fernandez, A., Manchanda, R., & McGoron, A. J. (2011). Theranostic applications of nanomaterials in cancer: Drug delivery, image-guided therapy, and multifunctional platforms. Applied Biochemistry and Biotechnology, 165(7–8), 1628–1651. https://doi.org/10.1007/s12010-011-9383-z
Fratoddi, I., et al. (2015). How toxic are gold nanoparticles? The state-of-the-art. Nano Research, 8(6), 1771–1799. https://doi.org/10.1007/s12274-014-0697-3
Garcia S.C., Guterres S.S., Bubols G.B., Bulcão R.P., Charão M.F., Pohlmann A.R. (2014) Polymeric Nanoparticles: In Vivo Toxicological Evaluation, Cardiotoxicity, and Hepatotoxicity. In: Durán N., Guterres S., Alves O. (eds) Nanotoxicology. Nanomedicine and Nanotoxicology. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8993-1_14
Gary-Bobo, M., et al. (2011). Pharmaceutical nanotechnology cancer therapy improvement with mesoporous silica nanoparticles combining targeting, drug delivery and PDT. International Journal of Pharmaceutics, 423, 509–515. https://doi.org/10.1016/j.ijpharm.2011.11.045
Gobbo, O. L., et al. (2015). Magnetic nanoparticles in cancer theranostics. Theranostics, 5(11), 1249–1263. https://doi.org/10.7150/thno.11544
Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. (2011). Biotechnology. Law Report, 30(5), 613–616. https://doi.org/10.1089/blr.2011.9814
Gulson, B., et al. (2010). Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicological Sciences, 118(1), 140–135. https://doi.org/10.1093/toxsci/kfq243
Guo, H., et al. (2014). pH-sensitive pullulan-based nanoparticle carrier for adriamycin to overcome drug-resistance of cancer cells. Carbohydrate Polymers, 111, 908–917. https://doi.org/10.1016/j.carbpol.2014.05.057
Guo, J., et al. (2017). Gold-nanoparticles-enlighten-the-future-of-cancer-thera. International Journal of Nanomedicine, 12, 6131–6152. https://doi.org/10.2147/IJN.S140772
Haque, E., & Ward, A. C. (2018). Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials, 8(7), 561. https://doi.org/10.3390/nano8070561
Hegde, K. et al. (2015). Environmental hazards and risks of nanomaterials, (November). https://doi.org/10.1061/9780784414088.ch14.
Hwang, H. H., & Lee, D. Y. (2016). Antiangiogenic actions of heparin derivatives for cancer therapy. Macromolecular Research, 24, 767–772. https://doi.org/10.1007/s13233-016-41111-8
Indoria, S., Singh, V., & Hsieh, M.-F. (2020). Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. International Journal of Pharmaceutics, 582, 119314. https://doi.org/10.1016/j.ijpharm.2020.119314
Jastrzebska, K., et al. (2015). Silk as an innovative biomaterial for cancer therapy. Reports of Practical Oncology and Radiotherapy, 20, 87–98.
Jesus, S., et al. (2019). Hazard assessment of polymeric nanobiomaterials for drug delivery: What can we learn from literature so far. Frontiers in Bioengineering and Biotechnology, 7(October), 261. https://doi.org/10.3389/fbioe.2019.00261
Jia, H. R., et al. (2019). Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery applications. Journal of Controlled Release, 311–312(July), 301–318. https://doi.org/10.1016/j.jconrel.2019.08.022
Jiang, X. M., et al. (2012). Gold nanomaterials: Preparation, chemical modification, biomedical applications and potential risk assessment. Applied Biochemistry and Biotechnology, 166(6), 1533–1551. https://doi.org/10.1007/s12010-012-9548-4
Jinyuan, C., et al. (2009). In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. Journal of Applied Toxicology, 29, 330–337.
Katriina Lappalainen, I. J., Syrjanen, K., & ArtoUrtti, S. S. (1994). Comparison of cell proliferation and toxicity assays using two cationic liposome. Pharm Res, 11(8), 1127–1131.
Kim, D., et al. (2019). Safety and photochemotherapeutic application of poly(γ-glutamic acid)-based biopolymeric nanoparticle. Acta Pharmaceutica Sinica B, 9(3), 565–574. https://doi.org/10.1016/j.apsb.2019.01.005
Kraegeloh, A., et al. (2018). Implementation of safe-by-design for nanomaterial development and safe innovation: Why we need a comprehensive approach. Nanomaterials, 8(4), 239. https://doi.org/10.3390/nano8040239
Kreyling, W. G., et al. (2009). Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhalation Toxicology, 21, 55–60. https://doi.org/10.1080/08958370902942517
Kuempel, E. D., Geraci, C. L., & Schulte, P. A. (2012). Risk assessment and risk management of nanomaterials in the workplace: Translating research to practice. Annals of Occupational Hygiene, 56(5), 491–505. https://doi.org/10.1093/annhyg/mes040
Kuhn, D. M., et al. (2003). Uses and limitations of the XTT assay in studies of Candida growth and metabolism. Journal of Clinical Microbiology, 41(1), 506–508. https://doi.org/10.1128/JCM.41.1.506-508.2003
Kumar, V., & Sharma, N. (2017). In vitro and in vivo toxicity assessment of nanoparticles. International Nano Letters, 7(4), 243–256. https://doi.org/10.1007/s40089-017-0221-3
Kumar, P. et al. (2019). Current status and future challenges of various polymers as cancer therapeutics, Elsevier. https://doi.org/10.1016/B978-0-12-816963-6.00001-7
Langdon, S. P. (2003). Cancer cell culture. Cancer Cell Culture, 731, 237–245. https://doi.org/10.1385/1592594069
Lei R, Wu C, Yang B, Ma H, Shi C, Wang Q, Wang Q, Yuan Y, Liao M. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: a rapid in vivo screening method for nanotoxicity. Toxicol Appl Pharmacol. 2008 Oct 15;232(2):292–301. https://doi.org/10.1016/j.taap.2008.06.026. Epub 2008 Jul 26. PMID: 18706438
Leif, R. C., Stein, J. H., & Zucker, R. M. (2004). A short history of the initial application of Anti-5-BrdU to the detection and measurement of S phase. Cytometry Part A, 58(1), 45–52. https://doi.org/10.1002/cyto.a.20012
Li, H., et al. (2015). PH-sensitive pullulan-DOX conjugate nanoparticles for co-loading PDTC to suppress growth and chemoresistance of hepatocellular carcinoma. Journal of Materials Chemistry B, 3(41), 8070–8078. https://doi.org/10.1039/c5tb01210d
Li, L., et al. (2019). Codelivery of DOX and siRNA by folate-biotin-quaternized starch nanoparticles for promoting synergistic suppression of human lung cancer cells Codelivery of DOX and siRNA by folate-biotin-quaternized starch nanoparticles for promoting synergistic suppression of human lung cancer cells. Drug Delivery, 26(1), 499–508. https://doi.org/10.1080/10717544.2019.1606363
Li, C., et al. (2020). Current multifunctional albumin-based nanoplatforms for cancer multi- mode therapy. Asian Journal of Pharmaceutical Sciences, 15, 1–12. https://doi.org/10.1016/j.ajps.2018.12.006
Liu, G., et al. (2013). Iron oxide nanoparticles applications and potential toxicity of magnetic Iron oxide nanoparticles. Wiley-VCH Verlag GmbH & Co. KGaA. https://doi.org/10.1002/smll.201201531
Lomis, N., et al. (2016). Human Serum albumin nanoparticles for use in cancer drug delivery: process optimization and in vitro characterization. Nanomaterials, 6(116), 1–17. https://doi.org/10.3390/nano6060116
Lu, J., et al. (2010). Tumor suppression biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Nano Small micro, 16. https://doi.org/10.1002/smll.201000538
Lungu, I. I., et al. (2019). Nanobiomaterials used in cancer therapy: An up-to-date overview. Molecules, 24(19), 3547. https://doi.org/10.3390/molecules24193547
Madhavan, H. N. (2007). Simple Laboratory methods to measure cell proliferation using DNA synthesis property. Journal of Stem Cells and Regenerative Medicine, 3(1), 12–14.
Malich, G., Markovic, B., & Winder, C. (1997). The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology, 124, 179–192.
Marchant, G. E., Sylvester, D. J., & Abbott, K. W. (2008). Risk management principles for nanotechnology. NanoEthics, 2, 43–60. https://doi.org/10.1007/s11569-008-0028-9
Micheletti, C., et al. (2017). Implementation of NaNoREG safe-by-design approach nanomaterial applications. IOP Conference series Journal of Physics, 838, 012019. https://doi.org/10.1088/1742-6596/838/1/012019
Michiko, W., et al. (2002). Pros and cons of apoptosis assays. Microscopy and Microanalysis, 8, 375–391. https://doi.org/10.1017/S1431927602010346
Mihail, G., et al. (2016). Chapter 2. Silver nanoparticles in cancer therapy. In Nanobiomaterials in cancer therapy (pp. 29–56). Oxford. https://doi.org/10.1016/B978-0-323-42863-7.00002-5
Mody, N., et al. (2016). Nanobiomaterials: Emerging platform in cancer theranostics. In Nanobiomaterials in cancer therapy: Applications of nanobiomaterials (7th ed., p. 146). Elsevier Inc.. https://doi.org/10.1016/B978-0-323-42863-7.00005-0
Montalban, M. G., et al. (2018). Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials, 8, 126. https://doi.org/10.3390/nano8020126
Movia, D., et al. (2014). A safe-by-design approach to the development of gold nanoboxes as carriers for internalization into cancer cells. Biomaterials, 35(9), 2543–2557. https://doi.org/10.1016/j.biomaterials.2013.12.057
Mueller, H., et al. (2004). Comparison of the usefulness of the MTT , ATP , and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines. Journal of Biomolecular Screening, 9(6), 506–515. https://doi.org/10.1177/1087057104265386
Murashov, V. (2015). Overview of risk management for engineered nanomaterials, 1–13. https://doi.org/10.1088/1742-6596/429/1/012062.Overview
Naatz, H., et al. (2017). Safe-by-design CuO nanoparticles via Fe-Doping, Cu-O bond length variation, and biological assessment in cells and zebrafish embryos. ACS Nano, 11(1), 501–515. https://doi.org/10.1021/acsnano.6b06495
Narang, J. K., et al. (2018). Nano-Oncologicals: Regulatory aspects and safety issues. Applied Clinical Research, Clinical Trials and Regulatory Affairs, 5(2), 122–131. https://doi.org/10.2174/2213476X05666180528094458
Narei, H., Ghasempour, R., & Akhavan, O. (2018). 7 – toxicity and safety issues of carbon nanotubes. In Carbon nanotube-reinforced polymers (pp. 145–171). Elsevier. https://doi.org/10.1016/B978-0-323-48221-9.00007-8
Noruzi, M. (2015). Biosynthesis of gold nanoparticles using plant extracts. Bioprocess and Biosystems Engineering, 38(1), 1–14. https://doi.org/10.1007/s00449-014-1251-0
Oksel, C. (2017). Risk management of nanomaterials.
Ovais, M., et al. (2016). Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine, 11(23), 3157–3177. https://doi.org/10.2217/nnm-2016-0279
Pandey, S., & Mishra, A. (2019). Rational approaches for toxicological assessments of nanobiomaterials. Journal of Biochemical and Molecular Toxicology, 33(7), e22335. https://doi.org/10.1002/jbt.22335
Pandurangan, M., & Kim, D. H. (2015). In vitro toxicity of zinc oxide nanoparticles: A review. Journal of Nanoparticle Research, 17(3), 1–8. https://doi.org/10.1007/s11051-015-2958-9
Pandurangan, A. K., et al. (2016). Nanobiomaterial-based delivery of drugs in various cancer therapies: Classifying the mechanisms of action (using biochemical and molecular biomarkers). In Nanobiomaterials in cancer therapy: applications of nanobiomaterials (7th ed., p. 365). Elsevier Inc.. https://doi.org/10.1016/B978-0-323-42863-7.00011-6
Park, H.-G., & Yeo, M.-K. (2016). Nanomaterial regulatory policy for human health and environment. Molecular & Cellular Toxicology, 12(3), 223–236. https://doi.org/10.1007/s13273-016-0027-9
Pastoris Muller, A., et al. (2017). Safety protocol for the gold nanoparticles administration in rats. Materials Science & Engineering C, 77, 1145–1150. https://doi.org/10.1016/j.msec.2017.04.027
Patricia I. Dolez. (2015). Nanoengineering global approaches to health and safety issues.
Pei, M., et al. (2017). Alginate-based cancer-associated, stimuli-driven and turn-on theranostic prodrug nanogel for cancer detection and treatment. Carbohydrate Polymers. https://doi.org/10.1016/j.carbpol.2017.12.013
Peng, N., et al. (2018). Novel dual responsive alginate- based magnetic nanogels for onco- theranostics. Carbohydrate Polymers. https://doi.org/10.1016/j.carbopol.2018.09.084
Pietroiusti, A. et al. (2018). Nanomaterial exposure, toxicity, and impact on human health. https://doi.org/10.1002/wnan.1513
Powell, A. C., Paciotti, G. F., & Libutti, S. K. (2010). Chapter 25 Colloidal gold: A novel nanoparticle for targeted cancer therapeutics. In Cancer nanotechnology, methods in molecular biology. Springer. https://doi.org/10.1007/978-1-60761-609-2_25
Prabhu, R. H., Patravale, B., & Joshi, M. D. (2015). Polymeric nanoparticles for targeted treatment in oncology: Current insights. International Journal of Nanomedicine Dovepress, 10, 1001–1018. https://doi.org/10.2147/IJN.S56932
Qu, J., et al. (2014). Silk fibroin nanoparticles prepared by electrospray as controlled release carriers of cisplatin. Material Science and Engineering C, 44, 166–174. https://doi.org/10.1016/j.msec.2014.08.034
Raja, G., et al. (2020). Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Materials Science and Engineering C, 107, 110303–110303. https://doi.org/10.1016/j.msec.2019.110303
Rauscher, H., Rasmussen, K., & Sokull-Klüttgen, B. (2017). Regulatory aspects of nanomaterials in the EU. ChemieIngenieur Technik, 89(3), 224–231. https://doi.org/10.1002/cite.201600076
Raychudhuri, R., et al. (2020). Pullulan based stimuli responsive and sub cellular targeted nanoplatforms for biomedical application: Synthesis, nanoformulations and toxicological perspective. International Journal of Biological Macromolecules, 169, 1189–1205.
Razavi, M., & Khandan, A. (2017). Safety, regulatory issues, long-term biotoxicity, and the processing environment, nanobiomaterials science, development and evaluation (p. 279). Elsevier Ltd. https://doi.org/10.1016/B978-0-08-100963-5.00014-8
Reijnders, L. (2020). Safer-by-design for nanomaterials. Nanotoxicity. https://doi.org/10.1016/B978-0-12-819943-5.00010-5
Rejinold, N. S., Jayakumar, R., & Kim, Y. C. (2015). Radio frequency responsive nano- biomaterials for cancer therapy. Journal of Controlled Release, 204, 85–97. https://doi.org/10.1016/j.jconrel.2015.02.036
Romar, G. A., Kupper, T. S., & Divito, S. J. (2016). Research techniques made simple: Techniques to assess cell proliferation. Journal of Investigative Dermatology, 136(1), e1–e7. https://doi.org/10.1016/j.jid.2015.11.020
Sanmartin, C., et al. (2012). Selenium compounds, appoptosis& other types of cell death: An overview for cancer therapy. International Journal of Molecular Science, 13, 9649–9672.
Santhosh, P. B., & Ulrih, N. P. (2013). Mini-review multifunctional superparamagnetic iron oxide nanoparticles: Promising tools in cancer theranostics. Cancer Letters, 336, 8–17. https://doi.org/10.1016/j.canlet.2013.04.032
Saralkar, P., & Dash, A. K. (2017). Alginate nanoparticles containing curcumin and resveratrol: Preparation, characterization, and in vitro evaluation against DU145 prostate cancer cell line. AAPS PharmSciTech, 18(7), 2814–2823. https://doi.org/10.1208/s12249-017-0772-7
Saravanan, M., et al. (2019). Emerging plant-based anti-cancer green nanomaterials in present scenario. Comprehensive Analytical Chemistry, 87, 291–318. https://doi.org/10.1016/bs.coac.2019.09.001
Schmutz, M., et al. (2020). A methodological safe-by-design approach for the development of nanomedicines. Frontiers in Bioengineering and Biotechnology, 8, 258. https://doi.org/10.3389/fbioe.2020.00258
Scholzen, T., & Gerdes, J. (2000). The Ki-67 protein: From the known and the unknown. Journal of Cellular Physiology, 182(3), 311–322. https://doi.org/10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9
Seeta, G., et al. (2019). Nanomaterials multifunctional behavior for enlightened cancer therapeutics. Seminars in Cancer Biology, 69, 178–189. https://doi.org/10.1016/j.semcancer.2019.08.013
Sharma, M., et al. (2016). Framework to evaluate exposure relevance and data needs for risk assessment of nanomaterials using in vitro testing strategies. Risk Analysis, 36(8), 1551–1563. https://doi.org/10.1111/risa.12581
Shmuel, A. (1992). Identification of programmed cell death in situ. Journal of Cell Biology, 119(3), 493–501. Shukla, A. K. (2019) Nanoparticles in Medicine.
Shukla, R., et al. (2019). Conclusion and future prospective of polymeric nanoparticles for cancer therapy. In Polymeric nanoparticles as a promising tool for anti-cancer therapeutics. Academic Press. https://doi.org/10.1016/B978-0-12-816963-6.00018-2
Si, A., et al. (2020). Sustainable preparation of gold nanoparticles via green chemistry approach for biogenic applications. Materials Today Chemistry, 17, 100327. https://doi.org/10.1016/j.mtchem.2020.100327
Singh, A., & Sahoo, S. K. (2013). Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discovery Today, 19, 474–481. https://doi.org/10.1016/j.drudis.2013.10.005
Singh, N., et al. (2010). Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Reviews, 1(1), 5358–5358. https://doi.org/10.3402/nano.v1i0.5358
Singh, S. P., et al. (2017). Silver nanoparticles: Biomedical applications, toxicity, and safety issues. International Journal of Research in Pharmacy and Pharmaceutical Sciences, 2, 2455–2698.
Singhal, N., Rishi, V., & Yadav, H. (2001). Cell proliferation assays. https://doi.org/10.1002/9780470015902.a0002566.
Sligo, T., Lane, A., & Yw, S. F. (2018). Toxicity of nanomaterials: Exposure, pathways, assessment, and recent advances. ACS Biomaterials Science & Engineering, 4, 2237–2275. https://doi.org/10.1021/acsbiomaterials.8b00068
Soni, M. et al. (n.d.) Green nanoparticles: Synthesis and applications, p. 7.
Steckiewicz, K. P., et al. (2019). Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. Journal of Materials Science: Materials in Medicine, 30, 22. https://doi.org/10.1007/s10856-019-6221-2
Stone, V., Johnston, H., &Schins, R. P. F. (2009). Development of in vitro systems for nanotoxicology: Methodological considerations. https://doi.org/10.1080/10408440903120975
Strober, W., & Diseases, I. (2019). Trypan blue exclusion test of cell viability, pp. 4–6. doi: https://doi.org/10.1002/0471142735.ima03bs111.Trypan
Sun, H., et al. (2018). Development of low molecular weight heparin based nanoparticles for metastatic breast cancer therapy. International Journal of Biological Macromolecules, 112, 343–355. https://doi.org/10.1016/j.ijbiomac.2018.01.195
Sun, B., et al. (2019). Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nature Communications, 10, 3211. https://doi.org/10.1038/s41467-019-11193-x
Svenson, S. (2013). Theranostics: Are we there yet? Molecular Pharmaceutics, 10(3), 848–856. https://doi.org/10.1021/mp300644n
Taguchi, K., et al. (2013). Safety of nanoparticles based on albumin-polymer conjugates as a carrier of nucleotides for pancreatic cancer therapy. Journal of Materials Chemistry B, 4, 1–3. https://doi.org/10.1039/C8TB01613E
Tang, H., et al. (2015). Stability, pharmacokinetics, biodistribution and safety assessment of folate-conjugated pullulan acetate nanoparticles as cervical cancer targeted drug carriers. Journal of Nanoscience and Nanotechnology, 15(9), 6405–6412. https://doi.org/10.1166/jnn.2015.10752
Tekade, R. K., Maheshwari, R., & Jain, N. K. (2018). Toxicity of nanostructured biomaterials, nanobiomaterials: nanostructured materials for biomedical applications (p. 256). Elsevier Ltd.. https://doi.org/10.1016/B978-0-08-100716-7.00027-1
Thomas, R., Park, I.-K., & Jeong, Y. Y. (2013). Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. International Journal of Molecular Sciences, 14, 14–14. https://doi.org/10.3390/ijms140815910
Torres Andón, F., & Fadeel, B. (2014). Nanotoxicology: Towards safety by design. Advances in Delivery Science and Technology. https://doi.org/10.1007/978-3-319-08084-0_14
Van De Poel, I., & Robaey, Z. (2017). Safe-by-design: From safety to responsibility. Nanoethics, 11, 297–306. https://doi.org/10.1007/s11569-017-0301-x
Veerapandian, M., et al. (2009). Biomaterial as nanobiopharmaceuticals. Thai Journal of Pharmaceutical Sciences, 33(1), 1–21.
WEBSTER, T. J., et al. (2013). Experimental methods and in vitro cytoxicity and genotoxicity of nanomaterials. Nano LIFE, 03(01), 1340008–1340008. https://doi.org/10.1142/s1793984413400084
WHO | Key statistics (2020) WHO. World Health Organization. Available at: https://www.who.int/cancer/resources/keyfacts/en/. Accessed: 27 Nov 2020.
Yahaya, J. W., & Zain, M. (2017). Safer by design strategies. Journal of Physics: Conference Series, 836, 012016. https://doi.org/10.1088/1742-6596/838/1/012016
Yamashita, T., et al. (2012). Carbon nanomaterials: Efficacy and safety for nanomedicine. Materials, 5(2), 350–363. https://doi.org/10.3390/ma5020350
Yan, L., et al. (2019). A safe-by-design strategy towards safer nanomaterials in nanomedicines. Advanced Materials, 31(45), 1–33. https://doi.org/10.1002/adma.201805391
Yang, X., et al. (2017). Redox- sensitive self-assembled nanoparticles based on alpha- tocopherol succinate-modified heparin for intracellular delivery of paclitaxel. Journal of Colloid and Interface Science, 496, 311–326. https://doi.org/10.1016/j.jcis.2017.02.033
Yu, C., et al. (2019). Hydroxyethyl starch-based nanoparticles featured with redox- sensitivity and chemo-photothermal therapy for synergized tumor eradication. Cancers, 11(207), 1–20. https://doi.org/10.3390/cancers11020207
Zhao, K., et al. (2017). Targeted hydroxyethyl starch prodrug for inhibiting the growth and metastasis of prostate cancer. Biomaterials, 116, 82–94. https://doi.org/10.1016/j.biomaterials.2016.11.030
Zhu, L. et al. (2016). Magnetic nanoparticles for precision oncology: theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine. https://doi.org/10.2217/nnm-2016-0316
Zielińska, A., et al. (2020). Nanotoxicology and nanosafety: Safety-by-design and testing at a glance. International Journal of Environmental Research and Public Health, 17(13), 1–22. https://doi.org/10.3390/ijerph17134657
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bhanushali, S., Tanna, V., Nimbalkar, Y., Ravikumar, P., Sawarkar, S.P. (2021). Safety of Nanobiomaterials for Cancer Nanotheranostics. In: Saravanan, M., Barabadi, H. (eds) Cancer Nanotheranostics. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-76263-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-76263-6_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76262-9
Online ISBN: 978-3-030-76263-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)