Abstract
Rickettsia is an infectious disease, caused by a small obligate intracellular gram-negative bacillus, which is transmitted to humans by blood-borne arthropods such as ticks, lice, mites, and fleas. It has a worldwide distribution, as an emerging and re-emerging febrile illness, accompanied by rash and eschar; its diagnosis is difficult, since its confirmation is made after the acute febrile episode, through serological tests. The suspicion of this infection should be high, since timely antibiotic treatment can lead to rapid clinical improvement. It is a life-threatening infection, with pulmonary manifestations, acute kidney injury, gangrene, and neurological manifestations such as delirium, seizures, stupor, and coma. During pregnancy, this infection can cause maternal mortality, with a high frequency of complications such as stillbirth, prematurity, and low birth weight. The use of the tetracyclines group, specifically doxycycline, is preferred as the first line, due to its efficacy and low toxicity to the mother as well as child, the use of quinolones remaining in the second line. Its prevention is based on vector control and measures to avoid contact with them.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Rickettsia
- Pregnant woman
- Rickettsia infections
- Rickettsia typhi
- Spotted Fever Group Rickettsiosis
- Rocky Mountain Spotted Fever
Introduction
Rickettsia is an infectious disease caused by small gram-negative microorganisms, obligated intracellular bacilli, transmitted to humans by hematophagous arthropod vectors such as ticks, lice, mites, and fleas [1, 2].
Due to their high prevalence in nature and that they have a large worldwide distribution, both in tropical and subtropical areas, they are a potential cause of emerging and re-emerging febrile illness, which unfortunately produces a febrile condition that is not differentiated with other diseases and that in many occasions is overlooked. This feverish picture can sometimes be accompanied with rash and eschar [3, 4].
The difficulty for the recognition of this infectious disease is very large, because a confirmatory test is not available during the acute phase of the disease and its diagnosis is usually confirmed, retrospectively by serological means.
The recognition of this infectious disease is important and at the beginning of the specific treatment is associated with a rapid clinical improvement and also with a decrease in mortality in severe cases [5, 6].
Etiology
This infectious disease is caused by bacteria of the genus Rickettsia, which, as mentioned, are obligated intracellular gram-negative bacilli, which have been classified into four categories, according to their genetic characteristics:
-
1.
Spotted fever group (SFG) rickettsiae: this group corresponds to the Rickettsia rickettsii, the cause of the Rocky Mountain spotted fever, which is responsible for one of the most severe and well-known presentations in North America, as well as others, such is the case of Rickettsia africae that produces the African tick bite fever in sub-Sahara Africa and Rickettsia conorii, which produces the Mediterranean spotted fever in Europe and North Africa. This group is currently responsible for at least 15 diseases.
-
2.
Typhus group rickettsiae, this group includes Rickettsia prowazekii and Rickettsia typhi.
-
3.
Ancestral group includes Rickettsia bellii and Rickettsia canadensis.
-
4.
Transitional group, named because it consists of members with genetic characteristics between the SFG and typhus group. In this group are Rickettsia akari, Rickettsia australis, and Rickettsia felis [7,8,9,10,11].
The List of Prokaryotic Names with Standing in Nomenclature [12] contains 27 species of Rickettsia, and of which 17 more are capable of producing infections in humans (Table 33.1) [4]. However, some Rickettsia, such as R. peacockii and R. buchneri, are symbiotic bacteria from ticks and have little capacity to cause infections in humans; on the other hand, others such as R. parkeri, R. slovaca, and R. massilliae were considered nonpathogenic to humans in the past and are now known to cause human infections. There are other isolated Rickettsia such as R. amblyommatis and R. philipii, which are associated with human infections [13,14,15,16,17,18,19,20].
Epidemiology
Rickettsia is an infection transmitted by ticks, lice, and fleas; hence, in many cases, humans are accidental guests.
The clinical picture will depend on the region in which the patient is, the type of vector, and the mechanism in which the disease is transmitted. Hence, it is that rickettsial infection is more common during the warmer months and people are exposed or do outdoor activities; as is the case, for example, of the Dermacentor variabilis (American dog tick), Dermacentor andersoni (Rocky Mountain wood tick), and Amblyomma americanum (lone star tick) that have been associated in many cases of Rocky Mountain spotted fever in the United States; Amblyomma cajennense, associated with spotted fever in South America; and Amblyomma hebraeum or Amblyomma variegatum in South Africa. Other pictures are related to poor hygiene conditions such as epidemic typhus, R. prowazekii transmitted by body lice, and murine typhus caused by R. typhi caused by flea bites in tropical and subtropical areas [21,22,23,24].
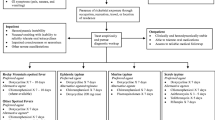
In the following figure (Fig. 33.1), the distribution of the most frequent Rickettsia infections is summarized, as well as the syndrome that causes and the vector or vectors involved in its transmission [25].
Major rickettsioses described by causative agent, clinical syndrome, and vector by region. (From Refs. [2, 14,15,16, 29, 64], and the CDC Yellow Book (https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/rickettsial-spotted-and-typhus-fevers-and-related-infections-including-anaplasmosis-and-ehrlichiosis). The map was created using mapchart.net
Table 33.2 [4] shows the heterogeneity of this infectious disease, since there is a lot of diversity in terms of severity, known name of the disease, distribution, the causative vector, and the clinical presentation of it.
Transmission and Pathophysiology
The transmission of this disease to the host requires of a vector. The mechanism of transmission will depend on the type of vector and the species of Rickettsia.
Four mechanisms for the transmission of this disease have been described:
-
1.
Transmission by saliva during the bite and feeding of ticks and mites, the cabbage is the most frequent mechanism in the rickettsias SFG.
-
2.
Transmission by entry of fecal material into bite sites and cuts in the skin of the host. This mechanism is characteristic of the flea- and louse-borne rickettsiae [26].
-
3.
TG rickettsiae can cause infection by inhalation via aerosolization or contamination of dust particles in the air [27].
-
4.
Finally, a rare route of inoculation is through the conjunctiva, through exposure of contaminated tick hemolymph on fingers from crushed ticks [26].
Rickettsia after entering the host organism has a rapid entry of the organisms into the cell and the downregulation of immune pathways allowing for persistence of infection. At the site of inoculation, a localized rickettsial infection, manifested by an eschar (“tache noir”), where a greater inflammatory reaction to achieve local control of the infection, can be observed [28] (Fig. 33.2) [1].
Maculopapular rashes caused by spotted fever group rickettsias (SFGR) are clinically and histologically identical with prominent perivascular mononuclear infiltrates. Likewise, the eschars produced by SFGR are both clinically and histologically identical, featuring mononuclear perivascular infiltrates in the deep dermis and microvascular fibrin thrombi-induced ischemic necrosis of superficial dermis with loss of epidermis. R. rickettsii (a and d), R. parkeri (b and e, h and j), R. akari (c and f, g and i), Photomicrographs: hematoxylin and eosin, 25×. (Image adapted from Denison et al. [58])
This infection produces in the vascular endothelium of the small and medium vessels of the organism, a disseminated inflammation, with loss of the barrier function and alteration in vascular permeability. This increase in vascular permeability is related to bacterial load and tumor necrosis factor, which produce disruptions of endothelial cell junctions [29, 30]. Vasculitis, and the endothelial damage produced, produces the clinical manifestations of fever, myalgia, symptoms in the central nervous system such as headache and confusion, rash and cardiovascular instability, cutaneous necrosis, digital gangrene, pneumonitis, meningoencephalitis, and multiorgan failure, which can cause death. A case of antineutrophil cytoplasmic autoantibody (ANCA)-positive vasculitis associated with Rocky Mountain spotted fever (RMSF) has been described [31].
Clinical Evaluation
Classically, patients have a triad of fever, headache, and a petechial or macular rash within 4–10 days after exposure to vectors, usually by bites of fleas or ticks. However, it is important to remember that patients may have flu-like symptoms in the summer months, where the possibility of contact between the host and the vector increases, since exposure to the latter may be brief or unnoticed by the patient, so it is very important to maintain a high index of suspicion.
Symptoms may include lymphadenopathy, changes at the level of the central nervous system such as confusion or nuchal stiffness, hearing at the inoculation site, myalgias, arthralgias, hepatitis, vomiting, and cardiovascular instability.
Although the classical triad is consistent with rickettsial species, the specific etiology must be defined according to the specific symptoms and geography where the exposure occurred. A detailed history about travel and outdoor exposure is important.
It is of interest that depending on the category of Rickettsia, the manifestations and severity of the disease can vary:
-
1.
Spotted Fever Group Rickettsioses (SFG rickettsiae):
This category has a broad presentation spectrum, from a disease with mild manifestations such as the case of R. slovaca to fatal cases such as those caused by R. rickettsii [32, 33]. In this group it is known for the seroconversion of patients, with very mild symptoms and even without them, in such a way that this infection can simulate other infections especially in tropical areas where other infections are prevalent [34].
Prominent symptoms include fever, headache, and myalgia. In addition, patients may experience nausea, vomiting, and abdominal pain, despite not being a gastrointestinal disease. The presence of rash is variable, being very frequent between 90 and 97% in RMSF and MSF, 46% in ATBF, and only 2% in TIBOLA [32, 35,36,37,38], which is usually macular or maculopapular; however it can vary, for example, in RMSF the rash can start on the wrists and ankles before the trunk or start on the trunk or be diffuse. The involvement of palms of the hands and feet is considered as characteristic of the RMSF. In ATBF the rash is papulovesicular and papulopustular.
It is also characteristic in the skin, the formation of the inoculation eschar or tache noir, which is observed in 95% in ATBF and in 72% in the MSF [35, 38].
Lymphadenopathy is observed in 27% of the RMSF; in other less severe diseases the nodal involvement can be observed in the drainage nodules of the eschar inoculation area.
In the case of TIBOLA, the clinical picture is less severe, and they even have asymptomatic seroconversion, with local symptoms such as eschar, with 100% local lymphadenopathy, with alopecia around the eschar and asthenia, with few constitutional symptoms [15, 39].
In severe cases such as RMSF, mortality has been reported between 4% and 30% in Mexico, and 40% have been reported in Brazil; the MSF is 2.5% [29, 33, 40].
In these cases, multiorgan compromise may occur manifested by:
-
(a)
Lung compromise manifested by cough, dyspnea, and respiratory failure, which requires mechanical ventilation
-
(b)
Acute renal injury due to prerenal azotemia that can cause acute tubular necrosis, requiring renal support
-
(c)
Neurological commitment, which can occur with delirium, coma, stupor, and seizures
-
(d)
Gangrene in limbs or fingers
The risk factors for severe manifestations are glucose-6-phosphate dehydrogenase deficiency, alcoholism, older age, and use of sulfonamide antibiotics.
-
(a)
-
2.
Typhus Group Rickettsioses:
The typical clinical manifestations of this group are the appearance of sudden fever, accompanied by myalgia and headache. They present rash in variable incidence, and it has also been described to listen in this group, although the latter is not recognized as a manifestation of the typhus group rickettsioses [41,42,43]. About half of the patients develop nausea and vomiting [41, 42].
Louse-borne typhus is the most severe manifestation, associated with neurological symptoms such as delirium, seizures, stupor, and coma, with a mortality between 13% and 50% [41]. Murine typhus has a mortality of 0.4–4%; the latter mortality is observed in severe cases that are not hospitalized; the less severe forms of this group are flying squirrel-associated typhus and recrudescent typhus (Brill-Zinsser disease), to which mortality is not associated [44, 45].
-
3.
Transitional Group Rickettsioses :
This group produces what has been called rickettsialpox; during the subsequent days of the mite bite, there is a papulovesicular lesion, which progresses to an eschar with induration and edema around. Then, between 1 and 2 weeks, the patient presents constitutional symptoms of fever, headache, and myalgia. Subsequently, days after the appearance of these symptoms, a skin rash occurs in the form of maculae, which evolves into papules that become papulovesicles and then crusted lesions [46].
The clinical presentation of Queensland tick typhus is similar to SFG rickettsioses with maculopapular rash in 90% and with eschar over 65% associated with regional lymphadenopathy, which are usually mild; however, severe and fatal cases have been reported [47, 48].
Flea-borne spotted fever is a disease that has mild manifestations compared to another rickettsioses SFG, in which rash has been reported in 75% and 13% eschar [49].
Clinical Evaluation and Diagnosis
The clinical diagnosis is based on a high suspicion of this disease in patients presenting with symptoms of fever, rash, and headache, with the history of possible exposure to infected arthropods or from trips to endemic areas.
Due to the limitations of laboratory data, empirical treatment should be initiated promptly, if there is a suspicion of this disease.
General laboratories may show thrombocytopenia, hyponatremia, and pleocytosis in the cerebrospinal fluid; in addition, leukograms can show high, normal, or low counts, unable to rule out a rickettsia infection.
Depending on the samples taken and the evolution of the symptoms, different diagnostic methods of this disease can be used, including immunohistochemical analysis, molecular detection, isolation and culture of pathogens, and serology (Fig. 33.3) [4].
A diagnostic algorithm for laboratory diagnosis of rickettsial diseases. ELISA enzyme-linked immunosorbent assay, FFPE formalin-fixed, paraffin-embedded, IFA immunofluorescence assay, IHC staining, immunohistochemical staining, LAMP loop-mediated isothermal amplification, OmpB outer membrane protein B, PCR polymerase chain reaction
-
1.
Detection of Rickettsial Antigen by Immunohistochemical Staining
It is based on the determination of rickettsial antigen present in the rash or eschar, by means of a skin biopsy, particularly in the acute phase of the disease. After the biopsy in formalin and paraffin embedded is established, immunohistochemical staining can show rickettsias, by means of antibodies directed or cross-reactive against these rickettsial species. [13, 49,50,51,52,53]
These techniques can be helpful in autopsy specimens, in tissues such as the skin, liver, spleen, lung, heart, kidney, and brain [54, 55].
However, this technique is not sensitive after 48 hours or more of the administration of antibiotic treatment [56]
-
2.
Molecular Genetic Approaches for Diagnosis
It has been possible to detect nucleic acid molecules of rickettsia, using techniques such as blood and skin PCR. However, the sensitivity and specificity are higher in the skin samples, due to the tropism of these intracellular bacteria to the endothelium; blood samples have poor sensitivity [57,58,59,60,61,62] U-Z).
The research and use of new molecular techniques have improved the sensitivity and specificity in the detection of this disease.
-
3.
Isolation and Culture
The culture and isolation of rickettsias can be performed by cell cultures of the skin, blood, and arthropod samples. However, this type of diagnostic technique requires expertise and special conditions.
Because small amounts of aerosolized rickettsia can cause disease, a laboratory with level 3 biosecurity is required. In addition, appropriate host cells for cultivation is required, and many skin samples or arthropods are not sterile, requiring processes or treatments to be cultured [4].
-
4.
Serology
The detection of antibodies in serum or plasma is the gold standard assay to confirm infection by Rickettsiae.
This detection can be performed by several methods such as enzyme-linked immunosorbent assay (ELISA), Western blot, and indirect immunofluorescence assay (IFA); the latter is the gold standard for the diagnosis of RMSF [56, 63, 64]
The antibody production response is after the clinical manifestations, usually after 7–10 days, but in some cases, it may be after 2–3 weeks. In the case of RMSF, the increase in IgM and IgG occurs almost simultaneously, in the second week of the disease [63]; IgM has a cross-reaction with nonrecreational antigens and therefore does not offer great sensitivity or specificity during the acute phase of the disease.
Seroconversion or a fourfold increase, from the acute phase to the convalescence phase, confirms the diagnosis of rickettsiosis [56].
Rickettsiosis in Pregnancy
In Southeast Asia, especially in rural areas, more than 1 million people a year suffer from scrub typhus and murine typhus, being one of the probable causes of treatable fever, with a mortality of 50–80,000 deaths per year.
97 cases of pregnant women with typhus have been described, of which 82 prognosis is known, with maternal death occurring in two cases. However, the neonatal prognosis was worse, occurring in more than 40% of pregnancies: stillbirth, prematurity, and low birth weight [65].
There are no large studies describing the infection during FMSR pregnancy, and it is unknown if it can cause infection in utero [66]. In addition, many laboratory abnormalities may be due to other diseases resulting from pregnancy such as preeclampsia and HELLP syndrome [66, 67].
In a report of four women with RMSF in Sonora, Mexico, between the years 2015 and 2016, it was found that the four pregnant women and one infant survived at 36 weeks’ gestation; however, in the other pregnancies that were in the first trimester, they suffered spontaneous abortion [68].
Treatment/Management
The onset of treatment for this disease is based on suspicion and clinical recognition, with early empirical treatment with effective antibiotic treatment [6].
The class of antibiotics of choice for all rickettsiosis are tetracyclines, and although there are few prospective studies to evaluate antibiotic treatments for rickettsiosis and none specific for RMSF, many decades of experience support the efficacy of these antibiotics [69, 70].
Other antibiotics, such as penicillins, cephalosporins, and sulfonamides, are ineffective for Rickettsia spp., and in the case of sulfonamides, poor prognosis has been associated [71, 72].
Quinolones are effective, in less severe conditions; however, their use in pregnancy or in pediatric patients is not recommended [73].
Doxycycline is not related to staining of permanent teeth in children, such as tetracycline. Therefore, doxycycline is recommended in pediatric patients [56].
As for pregnant women, their management is transformed into a real challenge, due to the following facts:
-
1.
Tetracyclines are deposited in the fetal skeleton and may cause temporary inhibition of bone growth [74].
-
2.
Tetracyclines are associated with pancreatitis and maternal hepatotoxicity [75].
-
3.
In advanced pregnancy, chloramphenicol has a high transplacental concentration, which can cause a gray baby syndrome (abdominal distention, pallor, cyanosis, and vasomotor collapse) [76].
-
4.
In pregnant women, with less severe disease, azithromycin can be considered as a safe but unproven option.
The treatment for this infection is outline[4] in Table 33.3.
Prevention
There is no vaccine for the prevention of SFG and typhus rickettsioses.
Prevention is based on avoiding contact with possible vectors and controlling them. The use of repellents or protective clothing that protects exposed skin is recommended.
Different strategies have been used with positive impacts in the control of some epidemics:
-
1.
The use of clothes treated with permethrin has been effective for the prevention of tick bites [77].
-
2.
In the 1940s DDT was used in rat harborages, with a decrease in the incidence of murine typhus in the United States.
-
3.
In some local outbreaks of louse-borne typhus, washing sheets and clothes with hot water kills lice and their eggs.
-
4.
WHO recommends mass treatment by compressed air dusting of permethrin on clothing [78].
-
5.
In Arizona, Brazil, and Sonora, Mexico, ticks have been reduced by treating animals and the environment with acaricides [79,80,81].
Bibliography
Adem PV. Emerging and re-emerging rickettsial infections. Semin Diagn Pathol. 2019;36(3):146–51.
Tello-Martin R, Dzul-Rosado K, Zavala-Castro J, Lugo-Caballero C. Approaches for the successful isolation and cell culture of American Rickettsia species. J Vector Borne Dis. 2018;55(4):258–64.
Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26(4):657–702.
Fang R, Blanton LS, Walker DH. Rickettsiae as emerging infectious agents. Clin Lab Med. 2017;37(2):383–400.
Drexler NA, Yaglom H, Casal M, et al. Fatal Rocky Mountain spotted fever along the United States-Mexico Border, 2013–2016. Emerg Infect Dis. 2017;23(10):1621–6.
Regan JJ, Traeger MS, Humpherys D, et al. Risk factors for fatal outcome from Rocky Mountain spotted fever in a highly endemic area–Arizona, 2002–2011. Clin Infect Dis. 2015;60(11):1659–66.
Dehhaghi M, Kazemi Shariat Panahi H, Holmes EC, Hudson BJ, Schloeffel R, Guillemin GJ. Human tick-borne diseases in Australia. Front Cell Infect Microbiol. 2019;9:3.
Diop A, Raoult D, Fournier PE. Paradoxical evolution of rickettsial genomes. Ticks Tick Borne Dis. 2019;10(2):462–9.
Laroche M, Raoult D, Parola P. Insects and the transmission of bacterial agents. Microbiol Spectr. 2018;6(5): https://doi.org/10.1128/microbiolspec.MTBP-0017-2016.
Hardstone Yoshimizu M, Billeter SA. Suspected and confirmed vector-borne rickettsioses of North America associated with human diseases. Trop Med Infect Dis. 2018;3(1):e2.
Gillespie JJ, Williams K, Shukla M, et al. Rickettsia phylogenomics: unwinding the intricacies of obligate intracellular life. PLoS One. 2008;3(4):e2018.
Parte AC. LPSN – list of prokaryotic names with standing in nomenclature (bacterio.net), 20 years on. Int J Syst Evol Microbiol. 2018;68:1825–9.
Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38(6):805–11.
Shapiro MR, Fritz CL, Tait K, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis. 2010;50(4):541–8.
Raoult D, Lakos A, Fenollar F, et al. Spotless rickettsiosis caused by Rickettsia slovaca and associated with Dermacentor ticks. Clin Infect Dis. 2002;34(10):1331–6.
Niebylski ML, Schrumpf ME, Burgdorfer W, et al. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int J Syst Bacteriol. 1997;47(2):446–52.
Kurtti TJ, Felsheim RF, Burkhardt NY, et al. Rickettsia buchneri sp. nov., a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int J Syst Evol Microbiol. 2015;65(Pt 3):965–70.
Vitale G, Mansuelo S, Rolain JM, et al. Rickettsia massiliae human isolation. Emerg Infect Dis. 2006;12(1):174–5.
Dahlgren FS, Paddock CD, Springer YP, et al. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg. 2016;94(1):35–42.
Walker DH, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med Clin North Am. 2008;92(6):1345–61, x.
Khamesipour F, Dida GO, Anyona DN, Razavi SM, Rakhshandehroo E. Tick-borne zoonoses in the Order Rickettsiales and Legionellales in Iran: a systematic review. PLoS Negl Trop Dis. 2018;12(9):e0006722.
Weitzel T, Aylwin M, Martínez-Valdebenito C, Jiang J, Munita JM, Thompson L, Abarca K, Richards AL. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
Eldin C, Parola P. Update on tick-borne bacterial diseases in travelers. Curr Infect Dis Rep. 2018;20(7):17.
Moreira J, Bressan CS, Brasil P, Siqueira AM. Epidemiology of acute febrile illness in Latin America. Clin Microbiol Infect. 2018;24(8):827–35.
Abdad MY, Abou Abdallah R, Fournier P-E, Stenos J, Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol. 2018;56:e01728–17.
Walker DH, Ismail N, Olano JP, Valbuena GA, McBride J. Chapter 2: pathogenesis, immunity, pathology, and pathophysiology in rickettsial diseases. In: Raoult D, Parola P, editors. Rickettsial diseases. New York: Informa Healthcare; 2007.
Raoult D, Woodward T, Dumler JS. The history of epidemic typhus. Infect Dis Clin N Am. 2004;18:127–40.
Parola P, Paddock C, Socolovschi C, Labruna M, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier PE, Raoult D. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657–702.
Valbuena G, Walker DH. Changes in the adherens junctions of human endothelial cells infected with spotted fever group rickettsiae. Virchows Arch. 2008;446:379–82.
Woods ME, Olano JP. Host defenses to Rickettsia rickettsii infection contribute to increased microvascular permeability in human cerebral endothelial cells. J Clin Immunol. 2008;28:174–85.
Nickerson A, Marik PE. Life-threatening ANCA-positive vasculitis associated with rickettsial infection. BMJ Case Rep. 2012;2012:bcr0320125993. https://doi.org/10.1136/bcr.03.2012.5993.
Silva-Pinto A, de Lurdes Santos M, Sarmento A. Tick-borne lymphadenopathy, an emerging disease. Ticks Tick Borne Dis. 2014;5(6):656–9.
Alvarez-Hernandez G, Roldan JFG, Milan NSH, et al. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect Dis. 2017;17(6):e189–96.
Traeger MS, Regan JJ, Humpherys D, et al. Rocky Mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area-Arizona, 2002–2011. Clin Infect Dis. 2015;60(11):1650–8.
Jensenius M, Fournier PE, Kelly P, et al. African tick bite fever. Lancet Infect Dis. 2003;3(9):557–64.
Helmick CG, Bernard KW, D’Angelo LJ. Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J Infect Dis. 1984;150(4):480–8.
Kaplowitz LG, Fischer JJ, Sparling PF. Rocky Mountain spotted fever: a clinical dilemma. In: Remington JB, Swartz HN, editors. Current clinical topics in infectious diseases, vol. 2. New York: McGraw-Hill; 1981. p. 89–108.
Raoult D, Weiller PJ, Chagnon A, et al. Mediterranean spotted fever: clinical, laboratory and epidemiological features of 199 cases. Am J Trop Med Hyg. 1986;35(4):845–50.
Dubourg G, Socolovschi C, Del Giudice P, et al. Scalp eschar and neck lymphadenopathy after tick bite: an emerging syndrome with multiple causes. Eur J Clin Microbiol Infect Dis. 2014;33(8):1449–56.
Walker DH. Changing dynamics of human-rickettsial interactions. Am J Trop Med Hyg. 2016;94(1):3–4.
Bechah Y, Capo C, Mege JL, et al. Epidemic typhus. Lancet Infect Dis. 2008;8(7):417–26.
Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16–24.
Blanton LS, Lea AS, Kelly BC, et al. An unusual cutaneous manifestation in a patient with murine typhus. Am J Trop Med Hyg. 2015;93(6):1164–7.
Pieracci EG, Evert N, Drexler NA, et al. Fatal flea-borne typhus in Texas: a retrospective case series, 1985–2015. Am J Trop Med Hyg. 2017;96(5):1088–93.
Dumler JS, Taylor JP, Walker DH. Clinical and laboratory features of murine typhus in South Texas, 1980 through 1987. JAMA. 1991;266(10):1365–70.
Kass EM, Szaniawski WK, Levy H, et al. Rickettsialpox in a New York City hospital,1980 to 1989. N Engl J Med. 1994;331(24):1612–7.
Stewart A, Armstrong M, Graves S, et al. Rickettsia Australis and Queensland tick typhus: a rickettsial spotted fever group infection in Australia. Am J Trop Med Hyg. 2017;97(1):24–9.
Graves SR, Stenos J. Tick-borne infectious diseases in Australia. Med J Aust. 2017;206(7):320–4.
Parola P. Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect. 2011;17(7):996–1000.
Walker DH, Feng HM, Ladner S, et al. Immunohistochemical diagnosis of typhus rickettsioses using an anti-lipopolysaccharide monoclonal antibody. Mod Pathol. 1997;10:1038–42.
Walker DH, Parks FM, Betz TG, et al. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am J Clin Pathol. 1989;91:720–4.
Paddock CD, Zaki SR, Koss T, et al. Rickettsialpox in New York City: a persistent urban zoonosis. Ann N Y Acad Sci. 2003;990:36–44.
Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12(9):1332–7.
Rutherford JS, Macaluso KR, Smith N, et al. Fatal spotted fever rickettsiosis, Kenya. Emerg Infect Dis. 2004;10(5):910–3.
Paddock CD, Greer PW, Ferebee TL, et al. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J Infect Dis. 1999;179(6):1469–76.
Chapman AS, Bakken JS, Folk SM, et al, Tickborne Rickettsial Diseases Working Group, CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55(RR-4):1–27.
La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715–27.
Denison AM, Amin BD, Nicholson WL, et al. Detection of Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin Infect Dis. 2014;59(5):635–42.
Sexton DJ, Kanj SS, Wilson K, et al. The use of a polymerase chain reaction as a diagnostic test for Rocky Mountain spotted fever. Am J Trop Med Hyg. 1994;50:59–63.
Mouffok N, Socolovschi C, Benabdellah A, et al. Diagnosis of rickettsioses from Es char swab samples, Algeria. Emerg Infect Dis. 2011;17:1968–9.
Kondo M, Akachi S, Kawano M, et al. Improvement in early diagnosis of Japanese spotted fever by using a novel Rick PCR system. J Dermatol. 2015;42(11):1066–71.
Znazen A, Sellami H, Elleuch E, et al. Comparison of two quantitative real time PCR assays for Rickettsia detection in patients from Tunisia. PLoS Negl Trop Dis. 2015;9(2):e0003487.
Clements ML, Dumler JS, Fiset P, et al. Serodiagnosis of Rocky Mountain spotted fever: comparison of IgM and IgG enzyme-linked immunosorbent assays and indirect fluorescent antibody test. J Infect Dis. 1983;148:876–80.
Jensenius M, Fournier PE, Vene S, et al. Comparison of immunofluorescence, Western blotting, and cross-adsorption assays for diagnosis of African tick bite fever. Clin Diagn Lab Immunol. 2004;11(4):786–8.
McGready R, et al. Pregnancy outcome in relation to treatment of murine typhus and scrub typhus infection: a fever cohort and a case series analysis. PLoS Negl Trop Dis. 2014;8(11):e3327.
Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial dis-eases in pregnancy. Obstet Gynecol Surv. 2013;68:635–49.
Stallings SP. Rocky Mountain spotted fever and pregnancy: a case report and review of the literature. Obstet Gynecol Surv. 2001;56:37–42.
Licona-Enriquez JD, et al. Case report: Rocky Mountain spotted fever and pregnancy: four cases from Sonora, Mexico Mexico. Am J Trop Med Hyg. 2017;97(3):795–8.
Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis – United States. MMWR Recomm Rep. 2016;65(2):1–44.
Dumler JS. Clinical disease: current treatment and new challenges. In: Palmer GH, Azad AF, editors. Intracellular pathogens II: Rickettsiales. Washington, DC: ASM Press; 2012. p. 1–39.
Rolain JM, Maurin M, Vestris G, et al. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother. 1998;42(7):1537–41.
Ruiz Beltran R, Herrero Herrero JI. Deleterious effect of trimethoprimsulfamethoxazole in Mediterranean spotted fever. Antimicrob Agents Chemother. 1992;36(6):1342–3.
Ruiz Beltran R, Herrero Herrero JI. Evaluation of ciprofloxacin and doxycycline in the treatment of Mediterranean spotted fever. Eur J Clin Microbiol Infect Dis. 1992;11(5):427–31.
Cohlan SQ. Teratogenic agents and congenital malformations. J Pediatr. 1963;63:650–9.
Herbert WN, Seeds JW, Koontz WL, et al. Rocky Mountain spotted fever in pregnancy: differential diagnosis and treatment. South Med J. 1982;75(9):1063–6.
Ross S, Burke FG, Sites J, et al. Placental transmission of chloramphenicol (Chloromycetin). J Am Med Assoc. 1950;142(17):1361.
Vaughn MF, Funkhouser SW, Lin FC, et al. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46(5):473–80.
Epidemic typhus risk in Rwandan refugee camps. Wkly Epidemiol Rec. 1994;69:259.
Straily A, Drexler N, Cruz-Loustaunau D, et al. Notes from the field: communitybased prevention of Rocky Mountain spotted fever – Sonora, Mexico, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(46):1302–3.
Brites-Neto J, Nieri-Bastos FA, Brasil J, et al. Environmental infestation and rickettsial infection in ticks in an area endemic for Brazilian spotted fever. Rev Bras Parasitol Vet. 2013;22(3):367–72.
Drexler N, Miller M, Gerding J, et al. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012-2013. PLoS One. 2014;9(12):e112368.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Silesky-Jiménez, J.I., Hidalgo, J. (2021). Rickettsiosis in Pregnant Women. In: Montufar, C., Hidalgo, J., Gei, A.F. (eds) Obstetric Catastrophes. Springer, Cham. https://doi.org/10.1007/978-3-030-70034-8_33
Download citation
DOI: https://doi.org/10.1007/978-3-030-70034-8_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70033-1
Online ISBN: 978-3-030-70034-8
eBook Packages: MedicineMedicine (R0)