Abstract
Percutaneous spine biopsy is an effective modality for the diagnosis and management of spinal infection when a microbiological diagnosis for a known associated organism has not been established by blood cultures or serological tests. It allows obtaining of specimens for microbiological and histopathological diagnosis. This safe and cost- and time-saving procedure is less painful and less invasive than open surgical biopsy. Several imaging-guidance methods are available, including fluoroscopy, computed tomography (CT), ultrasonography, and magnetic resonance imaging, but CT and fluoroscopy remain the most frequently used ones. A good knowledge of spinal anatomy, meticulous biopsy technique, and close collaboration among the interventional radiologist, pathologist, microbiologist, and clinician is essential to obtaining good results and avoiding complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Computed tomography (CT)
- Fluoroscopy
- Histology
- Microbiology
- Percutaneous biopsy
- Spinal biopsy
- Spinal infection
1 Introduction
An accurate diagnosis of spinal infection is essential for its successful management, but it remains a challenging exercise. Percutaneous spine biopsy is an effective modality for the prompt diagnosis and proper management of spinal infection when the other biological tests are negative. It allows obtaining of specimens for microbiological and histopathological diagnosis. One or more spinal biopsies are sometimes needed before the causative agent can be isolated from the specimen. Percutaneous imaging-guided biopsy has several advantages over surgical open biopsy. It is a safe and cost- and time-saving procedure and is less painful and less invasive than surgical biopsy. It usually does not require hospitalization or general anesthesia and results in fewer complications (Peh 2006; Colmenero et al. 2013).
Apart from investigating spinal infection, percutaneous spinal biopsy may be indicated to determine the nature of a solitary spine lesion or exclude malignancy by differentiating infections (most commonly tuberculosis) from malignant lesions (Aithala 2016). Several image-guidance technologies are available including fluoroscopy, computed tomography (CT), ultrasonography, and magnetic resonance imaging (MRI) (Wu et al. 2012; Liu et al. 2015) but CT and fluoroscopy remain the most frequently used ones. A good knowledge of spinal anatomy and a meticulous technique is important to obtain good results and to avoid complications.
2 General Principles, Indications, and Pre-biopsy Work-Up
First described by Coley and Martin in the early 1930s, percutaneous bone biopsy was used for spine lesions in 1935 by Robertson and Ball. The use of trephine and radiographs during the procedure was reported by Siffert et al. in 1949, and the use of CT for biopsy guidance was first reported by Adapon in 1981 (Nourbakhsh 2015). Percutaneous spinal biopsy is expected to serve as an effective modality for the prompt diagnosis and proper management of spinal infection (Colmenero et al. 2013). It is indicated in patients with suspected spinal infection, aiming to obtain samples of organism when a microbiological diagnosis for a known associated organism has not been established by blood cultures or serological tests (Peh 2006; Berbari et al. 2015). There are two other clinical scenarios of spinal infection that need biopsy, namely, to differentiate malignant lesion from infection (most commonly tuberculosis) and, secondly, treatment failure in a patient with clinical and imaging evidence of spinal infection (Berbari et al. 2015; Aithala 2016).
A well-planned and executed biopsy is however essential for accurate diagnosis and therefore appropriate treatment (Peh 2006). It should be performed in referral centers as it has been proven that the complication rates are lower (Nourbakhsh 2015). The only absolute contraindication of percutaneous spinal biopsy is bleeding diathesis with decreased platelet count <50,000/mm3 (Peh 2006; Nourbakhsh 2015). The pre-biopsy work-up should include routine spine radiographs, CT, and/or MRI of the spine, complete blood count, activated partial thromboplastin time (APTT), and prothrombin time (PT). The radiologist should carefully study the radiographs, CT, and/or MRI before the biopsy: firstly, to rule out some conditions that do not need biopsy such as spinal echinococcosis and degenerative disk disease and, secondly, to choose the most appropriate approach and therefore to avoid vital structures (Peh 2006; Ladeb et al. 2019). The diagnostic algorithm for patients with clinical suspicion of spinal infection (Fig. 1) is found in the chapter entitled “Diagnostic Algorithm of Spinal Infection”.
Percutaneous spinal biopsy is usually performed under local anesthesia. The need for sedation or antianxiety premedication should be evaluated and written informed consent obtained (Peh 2006; Nourbakhsh 2015). Anticoagulants, aspirin, and nonsteroidal anti-inflammatory drugs should be discontinued 7 days before the biopsy (Nourbakhsh 2015). Spinal biopsy should be performed before starting antibiotics, with samples sent for both pathological and bacteriological examinations (Rankine et al. 2004). Otherwise, antibiotics should be stopped at least 48 h before the procedure. However, apart from the lower success rate, antibiotic therapy should not be considered as a reason for not performing percutaneous biopsy in cases of suspected infection (Rehm et al. 2016). The diagnostic algorithm for management and etiological diagnosis of spinal infection (Fig. 2), including the role of imaging-guided percutaneous biopsy, is found in the chapter entitled “Diagnostic Algorithm of Spinal Infection”.
3 Biopsy Technique
3.1 Guidance Methods
A variety of imaging modalities, such as fluoroscopy, CT, CT fluoroscopy, ultrasonography, and MRI, may be used for biopsy guidance. The choice of imaging guidance modality to be employed is determined by individual operator preference and the availability of equipment and facilities (Peh 2006; Shrestha et al. 2015). CT is currently the modality of choice for guiding percutaneous biopsy of spinal lesions. It has been shown to be slightly superior to fluoroscopy for percutaneous biopsy, with regard to adequacy (92.6% in CT versus 90.1% in fluoroscopy), accuracy (90.2% in CT versus 88.1% in fluoroscopy), and complications (3.3% in CT versus 5.3% in fluoroscopy); although the differences were not statistically significant (Nourbakhsh 2015; Chaudhary et al. 2019).
Compared with fluoroscopy, CT more precisely shows the needle position and is potentially safer. Some lesions may be inaccessible under fluoroscopy guidance due to their small size or anatomical site. Additionally, visualization of the neural arch and paravertebral lesions is difficult by fluoroscopy, while CT ensures accurate biopsy of these lesions (Nourbakhsh 2015). However, a meta-analysis did not show any difference between percutaneous spinal biopsy performed under fluoroscopy compared to CT guidance (Pupaibool et al. 2015; Sahoo et al. 2019). With CT fluoroscopy, near real-time imaging is possible using a relatively small radiation dosage compared with conventional diagnostic CT (Peh 2006).
Ultrasonography has been advocated for guiding biopsy of lesions in the cervical spine and posterior elements of the thoracic and lumbar spine. The reasons for considering ultrasonography are cost-effectiveness, real-time monitoring, and avoidance of ionizing radiation. This modality cannot be used for deeply located bone lesions without invasion to the cortex. However, it can successfully be used for vertebral posterior elements or paraspinal lesions.
Independently of the chosen method of guidance, percutaneous spinal biopsy must be performed in aseptic conditions, identical to those achieved in the operating room. The decision also depends on the availability of imaging guidance and the expertise of the physician. There might be a steep learning curve to gain expertise in the wide variety of approaches used to obtain adequate specimens. The duration of biopsy depends on the radiologist’s expertise and lesion site. Rehm et al. (2016) observed a strong correlation between the anatomical level and the biopsy duration; following the rule that the closer to the sacrum, the faster the biopsy was carried out. An overview of CT-guided percutaneous biopsy is provided in the chapter entitled “Radiography and Computed Tomography of Spinal Infection”.
3.2 Patient Positioning and Biopsy Approach
The approach to be adopted by the interventional radiologist should be appropriately tailored for each patient. It depends on the involved spine level, the exact site of the lesion, and patient positioning (Peh 2006; Gallucci and D’Orazio 2015). A lateral extrapedicular approach may be used for the lumbar spine and the transpedicular approach (Fig. 1) or transcostovertebral approach (Figs. 2 and 3) for the thoracic and lumbar spine or posteriorly located cervical lesions (Peh 2006; Wiesner et al. 2018).
In biopsy of anteriorly located lesions in the mid- and lower cervical spine, an anterolateral approach is usually adopted (Fig. 4). The radiologist’s fingers are used to guard the carotid artery, the internal jugular vein, and adjacent nerves, while the needle is inserted and directed towards the vertebral lesion. The patient’s right side is preferably used, to avoid puncturing the esophagus and trachea (Peh 2006; Wiesner et al. 2018). The trans-oral approach is recommended for anteriorly located lesions of the first three cervical vertebrae, with this procedure preferably referred to the otolaryngological surgeon (Peh 2006; Nourbakhsh 2015).
For sacral lesions, either a posterior or posterolateral approach may be used (Nourbakhsh 2015).
The patient’s position depends on the guidance method and biopsy site and aims to facilitate needle access to the lesion and ensure as much comfort as possible to the patient while limiting movement. The prone position is usually used for CT-guided spinal biopsy of the thoracic and lumbosacral spine, as well as posterior elements of the cervical spine, whereas the supine position is used for anteriorly located cervical spinal lesions. Lateral decubitus and semi-prone or semi-supine position are rarely used. Care should still be taken to minimize irradiation to both the patient and the operator (Peh 2006). The patient’s vital signs should be continuously monitored during the procedure.
Following preliminary scanning, the most appropriate image is selected to plan the most optimal needle route (entry point, lesion depth, and angle), taking care to avoid vital anatomical structures, major blood vessels, nerves, peritoneal cavity, as well as the spinal canal and its contents. The skin is then cleaned and draped. After administration of local anesthesia (1% lignocaine), a small skin incision is made, and the biopsy needle is inserted and directed into the lesion under imaging guidance (Peh 2006; Ladeb et al. 2019). The determinants of the approach, instruments, and imaging method used for spinal biopsy include the lesion type, location of the lesion in the vertebrae, and the involved spinal level (Nourbakhsh 2015).
3.3 Biopsy Methods and Needles
Three main methods of percutaneous spine biopsy are currently used, namely, discovertebral core biopsy in which the operator performs a biopsy on the disk and adjacent vertebral end plates; fine needle aspiration or biopsy of the intervertebral disk; and biopsy of the paravertebral abscess wall (Fig. 5). International scientific societies recommend discovertebral biopsy, advising radiologists to obtain at least three specimens during biopsy (Spilf 2007; Berbari et al. 2015; Ladeb et al. 2019). The combination of aspiration and biopsy is preferred (Nourbakhsh 2015).
The type of needle used depends on the biopsy method, the nature of the lesion, and the operator’s personal preference. A large variety of needles are available: aspiration needles (e.g., spinal or Chiba) are mainly used for fine needle aspiration of the disk and cutting, or Tru-cut needles are mostly used in paravertebral soft tissue biopsy, and trephine needles are used for discovertebral biopsy (Fig. 6). They range in size from 11G to 22G. The needle should be long enough to reach the lesion and have the appropriate bore size to obtain an adequate amount of specimen (Peh 2006; Sahoo et al. 2019).
Whichever needle type and imaging method are employed, imaging should be continuously or at least intermittently done: firstly, to ensure that the needle tip is in a safe position during its insertion and, secondly, to confirm its placement within the lesion. Ideally, the needle should be placed into different parts of the lesion to ensure representative sampling (Peh 2006). Coaxial biopsy needle systems facilitate the procedure and allow easy and rapid obtention of multiple samples (Fig. 7).
3.4 Specimen Handling
All material, tissue or fluid obtained, including blood clots, should be sent for cytology, culture, and histopathological examination (Fig. 8). As there are different methods of specimen handling and fixation, pre-procedure consultation among the radiologist, microbiologist, and pathologist is required to ensure that the adequate number of specimens are obtained, and proper tests are ordered. The latter includes cultures, gram staining, gene amplification techniques, and histology tests (Nourbakhsh 2015). As many specimens as possible should be submitted to the laboratory and may include aspirates and/or tissue biopsies. However, there is no available data demonstrating which and how many tests should be utilized to be the ideal “combined reference standard” (Pupaibool et al. 2015).
Solid samples are usually sent for histopathological examination, but at least one of them should be collected in a sterile container and then submitted for culture. Liquid samples should ideally be immediately cultured in blood culture bottles in the procedure room (Fig. 9). If anaerobics are the expected causative organisms, rapid handling to the microbiologist is necessary. When an infection is suspected and no fluid could be obtained, several sterile saline injections and aspirations should be tried. Specimens should be transported immediately to the laboratory at room temperature.
All the specimens should be carefully labeled and dispatched promptly by a responsible person. The radiologist should also be familiar with smear preparation on glass slides and the various types of containers to be used for culture and histopathological specimens (Peh 2006) (Fig. 10). Clinical and epidemiologic information should be provided on the request form because additional testing may be required (Ladeb et al. 2019). The histological examination of the blood clots increases the accuracy of the biopsy and hence should be treated like a tissue specimen (Nourbakhsh 2015).
3.5 Follow-Up
Post-biopsy care monitoring of the patient may vary in duration from 1 h to overnight observation. Some authors recommend a chest radiograph after thoracic spine biopsy. In cases of wide-bore trephine use or high-risk patients, cardiopulmonary monitoring is recommended (Nourbakhsh 2015). Post-procedure blood cultures are recommended by some authors, but this practice is still controversial (Ladeb et al. 2019).
Negative results in the presence of high clinical suspicion necessitate a repeat biopsy before institution of prolonged antibiotic therapy (Ozsarlak et al. 2003; Kasalak et al. 2018). In cases of a negative initial biopsy, repeat CT-guided biopsy improves the overall yield (Terreaux et al. 2016; Husseini et al. 2020). Open biopsy should be considered when percutaneous needle biopsy yields a negative result, to enable administration of organism-specific antibiotics for a successful outcome. Furthermore, empirical antibiotics should be delayed until the results of cultures are available, unless the patient is severely septic, critically ill, neutropenic, or neurologically compromised (Nourbakhsh 2015).
4 Results
4.1 Microbiological Diagnosis of Spinal Infection
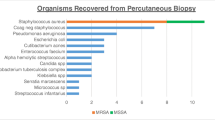
The epidemiology of the causative agents for spinal infection depends on the geographical area. Typical bacterial agents such as Staphylococcus aureus, Streptococcus spp., enterobacteria, and other gram-negative rods are the most common pathogens isolated in most regions of the world. However, Mycobacterium tuberculosis and Brucella spp. are among the common pathogens in certain endemic regions. Fungi are rare and are essentially reported in immunocompromised patients (Berbari et al. 2015).
Current Infectious Disease Society of America guidelines suggest the addition of fungal, mycobacterial, or brucellar cultures on image-guided biopsy and aspiration specimens in patients with suspected spinal infection, if epidemiological host risk factors or characteristic imaging clues are present. They also suggest the addition of fungal and mycobacterial cultures and bacterial nucleic acid amplification testing to appropriately stored specimens if aerobic and anaerobic bacterial cultures reveal no growth in patients with suspected spinal infection. Molecular diagnostic tools had been especially useful in the diagnosis of brucellar, mycobacterial, and fungal spinal infections (Berbari et al. 2015). When brucellar native vertebral osteomyelitis is suspected, the physician is advised to alert the microbiology laboratory personnel to mitigate the risk of laboratory-acquired brucellar infection and to use appropriate techniques (Berbari et al. 2015).
The reported overall accuracy of CT-guided spinal bone biopsy varies from 67 to 97% (Peh 2006; Nourbakhsh 2015). However, in cases of spinal infection, the reported accuracy is more variable, with positive microbiological results obtained in 29–70% of cases (Rankine et al. 2004; Sehn and Gilula 2012; Pupaibool et al. 2015; Joo et al. 2016; Ladeb et al. 2019; Özmen et al. 2019; Sertic et al. 2019). This is lower than the diagnostic yield of blood cultures which is approximately 59% and open biopsies estimated at 91%. This could be explained by the fact that more severe cases requiring surgical management could correlate to higher pathogen load (Sertic et al. 2019).
The location and type of the lesion, needle bore size, and imaging guidance modality have been mentioned as determinants of the accuracy of spinal biopsy (Nourbakhsh 2015; Aithala 2016). The wide range of success rates depends on the organism and multiple other factors, including inadequate amount of specimen, sampling error, and empirical antibiotics at the time of the biopsy, as well as experience and skill of radiologist and sometimes difficulty of the paraspinal approach (Nam et al. 2011; Pupaibool et al. 2015; Rehm et al. 2016). The puncture site and biopsy method do not seem to influence the results: no statistical difference was found among yields of end-plate disk, disk-only, and paravertebral soft-tissue biopsies, either for microbiological or histological study (Chang et al. 2015; Özmen et al. 2019).
However, improvements can be made in biopsy technique and specimen transfer to optimize culture yield and increase the clinical value of the procedure. Taking several cores seem to improve the diagnostic yield. However, obtaining more than three biopsies of the lesion does not increase the likelihood of positive results (Nourbakhsh 2015). Previous antibiotic therapy administration has been recognized as a major cause for negative results of microbiological examination of the specimen obtained. Some authors advise repeating the biopsy after at least 1 week without antibiotic therapy (Gallucci and D’Orazio 2015). Despite this, spinal biopsy is still worthwhile performing, even if the patient has been started on antibiotics (Rankine et al. 2004). In a recent meta-analysis, McNamara et al. (2017) did not find a significantly different biopsy yield between patients with and without prior antibiotic exposure.
4.2 Histopathological Diagnosis of Spinal Infection
In routine practice, fixation and decalcification are required for processing tissues obtained from the spine. The control of these key steps is crucial to preserve tissue morphology for histopathological examination and deoxyribonucleic acid (DNA) for molecular analysis. Fixation of core bone specimens in buffered formalin for at least 6 h is recommended. Ethylene diamine tetracetic acid (EDTA) is recommended for the decalcification of core bone specimens. However, for surgical specimens, decalcification with EDTA is slow and time-consuming. The histological examination of the blood clots increases the accuracy of the biopsy and should be treated as a tissue specimen (Nourbakhsh 2015). Although the diagnosis of infectious spondylodiscitis is mainly confirmed by microbiological investigations, histopathological examination of bone specimens is a key tool for the etiological diagnosis of the disease. Positive culture is not always obtained due to the low load of microorganisms and the difficulty of cultivating them. The etiological agent is never identified in approximately one-third of infectious spondylodiscitis (Sheikh et al. 2017).
Histopathology provides valuable information on cell morphology and tissue changes that allow the determination of the etiological diagnosis of infectious spondylodiscitis. Histopathological features show a large spectrum, which vary from neutrophil-rich infiltrate to granulomatous infection (Duarte and Vaccaro 2013). Positive histopathological results in patients with suspected infectious spondylodiscitis vary in the literature from 55 to 74% (Romdhane et al. 2020; Özmen et al. 2019), reaching more than 96% for spinal tuberculosis (Ladeb et al. 2019; Rammeh et al. 2021). The main goal of the histological study is to distinguish granulomatous from non-granulomatous spinal infections, and in the case of spinal tuberculosis, it may be sufficient to confirm the diagnosis, even in the absence of microbiological positivity (Romdhane et al. 2020). Pyogenic spinal infection is histologically characterized by edematous fibrosis and neutrophil-rich infiltrate (Li et al. 2016). The composition of the infiltrate depends on the stage of the disease. Neutrophils are numerous in the early stage (Fig. 11). But these features are not pathognomonic, as neutrophil-rich infiltrate is also observed in rare forms of tuberculous spondylodiscitis, in fungal spondylodiscitis, and even in brucellar spondylodiscitis (Rammeh et al. 2020).
Tuberculous spondylodiscitis is characterized by necrotizing epithelioid granulomas (Romdhane et al. 2020), which is observed in 59–76% of spinal tuberculosis (Cottle and Riordan 2008). Epithelioid granulomas are nodular-like focal collections of macrophage-derived cells called epithelioid cells, with elongated nuclei, abundant and eosinophilic cytoplasm, and poorly defined margins. Epithelioid granulomas include also lymphocytes and giant cells called Langhans cells, which are multinucleated cells characterized by numerous nuclei arranged peripherally in the shape of a horseshoe. As Langhans giant cells are seen in other granulomatous inflammations, they are not pathognomonic of tuberculosis. Caseous necrosis is highly suggestive of tuberculosis but is also not pathognomonic, as it may be also seen in other granulomatous diseases, e.g., fungal infection (Romdhane et al. 2020; Rammeh et al. 2021). Caseous necrosis is a distinctive form of coagulative necrosis. Macroscopically, it has a soft and white proteinaceous cheese-like appearance. Histologically, it appears as an eosinophilic, amorphous granular area, and often surrounded by a rim of epithelioid cells (Figs. 12, 13, and 14).
Granulomatous inflammation, in an appropriate clinical setting, is highly suggestive of tuberculous spondylodiscitis. Other granulomatous inflammations such as brucellosis, fungal spondylodiscitis, and sarcoidosis must be excluded (Duarte and Vaccaro 2013; Rammeh et al. 2021). In our series, tuberculosis represented 89.5% of granulomatous spondylodiscitis, while brucellosis represented 5.3%, fungi 3.5%, and sarcoidosis 1.7% (Rammeh et al. 2021). Suppurative forms of tuberculous spondylodiscitis mimicking pyogenic and fungal spondylodiscitis exist (Rammeh et al. 2021). The detection of acid-fast bacilli by Ziehl-Neelsen staining on formalin-fixed, paraffin-embedded tissues (FFPET) confirms the diagnosis of tuberculous spondylodiscitis. But due to the paucibacillary character of the disease in the spinal location, Ziehl-Neelsen staining often fails to visualize acid-fast bacilli. Immunohistochemistry using specific anti-Mycobacterium tuberculosis antibodies can be an alternative to Ziehl-Neelsen staining (Kohli et al. 2014).
Fungal spondylodiscitis is rare. Histologically, it displays suppurative and granulomatous forms, mimicking pyogenic and tuberculous spondylodiscitis. Many fungal microorganisms, such as Candida albicans, Aspergillus spp., and Cryptococcus neoformans, can be visualized by hematoxylin and eosin staining. Periodic acid Schiff and Gomori methenamine silver staining demonstrates spores and mycelial filaments in yeast infections and hyphae in fungal infections (Guarner and Brandt 2011). These stains must be systematically performed in the context of infectious spondylodiscitis to rule out fungal spondylodiscitis. Histopathological examination may suggest the nature of the fungal agent; for example, Candida albicans is a yeast-like fungus with budding and non-septated hyphae filamentous (pseudohyphal and hyphal) forms. Aspergillus spp. have septate hyphae with dichotomous branching. Cryptococcus neoformans is a yeast-like fungus. The yeast forms of cryptococcosis are usually widely separated by a thick mucoid capsule decorated by Periodic acid-Schiff and by mucicarmine stains (Figs. 15, 16, 17, and 18).
The diagnosis of brucellar spondylodiscitis is commonly based on clinical data and confirmed by the detection of specific antibodies (Wright, Rose Bengal, immunofluorescence) and/or the isolation of the causative agent from blood or tissues (Al Dahouk and Nöckler 2011; Bozbaş et al. 2016). The histopathological examination is carried out in two circumstances, namely, in cases with negative microbiological tests and high clinical suspicion of brucellosis and to exclude a tuberculous coinfection. In these circumstances, biopsy is advisable to obtain tissue samples for microbiological and histopathological investigations to confirm brucellar spondylodiscitis (Rammeh et al. 2020). The histopathological diagnosis of brucellar spondylodiscitis is challenging because it shows a large spectrum of histopathological lesions with nonspecific and granulomatous forms—each observed in about half of the cases (Fuentes Ferrer et al. 2012; Li et al. 2016; Rammeh et al. 2020).
The brucellar granuloma, highly suggestive of brucellar spondylodiscitis, is surrounded by a chronic inflammatory reaction composed of lymphocytes, plasmocytes, and neutrophils (Mondal and Misra 1994). It is typically small, not well-formed, noncaseating, and composed of aggregates of histiocytes with round nuclei without epithelioid appearance (Fig. 19). Granulomas with an epithelioid appearance and even caseous-like necrosis, although rare, are possible in brucellar spondylodiscitis (Rammeh et al. 2020). Epithelioid granulomas formed by eosinophilic cells with elongated nuclei are rarely observed in brucellar spondylodiscitis. They are typically associated with histiocytic-type granulomas and are not well-formed like those classically observed in tuberculous spondylodiscitis (Fig. 20). The association of histiocytic and epithelioid granulomas has also been reported in fungal and tuberculous spondylodiscitis (Cottle and Riordan 2008). Nonspecific forms of brucellar spondylodiscitis characterized by non-granulomatous infiltrate show typically a polymorphous infiltrate with the predominance of lymphocytes and plasmocytes (Figs. 21 and 22). As these forms mimic pyogenic spondylodiscitis, their distinction is histologically challenging. Pyogenic spondylodiscitis is characterized by predominant neutrophil infiltration (Landi et al. 2017). The predominance of the mononuclear cells should alert the pathologist to suggest the diagnosis of brucellar spondylodiscitis.
4.3 Cytological Diagnosis of Spinal Infection
CT-guided fine needle aspiration cytology (FNAC) is a simple and economical tool in the diagnosis of spinal infection, compared with core-needle biopsy or excision biopsy. It can provide an accurate diagnosis of spinal infection when it is used with a proper combination of experience and diligence (Mondal and Misra 1994). FNAC is cytologist- and operator-dependent procedure which requires experienced cytologists and radiologists, sufficient material, good sampling method, and coordination among the cytologist, radiologist, and physician (Jorda et al. 2000). It is recommended for paravertebral abscesses and for osteolytic lesions with cortical disruption (De Lucas et al. 2009). In countries with high tuberculosis endemicity, FNAC may be an alternative to histopathology and is also suitable for obtaining material for microbiology. Mycobacterium tuberculosis is easily isolated from these sites. The cytological diagnosis of spinal tuberculosis depends upon the demonstration of epithelioid granuloma with necrotic material which is observed in 50–68% of cases (Handa et al. 2010). The rate of noncontributory FNAC is one of the major limitations of this technique, which ranges from 10 to 31% (Phadke et al. 2001).
4.4 Molecular Diagnosis of Spinal Infection
With the advent of molecular techniques, the diagnosis of spinal infection has become prompt and accurate, allowing the initiation of adequate treatment and preventing neurological complications (Lecouvet et al. 2004; Gouliouris et al. 2010; Jacquier et al. 2019). These techniques are validated on fresh samples and can be helpful on FFPET but only a few studies have assessed the value of molecular diagnosis on FFPET. Molecular diagnosis provides a considerable gain in terms of sensitivity and rapidity. It is performed on biopsies frozen at −20 °C when cultures remain negative. It is particularly useful in case of decapitated infections or in case of infections due to microorganisms rarely isolated or with fastidious culture. It is based on specific or universal PCR techniques, which are currently accepted but whose indications are not well-established due to their lack of standardization (Ladeb et al. 2019).
Specific real-time PCR has shown good performance in the diagnosis of Staphylococcus aureus, brucellosis, and mycobacterial and fungal spinal infections (Berbari et al. 2015; Chebbi et al. 2019). PCR methods are widely used for the detection and the identification of Brucella spp. from peripheral blood or from fresh tissues (Kaden et al. 2017). They can be combined with clinical findings to differentiate between the different stages of brucellosis (Wang et al. 2014; Kaden et al. 2017). The efficacy of molecular methods depends mainly on the specificity of primers. Various targets used for the diagnosis of brucellosis include gene encoding BCSP 31, a sequence 16S rRNA of B. abortus, and a gene encoding omp2 (Wang et al. 2014). Colmenero et al. (2013) reported a sensitivity of 100% using the gene BCSP 31.
The utility of molecular diagnostic tools is also proved for diagnosing pyogenic spondylodiscitis. Several studies have demonstrated the advantage of targeting 16S rDNA for rapid identification of Staphylococcus aureus (Sheikh et al. 2017) and the gene mecA for the detection of methicillin-resistant Staphylococcus aureus (Kobayashi et al. 2009). The detection of galactomannan antigen by PCR is known to be a rapid tool for the diagnosis of invasive fungal infections. Given the rarity of this disease, the performance of PCR for the diagnosis of fungal infection has not been widely reported (Jorge et al. 2012). Molecular methods allow a rapid diagnosis of tuberculous spondylodiscitis. Most of the studies have used the insertion sequence IS6110 because Mycobacterium tuberculosis contains between 6 and 25 copies of this repetitive element. The majority of the studies reported the performances of uniplex, multiplex, and real-time PCRs. The sensitivity and specificity of PCR in fresh tissue for the diagnosis of spinal tuberculosis vary from 61 to 91% and from 63.7 to 93.7%, respectively (Rammeh et al. 2021).
The major limitation of molecular analyses on FFPET is the degradation of DNA due to fixation which are required for the histological processing of tissues. During the process of fixation, the formalin causes the formation of cross-linking between proteins and DNA which might interfere with the amplification process. Besides cross-linking, the oxidation of formalin to formic acid leads to the depurination, and strand DNA breaks (Dietrich et al. 2013). The control of these key steps is crucial to preserve tissue morphology for histopathological examination and nucleic acids for molecular analyses. Molecular techniques on FFPET with validated pre-analytic steps are useful tools for the diagnosis of tuberculous spondylodiscitis, especially for cases where microbiological investigations are negative or not carried out, mainly because clinical features were in favor of noninfectious pathology, e.g., metastasis and noninfectious disease (Choe et al. 2014; Jacquier et al. 2019).
5 Complications
The overall complication rate of spine biopsy varies from 0 to 10%, with serious complication rates being less than 1% (Peh 2006; Nourbakhsh 2015). The complication rate of CT-guided spinal biopsies (3.3%) is low, compared with 5.3% for fluoroscopy and compared to the open biopsy complication rate of 16% (Nourbakhsh 2015; Rehm et al. 2016). The types and incidence of complications depend on the anatomical level, the chosen approach, and the type of needle used (Peh 2006; Rehm et al. 2016). Potential complications include pneumothorax, hematoma, nerve root injury, transient paresis, transient spinal anesthesia, meningitis, radiculopathy, and paraplegia (Nourbakhsh 2015). Ultrasonography-guided biopsy has been used in the cervical and lumbar spine without any reported complications (Nourbakhsh 2015).
6 Conclusion
Percutaneous spine biopsy is an effective modality for assessment and management of spinal infection when a microbiological diagnosis for a known associated organism has not been established by blood cultures or serologic tests. It allows obtaining a specimen for microbiological and histopathological diagnosis in order to choose the appropriate treatment.
Adherence to biopsy guidelines, good planning, and execution of the procedure with accurate sample handling and close collaboration among the interventional radiologist, pathologist, microbiologist, and clinician are determinants of a successful percutaneous spinal biopsy. Compared to surgical open biopsy, percutaneous imaging-guided biopsy is a safe and cost- and time-saving procedure, which is less painful and less invasive, with good accuracy. CT and fluoroscopy remain the most frequently used imaging modalities to guide spinal biopsies. A good knowledge of the spine anatomy and of biopsy technique is essential to obtain good results.
Abbreviations
- CT:
-
Computed tomography
References
Aithala JP (2016) Role of percutaneous image guided biopsy in spinal lesions: adequacy and correlation with MRI findings. J Clin Diagn Res 10(8):RC11–RC15
Al Dahouk S, Nöckler K (2011) Implications of laboratory diagnosis on brucellosis therapy. Expert Rev Anti-Infect Ther 9:833–845
Berbari EF, Kanj SS, Kowalski TJ et al (2015) Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61:e26–e46
Bozbaş GT, Ünübol Aİ, Gürer G (2016) Seronegative brucellosis of the spine: a case of psoas abscess secondary to brucellar spondylitis. Eur J Rheumatol 3:185–187
Chang CY, Simeone FJ, Nelson SB, Taneja AK, Huang AJ (2015) Is biopsying the paravertebral soft tissue as effective as biopsying the disk or vertebral endplate? 10-year retrospective review of CT-guided biopsy of diskitis-osteomyelitis. AJR Am J Roentgenol 205:123–129
Chaudhary RK, Acharya S, Chahal RS, Kalra KL (2019) Fluoroscopy guided percutaneous transpedicular biopsy of vertebral body lesion. J Nepal Health Res Counc 17:163–167
Chebbi Y, Riahi H, Bouaziz MC, Romdhane E, Mhiri E, Rammeh S, Saidi LS, Achour W, Ladeb MF (2019) Mycobacterium bovis Spondylodiscitis: Report of 4 Cases. J Clin Rheumatol. https://doi.org/10.1097/RHU.0000000000001040. Online ahead of print
Choe H, Aota Y, Kobayashi N, Nakamura Y et al (2014) Rapid sensitive molecular diagnosis of pyogenic spinal infections using methicillin-resistant Staphylococcus-specific polymerase chain reaction and 16S ribosomal RNA gene-based universal polymerase chain reaction. Spine J 14:255–262
Colmenero JD, Ruiz-Mesa JD, Sanjuan-Jimenez R, Sobrino B, Morata P (2013) Establishing the diagnosis of tuberculous vertebral osteomyelitis. Eur Spine J 22(Suppl 4):579–586
Cottle L, Riordan T (2008) Infectious spondylodiscitis. J Infect 56:401–412
De Lucas EM, González Mandly A, Gutiérrez A et al (2009) CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol 28:315–320
Dietrich D, Uhl B, Sailer V et al (2013) Improved PCR performance using template DNA from formalin-fixed and paraffin-embedded tissues by overcoming PCR inhibition. PLoS One 8(10):e77771
Duarte RM, Vaccaro AR (2013) Spinal infection: state of the art and management algorithm. Eur Spine J 22:2787–2799
Fuentes Ferrer M, Gutiérrez Torres L, Ayala Ramírez O, Rumayor Zarzuelo M, del Prado González N (2012) Tuberculosis of the spine. A systematic review of case series. Int Orthop 36:221–231
Gallucci PM, D’Orazio F (2015) Image guided interventions in spinal infections. Neuroimaging Clin N Am 25:281–294
Gouliouris T, Aliyu SH, Brown NM (2010) Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 65(Suppl 3):iii11–iii24
Guarner J, Brandt ME (2011) Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 24:247–280
Handa U, Garg S, Mohan H, Garg SK (2010) Role of fine-needle aspiration cytology in tuberculosis of bone. Diagn Cytopathol 38:1–4
Husseini JS, Simeone FJ, Nelson SB, Chang CY (2020) CT-guided discitis-osteomyelitis biopsies: needle gauge and microbiology results. Skeletal Radiol 49:1431–1439
Jacquier H, Fihman V, Amarsy R et al (2019) Benefits of polymerase chain reaction combined with culture for the diagnosis of bone and joint infections: a prospective test performance study. Open Forum Infect Dis 6(12):ofz511
Joo EJ, Yeom JS, Ha YE et al (2016) Diagnostic yield of computed tomography-guided bone biopsy and clinical outcomes of tuberculous and pyogenic spondylitis. Korean J Intern Med 31:762–771
Jorda M, Rey L, Hanly A, Ganjei-Azar P (2000) Fine-needle aspiration cytology of bone: accuracy and pitfalls of cytodiagnosis. Cancer 90:47–54
Jorge VC, Cardoso C, Noronha C et al (2012) Fungal spondylodiscitis in a non-immunocompromised patient. BMJ Case Rep 2012:bcr1220115337
Kaden R, Ferrari S, Alm E, Wahab T (2017) A novel real-time PCR assay for specific detection of Brucella melitensis. BMC Infect Dis 17(1):230
Kasalak Ö, Adams HJA, Jutte PC et al (2018) Culture yield of repeat percutaneous image-guided biopsy after a negative initial biopsy in suspected spondylodiscitis: a systematic review. Skeletal Radiol 47:1327–1335
Kobayashi N, Inaba Y, Choe H et al (2009) Rapid and sensitive detection of methicillin-resistant Staphylococcus periprosthetic infections using real-time polymerase chain reaction. Diagn Microbiol Infect Dis 64:172–176
Kohli R, Punia RS, Kaushik R, Kundu R, Mohan H (2014) Relative value of immunohistochemistry in detection of mycobacterial antigen in suspected cases of tuberculosis in tissue sections. Indian J Pathol Microbiol 57:574–578
Ladeb F, Ben Aissa H, Tiouiri H et al (2019) Clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis. Tunis Med 97:14–92
Landi A, Grasso G, Iaiani G et al (2017) Spontaneous spinal discitis and spondylodiscitis: clinico-therapeutic remarks. J Neurosci Rural Pract 8:642–646
Lecouvet F, Irenge L, Vandercam B et al (2004) The etiologic diagnosis of infectious discitis is improved by amplification-based DNA analysis. Arthritis Rheum 50:2985–2994
Li T, Liu T, Jiang Z, Cui X, Sun J (2016) Diagnosing pyogenic, brucella and tuberculous spondylitis using histopathology and MRI: a retrospective study. Exp Ther Med 12:2069–2077
Liu M, Sequeiros RB, Xu Y et al (2015) MRI-guided percutaneous transpedicular biopsy of thoracic and lumbar spine using a 0.23t scanner with optical instrument tracking. J Magn Reson Imaging 42:1740–1746
McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A (2017) Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am J Neuroradiol 38:2021–2027
Mondal A, Misra DK (1994) CT-guided needle aspiration cytology (FNAC) of 112 vertebral lesions. Indian J Pathol Microbiol 37:255–261
Nam KH, Song GS, Han IH, Choi BK, Cha SH (2011) Diagnostic value of biopsy techniques in lumbar spondylodiscitis: percutaneous needle biopsy and open biopsy. Korean J Spine 8:267–271
Nourbakhsh A (2015) Percutaneous spine biopsy: a literature review. Int J Radiol Radiat Oncol 1:23–28
Özmen D, Özkan N, Guberina N et al (2019) Computed-tomography-guided biopsy in suspected spondylodiscitis: single-center experience including 201 biopsy procedures. Orthop Rev (Pavia) 11(1):7793
Ozsarlak O, De Schepper AM, Wang X, De Raeve H (2003) CT-guided percutaneous needle biopsy in spine lesions. JBR-BTR 86:294–296
Peh WCG (2006) CT-guided percutaneous biopsy of spinal lesions. Biomed Imaging Interv J 2(3):e25
Phadke DM, Lucas DR, Madan S (2001) Fine-needle aspiration biopsy of vertebral and intervertebral disc lesions: specimen adequacy, diagnostic utility, and pitfalls. Arch Pathol Lab Med 125:1463–1468
Pupaibool J, Vasoo S, Erwin PJ, Murad MH, Berbari EF (2015) The utility of image-guided percutaneous needle aspiration biopsy for the diagnosis of spontaneous vertebral osteomyelitis: a systematic review and meta-analysis. Spine J 15:122–131
Rammeh S, Romdhane E, Riahi H et al (2021) Granulomatous spondylodiscitis: a case series with focus on histopathological features. J Spinal Cord Med 44:282–287
Rammeh S, Romdhane E, Riahi H et al (2020) Brucellar spondylodiscitis: a case series with focus on histopathological features. J Clin Neurosci 78:360–364
Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA (2004) Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J 80:607–609
Rehm J, Veith S, Akbar M, Kauczor HU, Weber MA (2016) CT-guided percutaneous spine biopsy in suspected infection or malignancy: a study of 214 patients. Rofo 188:1156–1162
Romdhane E, Rammeh S, Riahi H et al (2020) The value of histology in the diagnosis of tuberculous spondylodiscitis. J Clin Rheumatol 26:63–66
Sahoo MM, Mahapatra SK, Sethi GC, Sahoo A, Kar BK (2019) Role of percutaneous transpedicular biopsy in diagnosis of spinal tuberculosis and its correlation with the clinico-radiological features. Indian J Tuberc 66:388–393
Sehn JK, Gilula LA (2012) Percutaneous needle biopsy in diagnosis and identification of causative organisms in cases of suspected vertebral osteomyelitis. Eur J Radiol 81:940–946
Sertic M, Parkes L, Mattiassi S et al (2019) The efficacy of computed tomography-guided percutaneous spine biopsies in determining a causative organism in cases of suspected infection: a systematic review. Can Assoc Radiol J 70:96–103
Sheikh AF, Khosravi AD, Goodarzi H et al (2017) Pathogen identification in suspected cases of pyogenic spondylodiscitis. Front Cell Infect Microbiol 7:60
Shrestha D, Shrestha R, Dhoju D (2015) Fluoroscopy guided percutaneous transpedicular biopsy for thoracic and lumbar vertebral body lesion: technique and safety in 23 consecutive cases. Skeletal Radiol 47:1327–1335
SPILF (2007) Primary infectious spondylitis, and following intradiscal procedure, without prothesis. Recommendations. Med Mal Infect 37:573–538
Terreaux W, Geoffroy M, Ohl X et al (2016) Diagnostic contribution of a second percutaneous needle biopsy in patients with spontaneous diskitis and negative blood cultures and first biopsy. Joint Bone Spine 83:715–719
Wang Y, Wang Z, Zhang Y et al (2014) Polymerase chain reaction-based assays for the diagnosis of human brucellosis. Ann Clin Microbiol Antimicrob 13:31
Wiesner EL, Hillen TJ, Long J, Jennings JW (2018) Percutaneous CT-guided biopsies of the cervical spine: technique, histopathologic and microbiologic yield, and safety at a single academic institution. AJNR Am J Neuroradiol 39:981–985
Wu HT, Chang CY, Chang H et al (2012) Magnetic resonance imaging guided biopsy of musculoskeletal lesions. J Chin Med Assoc 75:160–166
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chelli Bouaziz, M., Ladeb, M.F., Rammeh, S., Achour, W., Riahi, H. (2021). Percutaneous Biopsy of Spinal Infection. In: Ladeb, M.F., Peh, W.C. (eds) Imaging of Spinal Infection. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-030-70459-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-70459-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70458-2
Online ISBN: 978-3-030-70459-9
eBook Packages: MedicineMedicine (R0)