Abstract
Amongst the neglected tropical diseases, dengue remains a disease of concern, and the rise of global temperatures every year puts additional liabilities on the infrastructure of the health sector of various underdeveloped and developing countries. Increasing incidence puts additional stress on an already burdened healthcare infrastructures. This calls for an urgent need of discovering an inhibitor for Dengue virus (DENV). The success in targeting HIV and HCV proteases has driven considerable attention of researchers to explore the same towards the DENV NS2B-NS3 protease. Although most attempts yet remains futile, advancing computational power and biotechnology can certainly play a pivotal role in speeding up the drug discovery process. High-throughput Virtual Screening is one such frontier that could help screening of large libraries of chemical entities, making the in vitro pipeline efficient and cost effective. This chapter presents the DENV NS2B-NS3 protease inhibitors identified through High-throughput Virtual Screening and its impact on drug development against the Dengue virus.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Flavivirus
- NS2B-NS3
- DENV
- HTVS
- Protease inhibitors
- Dengue
- In silico
- Crystal structure
- Catalytic triad
- Docking
- Hydrophobic interactions
- Virtual screening
1 Introduction
Dengue has emerged as one of the most threatening arthropod-borne viral diseases in the past 50 years (WHO 2012) affecting nearly 390 million people (Bhatt et al. 2013) in tropical and the subtropical regions of the globe (Guzman et al. 2010). Dengue fever is caused by the Dengue virus (DENV), which belongs to the genus flavivirus of the Flaviviridae family. DENV is mainly transmitted by the mosquito species Aedes aegypti and Aedes albopictus. DENV has four serotypes, namely DENV-1, DENV-2, DENV-3, and DENV-4, whose clinical complexities in humans range from a milder dengue fever (DF) to fatal dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (Ebrahim 1993; Guzman et al. 2010). Interestingly, it was noted that being infected from one serotype did not provide immunity on cross-infection with other serotypes. A subsequent infection from a different serotype posed a potential risk of developing fatal dengue. The phenomenon is called the “antibody-dependent enhancement” effect (ADE) (Goncalvez et al. 2007). Vaccine development programs have suffered serious setbacks. The first vaccine developed by Sanofi Pasteur in 2015, despite being approved in twenty countries, carries a risk of causing ADE and has been limited for people in the age group of 9–45 years and who have had a previous viral infection at least once (Guy et al. 2017).
The dengue virus genome is a single strand of RNA, often referred to as positive-sense RNA, since it can be directly translated into proteins. The viral genome encodes ten genes. The genome is translated as a single, long polypeptide and then cut into ten proteins, three structural proteins, capsid (C), envelope (E), and membrane (M) proteins, and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). These nonstructural proteins play roles in viral replication and assembly (Mukhopadhyay et al. 2005).
With the establishment of HCV (Wyles 2013) and HIV (De Clercq 2009) protease as promising drug targets, researchers worldwide began investigating DENVNS3 protease to develop a possible inhibitor. NS3 protease is a trypsin-like serine protease harboring a classical serine protease catalytic triad (His51, Asp75, and Ser135) (Falgout et al. 1991). It resides at the N-terminal end of NS3 protein (Nestorowicz et al. 1994). The proteolytic activity of NS3 protease is witnessed in the presence of a cofactor NS2B, improves the catalytic function of NS3 (Falgout et al. 1991; Yusof et al. 2000). The NS2B-NS3 protease complex cleaves DENV polyprotein into its components, thereby promoting viral replication. Due to its crucial role in cleavage and assembling of viral proteins, it appears as a promising target for the design and development of anti-dengue drugs (Phong et al. 2011).
High Throughput Virtual Screening has now become a fundamental component of in silico studies. This approach uses computational algorithms to filter in potential bioactive molecules from large chemical compound libraries. A typical HTVS methodology involves docking and scoring millions of compounds from chemical databases against a protein binding site of interest. The binding site and the protein are sourced and prepared from crystal structures available in protein databases or by developing homology models. The chemical hits determined from this campaign are then screened in vitro using biochemical and biophysical assays. The in silico virtual screening approach has its limitations, e.g., when screening an extensive library, it is often required to ignore protein flexibility and other factors such as binding entropy and desolvation. However, a large number of studies have shown that HTVS had a significant impact on lead discovery.
In this chapter, we have compiled every attempts made by various groups searching for an anti-dengue therapeutics through the HTVS approach while stressing on factors such as uniqueness of the approach, merits, demerits and success metrics.
2 Structure of Dengue NS2B-NS3 Protease
Several protein structures of DENV NS2B-NS3 protease have so far been solved. Among these, only a few have so far been used for DENV protease in silico studies. Among these, the most prominent ones include 2FOM, 2VBC, and 3U1I.
Erbel et al. (2006) published the first reliable and enzymatically active, high resolution (1.5 Å) NS2B-NS3 protease structure that contained the catalytically important part of NS2B (47-residue core region) linked to the N-terminal of NS3 protease via a glycine linker. The NS3 protease domain adopted chymotrypsin-like folds with two β-barrels forming 6 β-strands. The two β-barrels formed a cleft where catalytic triad His51-Asp75-Ser135 were located. The catalytic triad is a classical feature of serine proteases responsible for their mechanism of action. This research held phenomenal importance in the understanding of the role of NS2B as a cofactor. The authors uncovered that, while a construct with NS2B residues 49–66 could produce a soluble enzyme, an active enzyme was only produced if the entire NS2B fragment was involved. When the structure was studied in the presence and absence of the ligand, the C terminus folds of NS2B adopted markedly different configurations. It was also noted that the integration of residues Arg78-Leu87 from the β-loop of NS2B into the protease-cofactor complex greatly influenced the active site. In summary, the association of 40 amino acid NS2B fragment with NS3protease is key to protease activity. The cofactor actively aids in S2 and S3 pocket formation in the protease active site.
A ligand-bound crystal structure of DENV3NS2B-NS3 protease published, where NS3 adopted a closed conformation and was wrapped by NS2B, which included the β-hairpin. They further studied the crystal structure in comparison with WNV protease. This study revealed the amino acid level changes that influence binding pocket and binding affinities (Noble et al. 2012).
3 High-Throughput Virtual Screening Against Dengue NS2B-NS3 Protease
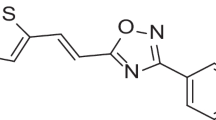
Ganesh et al. (2005) were amongst the first to perform virtual screening of compounds from the available chemical directory (ACD, MDL system, Foster city CA) against DENV2NS3 protease crystal structure (PDB ID:1BEF). The same structure was also used to model the West Nile Virus (WNV) protease using Modeller 4.0. Three biguanides and seventeen single guanidine containing groups were evaluated for their inhibitory activity against DENV and WNV protease, and five non-substrate-based inhibitors were identified. In their study, they observed how the modifications of the bridge group between two biguanidine arms affected the Ki value. Also, the effect of a single guanidino group on Ki was observed. As shown in Fig. 1, three compounds had Ki value below 50μM against both the proteases and thus served as effective lead for further modifications. Molecular modelling studies of compound 1showed that it existed in two confirmations (U shape and Z shape). Further RMS data (0.68 Å) on deviation and centroid to centroid distance (7.7 Å) between the two guanidine groups indicated that the inhibitor acted best in the U confirmation (Fig. 3). Residues Asp129, Tyr150, Ser163 showed electrostatic interactions with compound 1 (Figs. 2 and 3). Modeling data of Compound 2 & 3, which had a single guanidine group, showed that amide oxygen of the indolin ring in compound 2 and Oxygen atom of the phosphonic group in compound 3 could form a hydrogen bond with active site Ser135 of DENV NS3 protease, resulting in good inhibitor protease interaction.
DEN2NS3 protease-Compound 1 interaction (Ganesh et al. 2005). Reprinted with permission, Copyright © 2004 Elsevier Ltd.
U-conformation of compound 1 with ribbon drawing model of DEN2 NS3 protease (Ganesh et al. 2005). Reprinted with permission, Copyright © 2004 Elsevier Ltd.
Tomlinson et al. (2009) identified two small molecule inhibitors of DENV2 NS3 protease through Structure-based drug design. The chemical library was an in-house database of small molecules from Mayo Clinic that contained 2.5 million three-dimensional structure of compounds. Two filters were employed to screen the molecules, one being the selection of only non-zwitterionic compounds at physiological pH and the other being easy availability from reputed vendors. Further EUDOC program was used to computationally screen the filtered library against DENV2 NS3 pro (PDB ID: 1BEF, 1DF9, and 2FOM). Top 20 hits, identified from Molecular modeling studies were purchased and evaluated for in vitro inhibitory activities against NS2B-NS3 protease. Two compounds also inhibited viral replication in cell culture experiments. ARDP0006 (Fig. 4) was most efficacious in inhibiting DEN2V replication with EC50 of 4.2 ± 1.9 µM. Modeling studies were done, interactions were identified, and ARDP0006 was predicted to interact with the residues, Gln35 (a residue near active site), His51, and Ser135 of the active site and residues Gly151 and Gly153 of the P1 pocket. Other observed interactions were between the inhibitor and residues Ser131, Pro132, Gly133, Thr134, Asn152, and Val155 (Fig. 5). Images were generated with SWISS PDB.
Model of inhibitor-protease interaction (Tomlinson et al. 2009). Reprinted with permission, Copyright © 2009 Elsevier B.V.
Knehans et al. (2011) demonstrated the successful application of a structure-guided fragment-based in silico drug design approach for DENV protease inhibitor (Fig. 6). Applying retrosynthetic combinatorial synthetic analysis procedure, a library of the molecular fragment was derived from ZINC database. This library of about 1,50,000 fragments was docked to DENV NS2B-NS3 protease, which was developed by homology modeling. High scoring fragments were assembled and through an implicit linking approach, with a focus on interactions of the fragments with S1 and S2 pocket of the protease and similarity search methods, twenty-three compounds were selected, and inhibition assay was performed. The best compound obtained had an IC50 value of 7.7 µM. Figure 7, shows the predicted binding pose of the most potent compound. The inhibitor is shown in magenta color, and using GOLD (Version 4.1), it was docked into the homology modeled DENV2 substrate binding site. Docking study of the best inhibitor showed that the inhibitor was engaged in hydrogen bonding and electrostatic interactions with Asp129 of the S1 pocket, a π-π interaction was predicted between the phenyl guanidine group and Tyr161 and this arene-arene interaction was supported by hydrophobic interaction with Pro132 and thus Tyr161 and Pro132 served as a hydrophobic clamp in the S1 pocket. The opposite phenyl moiety interacted with His51 of the catalytic triad through π-π stacking interaction, and its guanidine group formed a hydrogen bond with Asn152 of the S2 pocket. Since S2 pocket has a negatively charged environment, the positive charge of the guanidine group compensates the same. Thus, this approach explains how a combination of homology modeling, fragment docking, chemical similarity and structural filters could pave the way for hit generation.
In another study, Deng et al. (2012) utilized the crystal structure of the DENV NS2B-NS3 complex (PDB ID: 2FOM) and screened ~600,000 compounds in the ACD database by molecular docking. Twenty-seven hits were purchased and evaluated for DENV2 NS2B-NS3 protease inhibition. As a result, three different scaffolds small molecules were found to be promising. One scaffold was chosen as a starting molecule for modification based on various parameters, including synthetic ease and its analogues were synthesized and evaluated (Table 1). Further, the scaffold hopping technique mediated structural modifications were done; eventually, the quinoline moiety replaced the core phenyl moiety and the resulting molecules were evaluated for DENV protease inhibitory activity (Table 2). A schematic representation of their work is shown in Fig. 8. Altogether thirty-five compounds were synthesized, and their inhibitory abilities against DENV2 NS2B-NS3 protease were evaluated. Of the thirty-five molecules, seventeen were potent inhibitors of protease, and eight molecules showed good antiviral activity against DENV2 in the cell line assay. The best molecule designed (compound A) had an IC50 value of 7.46 ± 1.15 µM against DENV2 NS2B-NS3 protease. For the Molecular modeling studies, screening was performed using DOCK 4.0 and docking was done using GOLD3.0. Visualization was done using PyMol. The quinoline ring of compound B showed hydrophobic interactions with Leu76 and Ile 165. The nitrogen of quinoline formed hydrogen bond with the side chain of Asn152. The first and the second nitrogen of hydrazone were involved in hydrogen bond formation with the side chain of Asn152 and the carbonyl group of Lys73, respectively. Bromobenzene portion occupied the hydrophobic region, P4 and showed hydrophobic interactions with the residue Ile123 and Val154. Further, hydrogen-bonding interaction was also predicted between Lys 74 and the carbonyl group of the amide linking the hydrazine on the right side of the structure and between amide-hydazine nitrogen and the main chain of Ile165. The remaining one phenyl portion was occupied between the alkyl portion of Glu88 side chain and the side chain of Ala166 (Fig. 10). The better inhibitory activity of compound A was attributed to the sulfonamide group present in its structure, which enabled more hydrogen-bonding interactions in the P4 region of it and the residues involved were Thr120 and Lys73 (Fig. 9).
3D Interaction of Docked pose of compound A with DENV2 NS2B-NS3 protease. Reprinted with permission from (Deng et al. 2012), Copyright © 2012 American Chemical Society
3D Interaction of Docked pose of compound B with DENV2 NS2B-NS3 protease. Reprinted with permission from (Deng et al. 2012). Copyright © 2012 American Chemical Society
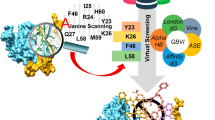
Yet in another study Pambudi et al. (2013), using structure based screening and cell-based viral replication assay identified a small molecule inhibitor, SK12, that interfered with the interaction between NS2B and NS3. They performed HTVS of a library of 661,417 compounds (derived from molecular operating environment lead-like database) against the X-ray crystal structure of DENV2 NS2B–NS3 protease (PDB ID: 2FOM). MOE site finder identified the docking region of the NS2B-NS3 interaction site. Thirty-nine compounds having the top score were identified and subjected to antiviral cell-based assay. Inhibitor SK12 (Fig. 11) was found to inhibit all four DENV serotypes with EC50 ranging between (0.74–2.43 µM). Insilico studies predicted that SK12 preoccupied the NS2B binding site of NS3, and it interacted with the NS2B binding site of NS3 through a hydrogen bond, and the residues involved were Lys26, Gln27, Met59, and His60. The ligand interaction studies were done using the MOE program. Figure 12, shows stereo view of SK-12 bound to NS3 (represented in pink color). Figure 13, shows the interaction of the inhibitor with the residues. Interestingly NS2B was also predicted to interact with NS3 through Arg24, Lys26, Gln27, Met59, and His 60 residues. Hence SK12 here interferes with the interaction between NS2B and NS3. Further, it was established that SK12 interfered with DENV protease non-competitively. Modification in the structure of SK12 to reduce the cytotoxicity and increase the inhibitory potential could give us a good lead for future drug development and a pan DENV agent.
Stereo view of SK12-NS3 interaction (Pambudi et al. 2013). Reprinted with permission, Copyright © 2013 Elsevier
SK12-NS3 residue interaction (Pambudi et al. 2013). Reprinted with permission, Copyright © 2013 Elsevier.
Additional experiments performed, employing High throughput virtual screening (HTVS) of about 300,000 compounds (obtained from ChemDiv Inc., San Diego, CA, USA) against DENV4 NS2B-NS3 proteases (PDB ID: 2VBC) identified few novel inhibitors of DENV4 NS2B-NS3 proteases. AutoDock 3.0.5 was used for computational molecular docking simulation. Grid Application Platform Virtual Screening Services (GVSS) for the batch process was employed for computation. Hydrophobic and hydrogen bond interactions with the active site residues were predicted for the active molecules. Of the thirty-six compounds selected for in vitro DENV NS2B-NS3 protease screening, seven molecules were identified as novel DENV4 protease inhibitors with IC50 in the micromolar concentration range. The best molecule identified, shown in Fig. 14, (Chemdiv ID: K286-0036), had binding affinity −11.9 kcal/mol and IC50 of 3.9 ± 0.6 µM against DENV4 NS2B-NS3 protease, and the mode of inhibition identified was competitive (Ki value of 3.4 ± 0.4 µM). Molecular docking studies predicted that the inhibitor (magenta color) was bound to the NS3 protease active site pocket (Fig. 15), hydrogen bonding interaction and hydrophobic interaction were responsible for the stabilization of the inhibitor (Fig. 16). The inhibitor formed hydrophobic interactions with Trp50, His51, Arg54, Arg73, Asn74, Asp75, Asn152, and Gly153. The pyrimidine group oxygen atom formed two hydrogen bonds, one with the hydroxyl group of Ser135 and the other with an amine group of Asn152. Thus here an attempt was made to correlate the interaction results obtained through molecular modeling studies to the in vitro assay result (Nguyen et al. 2013).
Inhibitor-protease Interaction (3D) (Nguyen et al. 2013), (Open Access)
Inhibitor-protease interactions (2D) (Nguyen et al. 2013), (Open Access).
In another study DENV NS2B-NS3 protease inhibitors were identified using the rational drug discovery approach. Using Modeller 9.11 software and WNV protease as a template, the homology model design of DENV NS2B-NS3 protease was realized. Pinostorbin was used as the standard reference ligand. AutoDock4.2 was used for the virtual screening of 13,341 molecules from the ZINC database, and the top hits were screened for in-vitro protease assay resulting in the identification of four small molecules as non-competitive inhibitors of DENV2 NS2B-NS3 protease. The best molecule (Fig. 17) had a binding affinity −6.17 kcal/mol and Ki of 69 µM against DENV2 NS2B-NS3 protease. Molecular modeling data (Figs. 18 and 19) predicted hydrogen bonding interaction of inhibitor with Asn167 (shown in pink color), hydrophobic interaction of the inhibitor was predicted with Ile78, Lys73 and Ile123 residues (shown in green color) these results were prepared using Ligplot and further Discovery Studio Visualizer 3.1 predicted pi-cation interaction between the inhibitor and Lys74 residue (shown in orange color) (Heh et al. 2013).
3D model of Interactions (Heh et al. 2013). Reproduced with permission, Copyright © 2013 John Wiley & Sons A/S
2D model of Interactions (Heh et al. 2013). Reproduced with permission, Copyright © 2013 John Wiley & Sons A/S
Viswanathan et al. (2014) introduced their newly constructed web-based drug discovery portal (DrugDiscovery@TACC) for structure-based drug discovery. In their HTVS study, screening was done using both the inhibitor bound protease structure (PDB ID: 3U1I & 3U1J) and inhibitor-free protease structure (PDB ID: 2FOM). Further, two virtual libraries were compiled, a library of 642,769 molecules, which was a subset of ZINC database and was named “collective” library; it was obtained after applying the Lipinski rule of 5 filters along with other filters. The second library, known as the focused library, obtained by applying the ClogP filter, included 45,458 small molecules. An independent Virtual screening was performed against DENV2 NS2B-NS3 protease (PDB ID: 2FOM) and DENV NS2B-NS3 protease (PDB ID: 3U1I, 3U1J). NS3 proteases of 2FOM and 3U1I, 3U1J share approximately 70% sequence identity and active site residues of the same shared approximately 90% sequence identity. Virtual screening yielded 179 hits from the collective library and 117 hits from the focused library. Thus, with its computational resources, this platform provided an option to screen multiple ligands for similar valid targets. Further, these hits were narrowed down by applying several filters, which included.
-
Discard of hits which did not form any hydrogen bond with catalytic side residue.
-
Discarding the one which had poor predicted solubility.
-
Discard of hits with fewer hetero atoms.
-
Discarding the polar hits or which contained formal charges.
Finally, they presented a proof of concept result for a validated compound ZINC04321905 (Fig. 20), which was a mixed non-competitive inhibitor and had Ki of 7 µM against DENV2 NS2B-NS3 protease. Drug-like properties of ZINC04321905 were calculated using ORIS property calculator, which indicated no adverse mutagenic, irritant effect, or tumorigenic effect associated with this pharmacophore. However, the cyclohexene ring was associated with an adverse reproductive effect. Hence future analog development approach would be to replace this unwanted fragment and substitute it with more drug-like molecules. For molecular modeling studies, the Vina docking program was used to calculate the orientation of the inhibitor to the DENV3 NS2B-NS3 protease. Figure 22 was generated using PyMol, and it showed the inhibitor (in magenta color) bound to the protease while the catalytic site was shown in green color. Three hydrogen bonding interactions (shown by green dashed lines) of the inhibitor with the amides of Gly133, Thr134, and Ser135 can be seen in Fig. 21. The hydrophobic interactions were shown by red hashed lines and residues involved were Val152, Val136, His51, Pro132, Asp129, Phe130, Tyr161, and Tyr150. The image was prepared using LigPlot+. An interesting study done by this group saw a comparison of NS3 protease sequence from 2211 strains spanning to the four DENV serotypes, and about 40% of the residues were conserved, more interestingly the active site residue conservation was 66% for all the 4 serotypes. Also, of the 11 residues, involved in the inhibitor ZINC04321905-protease interaction, 10 residues were conserved in all the 2211 strains of DENV examined, hence this molecule could be further exploited for the development of pan-dengue inhibitor (Viswanathan et al. 2014).
2D representation of inhibitor-protease interaction. Reprinted with permission from (Viswanathan et al. 2014). Copyright © 2014 American Chemical Society
3D representation of inhibitor-protease interactions. Reprinted with permission from (Viswanathan et al. 2014). Copyright © 2014 American Chemical Society
Subsequently in 2015, Li et al. attempted a structure-guided discovery of a small non-peptide as an inhibitor of DENV2 NS2B-NS3 protease. They carried out a multistep virtual screening campaign on a library of 5 million compounds obtained from 4 different commercial sources (Chembridge, Enamine, Life chemicals, and Maybridge). The pharmacophore-based preliminary filter narrowed the compounds to a few hundred, to this rigid docking, and induced fit docking was applied using AutoDock 4.2.5. Fourteen compounds based on docking scores were identified and subjected to biological screening. The protease inhibition assay was performed, and the EC50 of the top hit was around 5.0 µM in BHK21 cells (Fig. 23). Docking study of this compound further supported their concept of pharmacophore-based design as its results were in good agreement with the interactions seen in the known crystal structure (PDB ID: 3U1I) and the binding free energy being −10.65 kcal/mole. This hit occupied the pockets of the binding site (Fig. 24). The benzyl ring of the compound occupied the hydrophobic region between Pro 132 and Val 155. Also, Tyr 161 showed interaction with the lone pair of N atom, and an additional π-π interaction could also be seen with it. Further stabilization of the inhibitor-protease was seen when an additional water group linked the amino group of the inhibitor with Met184 and Gly153.
Binding mode of inhibitor (Li et al. 2015). Reprinted with permission, Copyright © 2014 John Wiley & Sons A/S
Subsequently, Timiri et al. (2015) performed HTVS of ZINC 8 database to identify an inhibitor of DENV2 NS2B-NS3 protease. Top hundred molecules were manually analyzed and they decided to synthesize phthalimide-sulphonamide analogues as they were encouraged by protease (HIV/HCV) (Lampa et al. 2014; Davis et al. 2017) inhibition activity of sulphonamides and antiviral (Selvam et al. 2012), antimycobacterial (Akgün et al. 2012) and anticancer (Matsushita et al. 2015) activities reported by phthalimide derivatives. Twenty derivatives of 4-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl) benzene- 1-sulphonamide were synthesized and screened for DENV2 protease inhibitory activity. The compound shown in Fig. 25 had an IC50 value of 48.2 µM against DENV2 NS2B-NS3 protease. Molecular docking and molecular dynamic simulation studies were carried out to understand the mechanism of action of the inhibitor. Using Modeller 9.15 and 3U1I as a template, the structure of DENV2 NS2B-NS3 protease was modeled and Induced–fit docking was performed using GLIDE of Maestro 9.2. The docking score of the most potent compound reported was −5.12. The interactions which were attributed for the DENV protease inhibition activities were π-π stacking interactions between ring A and Tyr200. Hydrogen bonding interaction between O atom of ring A and N atom of imidazole ring in Hip 90, hydrophobic interactions of =CH group of ring A with Asp168, Phe169, Pro171, Tyr189, Gly190 residues. Another hydrophobic interaction between 4-ethyl phenyl group attached to sulphonamide and Trp89 and Val111, A hydrogen bond interaction between N atom attached to sulphonamide and O atom of carbonyl group of Asn191, and π-cation interactions between electron cloud of ring B and cationic N of imidazole ring in Hip90 (Fig. 26). MD simulations revealed that NS2B/NS3 protease was more stable when it was bound with the active compound.
2D representation of Inhibitor-modelled DENV2 NS2B-NS3 protease interaction (Timiri et al. 2015). Reprinted with permission, Copyright © 2015 Elsevier Inc.
Subsequently Pelliccia et al. (2017). adopted a virtual screening approach to identify allosteric inhibitors against DENV NS2B-NS3 protease. The virtual screening mainly focused on the allosteric site of the enzyme. Based on their findings, they designed and synthesized compounds and evaluated their inhibitory potential by performing the cell-based and enzyme-based assay against DENV NS2B-NS3 protease. In the cell-based assay, the best compound showed an EC50 value of 6.7 ± 0.78 µM and in the enzyme-based assay, the EC50 value obtained was 4.7 ± 0.3 µM (Fig. 27). For the molecular modeling studies, crystal structure of DENV2 NS2B-NS3 protease (PDBID: 2FOM) was used. Docking was performed using PLANTS and scoring was done using ChemPLP scoring function (Korb 2009). Figure 28, shows the binding mode of the inhibitor (Green color) with the protease. PyMol was used to generate this structure. The carbonyl portion of the ester formed H-bond with Asp152 side chain and the ester was well-positioned inside the binding pocket, further hydrogen bond was predicted between indole, NH and Asn167. Residues Val146, Leu149 and Leu76 were predicted to form hydrophobic interaction with the indole ring, the methoxyphenyl portion also showed hydrophobic interactions with Trp83 and a pi-cation interaction with Lys74.
Inhibitor-protease interactions. (Pelliccia et al. 2017), (Open Access)
These studies were followed by, Hariono et al. (2019). efforts wherein they identified a hit (D0713) against DENV2 NS2B-NS3 protease by virtual screening of a library from the National Cancer Institute database. The binding free energy and the experimental IC50 against DENV2 NS2B-NS3 protease of this hit was −7.10 kcal/mol and 62 µM, respectively (Fig. 29). AutoDockVina (www.autodock.scripps.edu) was used for this virtual screening exercise. This hit had a thioguanine scaffold in it, and upon its modification, the best molecule obtained had an IC50 value of 0.38 µM against DENV2 NS2B-NS3 protease. Further, this molecule was docked to the DENV2 NS2B-NS3 protease model using AutoDock4.2 and the binding free energy obtained was −7.48 kcal/mol. Further, interactions associated with the inhibitory activity were identified, and residues Ser135, Tyr161, and Gly153 were predicted to form hydrogen bonding interactions with the inhibitor; other hydrophobic interactions identified across the length of the inhibitor were Pro132, Tyr154, Thr134, Ser131, Gly151, His51, Gly52, Asp51, Met34, Val72, Asp75, Tyr50, Asn152, Ser53 (Figs. 30 and 31). To get a better insight this inhibitor–protease interaction, Amber 14 (Le Grand et al. 2013) was used to perform molecular dynamics simulations, and key residues identified for hydrogen binding interactions were His51, Asp129, Ser135, Asn152, and Tyr 161. MMPBSA.py module of AMBER 14 was used to perform MM/PBSA calculations of this molecule, and the free binding energy obtained was −16.10 kcal/mol. MM/PBSA calculations indicated the role of polar and non-polar interactions contributing to this molecule’s low IC50 value.
The most potent compound of Hariono et al. (2019)
2D view of inhibitor-protease interaction. (Hariono et al. 2019), (Open Access)
3D view of inhibitor-protease interaction (Hariono et al. 2019), (Open Access)
In a very recent study, a multistep virtual screening of the Asinex database containing 590,859 compounds was performed against DENV2 NS2B-NS3 protease using crystal structure 2FOM. The first step of the virtual screening was done with the AutoDock Vina program. The set criteria narrowed the compound numbers to 3645 compounds. Further, using the virtual screening workflow of Schrodinger Suite and MM/GBSA based binding free energy estimation, the compounds were narrowed down to 102. In silico pharmacokinetic assessment further narrowed down this number and five compounds were proposed as potential NS2B-NS3 protease inhibitors. The best molecule identified had a docking score of −7.619 kcal/mol (Fig. 32) and the binding free energy of this molecule, calculated from MD simulation trajectories using MM/GBSA approach was 60.666 kcal/mol, which was better than the standard inhibitor (MB21) chosen for this study. MB 21 had binding free energy of −40.207 kcal/mol. The binding interaction of this inhibitor is shown in Fig. 33. Residues Lys73, Gly153, and Asn167 were predicted to form four Hydrogen bonds with the inhibitor. Lys74 and Leu76 showed hydrophobic interactions with the inhibitor and the only π-cation interaction was between Lys74 and the inhibitor. Figure 34, shows the binding pose of the inhibitor in 3D space (Bhowmick et al. 2020).
Inhibitor-protease binding interaction (Bhowmick et al. 2020). Reprinted with permission, Copyright © 2020 John Wiley & Sons Ltd.
3D view of inhibitor-protease interaction (Bhowmick et al. 2020). Reprinted with permission, Copyright © 2020 John Wiley & Sons Ltd.
4 Conclusion
As conclusion here we summarise the the important interactions chronologically reported by various groups from their HTVS studies. Ganesh et al. (2005) reported important electrostatic interaction involving residues Asp129, Tyr150, Ser163. Also they noted that, Ser135 was involved in hydrogen bonding. Tomlinson et al. (2009) reported P1 pocket interactions, which included residues Gly151, Gly153, some residues near to the catalytic triad (Gln35) and also the residues of the catalytic triad (His51, Ser135). Besides this, some other interactions involving residue Asn152 were also identified. Knehans et al. (2011) showed the engagement of residues Asp129 and Asn152 in hydrogen bonding in the S1 and S2 pocket of protease, respectively. His51 of the catalytic triad was involved in π-π interaction, Tyr161 was predicted to show pi-pi stacking interactions and Pro132 was involved in hydrophobic interaction. Deng et al. (2012) reported hydrogen bonding by residues Thr120, Lys73, Lys74, Asn152, and Ile165 with their potent compounds. Pambudi et al. (2013) reported hydrogen bonding interactions involving residues Lys26, Gln27, Met59, and His60. Nguyen et al. (2013) identified residues Ser135 and Asn152 to be involved in hydrogen bonding, and residues His51 and Asn152, along with few other residues, were found to be engaged in hydrophobic interactions. Heh et al. (2013) predicted hydrogen bond formation by residue Asn167 and a pi-cation interaction by Lys74. Besides this, Lys73, along with other residues, contributed to hydrophobic interactions. Li et al. (2015) reported important residue Tyr161 along with others for hydrophobic interactions. Timri et al. (2015) found residues Hip90 and Asn191 to be engaged in hydrogen bonding interactions and again residue, Hip90 was found to be involved in pi-cation interaction. Pelliccia et al. (2017) reported the involvement of residues Asp152 and Asn167 in hydrogen bonding and Lys74 in pi-cation interaction. This was followed by a report by Hariono et al. (2019) who found residue Tyr161 to be involved in hydrogen bonding interaction. Besides this, Asn152 and His51, along with other residues were involved in hydrophobic interactions. Finally, Bhowmick et al. (2020) reported residues, Lys73, Gly153, and Asn167 to be involved in hydrogen bonding, and Lys74 showing a pi-cation interaction.
Looking into these interactions, a commonality was exhibited by few residues pertaining to certain interactions, viz involvement of Lys74 in pi-cation interaction, the involvement of Asn152, and Asn167 in hydrogen bonding interaction, and involvement of Tyr161 in pi-pi stacking interactions. Understanding the role of these residues can certainly help in the designing of a suitable pharmacophore model.
Hence, it could be inferred from the above observations that HTVS has certainly helped the researchers in making a rational approach towards dengue drug discovery. It has given an option of speedily evaluating the big libraries available from different databases and has tactically provided a smarter approach for the drugdiscovery process. It may not be a surprise if in the near future we discover a pan-dengue inhibitor from the HTVS studies.
References
Akgün H, Karamelekoglu I, Berk B, et al. Synthesis and antimycobacterial activity of some phthalimide derivatives. Bioorganic Med Chem. 2012;20:4149–54. https://doi.org/10.1016/j.bmc.2012.04.060.
Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. https://doi.org/10.1038/nature12060.
Bhowmick S, Alissa SA, Wabaidur SM, et al. Structure-guided screening of chemical database to identify NS3-NS2B inhibitors for effective therapeutic application in dengue infection. J Mol Recognit. 2020;33:1–18. https://doi.org/10.1002/jmr.2838.
Davis BW, Diep JT, Jose S. (12) United States Patent 2. 2017.
De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents. 2009;33:307–20. https://doi.org/10.1016/j.ijantimicag.2008.10.010.
Deng J, Li N, Liu H, et al. Discovery of novel small molecule inhibitors of dengue viral NS2B-NS3 protease using virtual screening and scaffold hopping. J Med Chem. 2012;55:6278–93. https://doi.org/10.1021/jm300146f.
Ebrahim GJ. Dengue and dengue haemorrhagic fever. J Trop Pediatr. 1993;39:262–3. https://doi.org/10.1093/tropej/39.5.262.
Erbel P, Schiering N, D’Arcy A, et al. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat Struct Mol Biol. 2006;13:372–3. https://doi.org/10.1038/nsmb1073.
Falgout B, Pethel M, Zhang YM, Lai CJ. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol. 1991;65:2467–75. https://doi.org/10.1128/jvi.65.5.2467-2475.1991.
Ganesh VK, Muller N, Judge K, et al. Identification and characterization of nonsubstrate based inhibitors of the essential dengue and West Nile virus proteases. Bioorganic Med Chem. 2005;13:257–64. https://doi.org/10.1016/j.bmc.2004.09.036.
Goncalvez AP, Engle RE, St. Claire M, et al. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci USA. 2007;104:9422–7. https://doi.org/10.1073/pnas.0703498104.
Guy B, Noriega F, Ochiai RL, et al. A recombinant live attenuated tetravalent vaccine for the prevention of dengue. Expert Rev Vaccines. 2017;16:671–83. https://doi.org/10.1080/14760584.2017.1335201.
Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–16. https://doi.org/10.1038/nrmicro2460.
Hariono M, Choi SB, Roslim RF, et al. Thioguanine-based DENV-2 NS2B/NS3 protease inhibitors: virtual screening, synthesis, biological evaluation and molecular modelling. PLoS ONE. 2019;14:1–21. https://doi.org/10.1371/journal.pone.0210869.
Heh CH, Othman R, Buckle MJC, et al. Rational discovery of dengue type 2 non-competitive inhibitors. Chem Biol Drug Des. 2013;82:1–11. https://doi.org/10.1111/cbdd.12122.
Knehans T, Schüller A, Doan DN, et al. Structure-guided fragment-based in silico drug design of dengue protease inhibitors. J Comput Aided Mol Des. 2011;25:263–74. https://doi.org/10.1007/s10822-011-9418-0.
Korb O. Efficient ant colony optimization algorithms for structure- and ligand-based drug design. Chem Cent J. 2009;3:247–58. https://doi.org/10.1186/1752-153X-3-S1-O10.
Lampa AK, Bergman SM, Gustafsson SS, et al. Novel peptidomimetic hepatitis C virus NS3/4A protease inhibitors spanning the P2–P1′ region. ACS Med Chem Lett. 2014;5:249–54. https://doi.org/10.1021/ml400217r.
Le Grand S, Götz AW, Walker RC. SPFP: Speed without compromise—a mixed precision model for GPU accelerated molecular dynamics simulations. Comput Phys Commun. 2013;184:374–80. https://doi.org/10.1016/j.cpc.2012.09.022.
Li L, Basavannacharya C, Chan KWK, et al. Structure-guided discovery of a novel non-peptide inhibitor of dengue virus NS2B-NS3 protease. Chem Biol Drug Des. 2015;86:255–64. https://doi.org/10.1111/cbdd.12500.
Matsushita M, Ozaki Y, Hasegawa Y, et al. A novel phthalimide derivative, TC11, has preclinical effects on high-risk myeloma cells and osteoclasts. PLoS ONE. 2015;10:1–15. https://doi.org/10.1371/journal.pone.0116135.
Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the Flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. https://doi.org/10.1038/nrmicro1067.
Nestorowicz A, Chambers TJ, Rice CM. Mutagenesis of the yellow fever virus NS2A/2B cleavage site: effects on proteolytic processing, viral replication, and evidence for alternative processing of the NS2A protein. Virology. 1994;199:114–23. https://doi.org/10.1006/viro.1994.1103.
Nguyen TTH, Lee S, Wang HK, et al. In vitro evaluation of novel inhibitors against the NS2B-NS3 protease of dengue fever virus type 4. Molecules. 2013;18:15600–12. https://doi.org/10.3390/molecules181215600.
Noble CG, Seh CC, Chao AT, Shi PY. Ligand-bound structures of the dengue virus protease reveal the active conformation. J Virol. 2012;86:438–46. https://doi.org/10.1128/JVI.06225-11.
Pambudi S, Kawashita N, Phanthanawiboon S, et al. A small compound targeting the interaction between nonstructural proteins 2B and 3 inhibits dengue virus replication. Biochem Biophys Res Commun. 2013;440:393–8. https://doi.org/10.1016/j.bbrc.2013.09.078.
Pelliccia S, Wu YH, Coluccia A, et al. Inhibition of dengue virus replication by novel inhibitors of RNA-dependent RNA polymerase and protease activities. J Enzyme Inhib Med Chem. 2017;32:1091–101. https://doi.org/10.1080/14756366.2017.1355791.
Phong WY, Moreland NJ, Siew P, et al. Dengue protease activity: the structural integrity and interaction of NS2B with NS3 protease and its potential as a drug target. Biosci Rep. 2011;31:399–409. https://doi.org/10.1042/BSR20100142.
Selvam P, Lakra DR, Pannecouque C, De Clercq E. Synthesis, antiviral and cytotoxicity studies of novel N-substituted indophenazine derivatives. Indian J Pharm Sci. 2012;74:274–8. https://doi.org/10.4103/0250-474X.106077.
Timiri AK, Subasri S, Kesherwani M, et al. Synthesis and molecular modelling studies of novel sulphonamide derivatives as dengue virus 2 protease inhibitors. Bioorg Chem. 2015;62:74–82. https://doi.org/10.1016/j.bioorg.2015.07.005.
Tomlinson SM, Malmstrom RD, Russo A, et al. Structure-based discovery of dengue virus protease inhibitors. Antiviral Res. 2009;82:110–4. https://doi.org/10.1016/j.antiviral.2009.02.190.
Viswanathan U, Tomlinson SM, Fonner JM, et al. Identification of a novel inhibitor of dengue virus protease through use of a virtual screening drug discovery web portal. J Chem Inf Model. 2014;54:2816–25. https://doi.org/10.1021/ci500531r.
WHO. Treatment, prevention and control global strategy for dengue prevention and control 2. 2012.
Wyles DL. Antiviral resistance and the future landscape of hepatitis C virus infection therapy. J Infect Dis. 2013;207:33–9. https://doi.org/10.1093/infdis/jis761.
Yusof R, Clum S, Wetzel M, et al. Purified NS2B/NS3 serine protease of dengue virus type 2 exhibits cofactor NS2B dependence for cleavage of substrates with dibasic amino acids in vitro. J Biol Chem. 2000;275:9963–9. https://doi.org/10.1074/jbc.275.14.9963.
Acknowledgements
The Authors thank DST-SERB, Govt. of India for providing financial support through their project (EMR/2016/005711/) dated 7th August 2017 and Birla Institute of Technology, Mesra, Ranchi, India for providing the necessary infrastructure support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Murtuja, S., Shilkar, D., Sarkar, B., Sinha, B.N., Jayaprakash, V. (2021). Identification of Dengue NS2B-NS3 Protease Inhibitors Through High-Throughput Virtual Screening—Impacts on Drug Development Against the Dengue Virus. In: Ahmad, S.I. (eds) Human Viruses: Diseases, Treatments and Vaccines . Springer, Cham. https://doi.org/10.1007/978-3-030-71165-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-71165-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-71164-1
Online ISBN: 978-3-030-71165-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)