Abstract
Garlic, Allium sativum L., is a plant within the family Alliaceae that has been widely used for its culinary and medicinal properties. This plant contains organosulfur compounds with allyl groups such as allyl mercaptan (AM), S-allyl cysteine (SAC), diallyl trisulfide (DATS) and has been responsible for different health benefits such as antihypertensive, anticoagulant, anti-inflammatory, antimicrobial and anticancer. Especially some lipid-soluble allyl sulfur compounds can inactivate carcinogens and reduce cancer risk and regulate the cellular processes. Epidemiological studies have shown that garlic and its components can decrease the incidence of human stomach, colon, prostate, brain, skin, breast, lung, uterine, and esophagus cancers. These anticarcinogenic effects appear to be achieved by modifying common signaling pathways. But allyl sulfur compounds have different effect in supressing tumor proliferation. Therefore, the compounds that are responsible for the cellular and molecular effects, the stages which they suppress neoplasia and interactions with other drugs should be very well known. Tumor supression ability of allyl sulfur compounds of garlic is attributed the stimulation of detoxification enzymes, protection from oxidative stress, induction of cell apoptosis and cell cycle arrest, prevention of chromosomal damage, induction of immune system and supression of nitrosamine bioactivation. On the other hand, not only the genetic mechanisms, but also the epigenetic mechanisms can be associated with the cancer prevention. Garlic and its several allyl sulfur compounds can be modified by both DNA methylation and histon acetylation. In this chapter, preclinical and clinical studies on the effects of garlic consumption in reducing cancer prevalence will be presented in detail. Furthermore, studies involving the use of allyl sulfur compounds individually or in combination will be discussed and their mechanisms of action will be interpreted at cellular and molecular level.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Garlic (Allium sativum L.) is among the most widely used plants in the Alliaceae family, which has more than 850 different species (Sharifi-Rad et al. 2016). Bulbs of garlic are used as food and spice. Apart from its use as food, it has been used for centuries to treat various diseases such as gastrointestinal (Nicastro et al. 2015) and cardiovascular (Bradley et al. 2016) system disorders, diabetes (Bayan et al. 2014), Alzheimer’s disease (Borek 2006) and for wound healing (Srimuzipo et al. 2009). In addition, previous bioactivity studies have reported that garlic displays hepatoprotective (Ajayi et al. 2009), antihypertensive (Ried et al. 2008), antihelmentic (Worku et al. 2009), antimicrobial (Yin et al. 2003), antifungal (Kutawa et al. 2018), immune modulation (Kyo et al. 2001) and anticancer (Ejaz et al. 2003) effects. Epidemiological studies have shown that garlic consumption reduce the risk of disease development. This also supports the ethnobotanical use of the plant.
Phytochemical studies on garlic have shown that various types of chemical compounds are present especially in the bulbs of this plant, including a high water content (approximately 65%). Carbohydrates (28%) (mainly fructans), proteins (2%) (mainly alliin), amino acids (1.2%) (mainly arginine), fibers (1.5%), sulfur compounds (2.3%), trace elements and phenols (Butt et al. 2009) were reported (Fig. 11.1). According to USDA database, 63.535 search results are available on garlic containing food. The main ingredients mentioned above are in different amount for each type of food (USDA, https://fdc.nal.usda.gov). Several biological effects of garlic from wound-healing properties to anticancer effect are mainly attributed to allyl sulfur compounds and flavonoids (Putnik et al. 2018).
In vivo and in vitro preclinical studies on tumorigenesis have showed that garlic and its components are effective against human colorectal (Zhang et al. 2018), skin (Wang et al. 2010), prostate (Chu et al. 2006a), brain (Das et al. 2007), gastric (Ling et al. 2006), nasopharyngeal (Zhang et al. 2006), stomach (Fleischauer et al. 2000), lung (Li et al. 2012), breast (Kaschula et al. 2016), liver (Chu et al. 2013) and thyroid (Shin et al. 2010) cancers. Various mechanisms such as stimulation of detoxification enzymes, cells protection from oxidative stress, induction of cell apoptosis and cell cycle arrest, enhancement of immune system and epigenetic mechanisms are attributed to the anticancer activities of allyl sulfur compounds (Lea et al. 1999; Bruck et al. 2005; Melino et al. 2011; Upadhyay 2017). Understanding the mechanisms through which these compounds exert their biological activities is particularly important for the development of anticancer agents. It should be well known which compound or compounds are responsible for the cellular and molecular effects.
2 Organosulphur Compounds (OSCs)
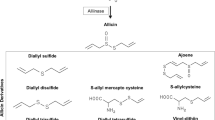
The characteristic aroma of garlic is due to sulfur-containing volatile compounds that compose 1% of its dry weight (Fenwick and Hanley 1985). These volatile compounds are produced from their non-volatile precursors namely γ-glutamyl-S-alk(en)yl-L-cysteines and S-alk(en)yl-L-cysteine sulfoxides (Butt et al. 2009). OSCs are generally divided into two groups as oil-soluble and water-soluble OSCs. Unharmed cells of garlic bulbs contain alliin (S-allylcysteine sulfoxide). When the garlic is crushed, chopped or chewed, thiosulfinates whose general formula is R1-S(O)-S-R2 (where R1 and R2 are methyl, allyl, 1-propenyl) are formed (Zalepugin et al. 2015). The half-life of thiosulfinates is about 5 min in the blood (Okada et al. 2005). It triggers biochemical transformations by reacting with thiols in the cells which are in the blood or plasma. It is thought that these transformations and additions of thiol functional groups to the proteins may be related to anticancer activity (Bhuiyan et al. 2015). With crushing, chopping or chewing of the fresh garlic an enzyme known as alliinase is released and converts alliin to allicin (diallyl thiosulfinate) which is a well-known thiosulfinate. Allicin is the main chemical compound of these family, however it is stability depends on its concentration, the temperature, and the solvent in the surrounding environment (Okada et al. 2005). It is rapidly metabolized in aqueous solutions into mono-, di- and trisulfides or other organosulfide compounds such as ajoene and vinilditins (Lanzotti 2006).
2.1 Water-Soluble Organosulphur Compounds: S-Allyl Cysteine (SAC), S-Allylmercaptocysteine (SAMC)
γ-Glutamyl-S-alk(en)yl-cysteine is converted to SAC through γ-glutamyl transferase during the long-term incubation of crushed garlic in aqueous solutions named as aged garlic extract (AGE) (Block 1985; Jiang et al. 2019) (Fig. 11.2). One of the major bioactive components in AGE is SAC. SAC is a stable compound that prevents cardiovascular (Chuah et al. 2007), neurodegenerative diseases (Ray et al. 2011), diabetes mellitus (Sathibabu Uddandrao et al. 2017) and cancer (Ho et al. 2018). In previous studies, it was reported that SAC acts as an antiproliferative agent against some cancer types in both in vitro and in vivo models (Tang et al. 2010). It has been revealed that another water-soluble organosulfur compound, SAMC, stimulates apoptosis in breast and gastric cancer cells (Sigounas et al. 1997; Yan et al. 2013), inhibits ROS formation and DNA damage in lung cancer cells (Wang et al. 2016), changes the expression of prostate biomarkers in prostate cancer cells (Pinto et al. 2000), activates JNK1 pathway and microtubule depolymerization in colon cancer cells (Xiao et al. 2003). Moreover, both SAC and SAMC were demonstrated to inhibit vascular endothelial cell growth and suppress the effect of colony-forming, development, and invasion rate of cancer cells (Chu et al. 2006b). In addition, SAC has a 30-fold lower toxicity than allicin and DADS (Amagase et al. 2001).
2.2 Oil-Soluble Organosulphur Compounds
Steam distillation of garlic produces an oil with different allyl sulfur components such as dialyl tetrasulfide (DATTS), dialyl trisulfide (DATS), dialyl disulfide (DADS), dialyl sulfur (DAS), allyl methyl trisulfide (AMT), allyl methyl disulfide (AMD), allyl methyl sulfide (AMS) and allyl mercaptan (AM). The oil obtained by maceration contains vinilditins such as 2-vinyl-4-H-1,2-dithiin and 2-vinyl-4-H-1,3-dithiin and ajoens such as E-Ajoene and Z-Ajoene (Yoo et al. 2014) (Fig. 11.2).
The allyl sulfur compounds, which are more commonly studied in anticancer studies, are DAS, DADS, DATS and DATTS. Therefore, in this chapter we will focus specifically on anticancer activities of these compounds.
3 Cancer Chemopreventive Effects of Organosulfur Compounds of Garlic
3.1 In Vitro Studies
Carcinogenesis , also called oncogenesis or tumorigenesis, consists of three different stages: initiation, promotion, and progression involving invasion and metastasis. In this process, cancer hallmarks including cell proliferation, inhibition of apoptosis, invasion and metastasis, angiogenesis, immortalization, inflammation, immunity, genome instability and mutation, cell energetics and metabolism are involved (Hanahan and Weinberg 2011). Therefore, agents with therapeutic effect focus on targeting these mechanisms.
The first study to suggest that garlic can prevent the growth of malignant cells belongs to Weisberger and Pensky (1958). Table 1 presents the results of biological activity of various allyl sulfur compounds on human cell lines. Allicin, one of the most studied compounds in cancer research, induce apoptosis and interfere with cell growth signaling pathways (Lawson et al. 1992; Rose et al. 2019). However, some studies have showed that the treatment of alliin alone does not exert an antiproliferative effect on the growth of tumor cells. Therefore, it has been considered that the alliin should be broken down for maximum tumor inhibition (Scharfenberg et al. 1990).
It has been reported that SAC and SAMC regulate the expression of E-cadherin and decrease the expression of Snail, E-cadherin suppressor, in prostate, ovarian, nasopharyngeal and esophageal cancer cells (Chu et al. 2006b). E-cadherin is a transmembrane protein that plays a role in cell adhesion and is an important factor for epithelial-mesenchymal transition (EMT). Decreased level of its expression is associated with an invasive phenotype. SAC and SAMC are shown as potential agents in suppressing invasive growth (Chu et al. 2006b). Ng et al. (2012) reported that SAC significantly suppresses the expression of proliferation markers Ki-67 and proliferation cell nuclear antigen (PCNA) and apoptosis-related B-cell lymphoma-extra large (Bcl-xL) and B-cell lymphoma 2 (Bcl-2), as well as stimulates the cell cycle arrest at S phase by decreasing cell division cycle-25 (Cdc25), cell division cycle-2 (Cdc2) and cyclin B1 expressions in the hepatocellular carcinoma cell line. They confirmed that SAC increases the level of E-cadherin and decreases the level of VEGF, similar to the studies of Chu et al. (2006b). In addition, SAC mediates the suppression of motility and invasion by stimulating E-cadherin and downregulation of MMP-2 in the breast cancer cell line, MDAMB231 (Gapter et al. 2008). According to a detailed study, DATS inhibits metastasis by inhibition of focal adhesion kinase (FAK), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 in colon cancer cell line (Lai et al. 2015).
It was previously mentioned that all allyl sulfur compounds obtained from garlic are not equally effective in reducing tumor proliferation (Dion et al. 1997; Sakamoto et al. 1997). Similarly, fat-soluble DATS has been shown to be more effective in suppressing cell growth than DAS and DADS in HCT-5 and DLD-1 (colorectal cancer cell lines) and A549 (lung cancer cell lines) (Seki et al. 2008; Wang et al. 2014). Furthermore, DATS treatment has been reported to reduce the activity of Wnt/β-catenin by stimulating apoptosis in colorectal cancer stem cells (Zhang et al. 2018). A study on proliferation and cell cycle progression by Shirin et al. (2001) showed that SAMC, but not SAC, stops the cell cycle in the G2/M phase and activates caspase-3, triggering apoptosis in colon cancer cell lines. In addition, coadministration SAC with sulindac sulfide (SS), a chemotherapy agent in colon cancer, apoptotic and growth-inhibiting effect increased. DADS has been reported to inhibit metastasis through the SRC/RAS/ERK signaling pathway by increasing the expression of miR-34a in the breast cancer cell line, MDM-MB-23 (Xiao et al. 2014). DATS inhibits gastric cancer cell growth by regulating the expression of MMP-9 and E-cadherin proteins in BALB/c(nu/nu) mice (Jiang et al. 2017). A recent study has shown that SAC reduces viability of MCF-7 cells by decreasing the 3-mercaptopyruvate sulfur transferase (MPST) expression, H2S/sulfane sulfur endogenous formation from L-cysteine, and sulfate sulfur level (Bronowicka-Adamska et al. 2020).
Garlic allylsulfide compounds also play a role in the cytotoxicity of cancer cells through ER stress. Ajoene, increases the level of GRP78 (Glucose-Regulated Protein 78 kDa) protein by activating the unfolded protein response (UPR) in the MDA-MB-231 breast cancer cell line and WHCO1 esophageal-cancer cells (Kaschula et al. 2016). In this way, it triggers ER stress by causing unfolded protein aggregates. A study on the colon cancer cell line showed that DATTS activates eIF2α and Nrf2/HO-1, one of the signal molecules associated with ER stress (Saidu et al. 2013). In the human malignant neuroblastoma cell line, SH-SY5Y, DAS and DADS have been demonstrated to stimulate Ca(2+)-dependent protease calpain (Karmakar et al. 2007). Wang et al. (2012b) showed that DATS increased intracellular Ca2+ mobilization and expression of ER stress sensors GRP78/Bip and CHOP (CCAAT-enhancer-binding protein homologous protein)/GADD153 (G1 arrest and DNA damage 153). These studies have revealed that allyl sulfur compounds have an anticancer effect through ER stress.
In addition, oil-soluble allyl sulfur compounds in garlic are possibly more toxic than water-soluble compounds. Indeed, studies have shown that oil-soluble allyl sulfur compounds including DADS reduce the growth of neoplasms, while a water-soluble compound SAC has no effect on established tumors (Sundaram and Milner 1993; Hong 2004). Detailed information about the possible activities of allyl sulfur compounds in various human cancer cell lines is presented in Table 11.1.
3.2 In Vivo Studies
Effective evidence has been obtained in animal models that allyl sulfur compounds can inhibit the tumor formation as well as cancer cell growth. The intraperitoneal (i.p.) application of raw garlic extract (RGE) completely improved the mice implanted with the murine sarcoma cancer cell S180 (100 mg of the RGE for 21 days), but the same findings could not be obtained in oral application (Li et al. 2018a). Although a meta-analysis of 18 studies found a negative association between garlic consumption and reduced risk of gastric cancer (OR = 0.51, 95% CI = 0.44–0.57), prospective study results were not significant (OR = 0.95, 95% CI = 0.66–1.24) (Li et al. 2018b). These results have shown that garlic extract should not pass through the gastrointestinal tract. Garlic treatment in mice with bladder cancer has been shown to inhibit tumor growth and reduce mortality (Rigs et al. 1997). It has been reported that allicin improved liver damage and increased chemotherapy response in tamoxifen-induced mice by i.p. injection at a dose of 45 mg/kg for 7 days (Suddek 2014). On the other hand, SAMC treatment inhibited hepatocarcinogenesis by targeting the LRP6/Wnt pathway in hepatocellular carcinoma (HCC) nude mice model by daily oral gastric lavage feeding at a dose of 300 mg/kg SAMC (Xiao et al. 2018).
Administration of DAS by orally at a dose of 200 mg/kg for 7 days with Se-methylselenocysteine or quercetin to animals with 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast tumor has been shown to have a greater antitumor effect than alone treatment (Ip and Ganther 1991). DAS also inhibits tumor formation in mice and rats with benzo[a]pyrene (BP) and 1,2-dimethylhydrazine (DMH)-induced colon tumors as well as inhibits lung tumor formation in mice by reducing the metabolic activation of nitrosamine. According to these results it can be said that DAS has shown antitumor effect by affecting defective signaling pathways of various types of cancer. Similarly, DADS has been reported to decrease the NF-κB phosphorylation in azoxymethane and dextran sulphate sodium (DSS)-induced mice (60 mg/kg for 5 weeks) and prevent colitis-induced colon cancer by inhibiting GSK-3β (Saud et al. 2016). However, when DADS was given orally to H-ras oncogene transformed tumors in mice at a dose of 33μmol for three times per week, H-RAS mutant cancer cells growth decreased (Singh et al. 1996). In addition, when U2OS cells were subcutaneously injected to BALB/c nude mice and then treated 100 mg/kg DADS with miR-134 inhibitor for 35 days, DADS was shown to suppress forkhead box protein M1 (FOXM1)-mediated proliferation and invasion by upregulating miR-134 in osteosarcoma (Li et al. 2018c).
According to the researchers, allyl sulfur compounds reduce or suppress the growth, proliferation, invasion and metastasis of cancer when administered alone. But little is known about its clinical implications, and it should be supported by epidemiological studies. First of all, the molecular mechanism studies, including the application of both alone and combination with chemotherapy drugs, should be investigated in detail in the prevention of metastasis, which is known as the primary cause of cancer deaths.
Lai et al. (2015) treated BALB/c (nu/nu) mice with 50 mg/kg DATS for 32 days after subcutaneously injected HT-29 cells. As a result of this study, DATS was found to reduced tumor growth, tumor weight, and angiogenesis. Ajoene significantly reduced the incidence of tumors in 12-O-tetradecanoylphorbol-13-acetate (TPA)-promoted mouse skin, treated with 250μg ajoene for 18 weeks (Nishikawa et al. 2002). In a similar study ajoene has been shown to reduce LPS-induced TNF-α and IL6 stimulation and inhibit lung metastasis with B16/BL6 melanoma tumor model in C57BL/6 mice by i.p. injection at a dose of 25μg/g for 28 days (Taylor et al. 2006). Another study indicated that it exerts antimetastatic effect by the suppression of viable circulating tumor cells (Howard et al. 2007). In addition, DATS (6μmol orally, thrice weekly) significantly reduced tumor growth without any side effects in PC-3 xenografts in athymic mice, as well as these results correlated with the increased expressions of Bax and Bak (Xiao et al. 2006a).
In DMBA-stimulated mouse skin tumor DAS (applied topically 10 mg/kg body-weight for 24 h) suppressed the growth of tumor cells by decreasing the expression of p21/Ras oncoprotein and H-RAS mRNA level (Arora et al. 2005). Similarly, it was reported that tumor size and number decreased and p53wt and p21/Waf1 were upregulated in mice where liposomized DAS formulation (250μg, three times a week for 12 weeks) was applied against DMBA-induced skin papilloma (Khan et al. 2007). In addition, DAS (applied topically 10 mg/kg body-weight for 48 h) provided effective protection against DNA strain fractures in the DMBA-stimulated skin tumor model (Nigam and Shukla 2007). In an in vivo study in androgen-independent prostate cancer xenografts, oral SMAC administration (300 mg/kg for 28 days) reduced the growth of primary tumors and the number of metastases to the lung and adrenal gland (Howard et al. 2007). In the study conducted by Ng et al. (2012), it was determined that treating SAC alone or in combination with cisplatin (1 mg SAC/kg/day + 1 mg cisplatin/kg/day for 6 weeks) in the in vivo xenograft liver tumor model suppressed the progression and metastasis of hepatocellular carcinoma.
4 Epidemiological Studies
Researchers or scientific authorities adopt the view that nutrition can reduce the risk of cancer. 80% of cancers are associated with environmental factors, only 1% are caused by cancer syndromes and up to 5% are caused by single gene mutations. Therefore, it is predicted that 35–40% of cancers can be prevented by nutrition and physical activity (Wilson et al. 2002; Tandon et al. 2008). Although several epidemiological studies provide evidence that garlic consumption changes the course of the disease by affecting the molecular pathogenesis of cancer, long-term intervention studies are lacking. For example, stomach cancer mortality was 13 times lower in those who consumed 20 g of garlic per day than those who consumed only 1 g/day in Shandong province of China (Han 1993). According to the Chinese Academy of Medical Sciences, there was a negative relationship between the consumption of garlic with the incidence of gastric cancer (Setiawan et al. 2005; Li et al. 2018a). Kodali and Eslick (2015) also reported a significant association with an elevated allium consumption and reduced gastric cancer risk in a meta-analysis study consisting of 8621 cancer cases and 14,889 controls. In a case-control and meta-analysis study of 230 cancer cases and 547 controls, a negative relationship was reported between increased garlic consumption and reduced risk of gastric cancer (Turati et al. 2015). However, in a study conducted in the Korean population, there was no significant relationship between garlic intake and decreased stomach cancer incidence (Kim et al. 2002). Dorant et al. (1996) and You et al. (2006) also adopted the same view. In another study in China, the intake of more than 10 g daily of allium vegetables in men reduced risk of prostate cancer compared to those who used less than 2.2 g daily (Hsing et al. 2002). Kirsh et al. (2007) reported that more than once intake per week was not associated with prostate cancer risk. In the case-control study conducted in China, it was observed that consumption of raw garlic more than twice [OR of 0.78 (95% CI: 0.62–1.01)] per week was negatively related to risk of liver cancer (Liu et al. 2019). These outcomes explain that daily dose should be determined according to cancer types in reducing the incidence.
Several studies have demonstrated that high garlic consumption is negatively related to the risk of prostate (Salem et al. 2011; Zhou et al. 2013), esophagus (Chen et al. 2004), larings, ovarian, renal and oral (Galeone et al. 2006), breast (Desai et al. 2019), multiple myeloma (Wang et al. 2012a), endometrium (Galeone et al. 2009), liver (Zhang et al. 2013), primary invasive epithelial ovarian and colon cancer (Steinmetz et al. 1994; Levi et al. 1999; Galeone et al. 2006). One of the most impressive studies have revealed that when garlic is consumed over 10 years, the incidence of hematological malignancy can lead to a 45% reduction (Nicastro et al. 2015). Nevertheless, there are studies claiming that there is no significant relationship between garlic consumption and cancer incidence. For instance, it was stated that the use of garlic is not related to the risk of colon (Tanaka et al. 2004; Giovannucci et al. 1994), lung (Dorant et al. 1995) and breast cancer (Galeone et al. 2006). In another study, after topical ajoene application in 21 patients with nodular or superficial basal cell carcinoma, it was reported that tumor size was reduced in 17 patients, Bcl-2 expression was significantly reduced, thus mitochondria-mediated apoptosis was stimulated (Tilli et al. 2003).
In some studies showing that garlic consumption has decreased cancer incidence, it was understood that the number of subjects was low, a low dose-control group was used instead of the placebo group, or the garlic intake was determined with qualitative questions without quantitatively measuring. On the other hand, the results may be directly related to the countries’ diet (cooked or raw) and consumption amounts, and the reason for the differences between studies. Therefore, there is a need for advanced epidemiological studies based on larger populations and quantitative data.
5 Mechanisms of Action
5.1 Stimulation of Detoxification Enzymes
One of the mechanisms mediating the anticarcinogenic effect of garlic and some allyl sulfur compounds is the induction of detoxification enzymes. In mammalian systems, these enzymes are generally divided into two classes, phase I and phase II enzymes. Phase I metabolism largely occurs through cytochrome P450 (CYP450s) enzymes. Xenobiotics including drugs, toxins, carcinogens, mutagenes and toxic chemicals are metabolized by the CYP450s. In the phase II, the metabolized products are conjugated with molecules such as glucoronic acid, sulfate and glutathione, so that they can be excreted from the body through gall and urine.
Various allyl sulfur compounds have been reported to suppress or activate the expression of CYP450 genes (Srivastava et al. 1997). This suppression or activation can provide some benefits, such as preventing DNA damage, removing various carcinogens from the body. In a study by Davenport and Wargovich (2005), it was determined that DAS and DADS decreased rat liver protein level, while propyl-derived compounds and water-soluble SAC were not effective. However, DAS has been shown to increase liver CYP1A1 and CYP1A2 protein levels in time and dose depending manner. In addition, Wargovich (2006) demonstrated that DAS and DADS are the compounds that block CYP2E1 protein synthesis but the compounds of propyl origin cannot show the same effect. DADS increased the expression levels of liver and intestine CYP2B1 and CYP2B2 in rat. Although DAS had similar effect with DADS in the liver, only CYP2B1/2 protein levels were increased in the intestine.
Nitrosamines (NA) are potential carcinogens that affect the risk of cancer in humans and play a role in increasing this risk. Suppression of nitrosamine formation has been proposed as one of the possible anticancer action mechanisms of garlic, and allyl sulfur compounds. (Atanasova-Goranova et al. 1997; Dion et al. 1997). They also regulate phase I and II enzymes and DNA repair (Wattenberg 1990). Several studies have shown that DAS is a competitive inhibitor of N-nitrosodimethylamine (NDMA), a highly carcinogenic NA (Yang et al. 2001; Fasolino et al. 2015). Studies have shown that SAC is more effective than DAS and DADS in suppressing nitrosamine formation (Dion et al. 1997; Milner 2001). These effects of allyl sulfur compounds can be associated with inhibition of carcinogen activation by the P450s.
Activation of detoxification pathways through the induction of phase II enzymes (glutathione S-transferase (GST), UDP-glucuronosyl transferase (UGT), quinone reductase) is suggested as one of the main anti-tumor mechanisms of allyl sulfide compounds (Hu et al. 1997; Andorfer et al. 2004). Although DADS significantly increases GST and glutathione (GSH) levels in rats, SAC does not show the same effect. It has been suggested that an allysulfur-rich diet can alter chemotherapy treatment by increasing the expression of genes associated with multiple drug resistance (Demeule et al. 2004). GSH activity has also been shown to increase in the DAS-treated mice stomach (Maurya and Singh 1991). There is a positive relationship between chemopreventive effects of the allyl sulfur compounds such as DAS, DADS and DATS, and increased NAD(P)H:quinone oxidoreductase (NQO) expression in benzo(a)pyrene (BP)-induced forestomach and lung cancer (Singh et al. 1998).
Garlic OSCs are H2S donors, gaseous signaling molecules, and release H2S through mainly GSH-dependent mechanism. Also, some OSCs such as DATS perform this secretion much faster than others (Liang et al. 2015). Under physiological conditions, while endogenous H2S or relatively low exogenous H2S takes part of maintaining homeostasis or limiting tissue damage, prolonged or high amount of H2S exposure is thought to cause cancer cell death due to cellular toxicity (Han et al. 2019). Therefore, exogenous H2S sources could be used as powerful therapeutic agents against a variety of diseases, including cancer.
As a result, some CYP450 enzymes and GSH mediate the anticancer effect of allyl sulfur compounds. However, compounds carrying allyl and oil-soluble groups are more effective in stimulation of detoxifying enzymes than those carrying propyl and water-soluble groups (Chen et al. 2004). Moreover, some components are not effective at the mRNA level, but at the protein level. This is an example of translational-level mechanisms of action, including epigenetic mechanisms.
5.2 Cell Protection from Oxidative Stress
Increased intracellular level of reactive oxygen species (ROS) causing oxidative stress is closely related to the pathogenesis of many diseases, including cancer (Liou and Storz 2010). Antioxidant system (involved in enzymes such as glutathione peroxidase (GPx), GST, catalase, superoxide dismutase (SOD)) neutralizes the oxidative damage. Garlic and its allyl sulfur compounds display free radical scarvening activity and protect the cell from lipid, protein and DNA damage (Sowjanya et al. 2009; Upadhyay 2017). Oral ingestion of garlic in animal models has been shown to reduce lipid peroxidation, increase circulating antioxidants, reduce glutathione and glutathione peroxidase (Balasenthil et al. 2000), and exhibit antimutation effect against gamma radiation (Chang et al. 2012). SAC has been found to have antioxidant properties in in vitro and in vivo models, improve K2Cr2O7-induced toxicity (Medina-Campos et al. 2007) and reduce DNA damage (You et al. 2006). Similarly, SAMC plays a role in reducing ROS formation, preventing DNA damage, increasing SOD activity, and preventing NF-κB activity (Wang et al. 2016). DADS and DATS have also been shown to fight cellular stress by activating antioxidant enzymes (Awan et al. 2019). However, DAS, DADS and DATS stimulate the production of ROS (Antosiewicz et al. 2006; Das et al. 2007) by triggering cellular apoptosis and arresting the cell cycle (Yang et al. 2009).
5.3 Induction of Cell Death and Cell Cycle Arrest
Possible anticancer activity mechanisms of garlic’s OSCs are summarized in Fig. 11.3. Cellular death and regulation of the cell cycle are among the most studied anticancer mechanisms of garlic and its OSCs. Although the only cell death mechanism is considered to be apoptosis (type I cell death), autophagy (type II cell death) and necrosis (type III cell death) are also included in this classification nowadays. In multicellular organisms, the main cellular death mechanism is apoptosis which is required for homeostasis in the development process from embryonic period to aging. However, apoptosis is triggered by intracellular (caspases) and extracellular pathways (death receptors) in immune response or cellular damage (Norbury and Hickson 2001). On the other hand, autophagy is a process for cellular homeostasis and cell survival that involved the remove of misfolded or aggregated proteins and damaged organelles as well as eliminated intracellular pathogens.
Molecular mechanisms of action of OSCs-induced cell cycle arrest and apoptosis in cancer cells. OSCs induce ROS generation and DNA damage. It results the activation of phospho-53 and p21, and p21 inhibits the regulatory proteins, and then blocks the cell cycle at G1/S, S/G2 and G2/M. Also, OSCs activates ERK and PI3K for transcription of some survival genes such as p21, Cyclin D1. And they activate the JNK and p38 MAPK, and then upregulate of Bax and downregulate of anti-apoptotic protein Bcl-2 gene. Decreasing of mitochondrial membrane potential trigger the release of Cyt C from mitochondria, and it results activation of caspase 9, caspase 3 and PARP that induce the caspase dependent apoptosis
In some studies, involving the effects of garlic allyl sulfur compounds on cell death, SAMC has been shown to inhibit cell growth in gastric cancer through apoptotic proteins (Katsuki et al. 2006) and trigger MAPK-induced apoptosis by TGF-β activation in colon and hepatocellular carcinoma cells (Tong et al. 2014). Although SAMC is more effective than SAC, it is reported that it suppresses the proliferation and invasion of prostate, ovarian, nasopharyngeal and esophagal cancer cells and rearranges the cell cycle (Chu et al. 2006a). However, SAC has been shown to upregulate caspase-3 in ovarian cancer lines and inhibit cell proliferation by stimulating DNA methylation via DNA methyltransferases (DNMTs) (Xu et al. 2014, 2018). After SAC treatment, it was demonstrated that the antiapoptotic proteins Bcl-2 and Bcl-xL expression decreased and apoptotic proteins Bak and PUMA expression increased (Velmurugan et al. 2005; Ng et al. 2012). In HepG2 cells, it stimulates the apoptosis and cell cycle arrest through p53/p21 and JNK/c-Jun pathways (Knowles and Milner 2003). Based on these data, water-soluble allyl sulfur compounds promote cell death through both intracellular and extracellular apoptotic proteins.
Hong et al. (2000) found that DAS, DADS, and ajoen direct cancer cells to apoptosis by increasing the expression of apoptotic proteins (such as p53 and Bax) and decreasing the expression of antiapoptotic Bcl-2 through DNA fragmentation and intracellular free calcium. In addition, DADS inhibits cell proliferation by inducing cell cycle arrest in G2/M phase by decreasing cyclin B, Cdc2 and Cdc25C in ECA109 esophageal squamous cell line. Then caspase-mediated apoptosis accompanied by Bcl-2 and Bax proteins and inhibition of MAPK/ERK pathway takes place (Yin et al. 2014). Kelkel et al. (2012) suggested that DATS exhibits anticancer properties by inhibiting tubulin polymerization, in particular, this effect is related to the number of sulfur atoms.
Xiao et al. (2009) showed that DATS stimulates apoptosis by arresting cell cycle in G2/M phase through checkpoint kinase 1 (CDK1) by phosphorylation of its Tyr 15 residue in LNCaP and HCT-116 human cancer cells. On the other hand, DATS has been shown to be effective in preventing the angiogenic properties of human umbilical vein endothelial cells (Xiao et al. 2006b), stimulating human epidermal growth factor receptor2 (HER2) or p53-induced apoptosis, cell growth, migration and cell viability in MCF-7 and MDA-MB-231 breast cancer cell line (Antony and Singh 2011; Chandra-Kuntal et al. 2013).
The integrity of the cell cycle is essential to maintain healthy cell proliferation. It is mainly regulated by cyclin dependent kinases (CDKs) and inhibitors. Any problem in cell division can initiate the tumor process by causing uncontrolled cell divisions. Therefore, agents that inhibit tumor growth at phases of the cell division are among the therapeutic targets. It has been reported that SAMC, DAS, and DADS cause an increase in the percentage of cells blocked in the G2/M phase (Knowles and Milner 2001). In another study, DADS has been shown to block the cell cycle in the G2/M phase on osteosarcoma cells, thereby stimulating apoptosis and autophagy (Knowles and Milner 2001). This anticancer effect of DADS is due to the blocking of the phosphoinositide 3-kinase/mammalian target of rapamycin (PI3K/Akt/mTOR) signal pathway, one of the major pathways involved in the growth and proliferation of many cancer cell types (Yue et al. 2019). Similarly, Chu et al. (2013) suggested that allicin inhibits cell viability by decreasing the level of Bcl-2, cytoplasmic p53 and PI3K/Akt/mTOR signaling pathway and stimulates autophagy by increasing tumor-suppressor AMP-activated protein kinase/tuberous sclerosis complex (AMPK/TSC2) expression and Beclin-1 signaling pathways.
5.4 Immune System Enhancement
Immune system is an incredibly complex host defence mechanism involved many biological structures and processes within an organism (Bourgeon et al. 2007). The host defense cells in the inflammatory system secrete many cytokines, chemokines and similar molecules to suppress malignant cells (Korniluk et al. 2017). When the defense system encounters a stimulus, it activates intracellular signaling pathways especially NF-kB, MAPK and JAK/STAT pathways, releasing inflammatory mediators (Chen et al. 2017). Expression of proinflammatory cytokines (such as IL1β, IL6, TNF-α) with the activation of these pathways supports tumor development. In addition, anti-inflammatory cytokines such as IL10 reverse this condition. Therapeutics that affect inflammatory pathways are being investigated in detail as they can potentially change the cancer process.
Evidence for both preventive and therapeutic effects of garlic on anticancer activity has been presented so far. Garlic and its OSCs have been shown to inhibit cancer progression by influencing inflammatory responses or by regulating cytokine production (Guan et al. 2018). For instance, garlic extract decreases the release of IL12, TNF-α, IL1α, IL6, IL8, IFNγ, IL2 cytokines, while increases the level of IL10 (Hodge et al. 2002).
It has been determined that SAC inhibits NF-κB activation in human T lymphocytes stimulated by TNF-α and H2O2 (Geng et al. 1997). In another study, it was reported that garlic extract inhibits NF-κB and the molecules in TLRs and LPS receptor signaling pathway cascades (Youn et al. 2008). DADS supresses NF-κB thought blocking the glycogen synthase kinase 3β (GSK-3β) activity, and inhibits tumor growth (Saud et al. 2016) in colitis-induced colorectal cancer, stimulates the release of IL1β, TNF-α and IL6 , inhibits the release of IL10 in LPS-stimulated human whole blood (Keiss et al. 2003; Chang et al. 2005). As a result, garlic and its allyl sulfur compounds can regulate inflammation by modulating cytokines and leading to inhibition of NF-κB activity.
5.5 Epigenetic Mechanisms
Not only genetic mechanisms, but epigenetic mechanisms also get involved in the cancer process. Epigenetic mechanisms are all changes in gene expression without modifying the DNA base sequence. There are three defined mechanisms involved epigenetic: histone modification (acetylation, methylation, phosphorylation, ubiquitination and sumolation), DNA methylation at the transcriptional level and noncoding ribonucleic acid regulation at the post-transcriptional level. These mechanisms affect the binding of transcription factors to DNA. For example; low level of methylation (hypomethylation) activates gene expression by DNA methyltransferase inhibitors (DNMTi) , while its high level suppresses gene expression by preventing transcription factors from binding to the promoter. Hypomethylation is a common condition in the early stages of cancer. Therefore, agents targeting epigenetic mechanisms in developing cancer therapeutics remain the focus of attention. In addition, histone deacetylases (HDAC) are considered as potential drug targets because they affect cellular processes such as differentiation, apoptosis, angiogenesis, invasion, and metastasis. Studies show that garlic and various allyl sulfur compounds act as HDAC inhibitors and activate epigenetically silenced genes, leading to apoptosis and cell cycle arrest. Indeed, the allyl mercaptan and DADS are examples of potent HDAC inhibitors (Nian et al. 2009; Druesne-Pecollo and Latino-Martes 2011). DADS has been shown to inhibit cell proliferation related with increased p21WAF1 expression, HDAC inhibition, and histone acetylation in colon cancer cell lines (Druesne et al. 2004a, b). DADS also stimulates cellular apoptosis as a result of increased histone acetylation in prostate cancer cells and inhibits the growth of H-RAS oncogene-transformed tumors (Singh et al. 1996). Apart from the fat-soluble allyl sulfur compounds, water soluble garlic extract has also been reported to inhibit tumor proliferation related with histone hyperacetylation in the T-cell lymphoma cell line (Bhuiyan et al. 2015).
6 Future Directions for Research on the Anticancer Effects of Garlic
Even though garlic and its organosulfur compounds have been used in food or pharmaceutical industry throughout history, detailed research has been undertaken for the past few decades involving mechanisms of action. It is very difficult to treat cancer after it has spread throughout the body which is called as metastasis. Various allyl sulfur type compounds were shown to have a decreasing effect on the frequency of cancer occurrence and progression. Therefore, they are potential agents in anticancer therapy, alone or in combination with antitumor drugs. Antioxidant, apoptotic, proliferative, cell cycle regulating, anti-inflammatory and detoxifying mechanism are among their described mechanisms of action. However, more studies are needed to understand the mechanism of action at both molecular and biochemical levels.
According to the literature, all isolated allyl sulfur compounds do not show the same anticancer effect when evaluated separately. In addition, garlic components, especially the lipophilic ones having allyl groups, exhibited higher anticarcinogenic activities via those mentioned mechanisms. However, there are many in vitro studies showing the anticancer properties of water soluble components, especially SAMC or allicin. Accordingly, when developing therapeutically effective compound/compounds from garlic or its preparations, this should be taken into consideration and its clinical implications should be evaluated. Due to the fact that many of the recent studies do not contain quantitative results the data on garlic’s effects on metabolism is limited. Although the studies carried out so far have provided sufficient data on a cellular basis, in clinical trials more attention should be drawn into the factors such as the applied dose, the route of administration and the type of the cancer as well as the diet style. It is important to know that individuals will have different respond to garlic intake, as the causes of cancers depend on variable factors including genetic and environmental. Detailed in vitro and in vivo studies on allyl sulfur compounds are still in need for further clarification between epigenetic mechanisms of action, especially in tumor inhibition, proliferation, invasion and metastasis.
Abbreviations
- AGE:
-
Aged garlic extract
- AM:
-
Allyl mercaptan
- AMD:
-
Allyl methyl disulfide
- AMPK/TSC2:
-
AMP-activated protein kinase/tuberous sclerosis complex
- AMS:
-
Allyl methyl sulfide
- Bak:
-
Bcl-2 homologous antagonist killer
- Bax:
-
Bcl-2-associated X protein
- Bcl-2:
-
B-cell lymphoma 2
- Bcl-xL:
-
B-cell lymphoma-extra large
- Bip:
-
Binding immunoglobulin protein
- BP:
-
Benzo[a]pyrene
- Cdc2:
-
Cell division cycle-2
- Cdc25:
-
Cell division cycle-25
- Cdk:
-
Cyclin dependent kinases
- CHOP:
-
CCAAT-enhancer-binding protein homologous protein
- DADS:
-
Diallyl disulfide
- DAS:
-
Diallyl sulfide
- DATS:
-
Diallyl trisulfide
- DATTS:
-
Dialyl tetrasulfide
- DMBA:
-
7,12-Dimethylbenz[a]anthracene
- DMH:
-
1,2-Dimethylhydrazine
- DNA:
-
Deoxyribonucleic acid
- DNMTi:
-
DNA methyltransferase inhibitors
- DNMTs:
-
DNA methyltransferases
- eIF2α:
-
Eukaryotic translation initiation factor 2α
- EMT:
-
Epithelial-mesenchymal transition
- ER:
-
Endoplasmic retikulum
- ERK1/2:
-
Extracellular signal–regulated kinases1/2
- FAK:
-
Focal adhesion kinase
- FOXM1:
-
Forkhead box protein M1
- GADD153:
-
G1 arrest and DNA damage 153
- GPx:
-
Glutathione peroxidase
- GRP78:
-
Glucose-regulated protein78
- GSH:
-
Glutathione
- GSK-3β:
-
Glycogen synthase kinase 3β
- GST:
-
Glutathione-S-transferase
- H2O2:
-
Hydrogen peroxide
- H2S:
-
Hydrogen sulfide
- HCC:
-
Hepatocellular carcinoma
- HDAC:
-
Histone deacetylase
- HER2:
-
Human epidermal growth factor receptor2
- H-RAS:
-
Harvey rat sarcoma viral oncogene homolog
- IFNγ:
-
Interferon-gamma
- IL10:
-
Interleukin 10
- IL12:
-
Interleukin 12
- IL1α:
-
Interleukin 1α
- IL1β:
-
Interleukin 1β
- IL2:
-
Interleukin 2
- IL6:
-
Interleukin 6
- IL8:
-
Interleukin 8
- JNK:
-
c-Jun terminal kinase
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MEK:
-
MAPK/ERK kinase
- MMP:
-
Matrix metallopeptidases
- NF-κB:
-
Nuclear factor kappa light chain enhancer of activated B cells
- NO:
-
Nitric oxide
- NQO:
-
NAD(P)H:quinone acceptor oxidoreductase
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- OSCs:
-
Organosulfur compounds
- P13k/Akt/mTOR:
-
Phosphoinositide-3-kinase/protein kinase B
- p38 MAPK:
-
p38 mitogen-activated protein kinases
- PCNA:
-
Proliferation cell nuclear antigen
- PUMA:
-
p53 upregulated modulator of apoptosis
- ROS:
-
Reactive oxygen species
- SAC:
-
S-allyl cysteine
- SAMC:
-
S-allylmercaptocysteine
- Slug (SNAI2):
-
Snail family transcriptional repressor 2
- SOD:
-
Superoxide dismutase
- TGF-β:
-
Transforming growth factor beta 1
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor-α
- TPA:
-
12-O-tetradecanoylphorbol-13-acetate
- UGT:
-
UDP-glucuronosyl transferase
- UPR:
-
Unfolded protein response
- VEGF:
-
Vascular endothelial growth factor
References
Ajayi GO, Adeniyi TT, Babayemi DO (2009) Hepatoprotective and some haematological effects of Allium sativum and vitamin C in lead-exposed wistar rats. Int J Med 1(3):64–67
Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y (2001) Recent advances on the nutritional effects associated with the use of garlic as a supplement. J Nutr 131(3s):951S–1123S
Andorfer JH, Tchaikovskaya T, Listowsky I (2004) Selective expression of glutathione S-transferase genes in the murine gastrointestinal tract in response to dietary organosulfur compounds. Carcinogenesis 25:359–367
Antony ML, Singh SV (2011) Molecular mechanisms and targets of cancer chemoprevention by garlic-derived bioactive compound diallyl trisulfide. Indian J Exp Biol 49(11):805–816
Antosiewicz J, Herman-Antosiewicz A, Marynowski SW, Singh SV (2006) c-Jun NH(2)-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res 66(10):5379–5386
Anwar A, Gould E, Tinson R, Iqbal J, Hamilton C (2018) Redox modulation at work: natural phytoprotective polysulfanes from alliums based on redox-active sulfur. Curr Pharmacol Rep 4(5):397–407
Arora A, Kalra N, Shukla Y (2005) Regulation of p21/ras protein expression by diallyl sulfide in DMBA induced neoplastic changes in mouse skin. Cancer Lett 242:28–36
Arunkumar A, Vijayababu MR, Gunadharini N, Krishnamoorthy G, Arunakaran J (2007) Induction of apoptosis and histone hyperacetylation by diallyl disulfide in prostate cancer cell line PC-3. Cancer Lett 251:59–67
Atanasova-Goranova V, Dimova P, Pevicharova G (1997) Effect of food products on endogenous generation of n-nitrosamines in rats. Br J Nutr 78(2):335–345
Awan KA, Butt MS, Ul Haq I, Suleria AR (2019) Investigating the antioxidant potential of garlic (Allium sativum) extracts through different extraction modes. Curr Bioactive Compd 15:45
Balasenthil S, Arivazhagan S, Nagini S (2000) Garlic enhances circulatory antioxidants during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. J Ethnopharmacol 72(3):429–433
Bayan L, Koulivand PH, Gorji A (2014) Garlic: a review of potential therapeutic effects. Avicenna J Phytomed 4:1–14
Bhuiyan AI, Papajani VT, Paci M, Melino S (2015) Glutathione-garlic sulfur conjugates: slow hydrogen sulfide releasing agents for therapeutic applications. Molecules 20(1):1731–1750
Block E (1985) The chemistry of garlic and onions. Sci Am 252:114–119
Borek C (2006) Garlic reduces dementia and heart-disease risk. J Nutr 136:810S–812S
Bourgeon S, Raclot T, Le Maho Y, Ricquier D, Criscuolo F (2007) Innate immunity, assessed by plasma NO measurements, is not suppressed during the incubation fast in eiders. Dev Comp Immunol 31:720–728
Bradley JM, Organ CL, Lefer DJ (2016) Garlic-derived organic polysulfides and myocardial protection. J Nutr 13:403S–409S
Bronowicka-Adamska P, Bentke A, Lasota M, Wróbel M (2020) Effect of S-allyl-L-cysteine on MCF-7 cell line 3-mercaptopyruvate sulfurtransferase/sulfane sulfur system, viability and apoptosis. Int J Mol Sci 21(3):1090
Bruck R, Aeed H, Brazovsky E, Noor T, Hershkoviz R (2005) Allicin, the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int 25(3):613–621
Butt MS, Sultan MT, Butt MS, Iqbal J (2009) Garlic: nature’s protection against physiological threats. Crit Rev Food Sci Nutr 49(6):538–551
Chandra-Kuntal K, Lee J, Singh SV (2013) Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res Treat 138(1):69–79
Chang HP, Huang SY, Chen YH (2005) Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. J Agric Food Chem 53(7):2530–2534
Chang HS, Endoh D, Ishida Y, Takahashi H, Ozawa S, Hayashi M et al (2012) Radioprotective effect of alk(en)yl thiosulfates derived from allium vegetables against DNA damage caused by X-ray irradiation in cultured cells: antiradiation potential of onions and garlic. Sci World J 2012:846750
Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S et al (2004) Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med 37(10):1578–1590
Chen XX, Liu XW, Zhou ZG, Chen XY, Li LD, Xiong T, Peng L, Tu J (2016) Diallyl disulfide inhibits invasion and metastasis of MCF-7 breast cancer cells in vitro by down-regulating p38 activity. Nan Fang Yi Ke Da Xue Xue Bao 36(6):814–818
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2017) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9(6):7204–7218
Cho O, Hwang HS, Lee BS, Oh YT, Kim CH, Chun M (2015) Met inactivation by S-allylcysteine suppresses the migration and invasion of nasopharyngeal cancer cells induced by hepatocyte growth factor. Radiat Oncol J 33:328e36
Chu Q, Lee DT, Tsao SW, Wang X, Wong YC (2006a) Sallylcysteine, a water-soluble garlic derivative, suppresses the growth of a human androgen-independent prostate cancer xenograft, CWR22R, under in vivo conditions. BJU Int 99:925–932
Chu Q, Ling M-T, Feng H, Cheung HW, Tsao SW, Wang X, Wong YC (2006b) A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis 27:2180–2189
Chu YL, Ho CT, Chung JG, Raghu R, Lo YC, Sheen LY (2013) Allicin induces anti-human liver cancer cells through the p53 gene modulating apoptosis and autophagy. J Agric Food Chem 61(41):9839–9848
Chuah SC, Moore PK, Zhu YZ (2007) S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am J Physiol Heart Circ Physiol 293(5):H2693–H2701
Chung JG, Lu HF, Yeh CC, Cheng KC, Lin SS, Lee JH (2004) Inhibition of N-acetyltransferase activity and gene expression in human colon cancer cell lines by diallyl sulfide. Food Chem Toxicol 42(2):195–202
Das A, Banik NL, Ray SK (2007) Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer 110:1083–1095
Davenport DM, Wargovich MJ (2005) Modulation of cytochrome P450 enzymes by organosulfur compounds from garlic. Food Chem Toxicol 43(12):1753–1762
Demeule M, Brossard M, Turcotte S, Regina A, Jodoin J, Beliveau R (2004) Diallyl disulfide, a chemopreventive agent in garlic, induces multidrug resistance-associated protein 2 expression. Biochem Biophys Res Commun 324:937–945
Desai G, Schelske-Santos M, Nazario CM, Rosario-Rosado RV, Mansilla-Rivera I, Ramírez-Marrero F, Nie J, Myneni AA, Zhang ZF, Freudenheim JL, Mu L (2019) Onion and garlic intake and breast cancer, a case-control study in Puerto Rico. Nutr Cancer 12:1–10
Dion ME, Agler M, Milner JA (1997) S-allyl cycsteine inhibits nitrosomorpholoni formation and bioactivation. Nutr Cancer 28(1):1–6
Dorant E, Van den Brandt PA, Goldbohm RA (1995) Allium vegetable consumption, garlic supplement intake, and female breast carcinoma incidence. Breast Cancer Res Treat 33:163–170
Dorant E, Van den Brandt PA, Goldbohm RA, Sturmans F (1996) Consumption of onions and a reduced risk of stomach carcinoma. Gastroenterology 110:12–20
Druesne N, Pagnies A, Mayeur C, Thomas M, Cherbuy C, Duee PH, Marter P, Chaumontet C (2004a) Repetitive treatments of colon HT-29 cells with diallyl disulfide induce a prolonged hyperacetylayion of histone H3 K14. Ann N Y Acad Sci 1030:612–621
Druesne N, Pagnies A, Mayeur C, Thomas M, Cherbuy C, Duee PH, Marter P, Chaumontet C (2004b) Diallyl disulfide (DADS) increases histone acetylation and p21 (waf1/cip1) expression in human colon tumor cell lines. Carcinogenesis 25:1227–1236
Druesne-Pecollo N, Latino-Martes P (2011) Modulation of histone acetylation by garlic sulfur compounds. Anti Cancer Agents Med Chem 11:254–259
Ejaz S, Woong LC, Ejaz A (2003) Extract of garlic (Allium sativum) in cancer chemoprevention. Exp Oncol 25:93–97
Fasolino I, Izzo AA, Clavel T, Romano B, Haller D, Borrelli F (2015) Orally administered allyl sulfides from garlic ameliorate murine colitis. Mol Nutr Food Res 59:434–442
Fenwick GR, Hanley AB (1985) The genus Allium--part 3. Crit Rev Food Sci Nutr 23:1–73
Fleischauer AT, Poole C, Arab L (2000) Garlic consumption and cancer prevention: meta analyses of colorectal and stomach cancers. Am J Clin Nutr 72:1047–1052
Galeone C, Pelucchi C, Levi F, Negri E, Franceschi S, Talamini R, Giacosa A, La Vecchia C (2006) Onion and garlic use and human cancer. Am J Clin Nutr 84:1027–1032
Galeone C, Pelucchi C, Dal Maso L, Negri E, Montella M, Zucchetto A, Talamini R, La Vecchia C (2009) Allium vegetables intake and endometrial cancer risk. Public Health Nutr 12(9):1576–1579
Gao Y, Liu YQ, Cao WK, Chen XF, Wan YY, Heng C, Xu LJ (2009) Effects of allicin on invasion and metastasis of colon cancer LoVo cell line in vitro. Zhonghua Yi Xue Za Zhi 89:1382e6
Gapter LA, Yuin OZ, Ng KY (2008) S-allylcysteine reduces breast tumor cell adhesion and invasion. Biochem Biophys Res Commun 367:446e51
Geng Z, Rong Y, Lau B (1997) S-allyl cysteine inhibits activation of nuclear factor kappa B in human T cells. Free Radic Biol Med 2:345–350
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC (1994) Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res 54:2390–2397
Guan MJ, Zhao N, Xie KQ, Zeng T (2018) Hepatoprotective effects of garlic against ethanol-induced liver injury: a mini-review. Food Chem Toxicol 111:467–473
Han J (1993) Highlights of the cancer chemoprevention studies in China. Prev Med 22:712–717
Han Y, Shang Q, Yao J, Ji Y (2019) Hydrogen sulfide: a gaseous signaling molecule modulates tissue homeostasis: implications in ophthalmic diseases. Cell Death Dis 10:293
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Ho JN, Kang M, Lee S, Oh JJ, Hong SK, Lee SE, Byun SS (2018) Anticancer effect of S-allyl-L-cysteine via induction of apoptosis in human bladder cancer cells. Oncol Lett 15(1):623–629
Hodge G, Hodge S, Han P (2002) Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry 48(4):209–215
Hong YS (2004) Chemoprevention by organosulfur compounds (from garlic, Allium Sativum) and garlic extracts. J Korean Assoc Cancer Prev 9(4):215–225
Hong YS, Ham YA, Choi JH, Kim J (2000) Effects of allyl sulfur compounds and garlic extract on the expression of Bcl-2, Bax, and p53 in non small cell lung cancer cell lines. Exp Mol Med 32(3):127–134
Hosono T, Hosono-Fukao T, Inada K, Tanaka R, Yamada H, Iitsuka Y, Seki T, Hasegawa I, Ariga T (2008) Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells. Carcinogenesis 29(7):1400–1406
Howard EW, Ling MT, Chua CW, Cheung HW, Wang X, Wong YC (2007) Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clin Cancer Res 13:1847–1856
Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G, Fraumeni JF (2002) Allium vegetables and risk of prostate cancer: a population-based study. JNCI J Natl Cancer Inst 94:1648–1651
Hu X, Benson PJ, Srivastava SK, Xia H, Bleicher RJ, Zaren HA, Awasthi S, Awasthi YC, Singh SV (1997) Induction of glutathione S-transferase pi as a bioassay for the evaluation of potency of inhibitors of benzo(a)pyrene-induced cancer in a murine model. Int J Cancer 73:897–902
Huang L, Song Y, Lian J, Wang Z (2017) Allicin inhibits the invasion of lung adenocarcinoma cells by altering tissue inhibitor of metalloproteinase/matrix metalloproteinase balance via reducing the activity of phosphoinositide 3-kinase/AKT signaling. Oncol Lett 14:468–474
Ip C, Ganther HE (1991) Combination of blocking agents and suppressing agents in cancer prevention. Carcinogenesis 12(2):365–367
Jiang XY, Zhu XS, Huang WZ, Xu HY, Zhao ZX, Li SY, Li SZ, Cai JH, Cao JM (2017) Garlic-derived organosulfur compound exerts antitumor efficacy via activation of MAPK pathway and modulation of cytokines in SGC-7901 tumor-bearing mice. Int Immunopharmacol 48:135–145
Jiang Q, Tian J, Liu G, Yin Y, Yao K (2019) Endoplasmic reticulum stress and unfolded protein response pathways involved in the health-promoting effects of allicin on the jejunum. J Agric Food Chem 67(21):6019–6031
Karmakar S, Banik NL, Patel SJ, Ray SK (2007) Garlic compounds induced calpain and intrinsic caspase cascade for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Apoptosis 12:671–684
Kaschula CH, Hunter R, Cotton J, Tuveri R, Ngarande E, Dzobo K, Schäfer G, Siyo V, Lang D, Kusza DA, Davies B, Katz AA, Parker MI (2016) The garlic compound ajoene targets protein folding in the endoplasmic reticulum of cancer cells. Mol Carcinog 55(8):1213–1228
Katsuki T, Hirata K, Ishikawa H, Matsuura N, Sumi S, Itoh H (2006) Aged garlic extract has chemopreventative effects on 1,2-dimethylhydrazine-induced colon tumors in rats. J Nutr 136:847S–851S
Keiss HP, Dirsch VM, Hartung T, Haffner T, Trueman L, Auger J, Kahane R, Vollmar AM (2003) Garlic (Allium sativum L.) modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J Nutr 133(7):2171–2175
Kelkel M, Cerella C, Mack F, Schneider T, Jacob C, Schumacher M, Dicato M, Diederich M (2012) ROS-independent JNK activation and multisite phosphorylation of Bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis 33:2162–2171
Khan A, Shukla Y, Kalra N, Alam M, Ahmad MG, Hakim SR, Owais M (2007) Potential of diallyl sulfide bearing pH-sensitive liposomes in chemoprevention against DMBA-induced skin papilloma. Mol Med 13:443–451
Kim HJ, Chang WK, Kim MK, Lee SS, Choi BY (2002) Dietary factors and gastric cancer in Korea: a case-control study. Int J Cancer 97:531–535
Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV (2007) Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther 6:1599–1609
Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, Hayes RB (2007) Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst 99:1200–1209
Knowles LM, Milner JA (2001) Possible mechanism by which allyl sulfides suppress neoplastic cell proliferation. J Nutr 131:1061S–1066S
Knowles L, Milner J (2003) Diallyl disulfide induces ERK phosphorylation and alters gene expression profiles in human colon tumor cells. J Nutr 133(9):2901–2906
Kodali RT, Eslick GD (2015) Meta-analysis: does garlic intake reduce risk of gastric cancer? Nutr Cancer 67:1–11
Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V (2017) From inflammation to cancer. Ir J Med Sci 186:57–62
Kutawa AB, Danladı MD, Haruna A (2018) Antifungal activity of garlic (Allium sativum) extract on some selected fungi. J Med Herbs Ethnomed 4:12–14
Kyo E, Uda N, Kasuga S, Itakura Y (2001) Immunomodulatory effects of aged garlic extract. J Nutr 131:1075S–1079S
Lai K, Hsu S, Yang J, Yu C, Lein J, Chung J (2015) Diallyl trisulfide inhibits migration, invasion and angiogenesis of human colon cancer HT-29 cells and umbilical vein endothelial cells, and suppresses murine xenograft tumour growth. J Cell Mol Med 19:474–484
Lanzotti V (2006) The analysis of onion and garlic. J Chromatogr A 1112:3–22
Lawson LD, Ransom DK, Hughes BG (1992) Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb Res 65(2):141–156
Lea MA, Randolph VM, Patel M (1999) Increased acetylation of histones induced by diallyl disulfide and structurally related molecules. Int J Oncol 15:347–352
Ledezma E, Apitz-Castro R, Cardier J (2004) Apoptotic and antiadhesion effect of ajoene, a garlic derived compound, on the murine melanoma B16F10 cells: possible role of caspase-3 and the alpha(4)beta(1) integrin. Cancer Lett 206:35–41
Lei XY, Yao SQ, Zu XY, Huang ZX, Liu LJ, Zhong M, Zhu BY, Tang SS, Liao DF (2008) Apoptosis induced by diallyl disulfide in human breast cancer cell line MCF-7. Acta Pharmacol Sin 29:1233
Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S (1999) Food groups and colorectal cancer risk. Br J Cancer 79:1283–1287
Li W, Tian H, Li L, Li S, Yue W, Chen Z, Qi L, Hu W, Zhu Y, Hao B, Gao C, Si L, Gao F (2012) Diallyl trisulfide induces apoptosis and inhibits proliferation of A549 cells in vitro and in vivo. Acta Biochim Biophys Sin Shanghai 44(7):577–583
Li Z, Le W, Cui Z (2018a) A novel therapeutic anticancer property of raw garlic extract via injection but not ingestion. Cell Death Discov 4:108
Li Z, Ying X, Shan F, Ji J (2018b) The association of garlic with Helicobacter pylori infection and gastric cancer risk: a systematic review and meta-analysis. Helicobacter 23(5):e12532
Li Y, Wang Z, Li J, Sang X (2018c) Diallyl disulfide suppresses FOXM1-mediated proliferation and invasion in osteosarcoma by upregulating miR-134. J Cell Biochem 120(5):7286–7296
Liang D, Wu H, Wong MW, Huang D (2015) Diallyl trisulfide is a fast H2S donor, but diallyl disulfide is a slow one: the reaction pathways and intermediates of glutathione with polysulfides. Org Lett 17(17):4196–4199
Ling H, Zhang LY, Su Q, Song Y, Luo ZY, Zhou XT, Zeng X, He J, Tan H, Yuan JP (2006) Erk is involved in the differentiation induced by diallyl disulfide in the human gastric cancer cell line MGC803. Cell Mol Biol Lett 11:408–423
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44(5):479–496. https://doi.org/10.3109/10715761003667554
Liu Z, Li M, Chen K, Yang J, Chen R, Wang T et al (2012) S-allylcysteine induces cell cycle arrest and apoptosis in androgen-independent human prostate cancer cells. Mol Med Rep 5:439–443
Liu Y, Zhu P, Wang Y, Wei Z, Tao L, Zhu Z et al (2015a) Antimetastatic therapies of the polysulfide diallyl trisulfide against triple-negative breast cancer (TNBC) via suppressing MMP2/9 by blocking NFkappaB and ERK/MAPK signaling pathways. PLoS One 10(4):e0123781
Liu Y, Yan J, Han X, Hu W (2015b) Garlic-derived compound S-allylmercaptocysteine (SAMC) is active against anaplastic thyroid cancer cell line 8305C (HPACC). Technol Health Care 23(Suppl 1):S89–S93
Liu X, Baecker A, Wu M, Zhou JY, Yang J, Han RQ, Wang PH, Liu AM, Gu X, Zhang XF, Wang XS, Su M, Hu X, Sun Z, Li G, et al (2019) Raw garlic consumption and risk of liver cancer: a population-based case-control study in Eastern China. Nutrients 11(9): pii: E2038
Maurya AK, Singh SV (1991) Differential induction of glutathione transferase isoenzymes of mice stomach by diallyl sulfide, a naturally occurring anticarcinogen. Cancer Lett 57(2):121–129
Medina-Campos ON, Barrera D, Segoviano-Murillo S, Rocha D, Maldonado PD, Mendoza-Patiño N, Pedraza-Chaverri J (2007) S-allylcysteine scavenges singlet oxygen and hypochlorous acid and protects LLC-PK(1) cells of potassium dichromate-induced toxicity. Food Chem Toxicol 45(10):2030–2039
Melino S, Sabelli R, Paci M (2011) Allyl sulfur compounds and cellular detoxification system: effects and perspectives in cancer therapy. Amino Acids 41(1):103–112
Milner JA (2001) Mechanisms by which garlic and allyl sulfur compounds suppress carcinogen bioactivation: garlic and carcinogenesis. Adv Exp Med Biol 492:69–81
Ng KTP, Guo DY, Cheng Q, Geng W, Ling CC, Li CX et al (2012) A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma. PLoS One 7:e31655
Nian H, Delage B, Ho E, Dashwood RH (2009) Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds. Environ Mol Mutagen 50:213–221
Nicastro HL, Ross SA, Milner JA (2015) Garlic and onions: their cancer prevention properties. Cancer Prev Res 8(3):181–189
Nigam N, Shukla Y (2007) Preventive effects of diallyl sulfide on 7,12-dimethylbenz[a]anthracene induced DNA alkylation damage in mouse skin. Mol Nutr Food Res 51:1324–1328
Nishikawa T, Yamada N, Hattori A, Fukuda H, Fujino T (2002) Inhibition by ajoene of skin-tumor promotion in mice. Biosci Biotechnol Biochem 66:2221–2223
Norbury CJ, Hickson ID (2001) Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol 41:367–401
Okada Y, Tanaka K, Fujita I, Sato E, Okajima H (2005) Antioxidant activity of thiosulfinates derived from garlic. Redox Rep 10(2):96–102
Pinto JT, Qiao C, Xing J, Suffoletto BP, Schubert KB, Rivlin RS, Huryk RF, Bacich DJ, Heston WD (2000) Alterations of prostate biomarker expression and testosterone utilization in human LNCaP prostatic carcinoma cells by garlic-derived S-allylmercaptocysteine. Prostate 45(4):304–314
Putnik P, Gabrić D, Roohinejad S, Barba FJ, Granato D, Mallikarjunan K et al (2018) An overview of organosulfur compounds from Allium spp.: from processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem 276:680–691
Ray B, Chauhan NB, Lahiri DK (2011) The “aged garlic extract:” (AGE) and one of its active ingredients S-allyl-L-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer’s disease (AD). Curr Med Chem 18:3306–3313
Ried K, Frank OR, Stocks NP, Fakler P, Sullivan T et al (2008) Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc Disord 8(13):1–12
Rigs DR, Dehaven JL, Lamm DL (1997) Allium staivum (garlic) treatment for murine transitional cell carcinoma. Cancer 79:1987–1994
Rose P, Moore PK, Whiteman M, Zhu YZ (2019) An appraisal of developments in allium sulfur chemistry: expanding the pharmacopeia of garlic. Molecules 24(21): pii: E4006
Saidu NE, Touma R, Asali IA, Jacob C, Montenarh M (2013) Diallyl tetrasulfane activates both the eIF2alpha and Nrf2/HO-1 pathways. Biochim Biophys Acta 1830:2214–2225
Sakamoto K, Lawson LD, Milner J (1997) Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr Cancer 29:152–156
Salem S, Salahi M, Mohseni M, Ahmadi H, Mehrsai A, Jahani Y, Pourmand G (2011) Major dietary factors and prostate cancer risk: a prospective multicenter case-control study. Nutr Cancer 63(1):21–27
Sathibabu Uddandrao VV, Brahmanaidu P, Saravanan G (2017) Therapeutical perspectives of S-allylcysteine: effect on diabetes and other disorders in animal models. Cardiovasc Hematol Agents Med Chem 15(2):71–77
Saud SM, Li WD, Gray Z, Matter MS, Colburn NH, Young MR, Kim YS (2016) Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-κB and prevents colitis-induced colorectal cancer by inhibiting GSK-3β. Cancer Prev Res 9:607–615
Scharfenberg K, Wagner R, Wagner KG (1990) The cytotoxic effect of ajoene, a natural product from garlic, investigated with different cell lines. Cancer Lett 53:103–108
Seki T, Hosono T, Hosono-Fukao T, Inada K, Tanaka R, Ogihara J et al (2008) Anticancer effects of diallyl trisulfide derived fromgarlic. Asia Pac J Clin Nutr 17(Suppl 1):249–252
Sengupta D, Chowdhury KD, Chatterjee S, Sarkar A, Paul S, Sur PK et al (2017) Modulation of adenylate cyclase signaling in association with MKK3/6 stabilization under combination of SAC and berberine to reduce HepG2 cell survivability, apoptosis. Int J Program Cell Death 22:1362–1379
Setiawan VW, Yu GP, Lu QY, Lu ML, Yu SZ, Mu L, Zhang JG, Kurtz RC, Cai L, Hsieh CC, Zhang ZF (2005) Allium vegetables and stomach cancer risk in China. Asian Pac J Cancer Prev 6(3):387–395
Sharifi-Rad J, Mnayer D, Tabanelli G, Stojanović-Radić ZZ, Sharifi-Rad M, Yousaf Z, Vallone L, Setzer WN, Iriti M (2016) Plants of the genus allium as antibacterial agents: from tradition to pharmacy. Cell Mol Biol 62(9):57–68
Shin HA, Cha YY, Park MS, Kim JM, Lim YC (2010) Diallyl sulfide induces growth inhibition and apoptosis of anaplastic thyroid cancer cells by mitochondrial signaling pathway. Oral Oncol 46(4):e15–e18
Shirin H, Pinto JT, Kawabata Y, Soh JW, Delohery T, Moss SF, Murty V, Rivlin RS, Holt PR, Weinstein IB (2001) Antiproliferative effects of S-allylmercaptocysteine on colon cancer cells when tested alone or in combination with sulindac sulfide. Cancer Res 61(2):725–731
Sigounas G, Hooker J, Anagnostou A et al (1997) S-allylmercaptocysteine inhibits cell proliferation and reduces the viability of erythroleukemia, breast, and prostate cancer cell lines. Nutr Cancer 27(2):186–191
Singh SV, Mohan RR, Agarwal R, Benson PJ, Hu X, Rudy MA, Xia H, Katoh A, Srivastava SK, Mukhtar H, Gupta V, Zaren HA (1996) Novel anti-carcinogenic activity of an organosulfide from garlic: inhibition of H-RAS oncogene transformed tumor growth in vivo by diallyl disulfide is associated with inhibition of p21H-ras processing. Biochem Biophys Res Commun 225:660–665
Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL (1998) Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun 244(3):917–920
Sowjanya BL, Devi KR, Madhavi D (2009) Modulatory effects of garlic extract against the cyclophosphamide induced genotoxicity in human lymphocytes in vitro. J Environ Biol 30(5):663
Srimuzipo P et al (2009) Effect of fresh garlic preparation on wound treatment and skin disease in dogs. In: International conference on the role of universities in hands-on Education Rajamangala University of Technology Lanna, Chiang-Mai, Thailand, pp 175–180
Srivastava SK, Hu X, Xia H, Zaren HA, Chatterjee ML, Agarwal R, Singh SV (1997) Mechanism of differential efficacy of garlic organosulfides in preventing benzo[a]pyrene-induced cancer in mice. Cancer Lett 118:61–67
Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD (1994) Vegetables, fruit, and colon cancer in the Iowa Women’s Health Study. Am J Epidemiol 139:1–15
Suddek GM (2014) Allicin enhances chemotherapeutic response and ameliorates tamoxifen- induced liver injury in experimental animals. Pharm Biol 52:1009–1014
Sundaram SG, Milner JA (1993) Impact of organosulfur compounds in garlic on canine mammary tumor cells in culture. Cancer Lett 74(1–2):85–90
Takeyama H, Hoon DS, Saxton RE, Morton DL, Irie RF (1993) Growth inhibition and modulation of cell markers of melanoma by S-allyl cysteine. Oncology 50:63–69
Tanaka S, Haruma K, Kunihiro M, Nagata S, Kitadai Y, Manabe N, Sumii M, Yoshihara M, Kajiyama G, Chayama K (2004) Effects of aged garlic extract (AGE) on colorectal adenomas: a double-blinded study. Hiroshima J Med Sci 53(3–4):39–45
Tandon M, Siddique RA, Arvind Singh NK, Ambwani T, Rai SN (2008) Anti-cancer diet: reviewing the role of nutrition in cancer prevention. Curr Top Nutraceut R 6(2):67–82
Tang FY, Chiang EP, Pai MH (2010) Consumption of S-allylcysteine inhibits the growth of human non-small-cell lung carcinoma in a mouse xenograft model. J Agric Food Chem 58(20):11156–11164
Taylor P, Noriega R, Farah C, Abad MJ, Arsenak M, Apitz R (2006) Ajoene inhibits both primary tumor growth and metastasis of B16/BL6 melanoma cells in C57BL/6 mice. Cancer Lett 239(2):298–304
Tilli CM, Stavast-Kooy AJ, Vuerstaek JD, Thissen MR, Krekels GA, Ramaekers FC, Neumann HA (2003) The garlic-derived organosulfur component ajoene decreases basal cell carcinoma tumor size by inducing apoptosis. Arch Dermatol Res 295(3):117–123
Tong D, Qu H, Meng X, Jiang Y, Liu D, Ye S et al (2014) S-allylmercaptocysteine promotes MAPK inhibitor-induced apoptosis by activating the TGF-β signaling pathway in cancer cells. Oncol Rep 32:1124–1132
Turati F, Pelucchi C, Guercio V, La Vecchia C, Galeone C (2015) Allium vegetable intake and gastric cancer: a case-control study and meta-analysis. Mol Nutr Food Res 59:171–179
Upadhyay RK (2017) Garlic induced apoptosis, cell cycle check points and inhibition of cancer cell proliferation. J Cancer Res Treat 5(2):35–54
USDA (n.d.). https://fdc.nal.usda.gov
Velmurugan B, Mani A, Nagini S (2005) Combination of S-allylcysteine and lycopene induces apoptosis by modulating Bcl-2, Bax, Bim and caspases during experimental gastric carcinogenesis. Eur J Cancer Prev 14(4):387–393
Wang HC, Yang JH, Hsieh SC, Sheen LY (2010) Allyl sulfides inhibit cell growth of skin cancer cells through induction of DNA damage mediated G2/M arrest and apoptosis. J Agric Food Chem 58(11):7096–7103
Wang Q, Wang Y, Ji Z, Chen X, Pan Y, Gao G, Gu H, Yang Y, Choi BC, Yan Y (2012a) Risk factors for multiple myeloma: a hospital-based case-control study in Northwest China. Cancer Epidemiol 36(5):439–444
Wang HC, Hsieh SC, Yang JH, Lin SY, Sheen LY (2012b) Diallyl trisulfide induces apoptosis of human basal cell carcinoma cells via endoplasmic reticulum stress and the mitochondrial pathway. Nutr Cancer 64(5):770–780
Wang Z, Xia Q, Cui J, Diao Y, Li J (2014) Reversion of P-glycoproteinmediated multidrug resistance by diallyl trisulfide in a human osteosarcoma cell line. Oncol Rep 31(6):2720–2726
Wang K, Wang Y, Qi Q et al (2016) Inhibitory effects of S-allylmercaptocysteine against benzo(a)pyrene-induced precancerous carcinogenesis in human lung cells. Int Immunopharmacol 34:37–43
Wargovich MJ (2006) Diallylsulfide and allylmethylsulfide are uniquely effective among organosulfur compounds in inhibiting CYP2E1 protein in animal models. J Nutr 136(3 Suppl):832S–834S
Wattenberg LW (1990) Inhibition of carcinogenesis by minor Anutrient constituents of the diet. Proc Nutr Soc 49:173–183
Weisberger AS, Pensky J (1958) Tumor inhibition by a sulfhydryl-blocking agent related to an active principle of garlic (Allium sativum). Cancer Res 18(11):1301–1308
Welch C, Wuarin L, Sidell N (1992) Antiproliferative effect of the garlic compound S-allyl cysteine on human neuroblastoma cells in vitro. Cancer Lett 63:211–219
Wilson S, Jones L, Couseens C, Hanna K (2002) Roundtable on environment health sciences, research, and medicine. Cancer and the environment: gene-enviroment interaction. National Academies Press, Washington, DC
Worku M, Franco R, Baldwin K et al (2009) Efficacy of garlic as an anthelmintic in adult boer goats. Arch Biol Sci 61(1):135–140
Wu CC, Chung JG, Tsai SJ, Yang JH, Sheen LY (2004) Differential effects of allyl sulfides from garlic essential oil on cell cycle regulation in human liver tumor cells. Food Chem Toxicol 42(12):1937–1947
Wu XJ, Kassie F, Mersch-Sundermann V (2005) The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat Res 579:115–124
Xiao DH, Pinto JT, Soh JW et al (2003) Induction of apoptosis by the garlic-derived compound S-allylmercaptocysteine (SAMC) is associated with microtubule depolymerization and c-Jun NH2-terminal kinase 1 activation. Cancer Res 63(20):6825–6837
Xiao D, Pinto JT, Gundersen GG, Weinstein IB (2005) Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol Cancer Ther 4(9):1388–1398
Xiao D, Lew KL, Kim YA, Zeng Y, Hahm ER, Dhir R, Singh SV (2006a) Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res 12:6836–6843
Xiao D, Li M, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, Zeng Y, Marynowski SW, Singh SV (2006b) Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr Cancer 55(1):94–107
Xiao D, Zeng Y, Singh SV (2009) Diallyl trisulfide-induced apoptosis in human cancer cells is linked to checkpoint kinase 1-mediated mitotic arrest. Mol Carcinog 48:1018–1029
Xiao X, Chen B, Liu X, Liu P, Zheng G, Ye F et al (2014) Diallyl disulfide suppresses SRC/Ras/ERK signaling-mediated proliferation and metastasis in human breast cancer by upregulating miR-34a. PLoS One 9:e112720
Xiao J, Xing FY, Liu YX, Lv Y, Wang XG, Ling MT, Gao H, Ouyang SY, Yang M, Zhu J et al (2018) Garlic-derived compound S-allylmercaptocysteine inhibits hepatocarcinogenesis through targeting LRP6/Wnt pathway. Acta Pharm Sin B 8:575–586
Xu Y, Feng J, Zhang D, Zhang B, Luo M, Su D et al (2014) Sallylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Acta Pharmacol Sin 35:267–274
Xu Y, Su D, Zhu L, Zhang S, Ma S, Wu K et al (2018) S-allylcysteine suppresses ovarian cancer cell proliferation by DNA methylation through DNMT1. J Ovarian Res 11:39
Yan JY, Tian FM, Hu WN et al (2013) Apoptosis of human gastric cancer cells line. SGC 7901 induced by garlic-derived compound S-allylmercaptocysteine (SAMC). Eur Rev Med Pharmacol Sci 17(6):745–751
Yang CS, Chhabra SK, Hong JY, Smith TJ (2001) Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J Nutr 131:S1041–S1045
Yang JS, Chen GW, Hsia TC, Ho HC, Ho CC, Lin MW, Lin SS, Yeh RD, Ip SW, Lu HF, Chung JG (2009) Diallyl disulfide induces apoptosis in human colon cancer cell line (COLO 205) through the induction of reactive oxygen species, endoplasmic reticulum stress, caspases casade and mitochondrial-dependent pathways. Food Chem Toxicol 47(1):171–179
Yin MC, Cheng WS et al (2003) Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci 63:23–28
Yin X, Zhang J, Li X, Liu D, Feng C, Liang R, Zhuang K, Cai C, Xue X, Jing F, Wang X, Wang J, Liu X, Ma H (2014) DADS suppresses human esophageal xenograft tumors through RAF/MEK/ERK and mitochondria-dependent pathways. Int J Mol Sci 15:12422–12441
Yoo M, Kim S, Lee S, Shin D (2014) Validated HPLC method and temperature stabilities for oil-soluble organosulfur compounds in garlic macerated oil. J Chromatogr Sci 52(10):1165–1172
You WC, Brown LM, Zhang L et al (2006) Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst 98:974–983
Youn HS, Lim HJ, Lee HJ, Hwang D, Yang M, Jeon R, Ryu JH (2008) Garlic (Allium sativum) extract inhibits lipopolysaccharide induced Toll-like receptor 4 dimerization. Biosci Biotechnol Biochem 72(2):368–375
Yue Z, Guan X, Chao R, Huang C, Li D, Yang P, Liu S, Hasegawa T, Guo J, Li M (2019) Diallyl disulfide induces apoptosis and autophagy in human osteosarcoma MG-63 cells through the PI3K/Akt/mTOR pathway. Molecules 24(14). pii: E2665
Zalepugin DY, Tilkunova NA, Chernyshova IV (2015) Stability of thiosulfinates from garlic (Allium sativum L.) supercritical extracts in polar and nonpolar solvents. Russ J Phys Chem B 9:1032–1042
Zhang YW, Wen J, Xiao JB, Talbot SG, Li GC, Xu M (2006) Induction of apoptosis and transient increase of phosphorylated MAPKs by diallyl disulfide treatment in human nasopharyngeal carcinoma CNE2 cells. Arch Pharm Res 29:1125–1131
Zhang W, Xiang YB, Li HL, Yang G, Cai H, Ji BT, Gao YT, Zheng W, Shu XO (2013) Vegetable-based dietary pattern and liver cancer risk: results from the Shanghai women’s and men’s health studies. Cancer Sci 104(10):1353–1361
Zhang Q, Li XT, Chen Y, Chen JQ, Zhu JY, Meng Y, Wang XQ, Li Y, Geng SS, Xie CF, Wu JS, Zhong CY, Han HY (2018) Wnt/β-catenin signaling mediates the suppressive effects of diallyl trisulfide on colorectal cancer stem cells. Cancer Chemother Pharmacol 81(6):969–977
Zhou XF, Ding ZS, Liu NB (2013) Allium vegetables and risk of prostate cancer: Evidence from 132,192 subjects. Asian Pac J Cancer Prev APJCP 14:4131–4134
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cetinkaya, S., Süntar, I. (2021). Garlic: Allyl Sulfur Compounds and Cancer Prevention. In: Jafari, S.M., Nabavi, S.M., Silva, A.S. (eds) Nutraceuticals and Cancer Signaling. Food Bioactive Ingredients. Springer, Cham. https://doi.org/10.1007/978-3-030-74035-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-74035-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74034-4
Online ISBN: 978-3-030-74035-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)