Abstract
Chronic obstructive pulmonary disease (COPD) includes emphysema and chronic bronchitis. These different phenotypes may introduce different characteristics that need to be known to improve COPD treatment. Thus, the objective of this study is to identify through the forced oscillations technique (FOT) the differences in respiratory mechanics in these phenotypes before and after bronchodilator (BD) use. In this cross-sectional study, the results of a sample of 30 volunteers are presented, being divided into control group (CG = 10), emphysema (ENF = 10) and chronic bronchitis (CB = 10). Spirometric and plethysmographic exams were also performed. Before BD, resistances were higher in CB than in controls, a fact that did not occur in a similar comparison in patients with ENF. It were observed increased resistances and impedance modulus in CB in comparison with ENF after BD use, which may be explained by secretion mobilization due to maximum expiratory effort during spirometry. These results provide evidence that the presence of CB results in greater respiratory work than that resulting from the presence of ENF. This new information on COPD phenotypes can contribute to improving the therapeutic strategies used, and consequently, to the assistance offered to these patients. The presented results also provide evidence that FOT is a useful tool in evaluation of the COPD phenotypes, adequately describing the disease’s physiology and clinical practice.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Responsible for approximately 8% of all deaths worldwide, chronic obstructive pulmonary disease (COPD) has a prominent role among the countless known respiratory diseases. The result of an abnormal inflammatory response of the lungs, usually caused by cigarettes, it presents itself through airflow limitation and comprises known diseases, such as emphysema and chronic bronchitis [1,2,3]. With different clinical manifestations, emphysema can be evidenced by the presence of increased lung and residual volume, chronic dyspnea, in addition to hyperinflation observed on chest radiography, with greater destruction of the lung parenchyma [4,5,6]. The patient with chronic bronchitis, on the other hand, experiences a productive cough with yellowish secretion and sputum for at least three months for two consecutive years, and dyspnea on medium exertion. Both present a long history of smoking [7]. It is known that the identification of these phenotypes provides a better therapeutic strategy [8].
Spirometry is considered the best method for diagnosing functional respiratory disorders in these patients [9]. However, it requires great effort and cooperation from the individual [10]. Complementary to the spirometry exam, whole body plethysmography assesses lung volumes and capacities, making it possible to measure other changes caused by the development of the disease [11].
The forced oscillation technique (FOT) is a non-invasive method based on the application of low amplitude oscillations to the individual’s respiratory system in spontaneous breathing [12]. It is currently under discussion whether FOT evaluates pulmonary mechanics in a more sensitive and detailed way, compared to spirometry [13,14,15,16]. Previous works by our group have shown that, through the parameters of reactance and resistance, this method is able to diagnose changes in the lungs of smokers early and assess obstructive respiratory disorders in detail, including parameters reflecting respiratory compliance [17, 18]. Thus, this method has great potential to expand our understanding of the differences between emphysema and chronic bronchitis, contributing to improve the treatment of COPD.
In this context, the objectives of this study were: (1) to identify possible differences in mechanical changes resulting from emphysema and chronic bronchitis; (2) to evaluate the possible differences in the response to the bronchodilator in these cases.
2 Materials and Methods
FOT, spirometry and plethysmography assessments were performed at the Biomedical Instrumentation Laboratory at the State University of Rio de Janeiro. This research is a cross-sectional study that was approved by the Research Ethics Committee of the Pedro Ernesto University Hospital (protocol 456—CEP/2018/HUPE), respecting the guidelines and regulatory standards established in resolution 466/2012 of the National Health Council (CNS) involving human beings, also meeting ethical and scientific foundations. All individuals signed an informed consent form (ICF) on the study and the examinations performed.
2.1 Analysis of the Individuals
A total of 30 individuals were evaluated. After medical evaluation through chest X-ray and interview, the individuals were allocated into the respective groups for the study: 10 in the emphysema group (ENF), 10 in the chronic bronchitis group (CB) and 10 in the control group (CG). Control consists of individuals over 40 years of age, with no smoking history, normal pulmonary function tests, and who had no history of respiratory or cardiac disease.
The use of a bronchodilator (400 μg of salbutamol in a sulfate spray) was performed after the first sequence of pulmonary function tests, as a way of quantifying the reduction in bronchial smooth muscle tone, waiting 15 min to repeat the same sequence of tests.
2.2 Instrumentation
For the FOT evaluation, the prototype of an impedance analyzer (OSCILAB 2.0) developed in our laboratory [19] has been used. The instrument evaluates the parameters from the resistance (Rrs) and reactance (Xrs) curves of the respiratory system in a frequency range between 4 and 32 Hz. These curves are interpreted by means of R0, which corresponds to the resistance of the intercept and represents the total resistance of the respiratory system, of the Rm that reflects the resistance of the central airways, and of the S and Xm that are related to the homogeneity of the respiratory system. By calculating the reactance measured at 4 Hz, the dynamic compliance of the Cdyn respiratory system is obtained (Cdyn = −1/2π.f.X4Hz). The resonance frequency parameter (fr) reflects the changes referring to inhomogeneity of the respiratory system that reduce compliance [20, 21]. A coherence function >0.90 is used as the test acceptance criterion [22, 23].
For spirometry and plethysmography, a computerized system with a pneumotachograph (nSpire Health, Inc., 1830 Left hand Circle, Longmont, CO 80501) is used, following the GOLD acceptance and reproducibility criteria [24]. The Pereira’s reference equations for spirometry were used to assess the forced expiratory volume in the first second (FEV1), the forced vital capacity (FVC) and the FEV1/FVC ratio. For full body plethysmography, Neder’s equations were used, evaluating the reserve expiratory volume (VRE), total lung capacity (CPT), functional residual capacity (CRF) and airway resistance (Rva) [25, 26].
2.3 Statistical Analysis
The results are presented using means ± SD. Statistical analysis was performed using the ORIGIN 8.0 program (Microcal Software Inc., Northampton, Massachusetts, United States). Considering that the groups are relatively small, a non-parametric test (Mann-Whitney) was used. The results were considered significant with p < 0.05.
3 Results
The anthropometric characteristics of the groups studied are shown in Table 1. Table 2 describes the results obtained by plethysmography. Two patients with CB did not obtain technically acceptable exams in plethysmographic assessments. The use of BD did not alter the plethysmographic parameters, both in the ENF and CB groups.
In Table 3, we observed that the pre-BD spirometric values dropped significantly in patients in the ENF group compared to the control group, with the exception of FVC. A similar analysis in CB showed a significant reduction in all spirometric parameters. Table 3 also shows that the use of BD did not significantly change the spirometric parameters in the three groups studied.
3.1 Resistive FOT Parameters
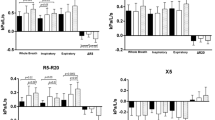
The changes in R0 and Rm (Figs. 1a, b) were not significant between the pre and post BD, when compared within the phenotypes and against the CG. There was a significant increase in R0 and Rm in CB compared to CG (p < 0.04). These parameters did not show differences between the ENF and CB groups before using BD. After using BD, R0 and Rm were significantly higher in CB (p < 0.05). S (Fig. 1c) did not change with the use of BD. The ENF and CB groups showed significantly more negative values than the CG (p < 0.05), and were not different from each other, both before and after using BD.
3.2 Reactive FOT Parameters
Xm, Cdyn, fr and Z4 did not change significantly with the use of BD (Fig. 2a–d, respectively). Xm was more negative in ENF and CB than in controls (p < 0.05), with no difference between phenotypes (Fig. 2a) before and after BD. Cdyn showed no differences between the groups studied (Fig. 2b) and fr was higher in the phenotypes than in the control group (p < 0.05), with no differences between the phenotypes (Fig. 2c). Z4 was significantly higher in the CB group compared to the CG (p < 0.05), with no significant changes in the comparison between the ENF group and the CG before the use of BD. After using bronchodilator medication, Z4 was significantly higher in patients with CB (p < 0.04).
4 Discussion
The biometric parameters of the groups under study (Table 1) did not show significant differences, which demonstrates the homogeneity of the studied groups.
Whole body plethysmography is indicated as a fast and effective method to assess obstructive diseases and better classify ventilatory disorders [25]. COPD patients often have increased total lung capacity due to loss of elastic retraction and consequent decrease in the capacity to empty the lungs, reflecting in greater airway resistance and increased residual volume. The plethysmography values before and after BD of the ENF and CB phenotypes (Table 2) are consistent with the pathophysiology of COPD [27].
The measures used by spirometry for the diagnosis of COPD such as FEV1 and CFV were within the normal range in the control group, as expected (Table 3). These parameters showed a significant reduction in the ENF and CB groups, confirming obstructive pulmonary disease. In addition, a significant reduction in the PFE value was observed.
The greatest resistances presented by R0 and Rm (Fig. 1a, b) before BD in CB contrast with the non-significant changes observed in the ENF. This can be explained, at least in part, by the physiological changes typical of this phenotype, which are related to increased bronchial secretion. These results indicate that this mechanism is more relevant to the increase in R0 and Rm than the typical reduction in radial retraction observed in patients with ENF [28].
The changes observed in S before the use of BD (Fig. 1c) confirmed the loss of homogeneity in ventilation usually observed in the phenotypes under study [28]. In this case, both phenotypes showed significant changes in relation to the control group, without a clear distinction between them.
The mean reactance values (Xm) were more negative in the phenotypes. According to Cavalcanti et al. [29] the loss of elastic retraction and destruction of the lung parenchyma in patients with COPD lead to a decrease in dynamic compliance, which results in more negative Xm values (Fig. 2a) and lower Cdyn values. In the present study, however, Cdyn did not change, either in the ENF group or in the CB group (Fig. 2b). This can be explained by the high standard deviation observed in the results.
The resonance frequency increased in both the ENF and CB groups (Fig. 2c). This can be explained by the increase in the non-homogeneity of the respiratory system of these patients, which occurs due to the increase of imbalances in the time constants in the different regions of the lungs of COPD patients [29].
Z4 presented a behavior consistent with that observed in R0 and Rm (Fig. 2d): the CB phenotype group showed a significant increase when compared to the CG, while the ENF phenotype did not show significant change. These results provide evidence that the presence of CB results in greater respiratory work than that resulting from the presence of ENF. This provides additional support for previous findings in the same direction [1].
Considering the responses resulting from the use of BD, we observed that the use of this medication did not result in changes, both in the resistive (Fig. 1) and reactive (Fig. 2) parameters. Previous studies have also shown no changes in FOT parameters due to the use of BD in controls [30]. These results are consistent with those observed in plethysmography (Table 2) and spirometry (Table 3), which did not show changes in the groups analyzed.
The most interesting results are, possibly, the presence of higher values of R0, Rm and Z4 after the use of BD in patients with CB, compared to those with ENF (Figs. 1a, b and 2d, respectively). These results are consistent with the observation that these parameters were higher in CB than in controls before BD, a fact that did not occur in a similar comparison in patients with ENF. One possible explanation is related to the presence of a greater number of women in the CB group than in the ENF group (Table 1). Previous studies using spirometry indicate that female patients respond less to the use of BD [31]. This explanation, however, does not seem to be adequate to the results obtained by the FOT, because in this case the most relevant biometric parameter is the height [32], and the height was similar between the CB and ENF groups (Table 1). From the point of view of the difference between the phenotypes, these findings can be explained by the greater amount of secretion present in patients with CB [1]. The spirometry test is performed before using BD and after using the medication. In the occurrence of efforts, such as those performed during the spirometry exam, which requires maximum effort on expiration, there is an increase in airway resistance due to the mobilization of secretion and a decrease in lumen [1]. Similar facts do not occur in ENF. Thus, the mobilization of secretion due to the maximum expiratory effort during spirometry appears to be a more plausible explanation for the differences observed in Figs. 1a, b and in Fig. 2d. Fujimoto et al. [33] investigated the association between phenotypes of COPD patients with or without predominance of emphysema and the efficacy of long-acting bronchodilators and β2-agonists. In agreement with the present study, the authors observed a greater change in resistance in the emphysema phenotype than in the phenotype that did not present emphysema predominance.
5 Conclusions
The results presented provide evidence that respiratory oscillometry is a useful tool in the diagnosis of changes caused by different COPD phenotypes. The system adequately described the physiology of the disease and clinical observations. After using BD, airway obstruction assessed by R0 and Rm is greater in CB phenotype than in ENF. This increase is reflected in greater respiratory effort in CB than in ENF, as assessed by Z4. This new information on the different phenotypes of COPD can contribute to improving the therapeutic strategies used, and, consequently, to the assistance offered to these patients.
Conflict of Interest
The authors report no conflict of interest.
References
Oliveira PC (2013) Clinical presentations of COPD. Pulmão RJ 22(2):15–18
Murray CJ, Lopez AD (1997) Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet 349(9064):1498–1504
Leung JM, Obeidat M, Sadatsafavi M, Sin DD (2019) Introduction to precision medicine in COPD. Eur Respir J
Izquierdo-Alonso JL, Rodriguez-Gonzalezmoro JM, de Lucas-Ramos P, Unzueta I, Ribera X, Anton E, Martin A (2013) Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD). Respir Med 107(5):724–731
Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, Trigueros JA, Cosío BG, Casanova C, Antonio Riesco J et al (2017) Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol 53(6):324–335
Cheng Y, Tu X, Pan L, Lu S, Xing M, Li L, Chen X (2017) Clinical characteristics of chronic bronchitic, emphysematous and ACOS phenotypes in COPD patients with frequent exacerbations. Int J Chron Obstruct Pulmon Dis 12:2069–2074
Koblizek V, Chlumsky J, Zindr V, Neumannova K, Zatloukal J, Zak J, Sedlak V, Kocianova J, Hejduk K, Pracharova S (2013) Chronic obstructive pulmonary disease: official diagnosis and treatment guidelines of the czech pneumological and phthisiological society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 157(2):189–201
Calle Rubio M, Casamor R, Miravitlles M (2017) Identification and distribution of COPD phenotypes in clinical practice according to Spanish COPD guidelines: the FENEPOC study. Int J Chron Obstruct Pulmon Dis 12:2373–2383
Trindade AM, Sousa TLF, Albuquerque ALP (2015) The interpretation of spirometry on pulmonary care: until where can we go with the use of its parameters? Revista Pulmão RJ 24(1):3–7
Qi GS, Zhou ZC, Gu WC, Xi F, Wu H, Yang WL, Liu JM (2013) Detection of the airway obstruction stage in asthma using impulse oscillometry system. J Asthma 50
Pereira CAC, Moreira MAF (2002) Pletismografia—resistência das vias aéreas. J Pneumologia 28:139–150
King GG, Bates J, Berger KI, Calverley P, de Melo PL, Dellaca RL, Farre R, Hall GL, Ioan I, Irvin CG et al (2019) Technical standards for respiratory oscillometry. Eur Respir J
Dubois AB, Brody AW, Lewis DH, Burgess BF Jr (1956) Oscillation mechanics of lungs and chest in man. J Appl Physiol 8(6):587–594
Ribeiro CO, Faria ACD, Lopes AJ, Melo PL (2018) Forced oscillation technique for early detection of the effects of smoking and COPD: contribution of fractional-order modeling. Int J Chronic Obstructive Pulm Dis 13:3281–3295
Kaczka DW, Dellacá RL (2011) Oscillation mechanics of the respiratory system: applications to lung disease. Crit Rev Biomed Eng 39(4):337–359
King GG (2011) Cutting edge technologies in respiratory research: lung function testing. Respirology 16(6):883–890
Ribeiro CO, Faria AC, Lopes AJ, Melo PL (2018) Forced oscillation technique for early detection of the effects of smoking and chronic obstructive pulmonary disease: contribution of fractional-order modeling. Int J COPD 13:3281–3295
Faria ACD, Dames da Silva KK, Costa GM, Lopes AJ, Melo PL (2012) Forced oscillation technique in the detection of smoking-induced respiratory changes. In: Hudak R, Penhaker M, Majernik J (eds) Biomedical engineering—technical applications in medicine vol 1. InTech, Croatia
Melo PLd, Werneck MM, Giannella-Neto A (2000) New impedance spectrometer for scientific and clinical studies of the respiratory system
Miranda IA, Dias Faria AC, Lopes AJ, Jansen JM, Lopes de Melo P (2013) On the respiratory mechanics measured by forced oscillation technique in patients with systemic sclerosis. PLoS ONE 8(4):
Navajas D, Farre R (2001) Forced oscillation technique: from theory to clinical applications. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace/Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Universita di Napoli, Secondo ateneo 56(6):555–562
Cataluña JJS, Cosio B, Izquierdo JL, Campos JLL, Marin JM, Aguero R, Baloira A, Carrizo S, Esteban C, Galdiz JB et al (2012) Documento de consenso sobre o fenótipo de asma-COPD misto no Documento de Consenso da DPOC sobre o Fenótipo de Sobreposição COPD-Asma na DPOC. Archivos de Bronconeumologia 48(9):331–337
Bártholo TP, da Costa CH (2017) Fenótipos da DPOC: há interesse prático nesta avaliação? COPD phenotypes: is it interesting in clinical practice? Revista Pulmão 26:23–28
Global Initiative For Chronic Obstructive Lung Disease—UPDATE (2019) Global strategy for the diagnosis, management, and prevention of chronic obstrutive pulmonary disease
Pereira CAC, Sato T, Rodrigues SC (2007) Novos valores de referência para espirometria forçada em brasileiros adultos de raça branca. J Brasileiro Pneumologia 33
Neder JA, Andreoni S, Lerario MC, Nery L (1999) Valores de referência para testes de função pulmonar. II. Pressões respiratórias máximas e ventilação voluntária. Revista Brasileira de Pesquisa Médica e Biológica 32(0100-879X)
Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M et al (2019) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report. Eur Respir J
Di Mango AM, Lopes AJ, Jansen JM, Melo PL (2006) Changes in respiratory mechanics with increasing degrees of airway obstruction in COPD: detection by forced oscillation technique. Respir Med 100(3):399–410
Cavalcanti JV, Lopes AJ, Jansen JM, Melo PL (2006) Detection of changes in respiratory mechanics due to increasing degrees of airway obstruction in asthma by the forced oscillation technique. Respir Med 100(12):2207–2219
Cavalcanti JA, Lopes AJ, Jansen JM, Melo PL (2006) Técnica de oscilações forçadas na análise da resposta broncodilatadora em voluntários sadios e indivíduos portadores de asma brônquica com resposta positiva. J Brasileiro de Paneumologia 91–98
Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke PS, Gulsvik A (2006) Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med 173(12):1316–1325
Ribeiro FCV, Lopes AJ, Melo PL (2018) Reference values for respiratory impedance measured by the forced oscillation technique in adult men and women. Clin Respir J 12(6):2126–2135
Fujimoto K, Kitaguchi Y, Kanda S, Urushihata K, Hanaoka M, Kubo K (2011) Comparison of efficacy of long-acting bronchodilators in emphysema dominant and emphysema nondominant chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 6:219–227
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this paper
Cite this paper
Teixeira, E.M., Lopes, A.J., Melo, P.L. (2022). Differences in Respiratory Mechanics in Emphysema and Chronic Bronchitis Evaluated by Forced Oscillations. In: Bastos-Filho, T.F., de Oliveira Caldeira, E.M., Frizera-Neto, A. (eds) XXVII Brazilian Congress on Biomedical Engineering. CBEB 2020. IFMBE Proceedings, vol 83. Springer, Cham. https://doi.org/10.1007/978-3-030-70601-2_44
Download citation
DOI: https://doi.org/10.1007/978-3-030-70601-2_44
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70600-5
Online ISBN: 978-3-030-70601-2
eBook Packages: EngineeringEngineering (R0)