Abstract
The diagnosis of acute myeloid leukemia (AML) requires timely treatment in order to avoid life threatening complications. In patients fit for intensive treatment, the realistic goal of treatment is the cure, which can be achieved in around 50% either by cytoreductive treatment alone or in combination with allogeneic stem cell transplantation. Adding novel agents to standard treatment improves its efficacy in certain subgroups. This chapter describes the current treatment standards, how they evolved, discusses concepts of fitness and time frame from diagnosis to treatment, and reveals open questions in conventional treatment as well as future options for first-line treatment with novel agents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Induction
- Postremission
- Consolidation
- Transplantation
- Targeted
- Treatment
- Fit
- Maintenance
- Stratification
- Algorithm
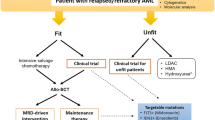
9.1 What Is Fit?
Untreated AML is a fatal disease. With the evolvement of treatment options beginning in the 1960s, it was demonstrated that a small proportion of patients can achieve long-term remissions, even beyond 5 years, indicating eradication of the disease and the potential of long-term cure. However, intensive cytoreductive treatment approaches had a rather high associated toxicity, in particular in old patients, leading to treatment-associated mortality during initial induction therapy around 20% (Atallah et al. 2007). In order to avoid that a potentially curative treatment results in a fatal outcome, researchers have continuously attempted to define and refine criteria and conditions associated with a high risk of life-threatening complications such as severe infections and sepsis often resulting in multi-organ failure. Patients fulfilling these criteria would rather not benefit from intensive treatment and would be considered ineligible for intensive treatment, “unfit,” or “frail.” Best supportive care plus/minus low-intensity treatments are offered to these patients with the goal to reduce the leukemic burden and prolong life while maintaining a reasonable quality of life in an outpatient setting (see Chap. 10). Not in all instances, the decision is straightforward since treatment-related mortality rates have been going down during the last years and so far, and it is still a matter of debate which patients benefit from receiving low-intensive treatments rather than intensive chemotherapy (Michaelis 2018).
Over time, several retrospective analyses from clinical trials using intensive therapy have identified factors associated with the risk of early death. Additionally, the chances of achieving a CR and long-term remission can be estimated by scores in order to balance benefits and risks in a shared decision-making process (Appelbaum et al. 2006; Walter et al. 2011; Krug et al. 2010; Wheatley et al. 2009; Klepin et al. 2013; Ossenkoppele and Löwenberg 2015; Valcárcel et al. 2012). There is no prospective evaluation or intervention-based study to validate scores and determine their predictive potential. Instead, items of the scores have been used and variably combined in catalogs and lists to determine eligibility for intensive treatment in guidelines and position papers (Michaelis 2018; Ferrara et al. 2013). There is no internationally agreed general set of criteria defining frailness or ineligibility of intensive treatment. However, most sets of criteria include:
-
age > 75–80 years,
-
significant comorbidities such as severe cardiac insufficiency or pulmonary disease, late-stage diabetes mellitus with signs of end-organ damage or an HCT-CI score ≥3,
-
geriatric assessment revealing high-risk features including poor cognitive function, and
-
a general clinical performance not related to AML of WHO/ECOG >2.
9.2 Time from Diagnosis to Treatment
Untreated AML is in general associated with a very limited remaining life span of only a few weeks as known from historic data (Southam et al. 1951). As a result, it has been a long-standing treatment paradigm to consider AML a hematologic emergency and to start treatment immediately after the establishment of the diagnosis. This paradigm was reinforced by retrospective data from 2009 showing that in young patients up to the age of 60 years with a time interval from diagnosis to treatment (TDT) of ≥5 days, the overall survival was significantly worse than in patients with a TDT <5 days (Sekeres et al. 2009). However, in a different cohort of newly diagnosed AML patients receiving a more homogeneous induction treatment and including patients with hyperleukocytosis, no difference in the overall prognosis could be found by several statistical methods (Fig. 9.1; (Bertoli et al. 2013). This finding was confirmed in the most recent and largest analysis in more than 2200 uniformly treated AML patients, which again failed to show differences neither in remission rates, early death rates nor overall survival when analyzing TDT durations of 0–5, 6–10, 11–15, and >15 days (Röllig et al. 2019). Based on these findings, it seems reasonable to wait for the results of the diagnostic and genetic workup in a clinically stable patient as the prognosis and clinical course seem to be determined by other factors than TDT. Clearly, no evidence is necessary to recommend immediate treatment start in patients with AML-related complications such as leukostasis, neutropenic fever, or deranged coagulation. Close clinical observation and blood monitoring are necessary in patients with delayed treatment start in order to detect the onset of potential AML-related complications as early as possible.
Association between overall survival (OS) and time from diagnosis to treatment (dichotomized at 5 days) (Bertoli et al. 2013)
9.3 Development of Current Standards
In 1971, James F. Holland, one of the pioneers of antineoplastic treatment in leukemias, stated three historic treatment phases of acute leukemia: (1) before 1947: the era of despair with no effective treatment; (2) from 1947 to 1963: the advent of chemotherapy, and with the failure to find a curative drug, the era of palliation; (3) since 1963, the appearance of new drugs, their use in intensive regimens and in combinations, which “have all made palliation too mean a goal” (Fairley 1971).
9.3.1 Induction
The first published attempts using cytarabine as a single agent in 1968 provided evidence of hematological remissions in 17–24% of patients (Ellison et al. 1968). Around the same time, daunorubicin was first used in pediatric and adult AML achieving hematologic remissions in 55% of patients (Boiron et al. 1969). Soon after, cytarabine and daunorubicin were combined in intermittent treatment intervals, followed by low-dose maintenance treatment with 6-MP and MTX or BCG. This first combination attempt was tested in 13 mostly younger patients aged 24–64 years and delivered a CR rate of 70% (Crowther et al. 1970). The combination of 7 days of cytarabine plus 3 days of daunorubicin was first published in 1973 (Yates et al. 1973). Later, four different variations of cytarabine (100 mg/m2) plus daunorubicin (45 mg/m2) combinations were prospectively evaluated in a randomized CALGB trial: 7 days of cytarabine continuous infusion plus 3 days of daunorubicin bolus versus delivered the highest CR rate (55%) and established the infusional 7 + 3 schema as a long-lasting treatment standard (Rai et al. 1981).
Continuous attempts were made to improve the efficacy of 7 + 3 by changing both the dose of its components, by substituting daunorubicin with other agents, by varying sequencing, and by the addition of other conventional cytoreductive agents.
The randomized comparison between 100 and 200 mg cytarabine provided no evidence of benefit in response or survival (Burnett et al. 2010a; Dillman et al. 1991). High doses of cytarabine (HDAC) delivered higher CR rates and prolonged RFS in randomized trials (Willemze et al. 2014; Burnett et al. 2013), whereas this could not be confirmed in other trials (Löwenberg et al. 2011; Röllig et al. 2018a; Niederwieser et al. 2016) or meta-analyses (Kern and Estey 2006). Subgroup analyses of one trial showed a survival benefit only in patients aged 15–45 years (Willemze et al. 2014). Apart from this, there is no significant evidence for an overall survival benefit associated with the use of HDAC in induction treatment in neither of these trials.
Doubling the traditional dose of daunorubicin to 90 mg/m2 led to a significant increase both in remission rates and OS in three randomized trials in patients up to the age of 65 years, which led to a departure from using 45 mg/m2. Two randomized comparisons of 60 mg versus 90 mg daunorubicin did not show significant differences in CR rates nor OS (Burnett et al. 2015; Röllig et al. 2018b). Subgroup analyses from one of these studies suggested a significant benefit of 90 mg daunorubicin in the subgroup of FLT3-ITD mutated patients (Burnett et al. 2016). Based on the mentioned results, most clinicians consider 60 mg daunorubicin as the standard dose. Furthermore, 7 + 3 containing 60 mg daunorubicin has been and is currently used as a backbone for the combination with novel agents (see Sect. 9.4).
The use of idarubicin as an alternative anthracycline instead of daunorubicin was associated with significantly higher remission rates, which did not translate into prolonged survival outcomes (Pautas et al. 2010). Other trials could not confirm a benefit in remission rates, and there is no evidence of a survival benefit by idarubicin (Lee et al. 2017; Gardin et al. 2013). In a meta-analysis, Teuffel et al. could show that the chances of remission are not different when the dose ratio of daunorubicin and idarubicin was ≥5 (Teuffel et al. 2013). Trials comparing the efficacy of mitoxantrone with daunorubicin showed no difference, neither in remission nor survival (Burnett et al. 2010a; Löwenberg et al. 1998; Mandelli et al. 2009).
A further 7 + 3 variation used a high-dose cytarabine–mitoxantrone combination and split it in two sequential halves (S-HAM) in order to increase efficacy and reduce toxicity. A comparison with two cycles of 7 + 3 showed a significantly reduced duration of leukopenia by S-HAM, but no difference in remission rates and no significant improvement in survival (Braess et al. 2018).
Various attempts have been made to improve the efficacy of 7 + 3 by the addition of other agents such as G-CSF or etoposide, but with no benefit (Krug et al. 2016; Burnett et al. 2010b; Estey et al. 1999). The addition of the purine analog cladribine to 7 + 3 in younger patients resulted in a significant OS benefit. It did not seem to benefit patients with poor-risk cytogenetics or age ≥50 years, and in general CR rates and OS in the control arm were relatively low (Holowiecki et al. 2012; Pluta et al. 2017).
9.3.2 Consolidation
After it had been shown that cytarabine and daunorubicin could induce complete hematologic remission as early as the late 1960s, it soon became clear that these remissions were not durable, even under low-dose cytarabine maintenance (Carey et al. 1975). Dose intensification of cytarabine to single doses of 3 g given repetitively over 5 days reduced the relapse rate significantly when compared with standard-dose cytarabine. However, this improvement was only seen in younger patients up to the age of 60 years (Mayer et al. 1994). Later it was shown that higher doses of cytarabine are able to significantly reduce the relapse rate also in patients older than 60 years (Röllig et al. 2018c). Attempts to improve the efficacy of consolidation treatment by adding other drugs were not superior to cytarabine alone, but associated with a higher risk of toxicity and no consistent survival benefit (Burnett et al. 2013; Schaich et al. 2013).
Whereas conventional consolidation treatment comprises 3–4 cycles of treatment, the administration of one cycle of myeloablative therapy followed by autologous stem cell rescue represents a more condensed and potentially equally effective treatment option. In comparative studies, autologous transplantation provided a benefit in RFS, mainly for favorable and intermediate risk patients. OS did not differ significantly between autologous transplantation and conventional high-dose cytarabine-based regimens (Vellenga et al. 2011; Pfirrmann et al. 2012; Cornelissen et al. 2015). The use of peripheral stem cells has reduced treatment-related mortality (TRM) enormously in comparison with bone marrow derived stem cells (SC), and hospital stay for one autologous transplantation is shorter than for 2–3 cycles of cytarabine.
There is evidence from several trials that a single dose of 1–1.5 g cytarabine may be equally effective as the original 3 g (Schaich et al. 2011). The only randomized comparison between 3 and 1.5 g shows a trend for better survival after 3 g in favorable and adverse and for 1.5 g in intermediate genetic risk (Burnett et al. 2013). Furthermore, data indicate that the third course of consolidation after double induction may not be necessary (Burnett et al. 2013; Löwenberg 2013).
9.3.3 Comparison of Standard Approaches for Induction and Consolidation
A large German intergroup study compared double induction chemotherapy using 7 + 3 (with 60 mg daunorubicin) followed by high-dose cytarabine consolidation with five different approaches for induction and consolidation including all variations of conventional induction and consolidation outlined above. The results of this 6-arm randomized trial assessing 3106 patients up to the age of 60 years showed significantly higher CR rates if patients with no response after one induction were treated with a combination of intermediate-dose cytarabine, idarubicin, and fludarabine (FLAG-Ida). However, as the main and sobering finding of the trial, no differences in relapse-free and overall survival were observed across all different induction-consolidation approaches (see Fig. 9.2) (Büchner et al. 2012).
Overall survival and relapse-free survival in over 3000 patients comparing standard 7 + 3 double induction followed by high-dose cytarabine consolidation with five alternative conventional induction and postremission strategies (Büchner et al. 2012)
The study gives a good overview of the results and the therapeutic potential of standard chemotherapy in a younger AML population with 70–82% CR/CRi rates, 5-year EFS of 27–39%, 5-year RFS of 35–47%, and a 5-year OS of 41–48%.
In elderly patients with intensive conventional treatment, trials produce 39–54% CR/CRi rates, a 5-year EFS of 10%, 5-year RFS of 10–25%, and 5-year OS of 15% (Röllig et al. 2018c; Löwenberg et al. 2009).
The inclusion and exclusion criteria of clinical trials create a positive selection of patients who are fitter than the general population (Estey and Gale 2017; Estey et al. 2018). Therefore, it is important to look at registry data to get a more comprehensive picture (Röllig et al. 2019; Nagel et al. 2017; Juliusson et al. 2012).
9.3.4 Maintenance
Historically, the first approach to keep patients in remission was the prolonged application of classic cytostatic agents. Whereas neither 6-MP, MTX, BCG nor low-dose cytarabine with or without thioguanine did turn out successfully (Crowther et al. 1970; Carey et al. 1975; Cassileth et al. 1992), the combination of 6-thioguanine, cytarabine, and daunorubicin given in low doses sequentially over 3 years was equally effective as one cycle of high-dose cytarabine-based consolidation (Büchner et al. 2003). However, with regard to time, effort, and convenience, this maintenance approach has not been widely implemented. Randomized trials exploring alternative substances for maintenance such as interferon, IL-2 with or without histamine or androgens for maintenance showed an improvement in relapse-free survival (RFS) for IL-2 plus histamine and for androgens, but all failed to show a significant improvement in survival for the entire patient population (Pautas et al. 2010; Goldstone et al. 2001; Brune et al. 2006; Pigneux et al. 2018).
Recently, a small randomized trial using azacitidine as maintenance for patients >65 years in CR after intensive induction showed a significant improvement in RFS which did not translate into an OS benefit, potentially due to differences in relapse treatments in the two patient groups (Huls et al. 2019). In a similarly designed larger randomized trial, the orally available hypomethylating compound CC-486 was used versus placebo for maintenance in CR patients >=55 years with intermediate or adverse cytogenetic risk after intensive pre-treatment not eligible for allogeneic stem cell transplantation. CC-486 reduced the risk for relapse or death by 35% and for death by 31%, resulting in an OS prolongation of 9.9 months (HR: 0.69) (Wei et al. 2020). As relapses occurred later but to a similar extent in the CC-486 arm, the long-term remission rate was still similar between both patient groups, indicating a prolongation of survival by CC-486 maintenance, but not an increase in the proportion of cured patients.
As new compounds with a more specific mode of action are evaluated in the first-line treatment and enter clinical practice (see Chaps. 17–19), their continuous use beyond induction may become a new mode of maintenance with the option not only to prolong remission, but also to increase the rate of cure.
9.4 Novel Agents and Treatment Stratification for Induction
Cytogenetic and molecular methods revealed that AML patients share the same clinical features and findings, but that on the biological and cellular level, there is a wide heterogeneity (see Chap. 5). However, conventional cytoreductive agents such as cytarabine and daunorubicin do not target differences in genetic cellular configurations. Patients with high genetic risk showed an adverse disease course with standard therapy, no matter which conventional agents were used (see Sect. 9.3.3). Due to a lack of other effective drugs, a “one size fits all” approach has been common practice in AML treatment for decades, using the standard 7 + 3 or one of its variations for all newly diagnosed fit AML patients.
The development of novel agents targeting cellular pathways that may be essential for leukemogenesis has led to improvements in treatment outcomes, accompanied by differential responses in different genetic subgroups. The approval and subsequent availability of some of these agents have changed the treatment landscape and have led to a diversification of AML therapy.
9.4.1 Tyrosine-Kinase Inhibitors
The presence of an internal tandem duplication mutation (ITD) in the gene coding for the FLT3 tyrosine kinase can drive hematopoietic cells toward leukemia and lead to increased proliferation and resistance to apoptosis in myeloid blasts, corresponding to a high relapse rate and limited long-term survival (Mizuki et al. 2003; Thiede et al. 2002). It was hypothesized that small molecules inhibiting FLT3 signaling could improve the course of the disease (Larrosa-Garcia and Baer 2017). First-generation tyrosine-kinase inhibitors (TKI) target several cellular kinases and have limited single-agent activity.
The first randomized evidence for the efficacy of TKIs in combination with intensive chemotherapy came from sorafenib, which prolonged EFS and RFS, but not OS significantly in a younger patient population ≤60 years irrespective of the FLT3 mutational status (Röllig et al. 2015). In elderly patients, sorafenib led to increased toxicity that prevented a survival benefit (Serve et al. 2013).
The RATIFY trial evaluated midostaurin in combination with standard induction and consolidation chemotherapy and as maintenance for 12 months in a randomized placebo-controlled design. While the addition of midostaurin did not increase the CR rates, RFS and OS were significantly prolonged, with an increase in median OS from 26 to 75 months (HR: 0.78). These results led to the approval of midostaurin for the first-line treatment of FLT3-mutated AML in combination with standard chemotherapy (Stone et al. 2017). Although the value of midostaurin in maintenance was not clear based on the study design, the EMA approved the drug also for maintenance.
The second-generation TKIs are more specific for FLT3 and inhibit fewer additional kinases (Larrosa-Garcia and Baer 2017). Furthermore, agents, such as quizartinib, gilteritinib, and crenolanib, show significant single-agent activity. Quizartinib and gilteritinib have been shown to be more effective than standard salvage treatment in relapsed/refractory FLT3 mutated AML, and gilteritinib has been approved for single-agent use in this clinical setting (see Chaps. 11 and 12). Currently, all three agents are evaluated in combination with standard intensive treatment for newly diagnosed fit AML patients.
9.4.2 Monoclonal Antibodies
As CD33 can be found on blasts of almost all AML types (Ehninger et al. 2014), targeting AML blasts with antibodies has been considered a promising treatment concept. Gemtuzumab ozogamicin (GO) is a humanized monoclonal CD33 antibody conjugated with the toxin calicheamicin. By binding to CD33 positive AML cells, the antibody–drug conjugate is internalized into the cell and broken down, releasing calicheamicin, which then binds to the DNA and causes apoptosis (Tsuchikama and An 2018). Several trials have shown proof of GO efficacy in relapsed and primary AML. For the combination of GO and standard intensive chemotherapy, meta-analyses of randomized trials have shown that (1) a low-dose fractionated administration results in the best tolerability, and (2) among AML subgroups, patients with favorable risk AML have the greatest benefit from GO in addition to standard therapy (Hills et al. 2014; Li et al. 2014). Results on the requirement of CD33 expression have been mixed (Walter et al. 2007; Khan et al. 2017); similarly, single-nucleotide polymorphisms (SNP) genotyping of large numbers of GO treatment patients disagree about its predictive ability (Lamba et al. 2017; Gale et al. 2018).
In the randomized open-label ALFA-0701 trial, GO was added to standard induction and consolidation treatment of newly diagnosed AML patients with mainly intermediate or adverse cytogenetic risk. The addition of GO led to a significant prolongation of event-free and relapse-free survival, whereas a benefit in OS did not reach statistical significance. Subgroup analyses revealed that the survival benefit was caused by patients with favorable or intermediate cytogenetics, whereas patients with adverse risk did not benefit from GO (Lambert et al. 2019). According to subgroup analyses, patients with NPM1mut and also FLT3-ITD showed a greater risk reduction by GO. A meta-analysis of five randomized trials identified the greatest survival benefit in patients with favorable risk (20% difference in 5-year OS), a smaller significant benefit in intermediate risk (6% difference in 5-year OS), and no benefit for adverse risk patients (Hills et al. 2014).
Based on the results of ALFA-0701, GO was approved by FDA and EMA for the treatment of newly diagnosed CD33 positive AML in combination with standard chemotherapy.
The effect of GO in addition to induction therapy with idarubicin, standard-dose cytarabine plus etoposide (ICE) in NPM1 positive AML patients, was assessed in the randomized open-label AML-SG 09-09 study. The use of GO was associated with a significant reduction in relapse risk, but the combination with ICE led to an increased early mortality rate in elderly patients, most likely due to the combination with etoposide and ATRA (Schlenk et al. 2019).
The impact of GO in postremission treatment is currently uncertain since there is no randomized evidence for a benefit in postremission (Burnett et al. 2011).
Several CD33 immunotherapy approaches are in clinical development. Also, CD123, CD70, and CD47 targets are in advanced clinical development and may become relevant for the first-line treatment in the future (see Chap. 19).
9.4.3 Liposomal Formulation of Cytarabine and Daunorubicin (CPX-351)
CPX-351 is a liposomal formulation of a fixed molar ratio (1:5) of daunorubicin and cytarabine. After cellular internalization, liposomes undergo degradation, releasing cytarabine and daunorubicin intracellularly to induce DNA damage resulting in cell death. In vitro studies demonstrated that the 1:5 ratio resulted in synergistic in vitro cytotoxicity in the majority of cancer cell lines evaluated (Krauss et al. 2019).
Study CLTR0310-301, a randomized, multicenter, open-label, active-controlled trial compared CPX-351 with a standard 7 + 3 combination of daunorubicin and cytarabine in 309 patients 60–75 years of age with newly diagnosed t-AML or AML-MRC. The results demonstrated higher remission rates (48% versus 33%), and an improvement in overall survival (HR: 0.69) by CPX-351 with an estimated median overall survival of 9.6 months compared with 5.9 months for the 7 + 3 control arm. The survival benefit was pronounced in patients who were able to proceed to allogeneic stem cell transplantation after receiving CPX-351 (HR: 0.46) compared with 7 + 3 induction (Lancet et al. 2018). Based on these results, CPX-351 was approved by FDA and EMA for newly diagnosed tAML or AML-MRC of all age groups.
9.5 Balancing Risks and Benefits in Postremission Treatment
Standard induction treatment without the addition of novel agents will bring around 60–80% of younger adults and 40–60% of older patients in complete morphologic remission, depending on prognostic factors, of which age and genetics are the most important (see Chap. 7). Still more than half of all intensively treated patients die from the disease (Dinmohamed et al. 2016), as relapse and subsequent treatment failure remain the biggest challenge in AML treatment (see Chaps. 11 and 12). As (1) the physical condition of patients in a relapsed situation after intensive first-line therapy may limit the option of salvage treatment, and (2) the relapsed disease is generally more difficult to treat, the primary goal of the first-line treatment is to prevent relapses. They will occur in almost 100% of CR patients if treatment is stopped after induction due to small quantities of residual leukemia cells (see Chap. 18).
In general, either dose intensive chemotherapy (“consolidation”) or allogeneic stem cell transplantation (allo-SCT) will be used for postremission treatment. Whereas autologous transplantation can be considered as being part of the first option, the graft versus leukemia immune mechanisms after allogeneic SCT introduce a different antileukemic mode of action (see Chap. 13). Allogeneic lymphocytes and the resulting immune mechanisms are at the same time boon and bane of allo-SCT. Whereas the graft versus leukemia effect eliminates chemoresistant leukemic cells and reduces the relapse rate compared with chemo-consolidation, the delayed immune reconstitution after SCT and the organ damage of graft versus host disease reduce the quality of life and increase the number of patients dying in remission (nonrelapse mortality).
The best way to balance the risks and benefits of consolidation chemotherapy versus allo-SCT is to weigh up the estimated relapse risk and the expected transplant-related mortality. The latter can be assessed by the EBMT score integrating age, disease stage, donor type, donor–recipient gender combination, and time interval from diagnosis to transplantation (Gratwohl 2012). Additionally, information on comorbidities contribute to the assessment of post-transplant mortality (Sorror et al. 2008). If the risk of non-relapse mortality (NRM) exceeds the risk of relapse after allo-SCT, the use of chemo-consolidation should be favored according to the guidelines of the ELN AML working party (Cornelissen et al. 2012). In fit patients in first CR with a good matched and readily available donor, the preferred postremission option for patients with favorable genetics would be chemotherapy, whereas allo-SCT would be recommended for an adverse risk constellation. In an intermediate-risk patient, a more detailed and individualized assessment is necessary (see Chap. 13).
Patients with FLT3-ITD at a low ITD-WT allelic ratio (FLT3-ITDlow) and co-occurring NPM1 mutation (NPM1mut) who have access to midostaurin represent a more complex scenario regarding relapse risk and postremission treatment decision. The low FLT3-ITD ratio, the NPM1 mutation, and midostaurin treatment reduce the relapse risk compared with other FLT3-ITD patients, who have a generally high risk of relapse compared with FLT3wt or FLT3-TKD and should be advised to undergo allo-SCT. If FLT3-ITDlow-NPNM1mut patients under midostaurin treatment are in hematologic CR and the level of minimal residual disease (MRD) is low as defined by NPM1mut/ABL levels or Multicolor Flow Cytometry (MFC), the relapse risk can be considered low based on studies on disease kinetics in NPM1mut patients after the end of consolidation (Krönke et al. 2011; Shayegi et al. 2013). Therefore, these patients can be advised to continue conventional treatment plus midostaurin, whereas allo-SCT should be recommended to patients with relevant MRD (see Fig. 9.3).
9.6 Treatment Stratification
Before discussing algorithms for treatment, the authors would like to emphasize the utmost importance of enrolling patients in clinical trials as the first priority whenever these are available. As clinical trials offer the standard of care as control treatment, patients are not put at risk of undertreatment. The development and availability of novel agents that may cause prolonged survival have been and will be only possible on the basis of clinical trials. The authors would therefore like to stress the necessity to reach out for clinical trials, ideally as part of an academic cooperative group and embedded in a general registry and biobanking infrastructure in order to continuously improve treatment options and outcomes for AML patients.
With midostaurin, GO and CPX-351 expanding the antineoplastic armamentarium by three agents with the potential for prolonged overall survival in certain subgroups of AML, the diagnostic workup at initial diagnosis is important not only for prognostication, but also for treatment stratification. As outlined in Sect. 9.2, the general prognosis of patients is not dependent on the time from diagnosis to treatment (TDT). Still, the turn-around time for genetic diagnosis should be as short as possible. In conclusion, the potential benefits of correct stratification seem to outweigh the risks of disease progression in clinically stable patients. High WBC counts do not automatically indicate an emergency as they can be managed by the use of hydroxyurea.
Patients with acute AML-related problems such as leukostasis syndrome (see Chap. 14), or disease-related coagulation disorders should start treatment immediately with 7 + 3 based standard induction. Patients presenting with leukocytosis without clinical signs of leukostasis should be treated with hydroxyurea to reduce the white blood cell (WBC) count until the start of intensive chemotherapy (Röllig and Ehninger 2015).
Based on the results of diagnostic tests, the treatment algorithms depicted in Fig. 9.4 can be recommended outside of clinical trials.
9.7 Open Questions and Future Perspectives
Although “standard” intensive treatment approaches have been around for several decades now, there are still open questions and issues, for which evidence is sparse and which may be worth clinical research. Many institutions aim for two induction cycles (double induction) in order to reduce the leukemic burden whereas others proceed to postremission treatment as soon as the blast count was reduced to <5% even after only one induction (Fernandez et al. 2009). Likewise, it is uncertain if the application of at least one cycle of high-dose cytarabine may be beneficial even for patients proceeding to allo-SCT or if allo-SCT should follow CR achievement directly. The dose and amount of cytarabine cycles in postremission treatment is the subject of an ongoing debate (Löwenberg 2013; Paul et al. 2020). Randomized trials will contribute to answering these questions, and new insights on the levels and behavior of measurable residual disease markers will help us optimizing the first-line treatment.
Standard intensive first-line treatment can cure a significant proportion of newly diagnosed patients. Due to advances in anti-infective prophylaxis and treatment and other supportive measures (see Chap. 16), the tolerability of intensive regimens has improved and early mortality is constantly going down (see Fig. 9.5) (Percival et al. 2015). Pilot studies suggest that it may be even feasible to complete a complete intensive induction course in an outpatient setting if patients are carefully selected and monitored on a daily basis (Mabrey et al. 2020). Although comprehensive and complex inpatient treatment is required for most patients, the cost of standard treatment is low in comparison with the prices of novel agents. Based on these considerations, intensive treatment will remain the backbone and reference of curative AML treatment for the time being.
Decline in early mortality in AML treatment from the SEER database (Percival et al. 2015)
Thanks to a promising pipeline of novel agents in advanced clinical development, treatment of AML will become not only more efficacious, more refined, individualized, and challenging, but also more expensive. We have seen that novel agents with limited single-agent activity can be successfully added to the standard cytoreductive treatment, but will they be able to replace standard approaches while still be curative? Will we maintain a less specific broad treatment backbone and add specific targeted agents, and how many conventional and novel agents can we combine at a tolerable level and with manageable toxicity? Finally, novel agents with low toxicity but high curative potential may blur the fit–unfit frontier and sever the connection fit = intensive = curative and unfit = nonintensive = palliative and replace it by “eligible for.”
References
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE et al (2006) Age and acute myeloid leukemia. Blood 107(9):3481–3485
Atallah E, Cortes J, O’Brien S, Pierce S, Rios MB, Estey E et al (2007) Establishment of baseline toxicity expectations with standard frontline chemotherapy in acute myelogenous leukemia. Blood 110:3547–3551
Bertoli S, Bérard E, Huguet F, Huynh A, Tavitian S, Vergez F et al (2013) Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood 121(14):2618–2626
Boiron M, Jacquillat C, Weil M, Tanzer J, Levy D, Sultan C et al (1969) Daunorubicin in the treatment of acute myelocytic leukaemia. Lancet:330–333
Braess J, Amler S, Kreuzer K-A, Spiekermann K, Lindemann HW, Lengfelder E et al (2018) Sequential high-dose cytarabine and mitoxantrone (S-HAM) versus standard double induction in acute myeloid leukemia-a phase 3 study. Leukemia 32(12):2558–2571
Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann WK et al (2006) Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood 108(1):88–96
Büchner T, Hiddemann W, Berdel WE, Wormann B, Schoch C, Fonatsch C et al (2003) 6-Thioguanine, cytarabine, and daunorubicin (TAD) and high-dose cytarabine and mitoxantrone (HAM) for induction, TAD for consolidation, and either prolonged maintenance by reduced monthly TAD or TAD-HAM-TAD and one course of intensive consolidation by seq. J Clin Oncol 21(24):4496–4504
Büchner T, Schlenk RF, Schaich M, Dohner K, Krahl R, Krauter J et al (2012) Acute myeloid leukemia (AML): different treatment strategies versus a common standard arm—combined prospective analysis by the German AML Intergroup. J Clin Oncol 30(29):3604–3610
Burnett AK, Hills RK, Milligan DW, Goldstone AH, Prentice AG, McMullin MF et al (2010a) Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol 28(4):586–595
Burnett AK, Hills RK, Green C, Jenkinson S, Koo K, Patel Y et al (2010b) The impact on outcome of the addition of all-trans retinoic acid to intensive chemotherapy in younger patients with nonacute promyelocytic acute myeloid leukemia: overall results and results in genotypic subgroups defined by mutations in NPM1, FLT3, and C. Blood 115(5):948–956
Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH et al (2011) Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol 29(4):369–377
Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J et al (2013) Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol 31(27):3360–3368
Burnett AK, Russell NH, Hills RK, Kell J, Cavenagh J, Kjeldsen L et al (2015) A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 125(25):3878–3885
Burnett AK, Russell NH, Hills RK (2016) Higher daunorubicin exposure benefits FLT3 mutated acute myeloid leukemia. Blood 128:449–452
Carey RW, Ribas-Mundo M, Ellison RR, Glidewell O, Lee ST, Cuttner J et al (1975) Comparative study of cytosine arabinoside therapy alone and combined with thioguanine, mercaptopurine, or daunorubicin in acute myelocytic leukemia. Cancer 36(5):1560–1566
Cassileth PA, Lynch E, Hines JD, Oken MM, Mazza JJ, Bennett JM et al (1992) Varying intensity of postremission therapy in acute myeloid leukemia. Blood 79(8):1924–1930
Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhauser M, Juliusson G, et al (2012) The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach [Internet]. Nat Rev Clin Oncol 9:579–90. https://doi.org/10.1038/nrclinonc.2012.150
Cornelissen JJ, Versluis J, Passweg JR, van Putten WLJ, Manz MG, Maertens J et al (2015) Comparative therapeutic value of post-remission approaches in patients with acute myeloid leukemia aged 40-60 years. Leukemia 29(5):1041–1050
Crowther D, Bateman CJ, Vartan CP, Whitehouse JM, Malpas JS, Fairley GH et al (1970) Combination chemotherapy using L-asparaginase, daunorubicin, and cytosine arabinoside in adults with acute myelogenous leukaemia. Br Med J 4:513–517
Dillman RO, Davis RB, Green MR, Weiss RB, Gottlieb AJ, Caplan S et al (1991) A comparative study of two different doses of cytarabine for acute myeloid leukemia: a phase III trial of cancer and leukemia group B. Blood 78(10):2520–2526
Dinmohamed AG, Visser O, van Norden Y, Blijlevens NMA, Cornelissen JJ, Huls GA et al (2016) Treatment, trial participation and survival in adult acute myeloid leukemia: a population-based study in the Netherlands, 1989-2012. Leukemia 30(1):24–31
Ehninger A, Kramer M, Röllig C, Thiede C, Bornhäuser M, Von Bonin M et al (2014) Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J 4:e218
Ellison RR, Holland JF, Weil M, Jacquillat C, Boiron J, Bernard J et al (1968) Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults. Blood 32(4):507–523
Estey E, Gale RP (2017) Acute myeloid leukemia therapy and the chosen people. Leukemia 31:269–271
Estey EH, Thall PF, Pierce S, Cortes J, Beran M, Kantarjian H et al (1999) Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin +/− all-trans retinoic acid +/− granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood 93(8):2478–2484
Estey EH, Gale RP, Sekeres MA (2018) New drugs in AML: uses and abuses. Leukemia 32:1479–1481
Fairley GH (1971) The treatment of acute myeloblastic leukaemia. Br J Haematol 20(6):567–570
Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM et al (2009) Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 361(13):1249–1259
Ferrara F, Barosi G, Venditti A, Angelucci E, Gobbi M, Pane F et al (2013) Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia 27(5):997–999
Gale RE, Popa T, Wright M, Khan N, Freeman SD, Burnett AK et al (2018) No evidence that CD33 splicing SNP impacts the response to GO in younger adults with AML treated on UK MRC/NCRI trials. Blood 131:468–471
Gardin C, Chevret S, Pautas C, Turlure P, Raffoux E, Thomas X et al (2013) Superior long-term outcome with idarubicin compared with high-dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. J Clin Oncol 31:321–327
Goldstone AH, Burnett AK, Wheatley K, Smith AG, Michael Hutchinson R, Clark RE (2001) Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood 98(5):1302–1311
Gratwohl A (2012) The EBMT risk score. Bone Marrow Transplant 47(6):749–756
Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M et al (2014) Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 15:986–996
Holowiecki J, Grosicki S, Giebel S, Robak T, Kyrcz-Krzemien S, Kuliczkowski K et al (2012) Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol 30(20):2441–2448
Huls G, Chitu DA, Havelange V, Jongen-Lavrencic M, van de Loosdrecht AA, Biemond BJ et al (2019) Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood 133(13):1457–1464
Juliusson G, Lazarevic V, Horstedt AS, Hagberg O, Hoglund M (2012) Acute myeloid leukemia in the real world: why population-based registries are needed. Blood 119:3890–3899
Kern W, Estey EH (2006) High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: review of three randomized trials. Cancer 107(1):116–124
Khan N, Hills RK, Virgo P, Couzens S, Clark N, Gilkes A, et al (2017) Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia [Internet] 31(5):1059–68. https://doi.org/10.1038/leu.2016.309
Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS et al (2013) Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 121(21):4287–4294
Krauss AC, Gao X, Li L, Manning ML, Patel P, Fu W et al (2019) FDA approval summary: (Daunorubicin and Cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res 25(9):2685–2690
Krönke J, Schlenk RF, Jensen KO, Tschurtz F, Corbacioglu A, Gaidzik VI et al (2011) Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol 29:2709–2716
Krug U, Röllig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M et al (2010) Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet 376(9757):2000–2008
Krug U, Berdel WE, Gale RP, Haferlach C, Schnittger S, Müller-Tidow C et al (2016) Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia 30(6):1230–1236
Lancet et al J Clin Oncol 2018. https://pubmed.ncbi.nlm.nih.gov/30024784/
Lamba JK, Chauhan L, Shin M, Loken MR, Pollard JA, Wang YC et al (2017) CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: report from randomized phase III children’s oncology group trial AAML0531. J Clin Oncol 35(23):674–2682
Lambert J, Pautas C, Terre C, Raffoux E, Turlure P, Caillot D et al (2019) Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 104(1):113–119
Larrosa-Garcia M, Baer MR (2017) FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther 16(6):991–1001
Lee JH, Kim H, Joo YD, Lee WS, Bae SH, Zang DY et al (2017) Prospective randomized comparison of idarubicin and high-dose daunorubicin in induction chemotherapy for newly diagnosed acute myeloid leukemia. J Clin Oncol 35(24):2754–2763
Li X, Xu SN, Qin DB, Tan Y, Gong Q, Chen JP (2014) Effect of adding gemtuzumab ozogamicin to induction chemotherapy for newly diagnosed acute myeloid leukemia: a meta-analysis of prospective randomized phase III trials. Ann Oncol 25:455–461
Löwenberg B (2013) Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood 121(1):26–28
Löwenberg B, Suciu S, Archimbaud E, Haak H, Stryckmans P, de Cataldo R et al (1998) Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy—the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report. European Organization. J Clin Oncol 16(3):872–881
Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A et al (2009) High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 361(13):1235–1248
Löwenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C et al (2011) Cytarabine dose for acute myeloid leukemia. N Engl J Med 364:1027–1036
Mabrey FL, Gardner KM, Shannon Dorcy K, Perdue A, Smith HA, Davis AM et al (2020) Outpatient intensive induction chemotherapy for acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood Adv 4(4):611–616
Mandelli F, Vignetti M, Suciu S, Stasi R, Petti MC, Meloni G et al (2009) Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA groups study AML-10. J Clin Oncol 27(32):5397–5403
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P et al (1994) Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med 331:896–903
Michaelis LC (2018) Cytotoxic therapy in acute myeloid leukemia: not quite dead yet. Hematology 2018(1):51–62
Mizuki M, Schwäble J, Steur C, Choudhary C, Agrawal S, Sargin B et al (2003) Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood 101(8):3164–3173
Nagel G, Weber D, Fromm E, Erhardt S, Lübbert M, Fiedler W et al (2017) Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol 96(12):1993–2003
Niederwieser D, Hoffmann VS, Pfirrmann M, Al-Ali HK, Schwind S, Vucinic V, et al (2016) Comparison of treatment strategies in patients over 60 years with AML: final analysis of a prospective randomized German AML Intergroup Study. Blood [Internet]. 128(22):1066. https://doi.org/10.1182/blood.V128.22.1066.1066
Ossenkoppele G, Lowenberg B (2015) How I treat the older patient with acute myeloid leukemia. Blood 125(5):767–774
Paul S, Rausch CR, Jabbour EJ (2020) The face of remission induction. Br J Haematol 188(1):101–115
Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S et al (2010) Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol 28(5):808–814
Percival M-EM, Tao L, Medeiros BC, Clarke CA (2015) Improvements in the early death rate among 9380 patients with acute myeloid leukemia after initial therapy: a SEER database analysis. Cancer 121(12):2004–2012
Pfirrmann M, Ehninger G, Thiede C, Bornhäuser M, Kramer M, Röllig C et al (2012) Prediction of post-remission survival in acute myeloid leukaemia: a post-hoc analysis of the AML96 trial. Lancet Oncol 13(2):207–214
Pigneux A, Bene MC, Salmi L-R, Dumas P-Y, Delaunay J, Bonmati C et al (2018) Improved survival by adding lomustine to conventional chemotherapy for elderly patients with AML without unfavorable cytogenetics: results of the LAM-SA 2007 FILO trial. J Clin Oncol 36(32):3203–3210
Pluta A, Robak T, Wrzesien-Kus A, Katarzyna BB, Sulek K, Wawrzyniak E et al (2017) Addition of cladribine to the standard induction treatment improves outcomes in a subset of elderly acute myeloid leukemia patients. Results of a randomized polish adult leukemia group (PALG) phase II trial. Am J Hematol 92(4):359–366
Rai KR, Holland JF, Glidewell OJ, Weinberg V, Brunner K, Obrecht JP et al (1981) Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood 58(6):1203–1212
Röllig C, Ehninger G (2015) How I treat hyperleukocytosis in acute myeloid leukemia. Blood 125(21):3246–3252
Röllig C, Serve H, Huttmann A, Noppeney R, Muller-Tidow C, Krug U et al (2015) Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol 16(16):1691–1699
Röllig C, Kramer M, Gabrecht M, Hänel M, Herbst R, Kaiser U et al (2018a) Intermediate-dose cytarabine plus mitoxantrone versus standard-dose cytarabine plus daunorubicin for acute myeloid leukemia in elderly patients. Ann Oncol 29(4):973–978
Röllig C, Steffen B, Herbst R, Noppeney R, Racil Z, Schäfer-Eckart K et al (2018b) Randomized comparison of 90 mg versus 60 mg daunorubicin in 7+3 standard induction for newly diagnosed acute myeloid leukemia: results from the SAL-DaunoDouble trial. HemaSphere 2(S1):Abstract S861
Röllig C, Kramer M, Gabrecht M, Hänel M, Herbst R, Kaiser U et al (2018c) Intermediate-dose cytarabine plus mitoxantrone versus standard-dose cytarabine plus daunorubicin for acute myeloid leukemia in elderly patients. Ann Oncol 29:973–978
Röllig C, Kramer M, Schliemann C (2019) Time from diagnosis to treatment does not affect outcome in intensively treated patients with newly diagnosed acute myeloid leukemia. Annu Meet Am Soc Hematol 134(Suppl 1):Abstract 13
Röllig C, Beelen DW, Braess J, Greil R, Niederwieser D, Passweg JR, et al (n.d.) Onkopedia-Leitlinie Akute Myeloische Leukämie [Internet]. https://www.onkopedia.com/de/onkopedia/guidelines/akute-myeloische-leukaemie-aml
Schaich M, Röllig C, Soucek S, Kramer M, Thiede C, Mohr B et al (2011) Cytarabine dose of 36 g/m2 compared with 12 g/m2 within first consolidation in acute myeloid leukemia: results of patients enrolled onto the prospective randomized AML96 study. J Clin Oncol 29(19):2696–2702
Schaich M, Parmentier S, Kramer M, Illmer T, Stolzel F, Rollig C et al (2013) High-dose cytarabine consolidation with or without additional Amsacrine and Mitoxantrone in acute myeloid leukemia: results of the prospective randomized AML2003 trial. J Clin Oncol 31(17):2094–2102
Schlenk RF, Weber D, Herr W, Wulf G, Salih HR, Derigs HG et al (2019) Randomized phase-II trial evaluating induction therapy with idarubicin and etoposide plus sequential or concurrent azacitidine and maintenance therapy with azacitidine. Leukemia 33:1923–1933
Sekeres MA, Elson P, Kalaycio ME, Advani AS, Copelan EA, Faderl S et al (2009) Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood 113:28–36
Serve H, Krug U, Wagner R, Sauerland MC, Heinecke A, Brunnberg U et al (2013) Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol 31:3110–3118
Shayegi N, Kramer M, Bornhäuser M, Schaich M, Schetelig J, Platzbecker U et al (2013) The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood 122:83–92
Sorror M, Storer B, Sandmaier BM, Maloney DG, Chauncey TR, Langston A et al (2008) Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer 112(9):1992–2001
Southam CM, Craver LF, Dargeon HW, Burchenal JH (1951) A study of the natural history of acute leukemia with special reference to the duration of the disease and the occurrence of remissions. Cancer 4:39–59
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD et al (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377:454–464
Teuffel O, Leibundgut K, Lehrnbecher T, Alonzo TA, Beyene J, Sung L (2013) Anthracyclines during induction therapy in acute myeloid leukaemia: a systematic review and meta-analysis. Br J Haematol 161(2):192–203
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U et al (2002) Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99:4326–4335
Tsuchikama K, An Z (2018) Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell 9(1):33–46
Valcárcel D, Montesinos P, Sánchez-Ortega I, Brunet S, Esteve J, Martínez-Cuadrón D et al (2012) A scoring system to predict the risk of death during induction with anthracycline plus cytarabine-based chemotherapy in patients with de novo acute myeloid leukemia. Cancer 118(2):410–417
Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ et al (2011) Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood 118(23):6037–6042
Walter RB, Gooley TA, Van Der Velden VHJ, Loken MR, Van Dongen JJM, Flowers DA et al (2007) CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 109(10):4168–4170
Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA et al (2011) Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol 29(33):4417–4423
Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al (2020) Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med 383(26):2526–2537
Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG et al (2009) Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol 145(5):598–605
Willemze R, Suciu S, Meloni G, Labar B, Marie JP, Halkes CJ et al (2014) High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol 32:219–228
Yates JW, Wallace HJ Jr, Ellison RR, Holland JF (1973) Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep 57:485–488
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Röllig, C., Ossenkoppele, G.J. (2021). Treatment of Newly Diagnosed AML in Fit Patients. In: Röllig, C., Ossenkoppele, G.J. (eds) Acute Myeloid Leukemia . Hematologic Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-030-72676-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-72676-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-72675-1
Online ISBN: 978-3-030-72676-8
eBook Packages: MedicineMedicine (R0)