Abstract
This chapter addresses the antibacterial activity of Carica papaya seeds due to their abundance in bioactive compounds and these seeds contain high levels of fatty acid methyl esters (FAMEs). However, no report is available to indicate (1) which FAMEs are potent against pathogens and (2) the effect of temperature on the distribution of FAMEs. Therefore, this study aims to evaluate the effect of temperature against the antibacterial activity of Carica papaya seed extract (CPSE) and its FAME profile via extraction of the seeds using methanol and the extract was subjected to test of antibacterial activity against Salmonella enteritidis, Bacillus cereus, Vibrio vulnificus, and Proteus mirabilis. FAME profiling was done using GC/MS incorporated with principal component analysis (PCA). The CPSE at 5.63 mg/mL was potent against these pathogens at < 40 °C. Although the CPSE was rich with FAMEs, the PCA result had identified individual FAMEs that inhibited the pathogen growth. Palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1n9c), and cis-vaccenic acid (C18:1n11c) had strongly inhibited V. vulnificus and P. mirabilis growths and moderately inhibit S. enteritidis growth. To avoid the formation of trans FAMEs, this study also suggested that the CPSE temperature should be held at < 150 °C.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Papaya or Carica papaya is a highly commercialized tropical fruit. Ripe Carica papaya is mainly eaten as a dessert and papain from the plant’s latex is commercialized as a meat tenderizer and is also been used as an enzyme in several enzymatic extraction works (Vasu et al., 2012). Not much attention has been paid to the fruit as a potential source of phytochemicals other than the flesh of the fruit itself. Out of 28 million metric tons of produced papaya worldwide, 5 million metric tons of the seeds were discarded in 2017 (FAOSTAT, 2019). Although Carica papaya seeds are discarded from other parts of the world, they are popularly consumed in India, Central Asia, and the Middle Eastern countries. They have been used to marinate meat, substitute for black pepper, and are added to salad dressings due to their spicy flavor (Lim, 2012). Several reports on antibacterial (Sani, 2018), antifungal (Chávez-Quintal et al., 2011) and anticancer (Nakamura et al., 2007) activities of Carica papaya seeds obtained from several solvent extracts could be found. Moreover, Sani et al. (2017b) found that the crude Carica papaya seed extract (CPSE) was active against S. enteritidis, V. vulnificus, P. mirabilis, and B. cereus, but reported potent antibacterial compounds. Since the yield and consumption of Carica papaya seeds are very high, it is worth investigating their compositions and potent antibacterial components that potentially inhibit these pathogenic bacteria.
The composition of CPSE was dominated by fatty acid methyl esters (FAMEs) (Sani et al., 2017b). However, there was limited information on which FAMEs had rendered bacterial inhibition. Also, trans FAMEs are of high concern because they have been linked to nutritional health issues (Tao, 2007), especially C18:1n9t (Preedy et al., 2011) while subjecting heat treatment. Cis and trans FAMEs have been stated to be hardly separated due to the same molecular weight except in the use of HP-88 column and GC/MS detection (Albuquerque et al., 2011). Thus, this study was performed in order to (1) investigate the efficacy of antibacterial CPSE and its distribution of FAMEs as heat-affected and (2) identify potential FAMEs that render antibacterial activity in CPSE.
8.2 Methodology
8.2.1 Plant Material
Carica papaya cv. Sekaki fruits were bought from D’Lonek Sdn. Bhd. Organic Farm, Rembau, Negri Sembilan, Malaysia. Carica papaya plant, flower, and fruit from this farm were deposited to Herbarium of the Institute of Bioscience, Universiti Putra Malaysia, and a plant voucher, numbered as SK 2368/14, was issued. The seeds of Carica papaya were removed from the fruit and treated as described by Sani et al. (2017a). The seeds were thoroughly washed in distilled water, oven-dried at 40 °C for three days, kept in airtight amber bottles, and stored at −20 °C until further analysis.
8.2.2 Extraction of Phytochemicals
Methanol (MeOH) was the solvent used for extraction. Dried seeds were ground to fineness for 5 min in a 240 W electrical blender (Panasonic MX-337, Malaysia) before extraction. A solvent-to-solid ratio of 10:1 was used in this study. Briefly, 50 g of dried ground Carica papaya cv. Sekaki seeds were weighed in a conical flask, and 500 mL of MeOH was added. The extraction was carried out at room temperature (27 °C) for 8 h in a shaker (100 rpm) followed by filtration through Whatman No.1 filter paper (GE Healthcare, UK). The filtrate was transferred to pre-weighed flat bottom flasks and concentrated using a rotary vacuum evaporator (Eyela N-1001, Japan) at 40 °C. The concentrated CPSE was kept at 4 °C until further use (Sani et al., 2017a).
8.2.3 Effect of Temperature on the Extract
About 29.5 mg of nitrogen-blown extract was heated at 60, 80, 100, 150, and 200 °C for 15 min and mixed with 1% Tween 80 and TSB for a final volume of 5 mL. The final extract concentration used in this study was 5.63 mg/mL, which was the MIC value determined by Sani et al. (2017b). Heated-extract solutions were subjected to the test of percentage growth inhibitions of S. enteritidis, V. vulnificus, P. mirabilis, and B. cereus.
8.2.4 Percentage of Growth Inhibition
The growth of Salmonella enteritidis (ATCC 13076), Vibrio vulnificus (ATCC 27562), Proteus mirabilis (ATCC 12453), and Bacillus cereus (ATCC 10875) was monitored using 96 well-microplates. A volume of 10 μL of TSB containing 106 CFU/mL of the tested pathogen was mixed with 190 μL of the TSB solutions in 96 well-microplates and assessed in a microplate spectrophotometer. Positive controls containing the tested solution was inoculated with the respective pathogens. Negative controls contained a mixture of crude extract, Tween 80, and TSB. The 96 well-microplate was incubated at 37 °C for 24 h on a Heidolph Inkubator and Titrama × 1000 (Germany) at 210 rpm to prevent adherence and clumping, after which the optical density was measured at 600 nm in Tecan Infinite® 200 Microplate Reader (Switzerland) before (T0) and after (T24) incubation.
The percentage growth inhibition (Patton et al., 2006) for TSB solutions was determined using Eq. (8.1)
8.2.5 Effect of Temperature on Fatty Acid Profile in Carica Papaya Seed Extract
The CPSE was heated at 40, 60, 80, 100, 150, and 200 °C for 15 min and subjected to pretreatment before GC/MS analysis.
8.2.6 Profiling of Fatty Acid Methyl Esters by GC/MS Analysis
8.2.6.1 Sample Preparation
An amount of 0.01 g/mL of the heated extract at 40, 60, 80, 100, 150, and 200°C for 15 min was re-dissolved in 0.6 mL of hexane and added with 0.4 mL of 1 M sodium methoxide and vortexed for 30 s. The top hexane layer (0.6 mL) was subjected to FAMEs quantification by gas chromatography-mass spectrometry (GC/MS) analysis.
8.2.6.2 Preparation of Calibration Curve
The linearity of the methods was evaluated using different concentrations of FAME standards, ranging from 0.0005 – 3 mg/mL and cis-vaccenic acid (0.0001 – 0.5 mg/mL). Linearity was assessed using the linear regression equation, where the correlation coefficient r > 0.98, indicated an acceptable identification (Fagundes & Caldas, 2012). The prepared standards were analyzed using GC/MS.
8.2.6.3 Quantification of Fatty Acids Methyl Esters
Characterization of FAMEs was performed using the Agilent-Technologies 7890A GC system equipped with the Agilent-Technologies 5975 mass selective detector (Agilent Technologies, USA). The compound separation was achieved using an HP-88 capillary column (100 m × 0.25 mm, film thickness 0.20 µm) with an oven temperature program at 150 °C for 5 min, heated to 240 °C at the rate of 4 °C/min and held for 15 min. Samples were injected in split mode with the injector temperature at 260 °C with helium as a carrier gas at a constant flow rate (1 mL/min). For MS detection, the electron ionization mode with ionization energy of 70 eV was used with a mass range of m/z 20–700 units. The MS transfer line and MS quadrupole temperature were set at 230 °C and 150 °C. The mass spectrometer was operated in both the scan and selected ion monitoring (SIM) modes for compound identification and quantification, respectively. In order to avoid the need to modify the retention times in the calibration tables due to column maintenance or column change, the calibration of standards was performed in the retention-time-lock mode (Caven-Quantrill & Buglass, 2007) where palmitic acid (C16:0) was chosen as the locking standard due to its stability. Compounds were identified by their retention times and mass fragmentation patterns of standards using the National Institute of Standard (NIST) Mass Spectral 11 offline library.
8.2.7 Statistical Analysis
8.2.7.1 ANOVA
Data were expressed as mean ± standard deviations of triplicate Rf and LC50 and residual methanol. One way analysis of variance (ANOVA) with Tukey’s test was conducted using XLSTAT-Pro (2014) statistical software (Addinsoft, Paris, France) to determine the significant difference between the means at 95% confidence level (p < 0.05) for bioautography, toxicity assay, and residual methanol.
8.2.7.2 Principle Component Analysis (PCA)
Principle component analysis (PCA) is the most commonly unsupervised pattern recognition technique used in the distribution of compounds in the food sample (Dorta et al., 2014). The PCA was employed to elucidate the data variance of inter-correlated variables and transform them into independent variables called a principle component (PC). PCA also excludes the less significant parameters.
In this study, PCA was applied to FAME’s contribution to the PI of tested pathogens as affected by temperature. From the calculation of the eigenvalue, a new set of groups called PCs was generated for each eigenvalue > 1. The PC was influenced by factor loading > 0.75, 0.74 - 0.50, and 0.49 – 0.30, which were considered as strong, moderate, and weak (Retnam et al., 2013). The profile of factor loadings and specific indicative FAMEs were used to deduce the FAMEs contribution on PI of tested pathogens as affected by temperature. PCA was conducted using XLSTAT-Pro (2014) statistical software (Addinsoft, Paris, France).
8.3 Discussion
8.3.1 Effect of Temperature on Antibacterial Activity and Fatty Acids Profile of Carica Papaya Seed Extract
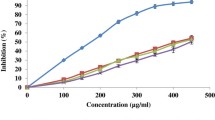
The effect of various heating temperatures on the antibacterial activity of CPSE indicated the stability of the extract as shown in Fig. 8.1. The potency of the extract against S. enteritidis and V. vulnificus had the same characteristics where the extract heated > 100 °C had a percentage inhibition of < 100%. Only the heated extract at 150 °C indicated a percentage inhibition < 100% (99.45%) against B. cereus. For P. mirabilis, only the heated extract at 40 °C was potent to the pathogen because the percentage inhibition > 100%, even though Nychas et al. (2003) reported that low temperatures reduced antibacterial activities. However, He et al. (2010) found that most antibacterial agents had lost their inhibitory efficiency at high temperatures. In general, all tested pathogens were sensitive to the extract in TSB at < 40 °C and this finding could be proposed for food incorporated with the extract to be handled below this temperature before consumption.
8.3.2 Effect of Temperature on Fatty Acid Methyl Esters Profile of Carica Papaya Seed Extract
Linear relationships between the ratios of the peak area signals and the corresponding concentrations of FAMEs content were observed in the analytical curves Fig. 8.2 when using different concentrations. The parameters of the analytical curves with the correlation of determination (R2) are shown in Table 8.1. The values of the analytical curve led to the conclusion that the linear regression model was adequate for the analytical determinations in this study, as R2 was higher than 0.98 (Fagundes & Caldas, 2012). In this study, we did not calculate trans vaccenic acid, and thus only cis-vaccenic acid (C18:1n11c) was recorded. The chromatogram of the FAMEs is shown in Fig. 8.3.
The PCA was used to establish the relationship between the FAMEs identified by GC/MS and PI of S. enteritidis, B. cereus, V. vulnificus, and P. mirabilis as affected by heat. The four main principal components (PCs) characterized were having an eigenvalue > 1 (Saiful et al., 2019) which was considered as significant factor loadings (FL) (p < 0.05). The four PCs also had a cumulative explained total variance of 100% which consisted of PC1 (47.99%), PC2 (22.01%), PC3 (20.23%), and PC4 (9.77%) (Table 8.2). The data variances were explained at 70% for PC1 versus PC2 and 68.22% for PC1 versus PC3.
The FL table showed the loading values between the FAMEs and the PI of tested pathogens (Table 8.2). Based on the strong FL limit (> 0.75), PC1 was mainly characterized for a higher content of C16:0, C16:1, C18:0, C18:1n9c, C18:1n11c, and lower content of C23:0, also had strongly contributed to the PI of V. vulnificus and P.mirabilis and moderate contribution to S. enteritidis. The PC2 was related principally to the higher content of C13:0 and C20:2. PC3, on the other hand, was dominated by a higher content of C10:0 and a lower content of C20:0 and moderately contributed to the PI of B. cereus.
The observation plot allowed exploring the correlations between the PCs and the extract as affected by temperature (Fig. 8.4). The extract heated at 40 °C clearly showed a significant antagonistic correlation in PC1 against 100 °C, 150 °C, and 200 °C, and significant antagonistic correlation against 80 °C in PC3 (Fig. 8.4b). In PC3 also, a significant antagonistic correlation was exhibited by 80 °C and 150 °C.
The variable plot in Fig. 8.5 helped to establish which FAMEs and PI of the tested pathogens discriminated against the heated extracts. The extract heated at 40 °C, as seen in Fig. 8.4a, had a strong PC1 score that has been related to higher content in C16:0, C16:1, C18:0, C18:1n9c, C18:1n11c (Fig. 8.5a). Nevertheless, the heated extract at 80 °C had a high PC3 score, as seen in Fig. 8.4b; therefore, had higher contents of C10:0 (Fig. 8.5b). The heated extract at 100 °C (Fig. 8.4a) had a higher content of C13:0 in Fig. 8.5a, whereas the extract heated at 150 °C had a lower content of C12:0 and C18:1n9t. However, the C23:0 was lower in 200 °C heated extracts.
These results suggest that the PI of the tested pathogens was addictively and antagonistically facilitated by different compositions of FAMEs. Besides, the individual FAMEs had different stability against different temperatures. In summary, from PC1 alone, we found that C16:0, C16:1, C18:0, C18:1n9c, and C18:1n11c from CPSE had strongly inhibited V. vulnificus and P. mirabilis growths and moderately inhibit S. enteritidis growth.
8.3.3 Profile of Cis and Trans Fatty Acid Methyl Esters as Affected by Temperature
FAMEs were dominant in CPSE (Sani et al., 2017b). Among the FAMEs, their trans form has received high concern due to its negative health impact, especially when the extract was used in the food and undergoes heat treatment, such as cooking and deep-frying. Thus, this study was done to identify the profile of the trans-FAMEs when the extract is heated. Also, the study of other FAMEs profile in non-heated CPSE (unheated) Carica papaya seed was done due to the capability of the HP-88 column to separate cis and trans-FAMEs, unlike the HP-5 ms column used in common plant metabolites analysis using GC/MS.
The profile of cis and trans-FAMEs affected by temperature is shown in Fig. 8.6. The highest concentration of oleic acid (C18:1n9c) was recorded at low temperatures (40 °C and 60 °C), whereas cis-vaccenic acid (C18:1n11c) was detected at each heating treatment and exhibited a reducing trend; C18:1n11c was the most stable FAMEs at 100 °C whereas other FAMEs were undetected.
C18:2n6t was not detected in each sample treatment, indicating that C18:2n6c was stable against heat and did not convert to trans FAMEs. Meanwhile, C18:1n9c showed a drastic reduction when heated at higher temperatures and producing its trans form (C18:1n9t) at 150 °C and 200 °C, thereby supports the finding of Gürdeniz et al. (2013) since all naturally occurring FAMEs of plant origin are in the cis form, and the trans form is generally generated when oils and fats are hydrogenated or heated at a high temperature (Tao, 2007). Thus, it can be proposed that the food incorporated with the CPSE could be handled at a temperature < 150 °C. However, C18:1n9c was still detected at 150 °C and 200 °C because of its high oxidative stability (Preedy et al., 2011) Fig. (8.6).
8.4 Conclusion
In summary, the crude of CPSE cv. Sekaki/Hong Kong had demonstrated antibacterial activity against S. enteritidis, V. vulnificus, P. mirabilis, and B. cereus. These tested pathogens were sensitive to the extract in TSB at < 40 °C. From the PCA, C16:0, C16:1, C18:0, C18:1n9c, and C18:1n11c from CPSE had strongly inhibited V. vulnificus and P. mirabilis growths and moderately inhibit S. enteritidis growth. The treatment of CPSE against heat had also caused the generation of the trans-FAMEs at 150 °C and 200 °C, thus indicating that CPSE should be handled at < 150 °C in food applications.
References
Albuquerque, T. G., Costa, H. S., Castilho, M. C., & Sanches-Silva, A. (2011). Trends in the analytical methods for the determination of trans fatty acids content in foods. Trends in Food Science & Technology, 22, 543–560. https://doi.org/10.1016/j.tifs.2011.03.009.
Caven-Quantrill, D. J., & Buglass, A. J. (2007). Determination of volatile organic compounds in English vineyard grape juices by immersion stir bar sorptive extraction gas chromatography-mass spectrometry. Flavour and Fragrance Journal, 22(November), 206–213. https://doi.org/10.1002/ffj.
Chávez-Quintal, P., González-Flores, T., Rodríguez-Buenfil, I., & Gallegos-Tintoré, S. (2011). Antifungal activity in ethanolic extracts of Carica papaya L. cv. Maradol leaves and seeds. Indian Journal of Microbiology, 51(1), 54–60. https://doi.org/10.1007/s12088-011-0086-5.
Dorta, E., González, M., Lobo, M. G., Sánchez-Moreno, C., & de Ancos, B. (2014). Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Research International, 57, 51–60. https://doi.org/10.1016/j.foodres.2014.01.012.
Fagundes, A. M., & Caldas, S. S. (2012). Development and validation of a method for the determination of fatty acid methyl ester contents in tung biodiesel and blends. Journal of the American Oil Chemists’ Society, 89, 631–637. https://doi.org/10.1007/s11746-011-1948-z.
FAOSTAT. (2019). Papaya crop yield. Retrieved May 30, 2019, from http://www.fao.org/faostat/en/#data/QC.
Gürdeniz, G., Rago, D., Bendsen, N. T., Savorani, F., Astrup, A., & Dragsted, L. O. (2013). Effect of trans fatty acid intake on LC-MS and NMR plasma profiles. PLoS ONE, 8(7), 1–11. https://doi.org/10.1371/journal.pone.0069589.
He, F., Yang, Y., Yang, G., & Yu, L. (2010). Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control, 21(9), 1257–1262. https://doi.org/10.1016/j.foodcont.2010.02.013.
Lim, T. K. (2012). Carica papaya. In Edible Medicinal and Non-Medicinal Plants (pp. 693–717). https://doi.org/10.1007/978-90-481-8661-7.
Nakamura, Y., Yoshimoto, M., Murata, Y., Shimoishi, Y., Asai, Y., Eun, Y. P., … Nakamura, Y. (2007). Papaya seed represents a rich source of biologically active isothiocyanate. Journal of Agricultural and Food Chemistry, 55(11), 4407–4413. https://doi.org/10.1021/jf070159w.
Nychas, G. J. E., Skandamis, P. N., & Tassou, C. C. (2003). Antimicrobials from herbs and spices. In S. Roller (Ed.), Natural antimicrobials for the minimal processing of foods (1st ed.). Woodhead Publishing Limited. https://doi.org/10.1016/B978-1-85573-669-6.50014-9.
Patton, T., Barrett, J., Brennan, J., & Moran, N. (2006). Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. Journal of Microbiological Methods, 64(1), 84–95. https://doi.org/10.1016/j.mimet.2005.04.007.
Preedy, V. R., Watson, R. R., & Patel, V. B. (2011). Nuts and seeds in health and disease prevention. In V. R. Preedy, R. R. Watson, & V. B. Patel (Eds.) (1st ed.). Elsevier.
Retnam, A., Zakaria, M. P., Juahir, H., Aris, A. Z., Zali, M. A., & Kasim, M. F. (2013). Chemometric techniques in distribution, characterisation and source apportionment of polycyclic aromatic hydrocarbons (PAHS) in aquaculture sediments in Malaysia. Marine Pollution Bulletin, 69(1–2), 55–66. https://doi.org/10.1016/j.marpolbul.2013.01.009.
Saiful, M., Azid, A., Iskandar, S., Shirwan, M., Sani, A., & Lananan, F. (2019). Comparison of prediction model using spatial discriminant analysis for marine water quality index in mangrove estuarine zones. Marine Pollution Bulletin, 141(February 2018), 472–481. https://doi.org/10.1016/j.marpolbul.2019.02.045.
Sani, M. S. A. (2018). Antibacterial activities of Carica papaya L. seed as food preservative. Universiti Putra Malaysia. Universiti Putra Malaysia. Retrieved from http://www.elib.upm.edu.my/cgi-bin/koha/opac-ISBDdetail.pl?biblionumber=574282.
Sani, M. S. A., Bakar, J., Rahman, R. A., & Abas, F. (2017a). In vitro antibacterial activities and composition of Carica papaya cv. Sekaki / Hong Kong peel extracts. International Food Research Journal, 24(June), 976–984.
Sani, M. S. A., Bakar, J., Rahman, R. A., & Abas, F. (2017b). The antibacterial activities and chemical composition of extracts from Carica papaya cv. Sekaki / Hong Kong seed. International Food Research Journal, 24(April), 810–818.
Tao, B. Y. (2007). Industrial applications for plant oils and lipids. In S.-T. Yang (Ed.), Bioprocessing for value-added products from renewable resources (1st ed., pp. 611–627). San Diego: Elsevier B.V. http://dx.doi.org/10.1016/B978-044452114-9/50025-6.
Vasu, P., Savary, B. J., & Cameron, R. G. (2012). Purification and characterization of a papaya (Carica papaya L.) pectin methylesterase isolated from a commercial papain preparation. Food Chemistry, 133(2), 366–372. https://doi.org/10.1016/j.foodchem.2012.01.042.
Acknowledgements
This work was supported by the Fundamental Research Grant Scheme (FRGS19-041-0649) of the Ministry of Higher Education Malaysia and Research University Grant (vot no. 9328200) of Universiti Putra Malaysia.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sani, M.S.A., Bakar, J., Rahman, R.A., Abas, F. (2021). Effect of Temperature on Antibacterial Activity and Fatty Acid Methyl Esters of Carica Papaya Seed Extract. In: Amid, A. (eds) Multifaceted Protocols in Biotechnology, Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-030-75579-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-75579-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-75578-2
Online ISBN: 978-3-030-75579-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)