Abstract
Ovarian cancer, one of the three leading malignancies in women, has high incidence and mortality worldwide. It is hard to diagnose until very late stages and the 5-year survival rate is very low, due mostly to its distant metastasis. Chemotherapy is currently the most common treatment to inhibit cancer growth, but long-term use could result in resistance and tumor recurrence in addition to damages to normal tissues and functions of the patients. In order to achieve safe and curative effects against cancers, many investigators have focused their attention on traditional Chinese herbal medicines. Paclitaxel, a natural antitumor agent, has significant effects on advanced malignancies including ovarian cancer and is in the standard front-line treatment. Additional natural anticancer substances have continually been discovered for their high effectiveness and low side-effects in ovarian cancer prevention and therapy. In this chapter, we summarize recent work on a selected group of natural components, including lignans, ellagic acid, luteolin, mangiferin, and Acanthopanax senticosus, which have all been demonstrated to reduce the progress of epithelial ovarian cancer in a dose-depend manner, by both in vitro and in vivo experiments. The mechanisms of the anticancer activities by these natural components involve expression suppression of MMP2 and MMP9.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Ovarian cancer is among the three leading causes of female cancers but ranks first in mortality worldwide [1]. In the United States, 22,820 new ovarian cancer cases and 14,240 deaths were reported in 2016 [2]. The five most common ovarian cancer types include high-grade and low-grade serous, endometrioid, mucinous, and clear cell carcinoma. All ovarian cancers have an insidious onset with hardly noticeable progress until advanced stages (Stages III and IV). As a result, at the time when the disease is finally diagnosed, peritoneal metastasis often has already occurred. The standard treatment comprises of surgery to remove all macroscopic tumors and systemic chemotherapy to clear or suppress remaining cancer cells [3]. Although ovarian cancers are generally sensitive to platinum agents, and so taxane/platinum combined regimens are often used as first-line chemotherapy, resistance to platinum reagents is common at advanced stages. Conventional chemotherapy is usually cytotoxic with a myriad of side effects. Therefore, more effective and less cytotoxic therapies to treat ovarian cancers are urgently required [4].
Many natural compounds provide health and anticancer benefits. Paclitaxel, a natural antitumor agent, is in the standard front-line treatment and has significant effects on advanced malignancies including ovarian cancer [12]. Substances like paclitaxel are good examples of how natural compounds may be used to treat cancers, inspiring the discovery of safe and effective approaches in ovarian cancer prevention and therapy.
In this chapter, we summarize the available evidence about the effects of plant components from selected fruits, vegetables, and herbs their potential applications as alternative therapeutics against ovarian cancer, with a focus on our recent work in this field.
4.1.1 Lignans
Phytoestrogens, especially lignans , are abundant in food materials and are considered to have preventive and therapeutic effects against various cancers [5, 6]. Enterodiol (END) and enterolactone (ENL) are extensively investigated lignans for their potential medical uses [7, 8]. We and other authors have reported the production of END and ENL by human intestinal microbiota through biotransformation from flaxseeds (seeds of Linum usitatissimum L.) [9,10,11,12]. END and ENL both can reduce the risk of hormone-dependent cancers in the breast [9, 13], uterus [14], and prostate [15]. The anticancer activities of flaxseed lignans have been attributed to two mechanisms, i.e., antioxidant and hormone receptor modulating effects [16, 17]. END and ENL act as antioxidants against DNA damage and lipid peroxidation in cancer and probably also contribute to the reduction of hypercholesterolemia, hyperglycemia, and atherosclerosis [18]. Of specific significance, END and ENL can mimic the structure of human estrogens to upregulate or downregulate the functions of estrogen receptors (ERs) [19]. At relatively low doses, END and ENL exhibit the estrogenic activity, while at higher doses they appear to be anti-estrogenic. The “biphasic effects” might be caused by protein kinase inhibitors at low doses and the topoisomerase activity at higher doses, respectively [7, 20].
There is a considerable body of evidence from epidemiological studies correlating high concentrations of lignans in body fluids with a low incidence of hormone-dependent tumors, in particular breast cancer [21, 22]. For example, a follow-up study showed that postmenopausal breast cancer patients having high enterolignan levels may have a better survival [23]. In another study on serum concentrations in correlation with dietary intake of flaxseed, postmenopausal women consuming flaxseeds had decreased serum 17β-estradiol and estrone sulfate concentrations and lowered breast cancer risks [24]. Additionally, numerous in vitro studies and in vivo animal experiments have demonstrated potent anticancer effects of END and ENL, such as work on breast cancer cell lines MCF-7 and MDA MB 231, which demonstrated the anti-metastatic activity of ENL, probably by inhibiting cell adhesion, cell invasion, and cell motility through downregulating MMP2, MMP9, and MMP14 gene expression [25]. Researchers measured the urinary ENL level in postmenopausal women as well as in breast cancer patients, who were treated with breast cancer removal surgery, and found that breast cancer patients had significantly lower ENL levels compared to the control group, suggesting that ENL might be involved in reducing the risk of breast cancer [26]. In another study, flaxseed, which is a rich source of END and ENL, administered in a basal high-fat diet reduced the nuclear aberration and epithelial proliferation in female rat mammary gland, suggesting a protective effect of flaxseed against breast cancer [27]. Similar results have been found in colon cancer, in which lignans inhibited cell proliferation and induced apoptosis [28].
Nude mouse models have been used to evaluate the therapeutic effects of END, ENL, and other phytoestrogens. A study based on a model of human breast cancers in nude mice showed that cancer animals treated with tamoxifen and fed with flaxseeds or ENL exhibited decreased IL-1β levels compared to controls, which would suppress tumor angiogenesis and reduce microvessel density in vivo [29]. Another breast cancer mouse model with MCF-7 cells showed that ENL had potent effects against breast cancer growth, whereas GEN (Genistein) as the control did not [30]. Additionally, compared to genistein, END and ENL are more suitable for prolonged treatment [9]. The effects of ENL on colon cancer growth and the involved mechanisms of action have been investigated by detecting apoptosis- and proliferation-related proteins and establishing colon cancer mouse models [31]. ENL at a dose of 10 mg/kg could suppress human colon cancer cell growth both in vitro and in vivo [31].
Numerous findings have been reported on END and ENL with different types of tissues or cancers, such as those of lignans on hen ovaries [32, 33], but work about the effects of END and ENL on human ovarian cancers is lacking. We found that both ENL and END performed excellent anticancer effects, although ENL exhibited higher efficacy and less side effects than END in ovarian cancer treatments [34].
4.1.2 Main Components of Pomegranate, Ellagic Acid, and Luteolin
Pomegranate has been used as medicine in many cultures throughout history but is usually consumed as fresh fruit or commercial fruit juice. It possesses many pharmacological effects, including anti-inflammatory, antioxidant, antibacterial, and estrogenic activities [35, 36]. All biological activities are generally attributed to the high phenol, flavonoid, anthocyanin, and tannin contents of the juice, seed, and peel [37]. Recent studies have demonstrated that pomegranate is a potent anti-carcinogenic agent that inhibits multiple signaling pathways, inducing apoptosis and cell cycle arrest [38,39,40,41]. Additionally, pomegranate can significantly inhibit angiogenesis and metastasis in cancer development progress [42].
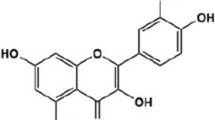
Luteolin (2-(3,4-dihydroxyphenyl) chromenylium-5,7-diol, L) is a nontoxic flavonoid compound that has been used in Chinese traditional medicine to treat various pathologies [43]. In different cancers, luteolin can act as an MMPs inhibitor, attenuating MMPs expression by suppressing the ERK/NF-κB pathway or directly inhibiting its activity [44, 45]. Ellagitannins , one subclass of hydrolyzable tannins, are broken down into free ellagic acid (2,3,7,8-Tetrahydroxy-chromeno[5,4,3-cde] chromene-5,10-dione, EA), which can be absorbed by stomach [46]. When the pomegranate juice is processed, each fruit produces a minimum of 2 g/L of ellagitannins [47]. EA has shown anti-proliferation activity in breast cancer and antioxidant activity through inhibiting inflammatory factors such as TNF-α [48]. However, controversial results have been reported on liver cancer, in which EA was demonstrated to promote hepatocarcinogenesis or perform no effect on hepatocarcinoma [49, 50].
While it is confirmed that pomegranate has significant effects on breast, prostate, and colon cancers [51,52,53], there are few detailed reports on ovarian cancer. The pharmacological effects and anticancer mechanisms of pomegranate fruit juice (PFJ), along with two of its main components, EA and L, on ovarian cancer provide theoretical basis for new anticancer drug development. We recently compared the efficacy of EA and L and found that EA performed stronger effects than L on ovarian cancer [54].
4.1.3 Mangiferin
Mangiferin (1,3,6,7-tetrohydroxyxanthone-c2-β-d-glucoside) is a kind of polyphenol extracted from the Anacardiaceae and Gentianaceae species [55], abundant in the leaves, bark of Mangiferin indica L. [56], and the roots of Salacia chinensis [57], and is commercially utilized in food and natural pharmaceutical industries [58]. Mangiferin has been shown to have promising chemotherapeutic and chemo-preventative potentials, such as antioxidant, anti-inflammatory, immunomodulatory, and anti-virial effects [59]. It also could mitigate the malignant progress of various cancers by suppressing proliferation, migration, and invasion. Furthermore, mangiferin could reverse epithelial-mesenchymal transition to exert anticancer activity in MCF7 breast cancer cell line by inhibiting Wnt/β-catenin pathway [60]. Mangiferin also suppressed expression levels of lung cancer associated enzymes (AHH, γ-GT, and 5′ND) in animal models [61]. Moreover, in some leukemia cases, mangiferin could suppress cyclin B1 and Akt phosphorylation levels, leading to cell arrest in G2/M phase, and activate Nrf2-reduced ROS reaction at a higher concentration [62, 63]. For other cancers, mangiferin could downregulate the Bcl-2/Bax ratio, which is involved in promoting cell apoptosis in nasopharyngeal carcinoma cells [64]. Additionally, it could also block methylmercury-induced DNA damage and oxidative stress in human neuroblastoma IMR-32 cells [65].
4.1.4 Acanthopanax senticosus
Acanthopanax senticosus is a small woody shrub that belongs to the Araliaceae family, distributed mainly over China, Korea, Japan, and Russia [66]. A. senticosus has a multitude of other names such as Siberian Ginseng, Eleutherococcus senticosus, and Ciwujia in China [66,67,68,69]. A. senticosus has been used in eastern Asia for over 2000 years [70], playing a vital role in traditional Chinese medicine. It is popular with its remarkable performance in the treatment of human cardiovascular diseases, diabetes, and neurasthenia [66, 67]. Recently, the possible anticancer activities of A. senticosus have attracted much interest in research, along with some other specific pharmacological effects such as immunostimulatory, immunomodulatory, radiation protection, and antioxidant functions [66]. The most active constituents of this plant are believed to come from the roots and stem, and the bioactivity of A. senticosus is attributed to the secondary compounds it synthesizes, such as lignans, saponins, coumarins, minerals, triterpenoid saponins, and various sugars [66]. This plant is processed into Herbal Tea and capsules and then dissolved in hot water. These compounds are suspected to interact with cancerous cells, the immune system, and protective enzymes to provoke anticancer and protective effects, which have been continually supported by experimental studies [71,72,73].
4.1.5 MMPs
Matrix metalloproteinases (MMPs) are a family of Ca2+-dependent Zn2+-containing endopeptidases, which are capable of degrading extracellular matrix proteins to promote cancer cell migration, invasion, and metastasis [74]. Among more than 20 members of MMPs, MMP2 and MMP9 are correlated with the aggressiveness of cancer [75]. MMPs are regulated by hormones, growth factors, and cytokines, which are all involved in ovarian cancer [76,77,78]. Thus, MMPs have been considered as significant targets for ovarian cancer therapy.
4.1.6 Natural Components in Ovarian Cancer Treatments
Ovarian cancer remains an overwhelming threat to the health and lives of women due to its high morbidity and mortality. Basic and clinical researchers are currently seeking effective antineoplastic agents without side effects for more accurate and efficient treatment of ovarian cancer and natural products from plants and other organisms provide hope. In this study, we demonstrated the activities of Pomegranate fruit juice (PFJ) and two of its main components, Ellagic acid (EA) and Luteolin (L), to suppress the migration and progression of ovarian cancer through downregulating the expression of MMP-2 and MMP-9.
4.2 Materials and Methods
4.2.1 Reagents
DMEM and McCoy’s 5A media were purchased from GE Healthcare Life Sciences, HyClone Laboratories. Histostain-Plus Kits (SP-9001, SP-9002) and Mouse Anti-β actin mAb (TA-09) were purchased from ZSGB-Bio, Beijing, China. Luteolin (≥98%, L9283) and ellagic acid (≥95%, E2250) were purchased from Sigma-Aldrich (USA). DAB Horseradish Peroxidase Color Development Kit (P0203), BeyoECL Plus (P0018), and Hematoxylin Staining Solution (C0107) were purchased from Beyotime Institute of Biotechnology. MMP2 (BMO569) and MMP9 (PB0709) were both purchased as primary antibodies from Boster Biological Technology Co., LTD [79]. HRP-linked rabbit- and mouse-anti IgG (7074s, 7076s) were chosen from CST (USA). Mouse MMPs ELISA Kit (DM-X6142, DM-X6008) was purchased from Baomanbio, Shanghai, China.
4.2.2 Cell Culture
A2780 and ES-2, two human epithelial ovarian cancer cell lines, were purchased from Procell, Wuhan, China. A2780 cells were cultured in DMEM supplemented with 10% FBS at 37 °C and 5% CO2 in a humidified incubator. Cells were passaged twice weekly using 0.05% trypsin. Similarly, ES-2 cells were cultured in McCoy’s 5A supplemented with 10% FBS at 37 °C and 5% CO2 in a humidified incubator.
4.2.3 MTT Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) , used to estimate the cytotoxicity of drugs, is a standard colorimetric assay for measuring cellular proliferation. For the cytotoxicity assay, cells were passaged into 96-well plates at 5000 (A2780) cells per well and grown to >80% confluence, before being treated with EA, L (5 μg/mL, 10 μg/mL, 15 μg/mL), or PFJ (5%, 10%). The viable cells were determined 12, 24, or 48 h later by the MTT assay. A total of 20 μL of MTT was added to each well at the indicated time points and 150 μL of DMSO was added to dissolve the formed formazan crystals. MTT has been validated to be an accurate measure of the viable cell population. DMSO at the concentrations used had no effect on cell viability.
4.2.4 Crystal Violet Assay
Crystal violet staining is a colorimetric indirect method to detect maintained adherence of cells. A2780 cells at 45,000 cells per 500 μL were seeded in 24-well plates and treated with different concentrations of PFJ (5%, 10%), EA (5 μg/mL, 10 μg/mL, 15 μg/mL), or L (5 μg/mL, 10 μg/mL, 15 μg/mL) overnight. After incubation for 48 h, the medium was aspirated from the wells and 300 μL 4% PFA was added per well to fix the cells. To remove the remaining liquid, invert the plate on filter paper, then dye cells with 1% crystal violet 300 μL/well for 5 min, and wash with a gentle stream of tap water. After added 1% SDS 300 μL per well, the plate was incubated at room temperature for 1–3 h on a bench rocker with a frequency of 20 oscillations/min, then measured the optical density of each well at 570 nm a plate reader.
4.2.5 Wound Healing Assay
In order to evaluate the migration ability, cells were passaged into 24-well plates at 300,000 (A2780) cells per well and grown to >80% confluence. Twenty microliter pipette tips were used to make a straight, 1-mm-wide scratch and the scattered cells were washed away by PBS twice. Next, cells were treated with serum-free medium (control), EA (5 μg/mL, 10 μg/mL, 15 μg/mL), L (5 μg/mL, 10 μg/mL, 15 μg/mL), or PFJ (5%, 10%) and cultured for another 24 h. The scratch gaps were photographed at time points of 0, 24 h under a light microscope and analyzed using the Digimizer Version 4.6.1 software.
4.2.6 Western Blot Analysis
The expression level of MMP2 and MMP9 in ovarian cancer cells were determined by western blot analysis, proteins extracted from A2780 cells were heat-inactivated and transferred to the PVDF membrane by electrophoresis (150 mA for 80 and 70 min for MMP2 and MMP9, respectively). 5% skim milk was used to block the other interfering proteins. The PVDF membrane was then washed with 1×TBST three times, dyed in a darkroom, and imaged. A 1:400 dilution of primary antibody in 5% skim milk and a 1:500 dilution of secondary antibody in 1×TBST were used. Finally, proteins grey-level was measured by Quantity One (Bio-Rad Quantity One version 4.6.2).
4.2.7 Nude Mice In Vivo Experiments
With the animal experiment approved by the Institutional Ethics Committee of Harbin Medical University, 24 female nude mice, weighing 16 ± 18 g and 4–8 weeks old, were purchased from VRL, Beijing, China. All mice were raised on purified, laminar air flow shelves in the sterile laboratory, at a constant temperature of 25 ± 2 °C. Humidity was maintained between 45% and 50%. We injected human ovarian cancer ES-2 cells (80 μL, 4.09 × 106/μL) into the right hind leg and monitored body weight every alternate day. All tumor mass could be touched 15 days after inoculation. The mice were randomly divided into four groups, to be treated with 50 mg/kg EA (n = 7), 50 mg/kg L (n = 7), 20 mL/kg PFJ (n = 5), or 1×PBS (n = 5). All mice were executed to examine tissue invasion around the tumor cells and the metastasis of superficial lymph node and viscera. The tumor tissues were used for histological examinations by HE and IHC staining. We also cut mice tails to draw blood for ELISA to detect the concentration of MMP2 and MMP9.
4.2.8 ELISA Analysis
Seven different concentrations of standard MMP2 and MMP9 were pipetted into pre-coated plates. Supernatant from A2780 cell culture and serum from nude mice were used as antigens. Next, biotin-labeled anti-human MMP2 and MMP9 were added to the plates and the reaction was allowed to continue for 60 min at 37 °C. After washing three times, Avidin-Biotin-enzyme Complex (ABC) was added to the stain. After a 15-min incubation at room temperature, a stop buffer was added and the optical density was measured at 450 nm.
4.2.9 HE Staining
Paraffin sections were dipped in xylene, followed by submersion in 100% and then in 80% ethanol solutions. Slides were washed with distilled water for 5 min, before being dyed with hematoxylin and eosin. The slides then underwent ethanol dehydration, being submerged in 85% and 90% ethanol, and then by carbol xylol. Finally, the slides were mounted using neutral balsam.
4.2.10 Immunohistochemical Staining
Previously prepared paraffin-fixed nude mice tissue sections (3 μm) (normal and tumor) were processed for peroxidase (DAB) immunohistochemistry. After deparaffinization and rehydration using xylene and a series of weakening concentrations of ethanol (95%, 80%, 70%), 50 μL of 1:200 dilution MMP2 and MMP9 primary antibody was added to each sample. The samples were stored overnight at 4 °C. After being washed with water for 5 min, addition of peroxidase-labeled polymer and substrate allowed the brown staining of the target proteins to be observed. The samples were counterstained by hematoxylin for 30 s.
4.2.11 Statistical Analysis
Data are presented as the mean of triplicate or quadruplicate determinants with standard error (s.e.). Assays were repeated at least three times. Statistical analysis was performed to assess the difference between the means of the untreated and treated samples using Student’s t-test, Chi-square test, and Spearman’s Rank correlation analysis with SPSS statistical software version 17.0 and GraphPad Prism software. P-value <0.05 was considered statistically significant.
4.3 Results
4.3.1 PFJ, EA, and L Could Inhibit the Proliferation of Human Ovarian Carcinoma Cell Line A2780 Cells
We examined different concentrations of PFJ , EA, and L to establish whether they might have the ability to inhibit the proliferation of cancer cells. As shown in Fig. 4.1, they (control, 5 μM/mL, 10 μM/mL, 15 μM/mL) all significantly suppressed the growth of A2780 cells in 12, 24, and 48 h, compared to the control group. Among the treatments (n = 3), dose- and time-dependent responses were observed in L (Fig. 4.1b), while EA showed a dose-dependent response only at 48 h (Fig. 4.1a). In order to confirm the results of MTT assays, we performed crystal violet assays to verify the proliferation inhibitive effects of PFJ, EA, and L. Cells were stained by crystal violet after 48 h treatments with PFJ, EA, or L at different concentrations and OD values were compared. We found that PFJ, EA, and L all could decrease the cell number of A2780 in a dose-dependent manner (Fig. 4.2). Furthermore, by triple repeats of each treatment, we found that the inhibitive effect of EA was more obvious than L after 48 h treatment (Fig. 4.2a, b), which was consistent and more favorable with the results from MTT assays at the 48 h time point.
Different concentrations of PFJ, EA, and L showed an inhibition effect on cell proliferation according to the MTT values. The inhibiting cancer cell proliferation activity of different concentration of EA (a), L (b), and PFJ (c). Treated after 12, 24, and 48 h, all of the three show obvious suppression features, both EA and L presented a desired dose- and time-dependent manner at 48 h. Results were obtained from three separate experiments. Student’s t-test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with ctrl
Cell numbers inhibition by different concentrations of PFJ, EA, and Lin A2780 cell line by Crystal Violet assay . After treating with different concentrations of EA, L, and PFJ, A2780 cells were stained with crystal violet (a–c). Cell number was determined by OD570 value after treating with different concentration of EA, L, and PFJ. After data analysis, each of the three compounds could reduce cell number remarkably; moreover, EA performed a most effective and does-dependent inhibit function compared to L. PFJ could also reduce cell numbers as the concentrations increased. Results were obtained from three independent experiments, # represents P < 0.01 and * represents P < 0.05 when compared with control
4.3.2 PFJ, EA, and L Could Inhibit the Migration of Human Ovarian Carcinoma Cell Line A2780 Cells
Migration is an initial step for a malignant tumor to make the disease rapidly deteriorating. As shown in Fig. 4.3, EA (Fig. 4.3a), L (Fig. 4.3c), and PFJ (Fig. 4.3e) significantly inhibited tumor migration in a dose-dependent manner. Consistent with MTT and crystal violet assay results, treatments with PFJ, EA, and L all inhibited cell motility into a wounded area of confluent cultures in a dose-dependent manner (Fig. 4.3b–f).
The migration of ovarian cancer cells, quantified by Wound Healing, was significantly suppressed by EA and L. Wound healing in the cell vitro experiments is typically characterized by the remaining distance of the scar after treated with three compounds in 24 h cells comparing to 0 h. EA (a, b), L (c, d), and PFJ (e, f) show dose- and time-dependent and preliminary demonstrate our hypothesis. Results were obtained from three separate experiments. Student’s t-test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with Ctrl
4.3.3 The Expression Levels of MMP2 and MMP9 Were Markedly Downregulated by PFJ, EA, and L
To elucidate the mechanisms of the three compounds to inhibit cancer cell migration, we used MMP2 and MMP9 as markers of cancer metastasis. MMP2 (72 kDa type IV collagenase) is intimately linked with the invasion and metastasis of ovarian cancer, while MMP9 (68 kDa type IV collagenase) is a useful serum marker of ovarian cancer [80]. To determine whether EA or L might regulate the expression of MMPs, we conducted western blot analysis and evaluated the expression intensity of MMPs in A2780 cells after treatment with the products for 24 h. Compared with the control (Fig. 4.4a–c), EA at concentrations of 10–15 μg/mL markedly downregulated MMP2 and MMP9 expression in a dose-dependent manner. L could slightly reduce MMP2 and MMP9 expression at the concentration of 5 μg/mL, but at increased concentrations (10–15 μg/mL) the inhibitory effects became much higher. Decreased expression levels of MMP2 (Fig. 4.4b) and MMP9 (Fig. 4.4c) were observed when the cells were treated with 5% and 10% PFJ. Decreased MMP2 and MMP9 expression was also observed as PFJ at different concentrations in western blot analysis (Fig. 4.5a–c).
The amount of MMP2 and MMP9 was significantly reduced by EA, L. To illustrate the metastasis inhibition mechanism of EA (a, b) and L (a, c), western blot show the downregulation of MMP2 and MMP9 in a dose-dependent tendency. But at the lower concentration (5 μg/mL) of EA (b), the inhibiting activity was not markedly compared with control group. Results were obtained from three separate experiments. Student’s t-test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with Ctrl
The expression level of MMP2 and MMP9 was inhibited by PFJ . At the same time, different concentrations of PFJ also exert an influence on inhibiting the expression of MMPs. Compared with corresponding ctrl group, both MMP2 and MMP9 expression levels were restrained by 5% and 10% PFJ at does-dependent manners. Results were obtained from three separate experiments. Student’s t-test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with Ctrl
The contents of MMP2 and MMP9 in the cell supernatant decreased in a dose-dependent manner upon treatment with EA, L, and PFJ as examined using ELISA assays (Fig. 4.6a).
The contents of MMP2 and MMP9 in the supernatant fluid of cultured cancer cells and mice serum were detected by ELISA. In cell supernatant ELISA assay (a), we measured the OD value at 450 nm, and it shows the same trend with western blot. In nude mice serum ELISA assay (b), each sample contains a corresponding number of mice group. And it also presents a trend that EA, L, and PFJ can decrease the expression level of MMP2 and MMP9. Cell supernatant ELISA assays were performed by three separate experiments. Student’s t-test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with Ctrl
4.3.4 PFJ, EA, and L Inhibited Tumor Growth In Vivo
To better understand the impacts of EA and L on ovarian cancer, we injected ES-2 cells into the right hind leg of female nude mice . Two weeks later, all mice could be found bearing a tumor and were randomly assigned into four experimental groups (EA, L, PFJ, and PBS). As the body weight curve shows, all animals gained body weight gradually (Fig. 4.7a). Interestingly, the body weight of PBS group suddenly dropped during 15–20 days and recovered as we improved the living environment. Compared with that in the PBS group, the tumor volumes increased more slowly in the other three groups (Fig. 4.7b). At the end of the experiment, all mice were sacrificed for histological examinations of the tumor tissues. We found that all three treatments reduced both tumor weight and volume (Fig. 4.8a, b, f, g) with no effects on body weight (Fig. 4.8d) or spleen weight (Fig. 4.8c, e). EA showed a greater reduction of tumor weight and volume than L, suggesting that it could be a better candidate for a future anticancer drug.
Tumor growth and body weight changes as time during EA, L, and PFJ treatments after the tumor mass could be touched. Treated three compounds bearing ovarian cancer nude mice to clarify the in vivo effect of them. In the everlasting 40 days, nude mice were treated with PBS , 50 mg/kg EA, 50 mg/kg L, and 20 mL/kg PFJ as experimental design. Body weight and tumor volume were started to measure after the tumor mass could be touched. At 15th–19th (a), due to the surrounding was worse, the body weight of PBS group dropped drastically before the living environment was improved, subsequently, the body weight of PBS group was recovered and performed as our result shown. All groups were stably increased during the 40 days. Tumor volume were measured once every 2 days and the tumor sizes were calculated according to the formula V = 0.5 ab2. As shown by the tumor volume curve (b), the tumor volumes were increasing slowly in these three compound groups compared with PBS
The final body weight, spleen weight, tumor weight, and tumor volume were measured and recorded at necroscopy (the 40th day). Nude mice were sacrificed then gathered the solid tumor and spleen at the end of the experiment (a–c). And contrast to the value of body weight, tumor weight, and tumor volume in different groups (d–g), EA shows more advantages and less side effect in vivo. It seems that EA could be used as a medicine in future treatment
4.3.5 PFJ, EA, and L Inhibited MMP2 and MMP9 Expression: Histological and Biochemical Evidence
Hematoxylin-Eosin staining showed dark and basophilic materials in the cytoplasm of most tumor cells compared to cells of control tissues (Fig. 4.9a). The HE staining results indicate that the three products all had anticancer effects through transforming cell structures. We then quantified the expression of MMPs in solid tumor paraffin sections by immunohistochemistry and found that the expression levels of MMP2 and MMP9 were significantly reduced compared to the PBS group (Fig. 4.9b, c). These results indicate that EA, L, and PFJ all had anticancer activities through downregulating MMPs expression. Staining of MMPs was strongest in the PBS groups but either “weak” or “moderate” after treatment, suggesting that all three treatments had therapeutic effects (Fig. 4.9c, d). Moreover, serum ELISA analysis demonstrated that EA, L, and PFJ had suppressive activities on MMP9 and MMP2 (Fig. 4.6b), further confirming the antitumor characteristics of the three products .
Immunohistochemistry staining for MMP2 and MMP9 and Hematoxylin-Eosin staining in different nude mice ovarian carcinoma tissues. The HE and immunohistochemistry staining in solid tumor paraffin sections. The result of MMP2: PBS (n = 5; moderate: 2, strong: 3), EA (n = 7; weak: 3, moderate: 3, strong: 1), L (n = 7; weak: 4, moderate: 2, strong: 1), and PFJ (n = 5; weak: 4, moderate: 1). And MMP9: PBS (n = 5; weak: 2, strong: 3), EA (n = 7; weak: 6, moderate: 1), L (n = 7; weak: 5, moderate: 2), and PFJ (n = 5; weak: 3, moderate: 2). It seems that all three compounds may inhibit MMP9 expression strongly than MMP2
4.4 Discussion and Conclusion
Ovarian cancer remains a serious threat to the health and lives of women due to its high mortality. Basic and clinical researchers are currently seeking effective antineoplastic agents without side effects for more accurate and efficient use in diagnosis, treatment, or prognosis of ovarian cancer. We demonstrated the ability of pomegranate fruit juice (PFJ) and two of its main components, ellagic acid (EA) and luteolin (L), to suppress the proliferation, migration , and progression of ovarian cancer through downregulating the expression of MMP2 and MMP9.
A previous study in prostate cancer showed that pomegranate could exert anticancer activity, which was attributed to its high content of polyphenols [81]. Another research group confirmed that EA, L, and ursolic acid extracted from pomegranate caused a concentration-dependent decrease in PANC-1 cell proliferation [82]. Consistently, a study in prostate cancer indicated that PFJ components EA, L, and punicic acid together inhibited the growth of both hormone-dependent and -independent prostate cancer cells and inhibited their migration, progression, and metastasis. Similarly, EA has also been demonstrated to exert in vivo anti-angiogenic effect and inhibit MMP2 activity, both obviously contributing to antitumor activities [83]. L acts as an anti-metastatic agent by suppressing MMP2 and MMP9 production and downregulating expression in azoxymethane-induced colorectal cancer [45]. Yuan-Chiang and colleagues first investigated the effects of EA on ovarian cancer and pointed out that EA may be a potential novel chemoprevention and treatment assistant agent for human ovarian carcinoma [84]. We sought to clarify the antitumor mechanism of EA, L, and PFJ in ovarian cancer; moreover, the efficacy of each treatment was compared as well.
We found EA and L to significantly reduce the proliferation, migration, and invasion of ovarian cancer both in vivo and in vitro. The growth of tumor cells was suppressed by PFJ, EA, and L and the inhibitory effect became even stronger with increasing concentrations of the fruit products. Both EA and L showed a time- and dose-dependent manner, EA performed a more obviously cell inhibitive effect comparing to L. Additionally, Wound Healing assays showed PFJ, EA , and L to have a dose-dependent inhibition of cell migration. MMP2 and MMP9, both important markers in tumor migration and invasion, showed the effects of treatments on protein levels. Intensity of MMP2 and MMP9 expression decreased with increasing concentrations of the compounds tested.
During our in vitro experiments, we noticed that EA seemed to have superior anticancer effects over L. To date, there is no publication comparing the antitumor activities of EA and L. Our publication may guide further study of the role played by EA in resisting ovarian cancer. In our experiment, PFJ was squeezed directly from fresh fruit, presumably containing anticancer substances such as anthocyanins. Therefore, the anticancer effect of PFJ should not be simply attributed to the effects of EA and L together. Because neither splenomegaly nor intense changes in body weight were observed, EA, L, and PFJ did not induce severe side effects in nude mice.
In addition to pomegranate, EA and L can be extracted from many other plants, including various berries, pineapple, broccoli, bird chili, and onion leaves [85, 86]. For further usages of these two compounds, our work encourages their dietary and medicinal applications.
Finally, the research demonstrated that EA, L, and PFJ suppressed the proliferation and migration of ovarian cancer through downregulating the expression of MMP2 and MMP9 , both in vivo and in vitro. We reported for the first time that EA had greater effects than L, suggesting that EA may be a promising candidate for further preclinical testing for the treatment of human ovarian cancer.
4.5 Further Direction of Natural Compounds in Ovarian Cancer
Natural plants or fruit-derived metabolites are of great resources for adjunct therapies to complement conventional treatment. Natural products markedly inhibited the metastasis of ovarian cancer cells by downregulating the expression of MMPs and slowed down the growth of solid tumors in our in vivo experiments. Our results indicate great potentials of using a broad variety of natural products to improve the prognosis of ovarian cancer. In conclusion, natural products are well on its way to improve the prognosis of ovarian cancer and have great potentials for further application as effective pharmacological treatments for ovarian cancer.
References
Collins, Y., Holcomb, K., Chapman-Davis, E., Khabele, D., & Farley, J. H. (2014). Gynecologic cancer disparities: A report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecologic Oncology, 133(2), 353–361.
Siegel, R. L., Miller, K. D., & Jemal, A. (2016). Cancer statistics, 2016. CA: A Cancer Journal for Clinicians, 66(1), 7–30.
Bhatt, A., & Glehen, O. (2016). The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer: A review. Indian Journal of Surgical Oncology, 7(2), 188–197.
Saucier, J. M., Yu, J., Gaikwad, A., Coleman, R. L., Wolf, J. K., & Smith, J. A. (2007). Determination of the optimal combination chemotherapy regimen for treatment of platinum-resistant ovarian cancer in nude mouse model. Journal of Oncology Pharmacy Practice, 13(1), 39–45.
Al-Anazi, A. F., Qureshi, V. F., Javaid, K., & Qureshi, S. (2011). Preventive effects of phytoestrogens against postmenopausal osteoporosis as compared to the available therapeutic choices: An overview. Journal of Natural Science Biology and Medicine, 2(2), 154–163.
Wietrzyk, J., Grynkiewicz, G., & Opolski, A. (2005). Phytoestrogens in cancer prevention and therapy—Mechanisms of their biological activity. Anticancer Research, 25(3c), 2357–2366.
Wang, L. Q. (2002). Mammalian phytoestrogens: Enterodiol and enterolactone. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 777(1–2), 289–309.
Martinchik, A. N., & Zubtsov, V. V. (2012). [Phytoestrogenis properties of flaxseed lignans]. Voprosy Pitaniia, 81(6):61–66.
Power, K. A., Saarinen, N. M., Chen, J. M., & Thompson, L. U. (2006). Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. International Journal of Cancer, 118(5), 1316–1320.
Tao, Y. L., Yang, D. H., Zhang, Y. T., Zhang, Y., Wang, Z. Q., Wang, Y. S., Cai, S. Q., & Liu, S. L. (2014). Cloning, expression, and characterization of the beta-glucosidase hydrolyzing secoisolariciresinol diglucoside to secoisolariciresinol from Bacteroides uniformis ZL1. Applied Microbiology and Biotechnology, 98(6), 2519–2531.
Zhu, H.-Y., Li, M.-X., Yang, D.-H., Tao, Y.-L., Zhang, Y., & Liu, S.-L. (2014). Biotransformation of the SDG in defatted flaxseed into END co-cultured by three single bacterial colonies. Process Biochemistry, 49(1), 19–24.
Wang, C. Z., Ma, X. Q., Yang, D. H., Guo, Z. R., Liu, G. R., Zhao, G. X., Tang, J., Zhang, Y. N., Ma, M., Cai, S. Q., et al. (2010). Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria. BMC Microbiology, 10, 115.
Mousavi, Y., & Adlercreutz, H. (1992). Enterolactone and estradiol inhibit each other’s proliferative effect on MCF-7 breast cancer cells in culture. The Journal of Steroid Biochemistry and Molecular Biology, 41(3–8), 615–619.
Power, K. A., Ward, W. E., Chen, J. M., Saarinen, N. M., & Thompson, L. U. (2006). Genistein alone and in combination with the mammalian lignans enterolactone and enterodiol induce estrogenic effects on bone and uterus in a postmenopausal breast cancer mouse model. Bone, 39(1), 117–124.
Denis, L., Morton, M. S., & Griffiths, K. (1999). Diet and its preventive role in prostatic disease. European Urology, 35(5–6), 377–387.
Hallund, J., Ravn-Haren, G., Bugel, S., Tholstrup, T., & Tetens, I. (2006). A lignan complex isolated from flaxseed does not affect plasma lipid concentrations or antioxidant capacity in healthy postmenopausal women. The Journal of Nutrition, 136(1), 112–116.
Zhou, Y., Liu, Y. E., Cao, J., Zeng, G., Shen, C., Li, Y., Zhou, M., Chen, Y., Pu, W., Potters, L., et al. (2009). Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clinical Cancer Research, 15(16), 5161–5169.
Prasad, K. (2000). Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. International Journal of Angiology, 9(4), 220–225.
Adlercreutz, H., Fotsis, T., Bannwart, C., Wahala, K., Makela, T., Brunow, G., & Hase, T. (1986). Determination of urinary lignans and phytoestrogen metabolites, potential antiestrogens and anticarcinogens, in urine of women on various habitual diets. Journal of Steroid Biochemistry, 25(5B), 791–797.
Landete, J. M., Arques, J., Medina, M., Gaya, P., de Las Rivas, B., & Munoz, R. (2016). Bioactivation of phytoestrogens: Intestinal bacteria and health. Critical Reviews in Food Science and Nutrition, 56(11), 1826–1843.
McCann, S. E., Thompson, L. U., Nie, J., Dorn, J., Trevisan, M., Shields, P. G., Ambrosone, C. B., Edge, S. B., Li, H. F., Kasprzak, C., et al. (2010). Dietary lignan intakes in relation to survival among women with breast cancer: The Western New York Exposures and Breast Cancer (WEB) Study. Breast Cancer Research and Treatment, 122(1), 229–235.
Guglielmini, P., Rubagotti, A., & Boccardo, F. (2012). Serum enterolactone levels and mortality outcome in women with early breast cancer: A retrospective cohort study. Breast Cancer Research and Treatment, 132(2), 661–668.
Buck, K., Zaineddin, A. K., Vrieling, A., Heinz, J., Linseisen, J., Flesch-Janys, D., & Chang-Claude, J. (2011). Estimated enterolignans, lignan-rich foods, and fibre in relation to survival after postmenopausal breast cancer. British Journal of Cancer, 105(8), 1151–1157.
Hutchins, A. M., Martini, M. C., Olson, B. A., Thomas, W., & Slavin, J. L. (2001). Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutrition and Cancer, 39(1), 58–65.
Mali, A. V., Wagh, U. V., Hegde, M. V., Chandorkar, S. S., Surve, S. V., & Patole, M. V. (2012). In vitro anti-metastatic activity of enterolactone, a mammalian lignan derived from flax lignan, and down-regulation of matrix metalloproteinases in MCF-7 and MDA MB 231 cell lines. Indian Journal of Cancer, 49(1), 181–187.
Adlercreutz, H. (1984). Does fiber-rich food containing animal lignan precursors protect against both colon and breast cancer? An extension of the “fiber hypothesis”. Gastroenterology, 86(4), 761–764.
Serraino, M., & Thompson, L. U. (1992). The effect of flaxseed supplementation on the initiation and promotional stages of mammary tumorigenesis. Nutrition and Cancer, 17(2), 153–159.
Bommareddy, A., Zhang, X. Y., Kaushik, R. S., & Dwivedi, C. (2010). Effects of components present in flaxseed on human colon adenocarcinoma Caco-2 cells: Possible mechanisms of flaxseed on colon cancer development in animals. Drug Discov Ther, 4(3), 184–189.
Lindahl, G., Saarinen, N., Abrahamsson, A., & Dabrosin, C. (2011). Tamoxifen, flaxseed, and the lignan enterolactone increase stroma- and cancer cell-derived IL-1Ra and decrease tumor angiogenesis in estrogen-dependent breast cancer. Cancer Research, 71(1), 51–60.
Saarinen, N. M., Abrahamsson, A., & Dabrosin, C. (2010). Estrogen-induced angiogenic factors derived from stromal and cancer cells are differently regulated by enterolactone and genistein in human breast cancer in vivo. International Journal of Cancer, 127(3), 737–745.
Danbara, N., Yuri, T., Tsujita-Kyutoku, M., Tsukamoto, R., Uehara, N., & Tsubura, A. (2005). Enterolactone induces apoptosis and inhibits growth of Colo 201 human colon cancer cells both in vitro and in vivo. Anticancer Research, 25(3B), 2269–2276.
Dikshit, A., Gao, C., Small, C., Hales, K., & Hales, D. B. (2016). Flaxseed and its components differentially affect estrogen targets in pre-neoplastic hen ovaries. The Journal of Steroid Biochemistry and Molecular Biology, 159, 73–85.
Dikshit, A., Gomes Filho, M. A., Eilati, E., McGee, S., Small, C., Gao, C., Klug, T., & Hales, D. B. (2015). Flaxseed reduces the pro-carcinogenic micro-environment in the ovaries of normal hens by altering the PG and oestrogen pathways in a dose-dependent manner. The British Journal of Nutrition, 113(9), 1384–1395.
Liu, H., Liu, J., Wang, S., Zeng, Z., Li, T., Liu, Y., Mastriani, E., Li, Q. H., Bao, H. X., Zhou, Y. J., et al. (2017). Enterolactone has stronger effects than enterodiol on ovarian cancer. J Ovarian Res, 10(1), 49.
Spilmont, M., Leotoing, L., Davicco, M. J., Lebecque, P., Mercier, S., Miot-Noirault, E., Pilet, P., Rios, L., Wittrant, Y., & Coxam, V. (2014). Pomegranate and its derivatives can improve bone health through decreased inflammation and oxidative stress in an animal model of postmenopausal osteoporosis. European Journal of Nutrition, 53(5), 1155–1164.
Costantini, S., Rusolo, F., De Vito, V., Moccia, S., Picariello, G., Capone, F., Guerriero, E., Castello, G., & Volpe, M. G. (2014). Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules, 19(6), 8644–8660.
Faria, A., & Calhau, C. (2011). The bioactivity of pomegranate: Impact on health and disease. Critical Reviews in Food Science & Nutrition, 51(51), 626–634.
Amin, A. R., Kucuk, O., Khuri, F. R., & Shin, D. M. (2009). Perspectives for cancer prevention with natural compounds. Journal of Clinical Oncology, 27(16), 2712–2725.
Syed, D. N., Afaq, F., & Mukhtar, H. (2007). Pomegranate derived products for cancer chemoprevention. Seminars in Cancer Biology, 17(5), 377–385.
Jurenka, J. S. (2008). Therapeutic applications of pomegranate (Punica granatum L.): A review. Alternative Medicine Review: A Journal of Clinical Therapeutic, 13(2), 128–144.
Seeram, N. P., Adams, L. S., Henning, S. M., Niu, Y., Zhang, Y., Nair, M. G., & Heber, D. (2005). In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. Journal of Nutritional Biochemistry, 16(6), 360–367.
Turrini, E., Ferruzzi, L., & Fimognari, C. (2015). Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxidative Medicine & Cellular Longevity, 2015, 1–19.
Lópezlázaro, M. (2009). Distribution and biological activities of the flavonoid luteolin. Mini Reviews in Medicinal Chemistry, 9(1), 31–59.
Amrutha, K., Nanjan, P., Shaji, S. K., Sunilkumar, D., Subhalakshmi, K., Rajakrishna, L., & Banerji, A. (2014). Discovery of lesser known flavones as inhibitors of NF-κB signaling in MDA-MB-231 breast cancer cells—A SAR study. Bioorganic & Medicinal Chemistry Letters, 24(19), 4735–4742.
Pandurangan, A. K., Dharmalingam, P., Sadagopan, S. K., & Ganapasam, S. (2014). Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Human & Experimental Toxicology, 33(11), 1176–1185.
Seeram, N. P., Henning, S. M., Zhang, Y., Suchard, M., Li, Z., & Heber, D. (2006). Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. Journal of Nutrition, 136(10), 2481–2485.
Gil, M. I., Tomásbarberán, F. A., Hesspierce, B., Holcroft, D. M., & Kader, A. A. (2000). Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. Journal of Agricultural & Food Chemistry, 48(10), 4581–4589.
Mehta, R., & Lansky, E. P. (2004). Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. European Journal of Cancer Prevention, 13(4), 345–348.
Tsuda, H., Uehara, N., Iwahori, Y., Asamoto, M., Iigo, M., Nagao, M., Matsumoto, K., Ito, M., & Hirono, I. (1994). Chemopreventive effects of beta-carotene, alpha-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo[4,5-f]quinoline in the rat. Japanese Journal of Cancer Research, 85(12), 1214–1219.
Tharappel, J. C., Lehmler, H. J., Srinivasan, C., Robertson, L. W., Spear, B. T., & Glauert, H. P. (2008). Effect of antioxidant phytochemicals on the hepatic tumor promoting activity of 3,3′,4,4′-tetrachlorobiphenyl (PCB-77). Food and Chemical Toxicology, 46(11), 3467–3474.
Shirode, A. B., Kovvuru, P., Chittur, S. V., Henning, S. M., Heber, D., & Reliene, R. (2014). Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Molecular Carcinogenesis, 53(6), 458–470.
Wang, L., & Martins-Green, M. (2014). Pomegranate and its components as alternative treatment for prostate cancer. International Journal of Molecular Sciences, 15(9), 14949–14966.
Jaganathan, S. K., Vellayappan, M. V., Narasimhan, G., & Supriyanto, E. (2014). Role of pomegranate and citrus fruit juices in colon cancer prevention. World Journal of Gastroenterology, 20(16), 4618–4625.
Liu, H., Zeng, Z., Wang, S., Li, T., Mastriani, E., Li, Q. H., Bao, H. X., Zhou, Y. J., Wang, X., Liu, Y., et al. (2017). Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Biology & Therapy, 18(12), 990–999.
Yoshimi, N., Matsunaga, K., Katayama, M., Yamada, Y., Kuno, T., Qiao, Z., Hara, A., Yamahara, J., & Mori, H. (2001). The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Letters, 163(2), 163–170.
Barreto, J. C., Trevisan, M. T. S., Hull, W. E., Gerhard, E., Brito, E. S., De Beate, P., Gerd, W., Bertold, S., & Owen, R. W. (2008). Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). Journal of Agricultural & Food Chemistry, 56(14), 5599.
Chavan, J. J., Ghadage, D. M., Kshirsagar, P. R., & Kudale, S. S. (2015). Optimization of extraction techniques and RP-HPLC analysis of antidiabetic and anticancer drug mangiferin from roots of ‘Saptarangi’ (Salacia chinensisL.). Journal of Liquid Chromatography & Related Technologies, 38(9), 963–969.
Burton-Freeman, B. M., Sandhu, A. K., & Edirisinghe, I. (2017). Mangos and their bioactive components: Adding variety to the fruit plate for health. Food & Function, 8(9), 3010.
Pinto, M. M., Sousa, M. E., & Nascimento, M. S. (2005). Xanthone derivatives: New insights in biological activities. Current Medicinal Chemistry, 12(21), 2517–2538.
Li, H., Huang, J., Yang, B., Xiang, T., Yin, X., Peng, W., Cheng, W., Wan, J., Luo, F., & Li, H. (2013). Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicology & Applied Pharmacology, 272(1), 180–190.
Rajendran, P., Rengarajan, T., Nishigaki, I., Ekambaram, G., & Sakthisekaran, D. (2014). Potent chemopreventive effect of mangiferin on lung carcinogenesis in experimental Swiss albino mice. Journal of Cancer Research & Therapeutics, 10(4), 1033–1039.
Zhang, B. P., Zhao, J., Li, S. S., Yang, L. J., Zeng, L. L., Chen, Y., & Fang, J. (2014). Mangiferin activates Nrf2-antioxidant response element signaling without reducing the sensitivity to etoposide of human myeloid leukemia cells in vitro. Acta Pharmacologica Sinica, 35(2), 257–266.
Peng, Z. G., Yao, Y. B., Yang, J., Tang, Y. L., & Huang, X. (2015). Mangiferin induces cell cycle arrest at G2/M phase through ATR-Chk1 pathway in HL-60 leukemia cells. Genetics & Molecular Research, 14(2), 4989–5002.
Pan, L.-L., Wang, A.-Y., Huang, Y.-Q., Luo, Y., & Ling, M. (2014). Mangiferin induces apoptosis by regulating Bcl-2 and Bax expression in the CNE2 nasopharyngeal carcinoma cell line. Asian Pacific Journal of Cancer Prevention, 15(17), 7065–7068.
Das, S., Rao, B. N., & Rao, B. S. S. (2011). Mangiferin attenuates methylmercury induced cytotoxicity against IMR-32, human neuroblastoma cells by the inhibition of oxidative stress and free radical scavenging potential. Chemico-Biological Interactions, 193(2), 129–140.
Davydov, M., & Krikorian, A. (2000). Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: A closer look. Journal of Ethnopharmacology, 72(3), 345–393.
Ho-Shan, N., I-Min, L., Juei-Tang, C., Che-Ling, L., & Feng-Lin, H. (2008). Hypoglycemic effect of syringin from Eleutherococcus senticosus in Streptozotocin-induced diabetic rats. Planta Medica, 74(2), 109–113.
Bahrke, M. S., Morgan, W. P., & Stegner, A. (2009). Is ginseng an ergogenic aid? International Journal of Sport Nutrition & Exercise Metabolism, 19(3), 298–322.
Eschbach, L. F., Webster, M. J., Boyd, J. C., Mcarthur, P. D., & Evetovich, T. K. (2000). The effect of Siberian ginseng (Eleutherococcus senticosus) on substrate utilization and performance. International Journal of Sport Nutrition & Exercise Metabolism, 10(4), 444–451.
Arouca, A., & Grassi-Kassisse, D. M. (2013). Eleutherococcus senticosus: Studies and effects. Health, 5(9), 1509–1515.
Nishibe, S., Kinoshita, H., Takeda, H., & Okano, G. (1990). Phenolic compounds from stem bark of Acanthopanax senticosus and their pharmacological effect in chronic swimming stressed rats. Chemical & Pharmaceutical Bulletin, 38(6), 1763–1765.
Linzhang, H., Hongfang, Z., Baokang, H., Chengjian, Z., Wei, P., & Luping, Q. (2011). Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Die Pharmazie, 66(2), 83–97.
Chen, L. I., Wang, X. Y., Xu-Wei, H. U., Fang, H. T., & Qiao, S. Y. (2008). [Determination of eleutheroside B in antifatigue fraction of Acanthopanax senticosus by HPLC]. Zhongguo Zhong yao za zhi, 33(23):2800–2802.
Verma, R. P., & Hansch, C. (2007). Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q)SARs. Bioorganic & Medicinal Chemistry, 15(6), 2223–2268.
Coussens, L. M., & Werb, Z. (1996). Matrix metalloproteinases and the development of cancer. Chemistry & Biology, 3(11), 895–904.
Cheung, L. W. T., Leung, P. C. K., & Wong, A. S. T. (2006). Gonadotropin-releasing hormone promotes ovarian cancer cell invasiveness through c-Jun NH2-terminal kinase-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Research, 66(22), 10902–10910.
Lu, Y. M., Rong, M. L., Shang, C., Wang, N., Li, X., Zhao, Y. Y., & Zhang, S. L. (2012). Suppression of HER-2 via siRNA interference promotes apoptosis and decreases metastatic potential of SKOV-3 human ovarian carcinoma cells. Oncology Reports, 29(3), 1133–1139.
Yu, Y., Li, H., Xue, B., Jiang, X., Huang, K., Ge, J., Zhang, H., & Chen, B. (2014). SDF-1/CXCR7 axis enhances ovarian cancer cell invasion by MMP-9 expression through p38 MAPK pathway. DNA & Cell Biology, 33(8), 543–549.
Langers, A. M., Verspaget, H. W., Hawinkels, L. J., Kubben, F. J., van Duijn, W., van der Reijden, J. J., Hardwick, J. C., Hommes, D. W., & Sier, C. F. (2012). MMP-2 and MMP-9 in normal mucosa are independently associated with outcome of colorectal cancer patients. British Journal of Cancer, 106(9), 1495–1498.
Mieszalo, K., Lawicki, S., & Szmitkowski, M. (2016). [The utility of metalloproteinases (MMPs) and their inhibitors (TIMPs) in diagnostics of gynecological malignancies]. Polski Merkuriusz Lekarski, 40(237):193–197.
Turrini, E., Ferruzzi, L., & Fimognari, C. (2015). Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxidative Medicine & Cellular Longevity, 2014, 1–19.
Nair, V., Dai, Z., Khan, M., & Ciolino, H. P. (2011). Pomegranate extract induces cell cycle arrest and alters cellular phenotype of human pancreatic cancer cells. Anticancer Research, 31(9), 2699–2704.
Huang, S. T., Wang, C. Y., Yang, R. C., Wu, H. T., Yang, S. H., Cheng, Y. C., & Pang, J. H. S. (2011). Ellagic acid, the active compound of Phyllanthus urinaria, exerts in vivo anti-angiogenic effect and inhibits MMP-2 activity. Evidence-Based Complementary and Alternative Medicine, 2011(5), 296–297.
Yuan-Chiang, C., Li-Cheng, L., Ming-Hsiu, T., Yu-Jen, C., Yi-Ying, C., Shih-Ping, Y., & Chih-Ping, H. (2013). The inhibitory effect of ellagic acid on cell growth of ovarian carcinoma cells. Evidence-Based Complementary and Alternative Medicine, 2013(2), 386.
Amakura, Y., Mai, O., Tsuji, S., & Tonogai, Y. (2000). High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. Journal of Chromatography A, 896(1–2), 87–93.
Miean, K. H., & Mohamed, S. (2001). Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. Journal of Agricultural & Food Chemistry, 49(6), 3106–3112.
Acknowledgments
This work was supported by grants of the National Natural Science Foundation of China (NSFC30970119, 81030029, 81271786, NSFC-NIH 81161120416, 81671980, 81871623) and College students’ Innovation & Entrepreneurship project in Heilongjiang Province (201410226047 J.J.K, D.Y.; 201510226020 D.S.L, L.Y., T.L., L.Q., L.L.G.; 201610226095 H.Y.W., Z.H.S. T.T.G., S.J.H., S.G.; 201610226094 Y.Y.Q., M.Y.; 201710226073 S.J.H., S.G.). H.D.L. is supported by a scholarship from China Scholarship Council, CSC No. 201508230143, for an academic visit to the University of Calgary (Univ. of Calgary ID number: 30016355). We also thank the Health and Family Planning Commission of Heilongjiang Province (2016-188), the Fundamental Research Funds for the Provincial Universities (2017JCZX57), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2018064), the China Postdoctoral Science Foundation (2018M630380), and the Heilongjiang Postdoctoral Financial Assistance (LBH-Z18198) program for their support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Liu, H., Liu, SL. (2021). Pharmacological Effects of Natural Components Against Ovarian Cancer and Mechanisms. In: Schatten, H. (eds) Ovarian Cancer: Molecular & Diagnostic Imaging and Treatment Strategies. Advances in Experimental Medicine and Biology, vol 1330. Springer, Cham. https://doi.org/10.1007/978-3-030-73359-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-73359-9_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73358-2
Online ISBN: 978-3-030-73359-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)