Abstract
Pandanus odorifer (Forssk.) Kuntze (kewda) is an industrially important aromatic plant currently having huge demand for the unique fragrance possessed by its essential oil. Phenyl ethyl methyl ether is the major component which imparts this exquisite odour to the kewda male flower essential oil. This distinctive aroma instigates its extensive usage in the cosmetic, pharmaceuticals and flavour and fragrance industries. Almost every part of the plant (flower, stem, root, leaves) possess numerous pharmacological and ethnic utilities. The plant propagation using elite genetic material is therefore imperative to produce improved quality kewda plants to meet the global need. The accelerated demand of kewda perfumes has resulted in a hike in the price of kewda oil. Hence, the farmers require its large-scale cultivation mainly in the coastal and sub-coastal regions. The present chapter focuses on the botanical, phytochemical, pharmacological, agronomical and biotechnological aspects of Pandanus odorifer. This comprehensive information will conclusively allow better utilization of this industrially important plant for various industrial uses and improve the socio-economic growth of low-income coastal villagers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pandanus odorifer
- Essential oil
- Perfume
- Chemotype
- Genotype
- Pharmacology
- Aromatic plant
- Flavour and fragrance industry
1 Introduction
Since ancient times plants have been an exorbitant source of natural products and have been widely used to treat various health-related disorders; natural products include numerous pharmaceutical compounds, colouring agents, dyes, and aromatic essential oils. The essential oils are the secondary metabolites stored in the glandular trichomes or cavities and provide a defence system to the plant against herbivores (Glas et al. 2012). Several aromatic plants have been identified and explored for their therapeutic essential oils which are used extensively in the flavor and fragrance industry as well as the pharmaceutical industries (Mohanty et al. 2017; Stringaro et al. 2018; Hanif et al. 2019; Manilal et al. 2020). About 90% of the essential oil is consumed by the flavour and fragrance industry in the production of perfumes, cosmetics, food flavouring agents and other healthcare products (Swamy and Sinniah 2016).
Pandanus odorifer (Forssk.) Kuntze (kewda) is a perennial evergreen dioecious monocotyledonous aromatic plant native to South Asia (Nadaf and Zanan 2012; Solomon Raju and Lakshminarayana 2020). The plant sees flowering during the rainy season and is highly valued for its fragrant male flowers. The essential oil from the male flowers is isolated by the hydro-distillation method, which possesses a unique fragrance (Nasim et al. 2018). The phytochemical analysis of kewda essential oil has revealed phenyl ethyl methyl ether (PEME) as the major component that imparts the characteristic smell to the oil (Naqvi and Mandal 1996). Very limited reports are available for the GC-MS analysis to identify the phytochemical composition of kewda flower essential oil (Naqvi and Mandal 1996; Misra et al. 2000; Raina et al. 2004; Nasim et al. 2017a, 2018). Because of its distinct aroma, of kewda oil has a massive demand in pharmaceutical, cosmetic and flavour and fragrance industry.
1.1 Botanical Description
1.1.1 Taxonomic Position
Pandanus odorifer (Forssk.) Kuntze (kewda) belongs to an ancient family Pandanaceae (Gallaher et al. 2015). The taxonomic classification of Pandanus odoriferis as follows:
Kingdom | Plantae |
|---|---|
Division | Angiospermae |
Class | Monocotyledons |
Order | Pandanales |
Family | Pandanaceae |
Genus | Pandanus |
Species | Pandanus odorifer |
The name Pandanus was derived from a Malayan vernacular name of the trees, “pandan” in 1743 by Rumphius (John 1960). In India, the genus is represented by 30–40 species, which are not well defined (Kirtikar et al. 1991). Among these, P. odorifer is a dominant species found mainly in Odisha's coastal regions (Nasim et al. 2017a). Commonly known as kewda, the plant has various synonyms (Table 15.1).
1.1.2 Morphological Description
The plant has a palm-like appearance, usually growing to a height of 3–5 m (Fig. 15.1A, B). The kewda leaves are deep green, glaucous, oblong, ensiform with coriaceous margins and tapering with spiny midribs, arranged spirally on the branches and measure 1–3 m long and 5–6 cm broad (Fig. 15.1C). The plant is dioecious with unisexual flowers. Male flowers appear as clusters with androecia having a unique fragrance and are surrounded by tender white bracts (Fig. 15.1 D). The male spadix is 25–50 cm in length and has numerous subsessile cylindrical spikes, which are 5–10 cm long (Padhy et al. 2016). The female flowers lack any fragrance and look like a pineapple. The female inflorescence consists of a single spadix (5 cm in diameter) and gynoecia without perianth and is made up of lots of carpels (Padhy et al. 2016). The male flowers last only a single day. The fruits of this plant are odourless with 15–25 cm width and are ellipsoid, ovoid, globose or subglobose. Unripen fruits are green, whereas mature fruits are yellow/red (Jose et al. 2016; Padhy et al. 2016). It has greyish- or reddish-brown smooth barks. Braches and stems are ringed with distinct leaf scars (Lim 2012). It has a spinous trunk (12–25 cm across) with the thick and strong prop or adventitious stilt roots that arise from the stems (Fig. 15.1E) (Panda et al. 2009). Ecologically, the plant has been reported to bear immense potential in its intricate root system, and thereby controlling soil erosion, fixing sand dunes and protecting from damage caused by tsunami (Tanaka et al. 2011; Thuy et al. 2018).
1.2 Industrial Importance of Pandanus Odorifer (Kewda)
Pandanus odorifer (kewda) is an industrially important essential oil-bearing plant with high priced flower essential oil. Economically, it is an important natural bio-resource for the perfumery industry due to its unique fragrance (Panda et al. 2009). The characteristic smell of the male flowers owes their application to the manufacturing of various perfumery products through hydro-distillation. Three types of end products are produced from the hydro-distilled kewda flowers, i.e. kewda oil, kewda attar and kewda water. Kewda oil has substantial demand in the perfumery industry, whereas kewda attar and kewda water are predominantly used for flavouring purposes in the food and cosmetic industry (Panda et al. 2012). Ganjam district of Odisha has been reported to have a superior quality of kewda essence (Raina et al. 2004). Hence, it provides 85–90% of kewda essence in India and about 50% of the world with an approximate turnover of Rs. Fifty crores (Padhy et al. 2016). The kewda products have seen an upsurge in their price due to the prompt demand of kewda perfume in the national and international markets, especially in the Arab countries (Sahu and Misra 2007). Kewda essential oil is approximately priced to be 2.5 to 4 lakh per litre, kewda attar is 0.2 lakh per litre and kewda water is Rs 300 per litre (Padhy et al. 2016).
Pandanus odorifer plant parts have multipurpose industrial applications and a broad ethnic value. Though the male flowers are extensively used for perfumery production, they also have broad pharmaceutical properties. Other plant parts such as leaves, roots and fruits are also employed in the food, fibre, handcraft and pharmaceutical industries. Kewda leaves are rigid and spiny and often used as fences across the crop fields to protect them from livestock. The low-income coastal villagers also use them in making various handicraft products such as ropes, mats, baskets, table lamps, files and wall hangings. (Abral et al. 2012; Teli and Jadhav 2017). The leaf extracts are also used for food colouring purposes. In Sri Lanka, the leaves are used for cooking (Takeda et al. 2008). The pulp and polyester composites of the leaves are used in the paper and fibre industry. The fibre obtained from P. odorifer leaves also has excellent potential for being used as a textile and composite material. The thick and strong prop roots are used as supports and fabrication of houses. The spinous trunks of mature plants are used in building thatched roof. It is also used for preparing glue and making string. The branches are used to make compost and wood fuel. In India and Sri Lanka, the flowers are used for decoration and are offered to God. Fruits are used as firewood and foodstuff (Nadaf and Zanan 2012; Baba et al. 2016).
Hence, cultivation practice and the marketing of kewda products have become an additional source of income for the deprived coastal villagers, resulting in their socio-economic growth. Thus, the plant has become an essential bio-resource with a positive impact on the local economy of Ganjam district, Odisha, India (Panda et al. 2007; Panda et al. 2009; Panda et al. 2010b; Jose et al. 2016).
2 Geographic Distribution
2.1 Origin
Pandanus is a pleiotropic genus, belonging to an ancient family Pandanaceae, representing dioecious monocotyledons having Gondwanan origin (Gallaher et al. 2015). Among all the genera of the Pandanaceae family, Pandanus is the largest genus and has the broadest geographical distribution with immense economic and medicinal importance (Buerki et al. 2012). The diversity of habitats included by the genus occupies the tropical and sub-tropical zones, riversides, rocky or sandy coasts, swamp forests, mangrove forests, savannas, lowland dipterocarp forest and mountain forest (Susanti et al. 2012).
2.2 Distribution
In India, Pandanaceae family represents about 30–40 species under three genera Pandanus, Benstonea and Freycinetia (Table 15.2). Pandanus odorifer is an important member of the genus Pandanus with a higher concentration in Andaman and Nicobar Islands and Northern and Southern India. It is an aromatic monocot species, native to Australia, Indonesia, South Asia and Philippines. It is widely distributed in South America, Micronesia, Papua New Guinea, Melanesia, Polynesia, India and Pacific Islands (Nadaf and Zanan 2012; Adkar et al. 2014; Nasim et al. 2020). In India, it is massively distributed in the Western Ghats zone and the coastal zone of Odisha, Kerala, Tamil Nadu, West Bengal, Andhra Pradesh, Gujarat and Uttar Pradesh (Padhy et al. 2016; Nasim et al. 2017a). In Odisha, the plant is found in Ganjam, Cuttack, Khorda, Jagatsinghpur, Bhadark, Puri and Balasore. Although the plant covers the entire coast of Odisha, the Ganjam district is the only growth centre where the plant is cultivated for commercial purposes (Nasim et al. 2017a).
3 Brief Phytochemistry
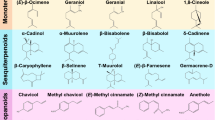
Aromatic plants have a wide range of chemical components that seeks the attention of pharmaceutical industries. A large number of phytoconstituents have been identified in the flower essential oil of Pandanus odorifer. The available reports showed a diverse chemical profile with a broad range of chemical constituents with a wide range of volatility, including ethers, esters, aldehydes, alcohol, ketones, acids, sulphur and nitrogen-containing compounds. The major constituent identified in the kewda oil was 2-phenylethyl methyl ether (75.0%) which is the key component responsible for the distinct aroma of kewda (Naqvi and Mandal 1996). Terpinen-4-ol (15.2%) was reported as the second major constituent for the first time by Naqvi and Mandal (1996). Raina et al. (2004) reported 2-phenyl ethyl methyl ether (37.7%), terpinen-4-ol (18.6%), α-terpineol (8.3%) and 2-phenyl ethyl alcohol (7.5%) as the dominating phyto-compounds in the hydro-distilled kewda flower oil obtained from Ganjam district of Odisha. The study also reported a comparative analysis of hydro-distilled kewda oil with oil purchased from the local market. Market kewda oil was reported to have 2-phenyl ethyl alcohol (33.2%) as the major compound followed by 2-phenyl ethyl methyl ether (16.1%), benzyl benzoate (11.0%), viridine (8.8%) and germacrene B (8.3%) (Raina et al. 2004). GC and GC-MS analysis of the kewda flower extract was reported by Rout et al. (2005). Rout et al. (2011) reported the chemical composition of the extract obtained by liquid CO2 extraction of the kewda flowers. The above studies show the better quality of kewda essential oil from Ganjam district, but there was unpredictability for the oil quality from different zones of Ganjam. In this context, a study was done by collecting kewda oil from twelve different zones of Ganjam district (Rushikulya River Bank, Kalipalli, Keluapalli, Markandi, Indrakhi, Mantridi, Kaliabali, Basanaputty, Chamakhandi, Podapadar, Chilika and Tampara) and GC-MS analysis was done. The study reported the PEME to be the major constituent highest in Rushikulya River Bank (81.86%) and lowest in Mantridi (58.03%). The second major compound reported was terpinen-4-ol (7.81–21.46%) (Nasim et al. 2018). The study also reported the role of soil factors for secondary metabolites of kewda. Among the five analysed parameters, i.e., soil pH, soil nitrogen (N), organic carbon (OC), potassium (K) and phosphorous (P), N was found to be the most influential factor for kewda oil yield and PEME content followed by OC, pH, P and K (Nasim et al. 2018). For a more detailed characterization of the volatile constituents present in kewda oil, GCxGC-TOFMS analysis was also done. The study identified 159 chemical compounds in kewda oil out of which kewda ether, ortho-cymene and terpinen-4-ol were the predominant constituents (Nasim et al. 2017a). The structures of some of the major chemical components found in kewda essential oil are shown in Fig. 15.2.
Other than Pandanus odorifer, very few reports are available on other Pandanus species (Table 15.3). MacLeod and Pieris (1982) analysed the essential oil of Pandanus latifolius leaves and reported the presence of mainly sesquiterpene hydrocarbons, and the only monoterpene that was reported was linalool. The chemical profile of the red fruit oil from Pandanus conoideus showed the presence of 1, 3-dimethylbenzene (27.46%), N-glycyl- L-alanine (17.36%), trichloromethane (15.22%) and ethane (11.43%) (Rohman et al. 2012). Pandanus amaryllifolius leaf oil chemical composition was reported by Jiang (1999). About twenty-two compounds were identified and the major components were 3-methyl-2-(5H)-furanone, 3-hexanol, 4-methylpentanol, 3-exanone and 2-hexanone. Isopentenyl and dimethylallyl acetates and cinnamates were reported as the dominant phytoconstituents in the essential oil of ripe fruit of Pandanus tectorius (Vahirua-Lechat et al. 1996). Pandanus tectorius root oil was analysed by Liu et al. (2012) and major compounds reported were asarone, longipinocarvone and 2-methyl-6-(4-methylphenyl) hept-2-en-4-one. The leaf extract of Pandanus odorus was analysed with GC–MS and α-tocopherol, β-sitosterol, hexadecanoic acid, campesterol, squalene, stigmasterol and 9,12,15-octadecatrien-1-ol were identified as major components by Rahman et al. (1999).
4 Medicinal Properties and Usage
Pandanus odorifer has a broad range of pharmacological properties. Different parts of the plant have been used as one of the ingredients in several Ayurvedic formulations (Nasim et al. 2020). In Ayurveda, kewda has been used for treated many human health problems such as indigestion, headache, anorexia, constipation, leprosy and rheumatism. (Udupa et al. 2011). Since ancient times kewda oil has been used for healing skin diseases, small-pox, earache, rheumatoid arthritis, headache, spasms and leprosy and as a laxative for colic infections (Adkar et al. 2014). The fruit is an excellent source of carotenoids and has been used to treat vitamin deficiencies and certain heart-related infections (Lim 2012). As a traditional practice, tablets made up of kewda leaf extracts are used for getting relief from pain and inflammation (Panda et al. 2009). The leaves are also used for curing syphilis, leprosy, leucoderma, scabies and small-pox (Padhy et al. 2016). The plant extract also possesses diuretic and anti-spasmodic properties (Rajeswari et al. 2012).
The biological potencies of Pandanus odorifer were reported as anti-inflammatory (Del mundo et al. 2020), anti-oxidant (Londonkar and Kamble 2011), anti-diabetic (Kumari et al. 2012), anti-cancer (Gowtham et al. 2014), anti-bacterial and thrombolytic (Penu et al. 2020), anti-fungal (Rahayu et al. 2013), cardio-protective (Sobhana et al. 2014), cytotoxic activity (Jitu et al. 2017), hepato-protective (El-Shaibany et al. 2016), protective effect on UV-B-induced DNA damage (Kaewklom and Vejaratpimol 2011), anti-stress (Adkar et al. 2014), anti-ulcer (Abirami et al. 2015), neuro-pharmacological activities (Kuber and Santhrani 2010), etc. Comprehensive information regarding pharmaceutical properties present in extracts of different parts of kewda is summarized in Table 15.4.
5 Agro-Technology/Cultivation/Domestication
5.1 Vegetative Propagation
Pandanus odorifer propagate by vegetative methods. The adventitious aerial roots and the branch cuttings are used widely for its propagation. About 60–80 cm long and 8–10 cm thick branch cuttings are implanted during the rainy season. The spacing between each plantlet is maintained at 3–7 m apart. It is highly salt-tolerant and can withstand strong winds (Rashmi and Nadaf 2017). The plant is adapted well to light and heavy well-drained soil. It is well acclimatized to saline, sandy and marshy wastelands. Among the different edaphic factors influencing the oil yield and PEME content of the kewda flower essential oil, nitrogen has been reported to be the most influential factor followed up by organic carbon, pH, phosphorous and potassium (Nasim et al. 2018).
5.2 Climate
The plant grows abundantly in high rainfall areas and is found profusely in a 45 × 15-km stretch in Ganjam district along the coast of the Bay of Bengal. The flowering starts after 3–4 years of the plantation and the rainy season (July to October) sees the maximum flowers. A mature kewda tress produces about 30–40 flowers spikes yearly. Branching, rainfall and nearness to the water body affect the flowering of the plant. The plant has a life span of 50–80 years which might last up to 100–150 years also. However, the fruiting stage is only for 20–25 years (Adkar et al. 2014).
5.3 Crop Nutrition
Nutritionally, the fruit of the plant is rich in provitamin A, vitamin C and total carotenoids. Foods rich in carotenoids are consumed to protect against anaemia, vitamin A deficiency and chronic diseases such as diabetes, cancer and heart diseases (Lim 2012). It is consumed in the form of a paste and contains protein, calcium, iron, thiamine and beta-carotene. The juice is also used in the form of a beverage (Englberger et al. 2009). The fruit seed oil has also been reported to be a promising source for non-edible biodiesel production (Mahlinda et al. 2017).
5.4 Diseases and Pests
The senescence of the plant is mainly due to the infection of insect pests such as bagworm, beetles and thrips causing economic losses. Diseases like foot rot of central shoot, leaf blight and fruit rot have also been reported in this plant (Jagadev et al. 2001). Arbuscular mycorrhizal fungi (AMF) association has also been reported in this plant which helps in strengthening the ecological efficacy in coastal regions (Kamble et al. 2013).
5.5 Harvesting
Kewda male flowers are the prime source of the essential oil and the harvesting time and extraction procedures of the essential oil play a significant role in maintaining its quality and yield. Depending on the flowering season which occurs thrice a year, the inflorescences are harvested. The male flowers are plucked manually early morning between 7–9 a.m. with the help of a long bamboo stick fitted with a hook and immediately subjected to essential oil extraction. Large quantities of flowers are collected and hydro-distilled by the traditional method of oil extraction employing copper vessel, copper lead and bamboo pipes. It has been reported that delay in the transportation of kewda flowers deteriorates the quality and aroma of kewda oil (Nasim et al. 2017a). This leads to decreased price of the oil and in turn causes a huge loss to the farmers. Hence, harvesting time and the extraction of essential oil have to be a rapid process to maintain the oil quality and quantity, thereby minimizing the loss to the kewda growers (Nasim et al. 2017a).
6 Biotechnological Approaches
6.1 Overview of Molecular Markers Technologies Employed in Kewda
Plant genetic resources are valuable and irreplaceable resources for current and future crop improvement strategies. Genetic diversity accounts for heritable genetic variability within and among populations of a species and forms the basis for survival, selection, adaptation and plant improvement (Rao and Hodgkin 2002; Laurentin 2009). Molecular markers have been extensively used to study genetic diversity (Agarwal et al. 2008; Omondi et al. 2016). Different types of molecular markers like RAPD, ISSR, AFLP, SSR and SNP have been developed and are being used for crop improvement (Nadeem et al. 2018). These molecular marker techniques can be employed to study genetic diversity within and among the species (Cervera et al. 2000; Zou et al. 2011). In comparison with other marker types, these DNA-based markers techniques can unmask the genetic diversity of almost all species at various developmental stages, remaining unaffected by environmental conditions (Shah et al. 2018). Molecular markers also provide information about the diversity at the nucleotide level (SNPs), population structure, frequencies of gene and allele (genotype information), distribution and range of genetic diversity. Besides, molecular markers can also be used for resolving taxonomic problems and help in providing exact taxonomic hierarchies important for phylogenetic studies (Sarwat et al. 2012). Characterization based on molecular markers is highly reliable and effective in studying variation in different genotypes (Kaur et al. 2015).
To date, there are only a few reports of the utilization of RAPD, ISSR and SSR for assessing genetic diversity in kewda. RAPD profile of three morphotypes (spinous, ketaki and spineless.) of Pandanus fascicularis was developed by Panda et al. (2007). Cluster analysis by the UPGMA method from their study resulted in a phylogenetic dendrogram which produced two groups, one separating ketaki morphotype while the second group was separating spinous and spineless morphotype suggesting ketaki as a distinct variety in the Pandanus genus. Panda et al. (2010a) utilized 30 RAPD primers to detect sex differences in Pandanus tectorius Parkinson (P. fascicularis, P. odorifer) from seven populations of Odisha. Dendrogram from their study divided the seven populations into two distinct groups of male and female plants. AMOVA show 3% molecular variance among populations and ~95% within populations. Molecular markers were also used for differentiating male and female genotypes in Pandanus fascicularis. Vinod et al. (2007) developed a male-specific SCAR marker for differentiating the sexes in Pandanus. Another report revealed a considerable amount of polymorphism at the interspecific level and a lower degree of polymorphism at the intraspecific level in the genus Pandanus when analysed by RAPD (Sarile and Menguito 2010). In a more recent study, genetic diversity analysis of Pandanus odorifer (Forssk.) Kuntze was carried out by utilizing 13 ISSRs and 30 SSRs (Nasim et al. 2020). A total of 84 accessions from different regions of Ganjam were utilized in the study which revealed SSRs being more effective than ISSR for genetic diversity evaluation in kewda accessions. Further studies in this direction will help in proper identification and characterization of kewda species and the data from genetic diversity analysis can be utilized for kewda improvement to meet its increasing demand in the perfumery industry.
6.2 Genomics Aspect of P. Odorifer
With the rapid advancement of genomics and transcriptomics, the identification of key genes involved in the synthesis of specialized metabolites in medicinal and aromatic plants has become much faster (Yamazaki et al. 2018). The next-generation sequencing strategies have enabled the researchers to sequence huge and complex genomes of medicinal plants effortlessly. Despite its medicinal and economic importance, there are only limited reports on the exploration of genes associated with Pandanus odorifer’s unique fragrance. Vinod et al. (2010) for the first time constructed a male-specific cDNA library in P. odorifer and identified specific transcripts involved in PEME biosynthesis. A total of 977 ESTs were generated from their study. Rashmi et al. (2019) utilized integrative transcriptomics and metabolomics approach for the understanding of salinity tolerance in P. odorifer. Results from their study revealed the up-regulation of Asparagine (Asn) biosynthesis genes. Higher transcript accumulation of genes, viz. glutamine synthetase, glutamine synthase, aspartate kinase, pyruvate kinase, aspartate aminotransferase, phosphoenolpyruvate, carboxylase and asparagine synthetase (AS) was observed under salt stress. Subsequently, the genomic resources generated from these studies will largely benefit our understanding of primary and secondary metabolite pathways and pave the way for metabolite engineering to increase essential oil yield and quality in P. odorifer.
7 Perspectives
In recent years, medicinal and aromatic plants have gained huge attention due to the stimulated demand from the pharmaceutical, cosmetic and aromatic industries in the local, national and international markets. Pandanus odorifer is an economically and industrially important aromatic plant. The exquisite fragrance owned by the kewda male inflorescence has captivated the perfumery industry. Different parts of the plant possess numerous pharmaceutical properties. It is an important natural bio-resource of Ganjam district, Odisha, and provides livelihood to the huge population there. The flower essential oil of kewda is in high demand for kewda perfumes, especially in the national and international markets, especially in the Arab countries.
In spite of so much economic value and numerous pharmaceutical properties in the oil and extracts, the plant still lacks proper exploration in the global market due to restricted plantation and high cost of kewda oil.
Use of elite chemotypes and elite genotypes of kewda is the need of the hour to improve the quality and quantity of kewda, thereby meeting the increased demand of this plant in the perfumery industry. Furthermore, future research is anticipated in developing efficient protocols for in vitro studies for maintaining the bioactive chemical entities naturally present in the essential oil. The plant is vegetatively propagated; hence, the development of different propagation strategies is needed for crop management with improved cultivation practices. Large-scale kewda cultivation can be achieved through the proper application of advanced biotechnological applications to study the genetic variation by various types of molecular markers to improve the quality of kewda cultivars and meet the global market requirement. Also, kewda growers should use high yielding kewda chemotypes as mass planting material and follow the rapid extraction of essential oil immediately after harvesting to improve the quality of different kewda products like kewda attar and kewda water. Different edaphic factors should also be taken care of with balanced nutrient application, weed control, timely planting, micronutrient use and timely harvesting to meet the future market demand.
References
Ab Rahman N, Hassan M, Omar A, Ab Kadir M (1999) Major chemical constituents of supercritical carbon dioxide extract of Pandanus odorus leaves. Nat Prod Sci 5(2):75–79
Abirami J, Sathya P, Joshi Eminal J, Brindha P (2015) Anti ulcer activity of Pandanus fascicularis (lam.) on ethanol induced ulcer in wistar albino rats. World J Pharm Pharm Sci 4(10):1698–1705
Abral H, Andriyanto H, Samera R, Sapuan S, Ishak M (2012) Mechanical properties of screw pine (Pandanus odoratissimus) fibers—unsaturated polyester composites. Polym-Plast Technol Eng 51(5):500–506
Adkar PP, Jadhav PP, Ambavade SD, Shelke TT, Bhaskar VH (2014) Protective effect of leaf extract of Pandanus odoratissimus Linn on experimental model of epilepsy. Int J Nutr, Pharmacol, Neurol Dis 4(2):81
Agarwal M, Shrivastava N, Padh H (2008) Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep 27(4):617–631
Andriani Y, Ramli NM, Syamsumir DF, Kassim MNI, Jaafar J, Aziz NA, Marlina L, Musa NS, Mohamad H (2019) Phytochemical analysis, antioxidant, antibacterial and cytotoxicity properties of keys and cores part of Pandanus tectorius fruits. Arab J Chem 12(8):3555–3564
Armayni UA, Ahmad MS, Yusof NM, Mohsin HF, Wahab IA (2015) The identification of biphenyl from Pandanus Pygmaeus. Procedia Environ Sci 30:68–72
Baba S, Chan HT, Kezuka M, Inoue T, Chan EW (2016) Artocarpus altilis and Pandanus tectorius: Two important fruits of Oceania with medicinal values. Emir J Food Agric 28(8): 531–539
Bhatt PR, Bhatt KP (2015) Neurobehavioral activity of Pandanus tectorius Parkinson (Pandanaceae) leaf extract in various experimental models. J Pharma Sci Tech 5(1):16–18
Buerki S, Callmander MW, Devey DS, Chappell L, Gallaher T, Munzinger J, Haevermans T, Forest F (2012) Straightening out the screwpines: A first step in understanding phylogenetic relationships within Pandanaceae. Taxon. 61(5):1010–20
Butaud J-F, Jacq F, Callmander MW (2019) Pandanus papateaensis (Pandanaceae): une nouvelle espècemenacée de l’atollsoulevé de Makatea (Tuamotu, Polynésiefrançaise). Candollea 74(2):137–144
Callmander MW, Laivao MO, Randrianaivo R (2010) A new species of Pandanaceae from northern Madagascar, Pandanus ankaranensis. Novon: J Bot Nomenclature/Missouri Bot Gard 20(3):243
Cervera MT, Remington D, Frigerio J-M, Storme V, Ivens B, Boerjan W, Plomion C (2000) Improved AFLP analysis of tree species. Can J for Res 30(10):1608–1616
Del Mundo CR, Castillo AL, An SS, Tan MA (2020). In vivo COX-2 modulation and metabolite profiling of Pandanus tectorius leaves extracts. 3 Biotech 10(3):1–10
Dutta P, Saxena H, Brahmam M (1987) Kewda Perfume Industry in India1. Econ Bot 41(3):403–410
El-Shaibany A (2014) Nocturnal enuresis, antioxidant and antimicrobial activities of Pandanus odoratissimus L. peduncle. Int J Pharmaceut Sci Res 5(3):811–818
El-Shaibany A, Molham AH, Al-Massarani S, El-Gamal A, Al-Ajami A, Al-Adhl A (2016) Hepatoprotective effect of Pandanus odoratissimus l inflorescence extracts in acetaminophen-treated guinea pigs. Trop J Pharm Res 15(2):259–267
Englberger L, Schierle J, Hofmann P, Lorens A, Albert K, Levendusky A, Paul Y, Lickaneth E, Elymore A, Maddison M (2009) Carotenoid and vitamin content of Micronesian atoll foods: Pandanus (Pandanus tectorius) and garlic pear (Crataevaspeciosa) fruit. J Food Compos Anal 22(1):1–8
Gallaher T, Callmander MW, Buerki S, Keeley SC (2015) A long distance dispersal hypothesis for the Pandanaceae and the origins of the Pandanus tectorius complex. Molecular phylogenetics and evolution. 83:20–32
Glas JJ, Schimmel BC, Alba JM, Escobar-Bravo R, Schuurink RC, Kant MR (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int J Mol Sci 13(12):17077–17103
Gowtham Raj G, Varghese HS, Kotagiri S, Swamy BM V (2014) Evaluation of anti-cancer potential of aqueous extract of Pandanus odoratissimus (Y. Kimura) Hatus. forma ferreus, by in vivo ascitic tumor model in swiss albino mice. Pharmacognosy J 6(1)
Hanif MA, Nisar S, Khan GS, Mushtaq Z, Zubair M (2019) Essential oils. In: Essential oil research. Springer, Cham, pp 3–17
Jagadev PN, Beura S, Maharana T (2001) Insect pests and diseases of Kewda (Pandanus fascicularis Lam.). Indian Perfumer 45(4):265–268
Jiang J (1999) Volatile composition of pandan leaves (Pandanus amaryllifolius). In: Flavor chemistry of ethnic foods. Springer, pp 105–109
Jitu KRM, Debnath D, Asad S, Das RC, Sultana A (2017) Phytochemical screening and evaluation of cytotoxic activity of Pandanus fascicularis L. (Fruits). Discov Phytomedicine 4(3):31
Jose B, Harikumar K, Krishnan PN, Satheeshkumar K (2016) In vitro mass multiplication of screw pines (Pandanus spp.)-an important costal bio-resource. J Coast Conserv 20(6):443–53
Kaewklom S, Vejaratpimol R (2011) In vitro protective effect of Pandanus ordoratissimus extract on ultraviolet B (UVB)-induced DNA damage. Afr J Biotechnol 10(45):9165–9169
Kamble VR, Kanoujiya LR, Rahate HL, Agre DG (2013) AM Fungal Status in Ketaka: Pandanus fascicularis From Coastal Region of Konkan, Maharahshtra. IOSR J Pharm Biol Sci 5(6):01–06
Kaur S, Panesar PS, Bera MB, Kaur V (2015) Simple sequence repeat markers in genetic divergence and marker-assisted selection of rice cultivars: a review. Crit Rev Food Sci Nutr 55(1):41–49
Kirtikar KR, Basu BD, Blatter E (1991) Indian Medicinal Plants, 2nd ed., vol 4. Indian Book Center, New Delhi, pp 2591–2593
Kuber BR, Santhrani T (2010) Assessment of Neuropharmacological Activities Of Pandanus Odoratissimus Root In Mice. J Pharm Chem 4: 114–117
Kumar D, Kumar S, Kumar S, Singh J, Sharma C, Aneja K (2010) Antimicrobial and preliminary phytochemical screening of crude leaf extract of Pandanus odoratissimus L. Pharmacol Online 2:600–610
Kumar S, Dagar S, Kumar P, Singh J, Kumar S, Kumar D (2017) Antifertility effect of hydroalcoholic extract of Pandanus odoratissimus L. leaves. Porto Biomed J 2(5):167–169
Kumari S, Wanjari M, Kumar P, Palani S (2012) Antidiabetic activity of Pandanus fascicularis Lamk-aerial roots in alloxan-induced hyperglycemic rats. Int J Nutr, Pharmacol, Neurol Dis 2(2):105
Laurentin H (2009) Data analysis for molecular characterization of plant genetic resources. Genet Resour Crop Evol 56(2):277–292
Lim TK (2012) Edible medicinal and non-medicinal plants, vol 1. Springer
Liu J, Peng L, Xian M, Cheng J-L, Chen W-W (2012) Supercritical carbon dioxide extraction of volatile oil from Pandanus tectorius Soland and analysis by GC-MS’J. Mod Chin Med 4:4–6
Londonkar R, Kamble A (2011) Hepatotoxic and invivo antioxidant potential of Pandanus odoratissimus against carbon tetrachloride induced liver injury in rats. Orient Pharm Exp Med 11(4):229–234
MacLeod AJ, Pieris NM (1982) Analysis of the volatile essential oils of Murrayakoenigii and Pandanus latifolius. Phytochemistry 21(7):1653–1657
Madhavan V, Nagar JC, Murali A, Mythreyi R, Yoganarasimhan S (2008) Antihyperglycemic activity of alcohol and aqueous extracts of Pandanus fascicularis lam. roots in alloxan induced diabetic rats. Pharmacologyonline 3:529–536
Mahlinda S, Husin H, Riza M, Muslim A (2017) A comparative study of biodiesel production from screw pine fruit seed: using ultrasound and microwave assistance in in-situ transesterification. J Eng Sci Technol 12(12):3412–3425
Manilal A, Sabu KR, Shewangizaw M, Aklilu A, Seid M, Merdekios B, Tsegaye B (2020) In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon 6(1):e03303
Mar A, Mar AA, Thin PP, Zin MM (2019). Study on the phytochemical constituents in essential oil of pandanus amaryllifolious Roxb. Leaves and their Anti-bacterial Efficacy. Yadanabon Univ Res J 10:1
Mishra G, Khosa R, Singh P, Jha K (2015) Hepatoprotective potential of ethanolic extract of Pandanus odoratissimus root against paracetamol-induced hepatotoxicity in rats. J Pharm Bioallied Sci 7(1):45
Misra R, Dash P, Rao Y (2000) Chemical composition of the essential oils of Kewda and Ketaki. J Essent Oil Res 12(2):175–178
Mohanty SK, Swamy MK, Sinniah UR, Anuradha M (2017) Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): botanical, agronomical, phytochemical, pharmacological, and biotechnological aspects. Molecules 22(6):1019
Murtini E, Yuwono S, Setyawan H, Nadzifah N Pandan(2020) Leaf powder: characteristics and its application in Pandan sponge cake making. IOP Conf Ser: Earth Environ Sci 475:012041. https://doi.org/10.1088/1755-1315/475/1/012041
Nadaf A, Zanan R (2012) Economical importance of Indian Pandanus species. In: Indian Pandanaceae-an overview. Springer, pp 127–137
Nadaf A, Zanan R, Wakte K (2011) A new endemic species of Pandanaceae from India: Pandanus palakkadensis. Kew Bull 66(1):183–186
Nadeem MA, Nawaz MA, Shahid MQ, Doğan Y, Comertpay G, Yıldız M, Hatipoğlu R, Ahmad F, Alsaleh A, Labhane N (2018) DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotechnol Biotechnol Equip 32(2):261–285
Naqvi AA, Mandal S (1996) Investigation of the essential oil of Pandanus fascicularis Lam. by GC/MS. J Essent Oil Res 8(5):571–572
Nasim N, Behera JK, Sandeep IS, Kar B, Ramarao V, Srivastava R, Nayak S, Mohanty S (2017a) Effect of harvesting time and storage on essential oil and PEME content of Pandanus fascicularis. J Appl Pharm Sci 7(10):185–189
Nasim N, Behera JK, Sandeep IS, RamaRao V, Kar B, Mishra A, Nayak S, Mohanty S (2018) Phytochemical analysis of flower from Pandanus odorifer (Forssk.) Kuntze for industrial application. Nat Prod Res 32(20):2494–2497
Nasim N, Ray A, Singh S, Jena S, Sahoo A, Kar B, Sandeep IS, Mohanty S, Nayak S (2017b) Characterization of Kewda volatile components by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. Nat Prod Res 31(7):853–856
Nasim N, Sandeep IS, Sahoo A, Das S, Panda MK, Acharya L, RamaRao V, Nayak S, Mohanty S (2020) Population genetic structure and diversity analysis in economically important Pandanus odorifer (Forssk.) Kuntze accessions employing ISSR and SSR markers. Ind Crops Prod 143:111894
Omondi EO, Debener T, Linde M, Abukutsa-Onyango M, Dinssa FF, Winkelmann T (2016) Molecular markers for genetic diversity studies in African leafy vegetables. Advan Biosci Biotechnol 7(3):188–197
Padhy S, Dash SK, Panda BB, Misra MK, Padhi NP, Sahu D, Das M, Nayak SP, Dash B, Rao VR (2016) Pandanus fasicularis Lamk. (Kewda): the prime vegetation in the hinterland biodiversity of coastal Odisha, with unique ethnic utility, genetic variation and economics-a review. J Biodivers 7(1):33–49
Panda KK, Das AB, Panda BB (2009) Use and variation of Pandanus tectorius Parkinson (P. fascicularis Lam.) along the coastline of Orissa, India. Genet Resour Crop Evol 56(5):629–637
Panda KK, Panigrahy RK, Das AB (2007) Analyses of chromosome number, nuclear DNA content and RAPD profile in three morphotypes. Plant Genet Resour Newsl 149–152:15212
Panda KK, Sahoo B, Das AB, Panda BB (2010a) Use of RAPD markers to detect sex differences in Pandanus tectorius Parkinson, an important bioresource plant in Orissa, India. Int J Biodivers Sci, Ecosyst Serv Manage 6(1–2):28–34
Panda P, Nayak S, Panda DP (2010b) Formulation and evaluation of cream prepared form Pandanus fascicularis Lamk. and their wound healing activity. Drug Invention Today 2(9)
Panda P, Panda D, Panda P, Nayak S (2008) Antinociceptive and anti-inflammatory activities of Pandanus fascicularis Lamk. leaves in animal models. Orient Pharm Exp Med 7(5):485–493
Panda K, Das AB, Panda BB (2012). Genomics of Pandanus: A useful plant resource of coastal Odisha, India. Lambert Academic Publishing
Penu F, Ivy S, Ahmed F, Uddin J, Hossain M, Labu Z (2020) In vitro assessment of antioxidant, thrombolytic, antimicrobial activities of medicinal plant Pandanus odoratissimus L. leaves extract. J Sci Res 12(3):379–390
Qu J, Hao Y, Zhu Y, Wu C, Wang W, Leng P (2011) Studies on germplasm of Juglans by EST-SSR markers. Acta Hortic Sinica 38(3):441–448
Rahayu SE, Handayani S, Noverita ISJ (2013) Antifungal and preliminary phytochemical screening of leaf extract of Pandanus odoratissimus L.f. Proceeding international conference pp 100–105
Rahman MK, Islam MF, Barua S, Rahman MM, Sayeed MA (2014) Comparative study of antidiarrheal activity of methanol extracts from leaf and fruit of Pandanus odoratissimus Linn. Orient Pharm Exp Med 14(4):363–367
Raina V, Kumar A, Srivastava S, Syamsundar K, Kahol A (2004) Essential oil composition of ‘kewda’ (Pandanus odoratissimus) from India. Flavour Fragr J 19(5):434–436
Rajeswari J, Kesavan K, Jayakar B (2011) Phytochemical and pharmacological evaluation of prop roots of Pandanus fascicularis Lam. Asian Pac J Trop Med 4(8):649–653
Rajeswari J, Kesavan K, Jayakar B (2012) Antidiabetic activity and chemical characterization of aqueous/ethanol prop roots extracts of Pandanus fascicularis Lam. in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 2(1):S170–S174
Raju S, Subbaiah NV, Reddy KS, Das A, Murugan KB (2011) Potential of Pandanus odoratissimus as a CNS depressant in Swiss albino mice. Braz J Pharm Sci 47(3):629–634
Rao VR, Hodgkin T (2002) Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell, Tissue Organ Cult 68(1):1–19
Rashmi D, Barvkar VT, Nadaf A, Mundhe S, Kadoo NY (2019) Integrative omics analysis in Pandanus odorifer (Forssk.) Kuntze reveals the role of Asparagine synthetase in salinity tolerance. Sci Rep 9(1):1–16
Rashmi D, Nadaf A (2017) Understanding the mechanism of salt tolerance in pandanus odorifer L. P Natl A Sci India B. 1–7
Rohman A, Sugeng R, Man YC (2012) Characterizaton of red fruit (Pandanus conoideus Lam) oil. Int Food Res J 19(2):563
Rout P, Misra R, Sahoo S, Sree A, Rao Y (2005) Extraction of kewda (Pandanus fascicularis Lam.) flowers with hexane: composition of concrete, absolute and wax. Flavour Fragrance J 20 (4):442–444
Rout PK, Naik S, Rao YR (2011) Liquid co2 extraction of flowers of Pandanus fascicularis Lam. and fractionation of floral concrete and comparative composition of the extracts. J Food Biochem 35(2):500–512
Rout PK, Rao YR, Prakash O, Khare P (2015) Adsorptive recovery of high value essential oil from kewda (Pandanus fascicularis Lam.) distillation condensate. Asia-Pac J Chem Eng 10(5):659–669
Sarile AS, Menguito CA (2010) Molecular differentiation of selected Pandanus spp. by Random amplified polymorphic DNA (RAPD) analysis. Acta Manilana 55:59–68
Sarwat M, Nabi G, Das S, Srivastava PS (2012) Molecular markers in medicinal plant biotechnology: past and present. Crit Rev Biotechnol 32(1):74–92
Shah UN, Mir J, Ahmed N, Fazili KM (2018) Assessment of germplasm diversity and genetic relationships among walnut (Juglans regia L.) genotypes through microsatellite markers. J Saudi Soc Agric Sci 17(4):339–350
Sobhana K, Nirosha K, Ravikumar A, Sravanthi R, Chandrkala P (2014) Evaluation of cardioprotective activity of Pandanus odoratissimus leaves against isoproterenol induced myocardial infarction in albino rats. Int J Novel Trends Pharm Sci 4(5):151–158
Solomon Raju A, Lakshminarayana G (2020) In: Prasada Rao C., Dileepu Kumar B, Santhi Kumari M, Prasad KBJ, Divyasree M, Suneetha Rani T. Pollination and fruit dispersal in the Fragrant Screw Pine, Pandanus odorifer (Forssk.) Kuntze (Pandanaceae), vol 21. Species
Son NT (2019) Secondary metabolites of genus Pandanus: an aspect of phytochemistry. Mini-Rev Org Chem 16(7):689–710
St John H (1960) Revision of the genus Pandanus stickman: part 1. Key to the sections
Stringaro A, Colone M, Angiolella L (2018) Antioxidant, antifungal, antibiofilm, and cytotoxic activities of Mentha spp. essential oils. Medicines 5(4):112
Susanti R, Suzuki E, Miyamoto J, Abe M, Uchiumi T (2012) Differences in the growth of beach Pandan, Pandanus odoratissimus, between tropical (Java) and subtropical (southern Japan) zones. Tropics 21(3):81–90
Swamy MK, Sinniah UR (2016) Patchouli (PogostemoncablinBenth.): botany, agrotechnology and biotechnological aspects. Ind Crops Prod 87:161–176
Takeda J, De Silva S, Muthuraman P, Rahman SM, Kawet L (2008) Spices in Sri Lanka, India and Bangladesh with special reference to the usages and consumptions. Bull Facul Agric (Saga Univ.) 93:1–25
Tanaka N, Jinadasa KB, Mowjood MI, Fasly MS (2011) Coastal vegetation planting projects for tsunami disaster mitigation: effectiveness evaluation of new establishments. Landscape Ecol Eng 7(1):127–135
Teli M, Jadhav A (2015) Mechanical extraction and physical characterization of Pandanus Odorifer Lignocellulosic fibre. Int J Sci Res (IJSR) ISSN 6
Teli M, Jadhav A (2017) Effect of mercerization on the properties of Pandanus odorifer lignocellulosic fibre. IOSR J Polym Text Eng (IOSR-JPTE) 4:7–15
Thuy NB, Nandasena NA, Dang VH, Tanaka N (2018) Simplified formulae for designing coastal forest against tsunami run-up: one-dimensional approach. Nat hazards 92(1):327–46
Tibpromma S, Daranagama DA, Boonmee S, Promputtha I, Nontachaiyapoom S, Hyde KD (2017) Anthostomelloideskrabiensis gen. et sp. nov.(Xylariaceae) from Pandanus odorifer (Pandanaceae). Turkish J Bot 41(1):107–116
Udupa AL, Ojeh N, Gupta S, Rathnakar UP, Rajput R, Shubha HV, Benegal D, Benegal A, Rao S, Rao S (2011) Analgesic activity of Pandanus fascicularis Lam. J Pharm Res 4(4):1234–1236
Vahirua-Lechat I, Menut C, Roig B, Bessiere J, Lamaty G (1996) Isoprene related esters, significant components of Pandanus tectorius. Phytochemistry 43(6):1277–1279
Vinod M, Raghavan PS, George S, Parida A (2007) Identification of a sex-specific SCAR marker in dioecious Pandanus fascicularis L. (Pandanaceae). Genome 50(9):834–839
Vinod M, Sankararamasubramanian H, Priyanka R, Ganesan G, Parida A (2010) Gene expression analysis of volatile-rich male flowers of dioecious Pandanus fascicularis using expressed sequence tags. J Plant Physiol 167(11):914–919
Wakte KV, Nadaf AB, Thengane RJ, Jawali N (2009) Pandanus amaryllifolius Roxb. cultivated as a spice in coastal regions of India. Genet Resour Crop Evol 56(5):735–740
Wakte KV, Zanan RL, Saini A, Jawali N, Thengane RJ, Nadaf AB (2012) Genetic diversity assessment in Pandanus amaryllifolius Roxb. populations of India. Genet Resour Crop Evol 59(7):1583–1595
Yamazaki M, Rai A, Yoshimoto N, Saito K (2018) Perspective: functional genomics towards new biotechnology in medicinal plants. Plant Biotechnol Rep 12(2):69–75
Zanan RL, Nadaf AB (2011) Collection, characterization and olfactory evaluation of Pandanus species in Southern India. Plant Genet Resour 1–3
Zanan RL, Nadaf AB (2012a) Pandanus mangalorensis: a new species of Pandanaceae from Southern India. Kew Bull 67(3):555–559
Zanan RL, Nadaf AB (2012b) Pandanus martinianus (Pandanaceae), a new endemic species from northeastern India. Phytotaxa 73(1):1–7
Zanan RL, Nadaf AB (2013) Conservation status of Indian pandanaceae. Am J Plant Sci 4(6A):51–56. https://doi.org/10.4236/ajps.2013.46A008
Zou Z, Gui Z, Yu L, Chen F, Xia B (2011) Genetic diversity within and among populations of Acarus siro L. revealed by inter simple sequence repeats (ISSR). Procedia Environ Sci 8:707–714
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nasim, N., Sandeep, I.S., Nayak, S., Mohanty, S. (2021). Cultivation and Utilization of Pandanus odorifer for Industrial Application. In: Ekiert, H.M., Ramawat, K.G., Arora, J. (eds) Medicinal Plants. Sustainable Development and Biodiversity, vol 28. Springer, Cham. https://doi.org/10.1007/978-3-030-74779-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-74779-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74778-7
Online ISBN: 978-3-030-74779-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)