Abstract
Blood pressure is one of the most commonly monitored values in managing critically ill and non-critically ill patients. In the critically ill it is the major value used by clinicians to adjust fluid use, inotropic, and vasopressor therapy. Proper use of blood pressure measurements requires first an understanding of factors that affect the reliability of what actually is measured. Next, one needs to understand the physics that determine the measured values; this includes the role of elastic, kinetic, and gravitational energy. Finally, it is important to understand the role of arterial pressure and how this relates to the distribution of resistances with the consequent distribution of flow. A paradoxical basic point that needs to be emphasized is that pressure does not indicate flow, but rather the total cardiac output and resistances determined the arterial pressure. However, regional flows depend upon the arterial pressure, the local resistance, and how that local resistance relates to resistances in other vascular beds. Finally, normal organ function ultimately depends upon adequate blood flow and not pressure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Resistance
- Gravity
- Elastic energy
- Kinetic energy
- Cardiac output
- Regional flows
- Coronary blood flow
- Critical closing pressure
- Impedance
- Tension
Blood pressure is measured by almost all health care professionals and is one of the most basic vital signs. However, little attention is given to the physics of the measurement and its physiological implications. As an example of a common error in reasoning, low values of arterial pressure frequently are used to identify inadequate tissue perfusion and then used to provide targets for titration of vasopressor therapy. However, arterial pressure does not predict cardiac output or indicate adequate tissue perfusion. The arterial pressure of a septic patient may be less than 80 mmHg, and cardiac output be twice normal. At peak exercise, a young male can have a cardiac output that is five times normal, but only have a small change, or even a fall, in mean arterial pressure. Blood pressure in patients with cardiogenic shock can be higher than normal even though cardiac output is critically low. Blood pressure in women often is 90 mmHg or less with a normal cardiac output and normal perfusion. To understand why these variations occur requires a better understanding of the determinants of arterial pressure, which is the subject of this chapter. Many of these concepts have been covered elsewhere (Magder 2014, 2018).
Physical Principles
The flow of a Newtonian fluid through a rigid tube, that is, a fluid that develops laminar flow, was described by Poiseuille as (Burton 1965a):
where ΔP is the difference between the inflow and outflow pressure, and R is the resistance to flow along the tube. R is defined as:
where l is length between the two pressure measurements, η is viscosity, and r is radius of the tube. When Poiseuille’s law is written the way it appears in Eq. 8.1, it appears that the pressure difference determines the flow. However, in the circulation, cardiac output injected into the aorta determines the arterial pressure, so that the relationship is:
More precisely, flow is determined by the product of cardiac output and systemic vascular resistance minus a downstream number, which will be discussed later because in Poiseuille’s law it is the difference in pressure, not just the upstream pressure. Equation 8.3 can be very useful diagnostically because it indicates that if blood pressure is decreased, it is either because the cardiac output is decreased or because the systemic vascular resistance is decreased. Since systemic vascular resistance is a derived value and cardiac output is either measured or estimated, when assessing a hypotensive patient the primary question should be: is the cardiac output normal or elevated, in which case the primary problem is a decrease in systemic vascular resistance; or is the cardiac output decreased, in which case the decrease in cardiac output is the primary problem. Causes of a decrease in systemic vascular resistance include a systemic inflammatory response due to infection or other causes of cytokine release, anaphylaxis, hepatic failure, endocrine conditions (hyperthyroid, adrenal insufficiency), spinal shock, spinal anaesthesia, a major arteriovenous fistula, vasodilating drugs, or Beriberi and thiamine deficiency. Treatment should then be directed at correcting the condition that caused the low resistance state, and if necessary, treating the decrease in systemic vascular resistance with a vasopressor to try to restore flow to regions that need a greater percent of total flow such as the heart, brain, kidney, and bowel. If the cardiac output is decreased, as discussed in Chap. 2 (Magder volume), this could be because of a decrease in pump function or a difference in the return function. If the problem is a decrease in pump function, treatment should be directed at correcting the pump problem by using therapies to increase pump function. If the problem is the return function, this usually means that a volume infusion is required. Abnormalities in the pump function versus return function often can be identified by examining the change that occurred in central venous pressure because this is where the cardiac and return functions intersect.
Poiseuille’s law makes it look like a pressure difference is the only force driving flow, but flow is really determined by the total fluid energy difference from the inflow to outflow of a vessel (Burton 1965a, b):
where ρ is density, g is the acceleration due to gravity, and v is the velocity of blood. All values are normalized to volume so that this represents energy per volume (ρ/volume = mass, and P × Volume = E). The first term of Eq. 8.4 is elastic energy. It is the lateral force that stretches the vascular walls and is the most important determinant of all vascular pressures. The second term, gravitational energy, becomes especially important in the upright posture and when making measurements with fluid-filled systems. The third term, kinetic energy, is the energy produced by the movement of blood. What counts for flow is the difference in total energy from the start of the system to the end. This point will become important when considering the kinetic and gravitational components.
Elastic Energy
Elastic energy is the primary type of energy in the cardiovascular system and the value we usually are referring to when we consider arterial pressure, venous pressure, capillary pressure, ventricular pressures, preload, afterload, and many others. It is based on Hooke’s law that says that an increase in length of a substance above a resting length produces a force that is proportional to the change in length (Fig. 8.1) (Burton 1965a). The proportionality constant is called elastance, and the units are force per length. If the substance is homogeneous, the relationship is linear. In the vasculature, we deal with curved structures so that the term pressure is used to describe the force per cross-sectional area. Pressure can be considered as a kind of “potential energy” in a fluid-filled vessel. Volume in a vessel distends the elastic wall and thus changes its length. This creates a recoil force that tends to push the volume out of the vessel when it is opened to atmosphere (Fig. 8.1). The walls of vascular structures are not homogeneous and thus their relationships of force per length, or pressure to volume, are not linear. At lower lengths elastin dominates, which is very “stretchable,” whereas collagen dominates at higher lengths, and it is much less stretchable so that the elastance greatly increases and then remains constant.
Length tension and pressure–volume relationships and elastic energy. (a) Hooke’s law : change in tension (T), or stress if normalized to cross-sectional area, with a change in length of an elastic substance. The slope of the line is elastance (E); V0 indicates the limit of unstressed length, that is, a length that does not cause tension. Tension only occurs above V0. (b) Pressure and volume are used for the length–tension relationship in a round structure; V is volume and P is pressure (force per cross-sectional area). When the structure is homogeneous, the relationship is linear. (c) Pressure–volume (P-V) relationship of vessels in the body. The lower end of the P-V relationship is curvilinear because of recruitment of parallel elements and differences in the elastance of the components of the vessel wall. At greater volumes, the relationship tends to be more linear, especially in veins, because collagen becomes the dominant factor. (d) The recoil of an elastic structure can produce flow
Kinetic Energy
Kinetic energy contributes only a small component to the arterial pressure, but it has important implications on how blood pressure is measured as well as the elastic force acting on vessel walls (Burton 1965b). The key to understanding this is the distinction between blood flow, which is volume per time, and blood velocity, which is volume per distance. Flow is related to velocity by the following (l = length):
Figure 8.2 shows a tube that is narrowed in its middle. Pressures are measured with two types of manometers. In one type, the opening of the tube faces the direction of flow and in the other the opening is perpendicular (lateral) to the flow. There only is a negligible resistive drop from the beginning to the end of the tube. In the first section, the pressure is higher (height of the manometer) when measured with the tube that has its opening facing the oncoming flow than the pressure measured with the tube that has its opening perpendicular to the flow (lateral pressure). This is because the fluid that directly hits the open part of the tube facing the flow is stopped, and the kinetic energy must be turned into elastic energy to maintain the conservation of energy. In the narrowed section, to conserve the movement of the same mass of blood (volume/time) from the beginning to the end of the tube, blood flow must speed up (volume/distance) to allow the same mass to pass through. This means that elastic energy must be converted into kinetic energy. This is evident by the decrease in the lateral elastic pressure with no change in the pressure measured with the tube opening facing the oncoming flow because it includes both the kinetic and elastic energies. When the tube again widens, the velocity of blood decreases and so does the kinetic energy. The lateral pressure is back to where it was before the narrowing, and this makes it look like the flow went from a lower to higher pressure. However, the total energy remained constant throughout the tube except for a trivial loss of energy due to friction against the walls of the tube and the “layers” of flowing fluid.
The importance of elastic and kinetic energies for measurements of pressure and for pathological processes. The schematic represents flow through a tube with minimal loss of energy due to friction over its length. The velocity (v) at the start is 100 cm/s and kinetic energy per volume (1/2 ρv2, where ρ is density and v is velocity) is 3.8 mmHg. This energy is detected by the catheter at P1 which has an opening facing the flow because it stops the flow and the kinetic energy is converted into elastic energy. P2 only measures the lateral pressure and thus gives a lower pressure value than P1. In the constricted region, velocity must increase to conserve the same volume over time as comes in as goes out (conservation of mass), which increases the kinetic energy component. P4 is thus much lower than P3. In the last section, the vessel is wider and velocity goes back to the initial rate, and the difference between P5 and P6 is the same as at the start. When just looking at the lateral measurements, it looks like the pressure is increasing from P4 to P6 but the total energy has not changed
From this example, it should be evident that blood pressure measured with an inflated cuff should be slightly lower than that measured with an arterial line in which the opening of the catheter faces the flow and thus “senses” the conversion of kinetic energy to elastic energy. The inverse of the example in Fig. 8.2, a tube that is wider in the middle as is the case with an arterial aneurysm, has the opposite consequence. Blood velocity in the dilated region is reduced, and kinetic energy is converted into lateral elastic energy. The lateral pressure in the aneurysm then becomes much higher than in the upstream and downstream regions. This effect is even worse when the person exercises and flow significantly increases because the velocity and thus kinetic component is even greater. The lateral force promotes further dilatation of the aneurysmal section and creates a vicious cycle. For this reason, the aneurysm must be repaired when the vessel diameter is beyond a critical size.
Kinetic energy contributes only about 3% to arterial systolic pressure (Burton 1965b). However, it potentially plays a larger role in some circumstances. In a septic patient who has a cardiac output that is twice normal, for example, 10 L/min, and the systolic blood pressure only is 80 mmHg, the kinetic component could be close to 10% of the pressure and could have a significant impact on how the pressure is measured and interpreted. As another example, the cross-sectional area of the pulmonary artery is similar to that of the aorta, and the blood flow is the same, so that the velocity of flow is the same. However, because pulmonary artery pressure is much lower than aortic pressure, the kinetic component makes up a larger proportion of the pressure. This is not measured with opening of most catheters facing away from the flow, whereas catheters measuring aortic pressure most often face the flow. If a catheter with a transducer at the tip or a fluid-filled catheter with a hole placed laterally, the kinetic component is not detected. Kinetic energy also makes up a greater proportion of venous pressures. This is because the sizes of the inferior and superior venae cavae are similar to the descending aorta. This means that flows and velocities are similar and thus the kinetic energy is the same, but the lateral pressures are lower.
Gravitational Energy
The third energy type is gravitational energy (Burton 1965a). Our bodies always are being accelerated towards the centre of the earth by gravity, but this force is resisted by the structures below us. This force is all around us all the time but we do not think about it because it is our baseline state. However, it is very noticeable to astronauts returning from space. Gravity does not have a major impact on haemodynamics when in the supine position because the height differences are small and all parts of the body are affected fairly equally. However, even when supine, there still is a significant force difference between the top and bottom of the body that can affect fluid filtration from capillaries. Where gravity really counts is in the upright posture, which is how we as bipeds spend most of our time. The key variable in the force of gravity is height. Figure 8.3 shows a man in the supine position. The mean pressure at the level of the heart is 100 mmHg. There is only a small pressure drop due to resistance along the major vessels so that in this case, the pressure in the arteries of the feet and head only are 5 mmHg lower than at the level of the heart. Gravity plays no significant role. However, things are dramatically different when the person stands up. In this example, the man is 180 cm in height (71 inches). The height of a column of fluid has a weight because of the acceleration due to gravity of the mass of the fluid in the column. The density of water is 1 (mass/volume) so that the force is simply the height in cm of water times the gravitational constant. This can be converted to the usual mmHg unit by multiplying by the density of mercury which is 13.6 times the density of water and accounting for the conversion from cm to mm which gives 1.36. Accordingly, the addition of the gravitational force to the elastic force gives an arterial pressure of 183 mmHg in the foot. More strikingly, the pressure in the arteries perfusing the top of the head only is 51 mmHg. In someone who is 198 cm tall (6′ 6″) and has a pressure of 120/80 mmHg at the level of the heart, the systolic pressure at the top of the head only is 69/29 mmHg. This likely imposes a limit on how tall humans can be.
Gravitation effect on arterial and venous pressures. On the left side , in the supine position, the effect of gravity is minimal. The dotted lines indicate the reference level at the midpoint of the right atrium. The arterial pressure is 100 at the level of the heart and the venous pressure is 2 mmHg. There is a 5 mmHg arterial pressure drop from the heart to the head and foot due to resistance and 2 mmHg for the returning venous blood. The right side of the figure indicates the person in the upright posture. His height is 182 cm. The dotted line again indicates the references at the heart. The numbers on the right in mmHg indicate added gravitational energy to the pressures in the feet and loss of energy to vessels in the head with the same arterial pressure of 100 mmHg at the level of the heart and 2 mmHg of venous pressure. The arterial pressure is this example is 183 mmHg in the foot and 51 mmHg in the head. See text for further details. (From Magder 2014. Used with permission of Wolters Kluwer Health, Inc.)
Why Is Mammalian Arterial Pressure Set So High Compared to Lower Species?
After the previous discussion, it may seem that at high pressure is needed to perfuse the head. However, the arterial pressures of mice and rats are similar to humans, and they do not have a gravitational challenge. It is higher in all mammals, and even higher in birds, than any other animals. A high pressure also is not simply neccessary to maintain cardiac output in the range of 5 L/min. The right heart of the averaged-sized male heart pumps 5 L/min through the lungs with a systolic pressure of less than 20 mmHg. Blood pressure also is not higher in whales and elephants. There are two important advantages for high aerobic mammals and birds to have high systemic arterial pressures. First, by starting with a high arterial pressure, blood flow can be selectively increased to different regions of the body by decreasing the local resistance in the area of need (Magder 2018; Burton 1965a). The alternative approach would have required decreasing the already very low resistances in the areas that need more flow. This would have required producing a very large increase in the vessel diameters of the regions in need of more flow. More space would be needed for the dilated vessels and blood volume would be sequestered thereby decreasing the effective volume for venous return. The pulmonary circuit actually increases its flow through recruitment and dilatation of pulmonary vessels when flow increases but this works because the whole organ dilates with only a modest redistribution of blood flow; the net effect is an increase in flow with little change in pressure. Another strategy could have been to significantly vasoconstrict all regions except for the region that requires more flow. However, this would compromise local metabolic regulation of the non-working regions and require a lot more central neural coordination. The second advantage of starting with a high arterial pressure is that the load on the left ventricle can remain relatively constant. This is important because generating pressure is a much greater stress on heart muscle than ejecting volume. Even at peak exercise and the need for high tissue flow the arterial pressure of a healthy young person does not increase much above the normal value. This increase in flow can occurt because the major vasodilation in the working muscles. This brings us to the next topic, and the most difficult one, which is the distribution of regional flows.
Regional Distribution of Flow
I will first begin with some basic principles. When resistances are in series, the total resistance is the sum of all the resistances in the series (Ross 1985):
For example, the total resistance from the aorta to the capillaries is the sum of the resistance in the aorta, large arteries, smaller arteries, arterioles, and pre-capillary sphincters. The significance of this is that the narrowest region dominates the pressure drop. The major resistance, and thus major pressure drop, occurs at the level of small arteries and arterioles, which are in the 100–200 u size. The significance of this is very evident in coronary artery disease (Fig. 8.4). Coronary stenoses occur in the large epicardial conductance vessels, which normally contribute very little to the pressure drop along the coronary arteries. Thus, a major narrowing of epicardial vessels can be readily compensated by dilation of the major downstream resistance vessels (Ross 1985; Gould et al. 1975; Gould and Lipscomb 1974; Gould 2009). Because of this, a coronary stenosis of 50% only has a minimal effect on maximum coronary flow, and coronary flow does not become limited enough to be symptomatic until there is a greater than 70% stenosis. Resting symptoms are not present until the proximal stenosis is >90%.
Effect of stenosis in epicardial coronary artery on coronary blood flow (CBF). The bottom of figure indicates that the epicardium contributes only 5% of the total coronary arterial resistance and the arterioles (100–200 μm) make up ~95%. Thus, a proximal stenosis can be adequately compensated by downstream dilation until there is a >75% proximal stenosis. Resting flow (dotted line) is not compromised when the stenosis is >95%
Blood flows to the different organs are in parallel. Total resistance through a vascular bed with parallel resistances is given by (8.5):
The rationale is that the greater the number of parallel channels, the greater the total cross-sectional area and the lower the total resistance.
The greatest flow occurs in the path of least resistance (i.e., greatest cross-sectional area). The distribution of blood flow to different parts of the body is based on the relative vascular resistances of the different parts as represented in Fig. 8.5a by their individual pressure vs flow lines, which represent conductance or 1/resistance (Ross 1985). At rest, the greatest proportion of flow goes to the muscle region because it comprises the large proportion of total body mass, and its vessels have the largest cross-sectional area. The next largest proportion is the splanchnic region because it is metabolically active and has a large area. Although the kidneys are small, only about 250 g each, they have a large blood flow because of their role in filtering blood. Brain blood flow accounts for ~15% of the total because of its active metabolic rate. In comparison, at resting heart rates of around 70 b/min, the heart only takes up about 5% of cardiac output because of its small mass.
Hypothetical pressure flow relationships of major vascular regions. (a) Regional pressure–flow (P-F) relationships for muscle, splanchnic region, kidney, brain, and heart (based on data from Magder 1986; Hoffman 1984) based on actual flow. The slope of the lines is conductance or 1/resistance. (b) The same relationships with flow are normalized by the weight (100 g) of the organ. The slope of the muscle P-F now is small; it increases markedly with exercise (curved arrow) as does that to heart with an increase in heart rate from 70 to 180 b/min (curved arrow). The heart has the highest flow capacity per weight of tissue of the major vascular beds and has a marked capacity to increase vascular conductance (decrease in resistance)
The pattern looks quite different when the flows are normalized to tissue mass; this allows the importance of local metabolic activity of the tissues to be more evident (Fig. 8.5b). In this analysis, resting flow to the muscle is very low and flow to the heart is proportionally higher. Proportional flows per weight to the brain and splanchnic regions are not as high because although they are active regions, they are not as metabolically active as the heart and the flow to the kidney is not related to metabolic activity.
Flows to regions can increase by decreasing their local resistances. The minimal resistance (or greatest conductance) of a region determines the maximum possible flow for a given blood pressure (Magder 1986). Renal resistance is close to its minimum at baseline and it thus only has a small reserve to increase its flow (Figs. 8.5 and 8.6). This makes it very vulnerable to a fall in pressure. The splanchnic bed and brain, too, change little with increased metabolic activity because the range of changes in their metabolic activity is small. Skeletal muscle has a very large capacity to increase its flow (Fig. 8.5b). During exercise, blood flow to large working muscles can go from less than 5–10 ml/min/100 g to 200 ml/min/100 g. The heart is the most impressive of all. Its resting flow is in the range of 80 ml/min/100 g of tissue and can reach 500 ml/min/100 g of tissue at peak heart rate (Figs. 8.4, 8.5, and 8.6) (Hoffman 1984). It thus has a very large vasodilatory reserve, which is far larger than any other tissue (Magder 1986). Of course, this assumes that there is no proximal coronary artery stenosis.
Pressure-flow (P-F) lines for kidney (left) and heart (right) obtained in dogs under baseline condition and after maximal dilatation of the vasculature with nitroprusside. P-F relations were obtained by haemorrhaging the animals and blood flow was measured with radio-labelled microspheres. The solid line indicates the P-F for the maximally dilated state and the hashed lines the non-dilated state. The peak flows at a given pressure are higher in the kidney than in the heart. However, the kidney starts close its maximal P-F line and thus has much less reserve. The coronary vasculature heart has a very large capacity to dilate. Note that the dilated coronary flow was still above the non-dilated value at very low arterial pressures. (From Magder 1986, APS journal – no permission needed)
The issue of relative resistances brings up a very important point for resuscitation. Equation 8.3 indicates that arterial pressure is approximated by the product of cardiac output and systemic vascular resistance. If arterial pressure falls because cardiac output falls (Fig. 8.5), and only a vasopressor is given to increase arterial pressure, total flow does not change, nor will the flow in any region change unless the distribution of flow changes (Fig. 8.7) (Magder 2011; Thiele et al. 2011a, b). This emphasizes the earlier point that pressure does not indicate flow, and vasoconstriction by itself only produces what is called a “tangible bias” in that the pressure on the monitor increases but O2 delivery to tissues does not change. This is most evident with the use of phenylephrine, a pure alpha agonist with no significant effect on cardiac contractility (Thiele et al. 2011a, b). The only way that a region can benefit by vasopressor therapy is for that region to constrict less proportionally than other regions. This could happen if its local regulatory mechanisms override the effect of the exogenous vasoconstrictor (Berne 1964a, b; Guyton et al. 1964), but this is hard to predict and less likely to be the case at high doses of vasopressors. It also means that some other regions lost out. The message is that if the blood pressure falls because of a fall in cardiac output, cardiac output must rise to correct the perfusion deficit. At the other extreme, if all regional resistances decrease proportionately, the same cardiac output can be delivered at a lower pressure (Fig. 8.8). As an example, as noted at the start of this chapter, it is not uncommon to see women, and sometimes men, with systolic pressures less than 90 mmHg and still be perfused normally. This indicates that all their regional resistances are proportionally lower than normal and this allows their normal distribution of blood flow. This has important implications for the management of the markedly reduced systemic vascular resistance in patients with septic shock. If resistances have decreased proportionally in all regions, even low blood pressures can be tolerated. However, it is more likely that resistance does not decrease proportionally and that the greatest decrease is in the peripheral muscle bed because it has a large proportional mass and large dilatory reserves. It also is possible that mitochondrial dysfunction sends signals which are equivalent of normal metabolic signals. In contrast to the muscle bed, renal resistance starts close to its minimum value and cannot dilate much more (Fig. 8.6). The kidney, thus, is the most vulnerable of all organs and usually the first to fail. Because it is so sensitive, renal function may not be the best target in resuscitation protocols because trying to protect it may compromise other more important regions for survival.
Hypothetical regional pressure–flow relationships during cardiogenic shock and in response to a vasopressor. Regional flows are based values from ref. (Magder 1986). The x-axis is arterial pressure (mmHg) and the y-axis flow (L/min). The slope of the lines is conductance, the inverse of resistance (1/R). (a) The initial values are at (1). At (2) cardiac output falls without any reflex adjustment. The graph (b) shows what would happen if resistance increased by a similar amount in all regions but without any change in cardiac output. (3) The vasopressor restored the mean pressure to 90 mmHg but flows in each region remain the same as indicated by the dotted lines. The downward arrows mark the decrease in flow from baseline for the muscle (M), kidney (K), and heart (H) that would occur without any local autoregulation
Hypothetical regional P-F relationships with a proportionally equal dilation of all vascular regions. The left side shows the same starting P-F relationships as in Fig. 8.5. On the right side, all the P-F slopes have been increased (decreased resistance) proportionately. The dotted lines show that the same initial resting flows can be maintained at 55 mmHg (the splanchnic and cardiac flows are marked by dotted lines). However, it is worth noting that the kidney likely does not have the vasodilatory reserve to reach this value
The concept of regional resistances potentially is important when considering use of high doses of vasoactive drugs. The typical baroreceptor response to hypotension is an increase in systemic vascular resistance. This vasoconstriction is greater in peripheral muscle beds than in the splanchnic region (Hainsworth et al. 1983), which from an evolutionary point of view makes sense because the constriction will have less consequences for muscles than on the metabolically active and more delicate splanchnic region. The difference responses in the two regions is most likely due to differences in receptor densities. Presumably, the same selectivity occurs when exogenous vasoconstrictors are given at moderate doses, which provides a more physiologically appropriate response. However, although speculative, it is quite possible that this selectivity is lost at higher vasopressor doses and consequently there is increased vasoconstriction everywhere which disturbs normal flow distribution and compromise perfusion of vital organs, especially if there is no increase in cardiac output. Furthermore, vasoconstrictors also can increase the resistance to venous return which decrease cardiac output and makes matters worse. The problem for clinicians is that it is currently not known what constitutes a “high” dose of vasopressors which would produce a loss of vasopressor selectivity.
Critical Closing Pressure
Systemic vascular resistance classically is calculated from the difference between mean aortic pressure and central venous pressure. However, it has been shown that there is a critical closing pressure (Permutt and Riley 1963), or flow limitation, at the arteriolar level. Starling understood the importance of this for the regulation of flow and the constancy of the load on the heart (Patterson and Starling 1914) (Fig. 8.9). He inserted a floppy tube, which is now called a Starling resistor, in the arterial circuit of his heart-lung preparation. By controlling the pressure around the floppy tube, he could regulate the downstream pressure faced by the heart and thereby study the effects of changes in afterload. The value of critical closing pressures varies throughout the vasculature. A critical closing pressure of about 25–30 mmHg has been demonstrated in the coronary circulation under baseline conditions (Bellamy 1978; Kloche et al. 1981; Dole et al. 1984) and a value greater than 60 mmHg in resting skeletal muscle (Magder 1990). An average for the whole body has be demonstrated in dogs by obtaining pressure–flow relationships of cardiac output versus arterial pressure; the value was around 30 mmHg (Sylvester et al. 1981). The critical closing pressure in the hind limb of a dog has been shown to decrease with exercise (Magder 1990), reactive hyperemia (Magder 1990), adenosine (Shrier and Magder 1997), calcium channel blockers (Shrier and Magder 1995a), and is raised by alpha agonists (Shrier and Magder 1995b), a reduction in baroreceptor tone (Shrier et al. 1991), by myogenic response in response to a local increase in arterial pressure (Shrier and Magder 1993) which thereby tries to keep flow constant, and by inhibition of nitric oxide synthase (Shrier and Magder 1995b).
Hypothetical P-F lines and significance of a downstream critical closing pressure on the measurement of vascular resistance. The vertical dotted line indicates three pressure values with the same arterial pressure. In A, resistance is measured in the standard way as the inverse of P-F line from the arterial pressure to the central venous pressure (CVP). In B, there is a critical closing pressure which means that the resistance (i.e., inverse of the slope of the P-F line) is actually much lower than measured by calculating the pressure difference from the arterial pressure to the CVP. The right upper figure shows how the error in the slope of the P-F relationship increases as the pressure and flow fall and the bottom right shows how the resistance “appears” to increase even without any actual vasoconstriction. At C, the critical closing pressure increased without a change in the slope of the P-F line (1/resistance); the flow falls for the same arterial pressure. At D, the arterial resistance increased with the same critical closing pressure, and there is a further fall in flow at the same arterial pressure
When a circuit has a critical closing pressure, the pressures and the resistances downstream from the site no longer affects total flow into the region. However, factors downstream from the critical closing pressure still can affect capillary filtration and the distributions of flow in the microcirculation. The consequence of a critical closing pressure in systemic arteries is that total systemic vascular resistance (SVR) should be calculated from mean aortic to mean critical closing pressure and not to the central venous pressure because factors below the critical pressure do not affect the flow into the region. The standard calculation of SVR gives a resistance value that is much higher than the SVR calculated in the usual way and introduces an important artefact when the true resistance changes. If the critical closing pressure does not change, and the difference in pressure between the CVP and critical closing pressure do not change, the error produced by neglecting the critical closing pressure as the true downstream pressure increases as the arterial pressure or cardiac output decrease. This makes it look like the arterial resistance is increasing when flow or pressure decrease, which would make sense physiologically, but because of the error it also could just be an artefact. As an example, milrinone has been considered by some to have no inotropic effect and to only act as a vasodilator because when milrinone is administered the SVR falls with the rise in cardiac output. However, based on the artefact just described, any increase in cardiac output will produce a decrease in calculated SVR without any actual dilation. This is not to say that milrinone does not dilate vessels, or for that matter, lower the critical closing pressure, but rather that the effects cannot be distinguished without having a number of points on a pressure-flow line. The presence of critical closing pressures also impacts on measurements of impedance and dynamic aortic elastance.
What Determines Pulse Pressure?
As discussed in Chap. 2, a pressure exists throughout the vasculature even without cardiac contractions, although it only is in the range of 8 to 10 mmHg. With each cardiac contraction, the heart pumps a stroke volume into the aorta which stretches its walls and produces a rising pressure that is modified by the run-off of volume to downstream regions with a lower pressure. The distended elastic walls of the aorta recoil after the end of systole and allow aortic flow to continue in diastole and lengthen the pulse pressure. This is called a Windkessel effect based on the mechanism in early steam engines. When the elastance of the aorta is normal, during early diastole a pressure wave moves in the opposite direction to blood flow because of reflected waves from downstream bifurcation points. These backward waves further augment aortic pulse pressure. When aortic elastance is increased by hypertension or aging, the speed of reflected waves increase and they can come back to the heart during the ejection phase and increase the load on the left ventricle.
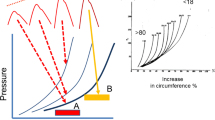
The primary determinants of systolic pressure in the aorta are the amount of volume entering per beat, that is, the stroke volume, and the elastance of the aortic wall. Because the elastance of the aorta is curvilinear, another variable is the volume remaining in the aorta at the onset of systole (Fig. 8.10). The greater the initial volume, the greater the pressure for a given stroke volume. The volume left in the aorta at the end of diastole is dependent upon the downstream resistance and critical closing pressure. It also can be affected by heart rate because a faster heart rate reduces the diastolic time for aortic emptying and increases the aortic volume at the end of diastole much the same way that a rapid breathing rate can lead to hyperinflation in the lungs, especially if arterial resistance is high (see Chap. 7, Heart Rate). Higher pressures also can produce a myogenic effect and increase critical closing pressures (Shrier and Magder 1993). The shape of the elastance curve also is important. Although true aortic elastance likely does not change acutely, the aorta becomes stiffer with age, with chronic hypertension, and potentially, with other chronic processes (Nakashima and Tanikawa 1971). A stiffer aorta produces a larger pulse pressure and the increased pressure leads to further increases in aortic elastance by the induction of compensatory transcriptional mechanisms in vascular walls. For all these reasons, pulse pressure is related to stroke volume, but the relationship is not direct.
Effect of age and initial volume on thoracic aortic elastance. Schematic pulse pressures are shown above a graph of hypothetical pressure–volume relationships in the aorta on the left and actual data of the aorta showing an increase in circumferential tension versus increases in aortic circumference in % from age <18 to >80 mmHg. (From Nakashima and Tanikawa 1971, with permission of SAGE Publications). The slope of lines in the graphs is elastance or 1/compliance. With aging, the aorta becomes stiffer and shifts to the left. This results in increasing pulse pressure (top) for the same size SV (A) and from the same staring diastolic volume. The SV at B is the same size but starts from a higher initial volume diastolic volume and thus is on a steeper part of the aortic P-F curve. This results in a much larger pulse pressure for the same SV
Impedance
The arterial waveform is determined by a pressure wave, a velocity wave, downstream resistance, the capacitance effect from the arterial elastance, and reflected waves (O’Rourke 1971, 1990). It thus is argued that the load on the heart is best evaluated by assessing the impedance to flow in the frequency domain instead of the time domain. This allows a decomposition of the various components of the waveform and is discussed in detail in Chap. 9. However, in the intensive care unit, we mostly are concerned with determinants of cardiac output, and the major determinant of this in the impedance analysis is the arterial resistance and the downstream critical closing pressure. The aortic elastance is a major determinant of the wave patterns, but elastance is a function of the make-up of the walls of the vessels and does not change rapidly in acute illness because changes are required in the composition of the vascular walls. In some studies, attempts have been made to assess arterial elastance under acute conditions, but what most likely is being observed is a change in downstream resistance or the critical closing pressures. Factors in the impedance analysis can affect the magnitude of the pulse pressure, shape of the pulse, and the pattern of downstream transmission, but these only have a small impact on cardiac output and thus on O2 delivery, which is the primary concern for tissue perfusion. I thus have not included impedance as a major factor in this chapter (for discussion see Chap. 9). Its analysis also is not feasible in most critically ill patients.
Where Should Pressure Be Measured and Which Pressure?
Because of reflected waves, the pulse pressure increases the farther away the pulse is from the aortic valve. It thus has been argued that aortic pressure ideally should be measured at the aortic valve, and a tonometric technique has been developed to estimate this value. This central aortic pressure likely is important for understanding the physical forces that induce cardiac hypertrophy, but this pressure likely is not very significant determinant of regional flows. The assumptions required to estimate the central pressure in critically ill also likely are not valid in patients with distributive shock.
More proximal sites for pressure measurement, such as the femoral artery or brachial artery, often are recommended for monitoring patients in shock. This is based on the potential for the more peripheral radial artery pressure to be damped. There also is data indicating that when these more proximal sites are used, less catecholamines are needed, especially in cardiac surgery patients (Lee et al. 2015; Dorman et al. 1998; Kim et al. 2013). However, I have found that this often is just another tangible benefit. If it is known that proximal values are higher than a radial artery pressure, then why not just accept the lower radial artery value and save potential complications from the more proximal sites!
The question also arises as to which pressure to use: systolic, mean, or diastolic. The mean is the most frequently used with indwelling catheters and today the mean is commonly obtained with automated cuff-compression techniques. Use of the mean avoids under or over-damping errors with indwelling catheters (Gardner 1981). It also is thought that it better indicates organ perfusion pressures. Much of this has come from studies examining the ideal perfusion pressure for the kidney (Bersten and Holt 1995), and most from studies that were performed with some kind of controlled renal blood flow with a relatively non-pulsatile pump. However, most tissues in the body receive the largest percent of their flow during systole. Furthermore, the mean is very much affected by diastolic run-off and a low diastolic pressure lowers the mean. However, for the diastolic pressure to be disproportionately lower than expected based on the observed systolic pressure, flow must continue to be emptying from the aorta during diastole and thus some regions are still being perfused.
I thus prefer the use of systolic pressure. To begin, this was the traditional measure in the emergency department or on the ward that triggered the call for the patient to be assessed when blood pressure was measured by a sphygmomanometer. There are few caveats though. When using systolic pressure with an arterial cannula, it is essential to ensure that the waveform is valid. When I am concerned about the systolic pressure, especially if it is low, I compare it to a cuff pressure. For me the gold standard is not an oscillometer, which calculates the systolic pressure, but rather the auscultated pressure or palpated pressure with a cuff. I will often use the cuff pressure as my reference and use the arterial line to rapidly detect changes in the patient’s condition, which likely is the most important thing to know. In my experience, this leads to less catecholamine use than occurs with use of the mean pressure. However, there is no outcome data to know if this is valid.
There is a school of thought that argues that diastolic pressure is an important value to monitor (Hamzaoui and Teboul 2019). The basis of the argument is that diastolic pressure is important for coronary blood flow, and therefore there is a minimal coronary perfusion pressure that needs to be maintained. On the other hand, coronary flow reserves are very large and can be maintained with very low pressures (Magder 2019). However, this assumes that there are no significant proximal coronary lesions which then require higher perfusion pressures. My approach is to not follow the diastolic pressure because this leads to higher concentrations of vasopressors, which also have been found to be harmful. However, if signs of myocardial ischemia appear, arterial pressure likely should be increased. Of note, an increase in troponin is not a reliable measure of this. These issues were recently reviewed in an online debate (Hamzaoui and Teboul 2019; Magder 2019).
Conclusion
I have not dealt in this chapter with the subject of what is the best clinical arterial target for blood pressure in the critically ill. This is because currently there is no satisfactory empiric data. An appropriate physiological prediction also cannot be made because of the complex determinants of what we measure. A crucial point is that pressure is not flow and what counts for tissue function is the flow that they receive. A central factor in the assessment of the ideal pressure is how flow is distributed based on arterial resistances feeding different organs. Unfortunately, this currently cannot be obtained in an intact person. It is likely that only carefully planned empiric studies which take into account the physiological principles linking pressure and flow will allow recommendations for best targets for blood pressure. These likely also will have to be specific for different patho-physiologies because best pressure targets will be different for haemorrhagic shock, septic shock, and cardiogenic shock. Even with general recommendations, target values will likely still need to be individualized based on the individual patient’s needs. In difficult cases, an estimate of cardiac output as well as tissue perfusion should help individualize care.
References
Bellamy RF. Diastolic coronary artery pressure-flow relations in the dog. Circ Res. 1978;43(1):92–101.
Berne RM. Metabolic regulation of blood flow. Circ Res. 1964a;15(Suppl):261–8.
Berne RM. Regulation of coronary blood flow. Physiol Rev. 1964b;44:1–29.
Bersten AD, Holt AW. Vasoactive drugs and the importance of renal perfusion pressure. New Horiz. 1995;3(4):650–61.
Burton AC. Total fluid energy, gravitational potential energy, effects of posture. Physiology and biophysics of the circulation: an introductory text. Chicago: Year Book Medical Publishers Incorporated; 1965a. p. 95–111.
Burton AC. Kinetic energy in the circulation. Physiology and biophysics of the circulation: an introductory text. Chicago: Year Book Medical Pulblishers Incorporated; 1965b. p. 102–12.
Dole WP, Alexander GM, Campbell AB, Hixson EL, Bishop VS. Interpretation and physiological significance of diastolic coronary artery pressure-flow relationships in the canine coronary bed. Circ Res. 1984;55(2):215–26.
Dorman T, Breslow MJ, Lipsett PA, Rosenberg JM, Balser JR, Almog Y, et al. Radial artery pressure monitoring underestimates central arterial pressure during vasopressor therapy in critically ill surgical patients. Crit Care Med. 1998;26(10):1646–9.
Gardner RM. Direct blood pressure measurement--dynamic response requirements. Anesthesiology. 1981;54(3):227–36.
Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2(8):1009–23.
Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34(1):48–55.
Gould KL, Lipscomb K, Calvert C. Compensatory changes of the distal coronary vascular bed during progressive coronary constriction. Circulation. 1975;51(6):1085–94.
Guyton AC, Carrier O Jr, Walker JR. Evidence for tissue oxygen demands as the major factor causing autoregulation. Circ Res. 1964;15(Suppl):60–9.
Hainsworth R, Karim F, McGregor KH, Rankin AJ. Effects of stimulation of aortic chemoreceptors on abdominal vascular resistance and capacitance in anaesthetized dogs. J Physiol. 1983;334:421–31.
Hamzaoui O, Teboul JL. Importance of diastolic arterial pressure in septic shock: PRO. J Crit Care. 2019;51:238–40.
Hoffman JI. Maximal coronary flow and the concept of coronary vascular reserve. Circulation. 1984;70(2):153–9.
Kim WY, Jun JH, Huh JW, Hong SB, Lim CM, Koh Y. Radial to femoral arterial blood pressure differences in septic shock patients receiving high-dose norepinephrine therapy. Shock. 2013;40(6):527–31.
Kloche FJ, Weinstein IR, Klocke JF, Ellis AK, Kraus DR, Mates RE, et al. Zero-flow pressures and pressure-flow relationships during single long diastoles in the canine coronary bed before and during maximum vasodilation. J Clin Invest. 1981;68:970–80.
Lee M, Weinberg L, Pearce B, Scurrah N, Story DA, Pillai P, et al. Agreement between radial and femoral arterial blood pressure measurements during orthotopic liver transplantation. Crit Care Resusc. 2015;17(2):101–7.
Magder SA. Pressure-flow relations of diaphragm and vital organs with nitroprusside-induced vasodilation. J Appl Physiol. 1986;61:409–16.
Magder S. Starling resistor versus compliance. Which explains the zero-flow pressure of a dynamic arterial pressure-flow relation? Circ Res. 1990;67:209–20.
Magder S. Phenylephrine and tangible bias. Anesth Analg. 2011;113(2):211–3.
Magder SA. The highs and lows of blood pressure: toward meaningful clinical targets in patients with shock. Crit Care Med. 2014;42(5):1241–51.
Magder S. The meaning of blood pressure. Crit Care. 2018;22(1):257.
Magder S. Diastolic pressure should be used to guide management of patients in shock: PRO. J Crit Care. 2019;51:241–3.
Nakashima T, Tanikawa J. A study of human aortic distensibility with relation to atherosclerosis and aging. Angiology. 1971;22(8):477–90.
O’Rourke MF. The arterial pulse in health and disease. Am Heart J. 1971;82(5):687–702.
O’Rourke RA. The measurement of systemic blood pressure; normal and abnormal pulsations of the arteries and veins. In: Hurst JW, Schlant RC, Rackley CE, Sonnenblick EH, Kass Wenger N, editors. The heart. 7th ed. New York: McGraw-Hill; 1990. p. 158–60.
Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol. 1914;48(5):357–79.
Permutt S, Riley S. Hemodynamics of collapsible vessels with tone: the vascular waterfall. J Appl Physiol. 1963;18(5):924–32.
Ross J Jr. Dynamics of the peripheral circulation. In: West J, editor. Best and Taylor's physiological basis of medical practice. 11th ed. London/Baltimore: Williams and Wilkins; 1985. p. 119–31.
Shrier I, Magder S. Response of arterial resistance and critical closing pressure to change in perfusion pressure in canine hindlimb. Am J Physiol. 1993;265:H1939–H45.
Shrier I, Magder S. The effects of nifedipine on the vascular waterfall and arterial resistance in the canine hindlimb. Am J Physiol. 1995a;268:H372–H6.
Shrier I, Magder S. N G -nitro-L-arginine and phenylephrine have similar effects on the vascular waterfall in the canine hindlimb. Am J Physiol. 1995b;78(2):478–82.
Shrier I, Magder S. Effects of adenosine on the pressure-flow relationships in an in vitro model of compartment syndrome. J Appl Physiol. 1997;82(3):755–9.
Shrier I, Hussain SNA, Magder S. Carotid sinus stimulation influences both arterial resistance and critical closing pressure of the isolated hindlimb vascular bed. Clin Invest Med. 1991;14(4):A13.
Sylvester JL, Traystman RJ, Permutt S. Effects of hypoxia on the closing pressure of the canine systemic arterial circulation. Circ Res. 1981;49:980–7.
Thiele RH, Nemergut EC, Lynch C III. The physiologic implications of isolated alpha 1 adrenergic stimulation. Anesth Analg. 2011a;113(2):284–96.
Thiele RH, Nemergut EC, Lynch C III. The clinical implications of isolated alpha 1 adrenergic stimulation. Anesth Analg. 2011b;113(2):297–304.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Magder, S. (2021). Physiological Aspects of Arterial Blood Pressure. In: Magder, S., Malhotra, A., Hibbert, K.A., Hardin, C.C. (eds) Cardiopulmonary Monitoring. Springer, Cham. https://doi.org/10.1007/978-3-030-73387-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-73387-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73386-5
Online ISBN: 978-3-030-73387-2
eBook Packages: MedicineMedicine (R0)