Abstract
In the past few decades, an increasing number of fungal infections in animals as well as in human have been observed. Of these, some severe diseases cause die-off and extinctions of animal species and are responsible for higher morbidity and mortality rate. Dermatophytosis is a superficial infection of skin caused by three genera of fungi including Epidermophyton, Microsporum, and Trichophyton collectively called as dermatophytes. It is the most frequent fungal infections of animals including pets and livestock and even in wild species. The chief causal fungal species included M. canis, M. nanum, M. gypseum, T. terrestre, T. equinum, T. verrucosum, and T. mentagrophytes. Dermatophytes are distributed globally with its specific species and clinical form. The fungi are found in soil (geophilic organisms), animals (zoophilic), and humans (anthropophilic). Dermatophyte spores can survive more than a year under humid and mild temperature conditions. The animals may infect by close contact with other infected animals or soil fungi during digging or rooting. Dermatophytes require keratin for growth so they infect in keratinized tissue only. While, fungal exposure to animals does not always cause infection. There are multiple factors involved in infection such as fungal species, surface and exposure of animal skin, immunocompetence, age, and animal nutritional behavior. The disease diagnosis is based on the history, physical examination, and much more microscopic study, histology, and cultural characteristics of fungi. For the treatment of disease itraconazole, fluconazole, terbinafine, ketoconazole, and griseofulvin have been effectively used in animals. But, in few years, these treatments have become ineffective which may be due to antifungal resistance or tolerance. Therefore, use of new antifungal compounds from natural/plant-derived products can be an alternative approach to combat such situation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antifungal compounds
- Animals
- Dermatophytosis
- Drug resistance
- Plant-derived products
- Superficial infection
7.1 Introduction
Fungi are ubiquitous, free-living saprophytes and depend parasitically on its host. Animal fungal disease is based on host-pathogen interaction , immunocompetency of host, and constant exposure of fungal propagules. For disease establishment, fungi take advantages of host like nutritional requirement, niche establishment, reproduction, growth, and completion of life cycle (Seyedmousavi et al. 2018). Both adaptation and propagation mechanisms are equally responsible for fungal disease facilitation. Although the host defense system is sufficient at primary level as physical barrier, other than this fungal virulence mechanisms contribute to cause disease. The literature data estimated that fungi are the highest threat for animal-host and plant-host species and cause approximately 65% host loss (Fisher et al. 2012). In global scenario, the increased incidence of fungal disease may be worse due to climatic change (Garcia-Solache and Casadevall 2010) and antifungal resistance or tolerance (Kontoyiannis 2017).
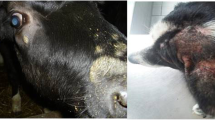
Dermatophytosis is a superficial infection of keratinized epithelium and the most common fungal disease of broad range of domestic and wild animals and as well as in humans (Moretti et al. 2013). It belongs to three fungal genera including Epidermophyton , Microsporum , and Trichophyton together called as dermatophytes. Dermatophytes are similar to filamentous fungi and cause damage and utilization of the keratin of skin, hair, and nails (Shalaby et al. 2016). Nearly 40 species of dermatophytic fungal organisms have been identified (Weitzman and Summerbell 1995). The spread of disease happens through the soil (geophilic), humans to human (anthropophilic), and animal to humans (zoophilic). The hot and humid climate favors the growth of dermatophytes. The animals generally show combination of symptoms like hair loss with patchy or circular, reddish or dark skin, itchiness, etc. Currently, topical azoles and allylamines are group of synthetic drugs that are used for treating this disease. Furthermore, nanoparticle-based remedies have been tested significantly and introduced in treatment of dermatophytosis. The metal oxide nanoparticles have substantial advantages because of their exclusive property of a large surface-to-volume ratio (Babu et al., 2019).
The synthetic drugs have high rates of clinical efficacy but the disease reoccurrence is another issue. The reoccurrence may be due to antifungal resistance or tolerance . The antifungal drug resistance consists of various mechanisms including nonsynonymous point mutations within the gene encoding the target enzyme leading to alterations in the amino acid sequence, high expression of the target enzyme through transcription of their respective genes, decreased concentrations of the drug due to drug efflux, changes in the biosynthetic pathway of the target of the antifungal drugs , etc. (Seyedmousavi et al. 2018). Therefore, using natural/plant-derived products based on antifungal drugs can be an alternative approach for such situation.
7.2 Etiological Agents and Taxonomy
The dermatophyte word is derived from Greek derma “skin” and phyton “plant.” The causal agents of dermatophytes are distributed into three asexual genera, namely, Epidermophyton , Microsporum , and Trichophyton belonging to the class Deuteromycetes (imperfect fungi) and order Moniliales (Maldonado 2011). The disease has been classified as geophilic (soil), anthropophilic (humans to human), and zoophilic (animal to humans) based on their habitat. In the above three, the zoophilic fungal organisms occur primarily in animals and cause infection to human via contact with infected animals. The M. canis affects to cat and dog, M. equinum to horse, M. persicolor to voles, M. nanum to pig, T. verrucosum to cattle, and species of T. mentagrophytes to rodents, rabbits, and hedgehogs (Moriello et al. 2017).
Dermatophytes have their own classification history suggested by many workers. Today, the five epidemiological importance dermatophytes including M. audouinii, E. floccosum, T. schoenleinii, T. tonsurans, and T. mentagrophytes had been described over the period of 1841–1875 (Seeliger 1985) on the basis of colony and microscopic morphology and nutritional and biochemical characteristics (Refai et al. 2013). Currently, the classification is based on genetics and molecular characteristics by targeting the ITS region of rDNA of three genera, i.e., Trichophyton, Microsporum, and Epidermophyton (Makimura et al. 1999; Kawasaki 2011). A recent multilocus phylogenetic study has restructured the taxonomy of dermatophytes into seven genera including Trichophyton (16 species), Epidermophyton (1 species), Nannizzia (9 species), Microsporum (3 species), Lophophyton (1 species), Arthroderma (21 species), and Ctenomyces (1 species), respectively (de Hoog et al. 2017).
7.2.1 Morphology
Dermatophytes produce colonies that differ in texture, color, and growth rate. There are three forms of colonies including membranous form, filamentous form, and granular-powdery form (Refai et al. 2013) as mentioned below:
7.2.1.1 Membranous Form
The colonies appeared as glabrous, waxy, humid, and faviform. It lacks the aerial mycelium, e.g., M. ferrugineum, T. concentricum, T. violaceum, and T. verrucosum.
7.2.1.2 Filamentous Form
The colonies look like cottony, fluffy, hairy, and velvety with aerial mycelium, e.g., M. canis, M. nanum, T. rubrum, and E. floccosum.
7.2.1.3 Granular-Powdery Form
This form shows excessive conidia and aerial mycelium absent, e.g., M. equinum, T. mentagrophytes, and T. megnini.
The microscopic structure of dermatophytes has the micro- and macroconidia, elongated and septate hyphae, undulant, and branching. Members of the genus Epidermophyton have macroconidia with broad clavate, thin or thick wall, and one to nine septa. In the genus Microsporum, macroconidia have rough wall and are spindle or fusiform and obovate. Microconidia are sessile or stalked with hyphae. In the genus Trichophyton, macroconidia have smooth, thin wall, with 1–12 septa, and are elongated, clavate, fusiform, or cylindrical. Microconidia may be globose, pyriform , clavate, sessile, or stalked (Fig. 7.1.).
7.3 Epidemiology
Dermatophytosis is significant in view of their global distribution, recurrence, individual to individual transmission, and morbidity (Kelly 2012). Even though dermatophytes are specific to its species, subsequently the clinical presentation may differ from region to region (Kelly 2012). For survival and growth, they infect only in keratinized epithelium not in mucosal membranes. In most of cases, anthropophilic organisms are responsible for the infection. The disease transmission could take place via exposure to desquamated cells and direct contact or inoculation of fungal propagules from infected animals. In aged or immune-compromised individuals , disease symptoms appear earlier on superficial layers of the skin (Hainer 2003). Dermatophytes are distributed globally but very frequent in tropical regions and may reach to outbreak proportions in other regions with higher humidity and overpopulation and with poor hygienic conditions. The major species with worldwide occurrence is M. canis, T. verrucosum, A. benhamiae, T. equinum, M. equinum, T. gallinae, M. nanum, T. quinckeanum, and M. gypseum (Moriello et al. 2017). In cattle dermatophytosis, the prevalence rate in Plateau State has been observed 11.0% of T. verrucosum (54.2%) and T. mentagrophytes (45.8%), respectively (Table 7.1). The animal age, breed, management practice, and season can be significantly associated with the occurrence of the disease (p < 0.05) (Dalis et al. 2019). Moreover, India has very hot and humid climate, and dermatophytosis is also prevalent in several states including Chennai, Madhya Pradesh, Andhra Pradesh, West Bengal, Gujarat, Chandigarh, and Karnataka (Bhatia and Sharma 2014).
7.4 Cultural Characteristics
For isolation and identification of dermatophytes , Sabouraud’s dextrose agar (SDA) or potato dextrose agar (PDA) supplemented with antibiotics, i.e., chloramphenicol , gentamicin , or cycloheximide , are generally used. The fungi can be incubated at 20–25 °C for 4–6 weeks. For T. verrucosum, the temperature should higher at 30–32 °C. Similarly, Pal (2017) has been isolated T. verrucosum from the 4-year-old cow by culturing on SDA medium with chloramphenicol and actidione and small, compact, heap-shaped, white to gray color colonies observed after 14 days incubation at 37 °C. Some specific and enrichment media, i.e., Borelli lactritmel agar , brain heart infusion agar, Baxter’s medium , Takasio medium , malt agar , bromocresol purple milk solid glucose agar, urea indole broth, Christensen’s urea agar medium (for differentiation of T. mentagrophytes from T. rubrum and T. soudanense), etc., are used for the study of fungal morphology, conidiation, and pigments of dermatophytes (Rudramurthy and Shaw 2017).
7.5 Transmission
Dermatophytosis is a superficial infection of keratinized epithelium including skin, hairs, and nails. It can be suspected in animals with lesions of erythema, papules, scaling, and crusting (Bond 2010; Pollock 2003). The disease transmits through the soil (geophilic), human to human (anthropophilic), and animal to human (zoophilic). The geophilic species are found in the soil and associated with hair keratin, feathers, and horn. The animals and human can get infected after contact with contaminated soil (Moriello et al. 2017). M. canis is the most common infectious fungal agent in cat globally causing skin diseases. The fungal arthrospores can survive in environment for about a year. The dermatophytes can transmit through contact with sick cats or healthy carriers, soil, clothes, and other fomites (Frymus et al. 2013). Pal (2017) has been suggested to apply 2% solution of tincture iodine regularly for 2–3 weeks on lesions and removal of skin crusts with disposable spatula. The zoophilic dermatophytes are adapted to animal hosts. Recent report also showed the transmission of dermatophytes by Demodex mites (Demodex folliculorum) a group of obligate parasites of the skin of mammals (Vanam et al. 2018).
7.6 Pathogenesis
The fungal arthrospores or hyphae give rise to threadlike hyphae and initiate the infection in the growing hair or in stratum corneum. The infection is followed by penetration in hair shaft and leads to inflammation and finally hair loss. At matured stage, clusters of arthrospores developed on the outer surface of hair shafts. The arthrospores are the chief source of reoccurrence of disease. The spores are highly resistant and can survive in a dry environment more than 12 months. In cat circular alopecia, desquamation and occasionally erythematous margin with central healing have appeared (Frymus et al. 2013). During host interaction , the dermatophytes also produce multiple enzymes with potential roles including polyketide synthases, nonribosomal peptide synthetases, LysM, proteases, kinases, and pseudokinases. These enzymes enhance the interaction and pathogenicity of fungi with host (White et al. 2014). Other mechanisms may be described as changing in host immune response, adaptability to skin pH, fungal adhesion, sensation, and adaptation to host surface and tissues (Martinez-Rossi et al. 2016). The colonization of dermatophytes is limited to the dead keratinized tissues of stratum corneum and responsible for mild or intense inflammatory reaction. The dermatophytes produce keratinases which help skin invasion and cause delayed-type hypersensitivity responses after injecting into the skin of animals (Weitzman and Summerbell 1995).
7.7 Laboratory Diagnosis
Dermatophytosis is sometimes difficult to treat due to lack of proper diagnosis. Therefore, accurate diagnosis is essential step for management of this disease and prevention of relapse or recurrence (Rudramurthy and Shaw 2017). The isolation and identification of the dermatophytes and the antifungal susceptibility test can be helpful to initiate suitable therapy. For laboratory diagnosis, localization, and characterization of lesions, skin scrapings are collected and studied by direct microscopic examination, and furthermore, cultural characteristics can be done. The detailed methods are mentioned below.
7.7.1 Wood’s Lamp
This method employs a handheld lamp having the source that emits ultraviolet radiations through the filters made up of nickel or cobalt glass. Florescence occurs due to the absorption of shorter wavelength light and the emission of longer wavelength light. This method is not very useful for the diagnosis in small animals as it is only a screening tool (Moriello et al. 2017).
7.7.2 Direct Visualization Under the Microscope
In order to visualize sample, various keratin digesting solutions are needed such as 10% or 20% potassium hydroxide (KOH) with or without dimethyl sulfoxide, 10% sodium hydroxide, Amann’s chloral lactophenol, and detergents, i.e., sodium dodecyl sulfate (SDS) . Several stains including cotton blue C4B or blue–black ink permanent or chlorazol black E stain which imparts deep blue or black color to fungal element are used. Additionally, several other stains such as periodic acid Schiff (PAS) are generally utilized to stain polysaccharide glycosaminoglycans and Congo red which stains ß-D-glucans and fluorochromes (Rudramurthy and Shaw 2017).
7.7.3 Molecular Methods
The molecular approaches are highly specific and makes easier for dermatophytes detection in clinical samples. The multiplex RT-PCR (real-time PCR) was developed by Arabatzis et al. (2007) for the direct detection of the fungi in the clinical specimens. It limits the risk of contamination and helps in recognition of multiple species of dermatophytes using diverse species-specific probes. A real-time PCR can distinguish both dermatophytes and non-dermatophytes with a sensitivity of 97% when compared with culture (Sharma et al. 2006). Hayette and Sacheli (2015) identified several dermatophytes which were negative on microscopy. In 2008, PCR reverse line blot assay targeting ITS sequences has been utilized in order to identify the nine relevant dermatophytes of hair, nails and skin samples. Immobilized oligonucleotide probe that is present on the membrane was exposed to denatured PCR products followed by hybridization and washing and detected by using streptavidin peroxidase and chemiluminescence (Bergmans et al. 2008). PCR–ELISA-based method has been used for direct detection of clinical samples of dermatophytes including T. rubrum, T. interdigitale, T. violaceum, M. canis, and E. floccosum (Beifuss et al. 2011). PCR-RFLP has higher specificity for detection of dermatophytes by targeting the ITS region, 18S ribosomal DNA from nails, hairs, and skin (Elavarashi et al. 2013).
7.8 Treatment
Traditionally, topical therapy is recommended for the treatment of dermatophytosis. Azoles and allylamines has high efficacy. The azoles include econazole, ketoconazole, clotrimazole, miconazole, oxiconazole, sulconazole, and sertaconazole, respectively. The azoles interfere with cytochrome P450-dependent ergosterol synthesis and stop fungal cell proliferation (Refai et al. 2013). The systemic medication included the itraconazole, fluconazole, griseofulvin, and terbinafine. Chiefly, itraconazole, fluconazole, terbinafine, ketoconazole, and griseofulvin have been effectively used in animals. Vaccination is also available for certain species, i.e., T. verrucosum and T. mentagrophytes, in livestock, farmed foxes, and rabbits, T. equinum in horses (Chollet et al. 2015), and M. canis in cats and dogs (Refai et al. 2013). But, in few years, these treatments have become ineffective which may be due to climate change, antifungal resistance, or tolerance . The hot and humid climate also supports the survival of dermatophytes on host body. The fungi easily travel from soil to animal/human or human to animal, and animal to human sequentially. The mechanisms behind drug resistance may be alteration of drug target sites, increased drug efflux, biofilm formation, etc. (Shivanna and Inamadar 2017). Finally, the synthetic drugs become ineffective after a while due to reoccurrence of disease .
Thus, natural/plant-derived products can be applied as an alternative approach to combat such situation. Ibrahim and El-Salam (2015) had reported 26 plant-derived essential oils against four dermatophytes, i.e., M. canis, E. floccosum, T. rubrum, and T. mentagrophytes. The oils of Prunus armeniaca, P. dulcis var. amara, Olea europaea, and Mentha piperita were found most effective. The Juniperus oil had antifungal activity against M. canis, M. gypseum, T. rubrum, T. mentagrophytes, and E. floccosum (Cavaleiro et al. 2006). Kakande et al. (2019) had reported the efficacy of Tetradenia riparia ethanol crude extract against three dermatophytes including T. tonsurans, T. mentagrophyte, and M. audouinii. A recent review paper by Sepahvand et al. (2018) has listed 24 medicinal plants against dermatophytes. These plants are Azadirachta indica, Capparis spinosa, Anagallis arvensis, Juglans regia, Inula viscosa, Phagnalon rupestre, Plumbago europaea, Ruscus aculeatus, Ruta chalepensis, Salvia fruticosa, Artemisia judaica, Ballota undulate, Cleome amblyocarpa, Peganum harmala, Teucrium polium, Aegle marmelos, Artemisia sieberi, Cuminum cyminum, Foeniculum vulgare, Heracleum persicum, M. spicata, Nigella sativa, and Rosmarinus officinalis, respectively. The antifungal properties of medicinal plants are due to presence of secondary active constituents, i.e., alkaloids, phenols, terpenes, flavonoids, phytosterols, anthocyanins, etc.
Furthermore, metal nanoparticles can enhance the currently available schemes to overcome dermatophytosis as they can deliver drug on the target site. These include the phospholipid vesicles , non-phospholipid vesicles , solid lipid nanoparticles , nanostructured lipid carriers , polymeric nanoparticles , polymeric micelles , nanoemulsions , and dendrimers . Among these lipid-based nanoparticles are considered as the most suitable carriers for the drugs (Bangia et al. 2019). Similarly, the antidermatophytic properties of silver nanoparticles has been reported against T. rubrum (Pereira et al. 2014), Microsporum, and Trichophyton (Ronavari et al. 2018) synthesized from fungal origin. Other therapies, i.e., photodynamic and adjuvant therapy , have their own importance against dermatophytosis. The photodynamic therapy involves the selective illumination of the target site with the light of a specific wavelength causing free radical generation, which causes the death of the cell. The aminolevulinic acid and methylene blue are well-known examples of photosensitizing agents and most frequently used (Sahni et al. 2018), while adjuvant therapy may cause excessive proliferation of epidermal cells leading to thickening and scaling due to hyperkeratosis, which obstructs the absorption of the antifungal drug . Therefore, the adjuvant-based treatment is recommended where the moisturizing agent salicylic acid (3–6%) is used most of the time with the drug which helps to prevent transepidermal loss of water and helps the antifungal drug in absorption (Rajagopalan et al. 2018).
7.9 Conclusion and Future Prospects
Dermatophytosis is a contagious fungal disease of animals as well as in human. Currently, this disease has global impact especially in tropical and subtropical countries. The hot and humid conditions favor the transmission, growth, and survivability of dermatophytes. The fungi have well-adapted survival and growth mechanism in host. Proper sanitation and good hygienic condition may minimize the fungi. Animals should keep away from infected animals and humans and be checked routinely. Only successful treatment can control the disease. Synthetic drugs are prescribed for long duration use for complete removal of fungi inside animal body. The overuse may cause side effects and develop drug resistance in fungi. Resistance and tolerance are other survival routes for the dermatophytes. Therefore, naturopathy and plant-based products may be an effective alternative to overcome this problem.
References
Abarca ML, Castella G, Martorell J, Cabanes FJ (2017) Trichophyton erinacei in pet hedgehogs in Spain: occurrence and revision of its taxonomic status. Med Mycol 55(2):164–172
Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, De Hoog GS, Lavrijsen AP, Templeton K, vander Raaji-Helmer EM, Velegraki A, Summerbell RC (2007) Diagnosis of common dermatophyte infections by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol 157(4):681–689
Babu N, Pathak VM, Singh A, Navneet (2019) Sonchus asper leaves aqueous extract mediated synthesis of titanium dioxide nanoparticles. Pharma Innov 8(4):817–822
Bangia R, Sharma G, Dogra S, Katare OP (2019) Nanotechnological interventions in dermatophytosis: from oral to topical, a fresh perspective. Expert Opin Drug Deliv 16(4):377–396
Bartosch T, Frank A, Gunther C, Uhrla S, Heydel T, Nenoff P et al (2019) Trichophyton benhamiae and T. mentagrophytes target guinea pigs in a mixed small animal stock. Med Mycol Case Rep 23:37–42
Beguin H, Goens K, Hendrickx M, Planard C, Stubbe D, Detandt M (2013) Is Trichophyton simii endemic to the Indian subcontinent? Med Mycol 51(4):444–448
Beifuss B, Bezold G, Gottlober P, Borelli C, Wagener J, Schaller M, Korting HC (2011) Direct detection of five common dermatophyte species in clinical samples using a rapid and sensitive 24-h PCR–ELISA technique open to protocol transfer. Mycoses 54(2):137–145
Bergmans AMC, Schouls LM, Van Der Ent M, Klaassen A, Bohm N, Wintermans RGF (2008) Validation of PCR–reverse line blot, a method for rapid detection and identification of nine dermatophyte species in nail, skin and hair samples. Clin Microbiol Infect 14(8):778–788
Bhatia VK, Sharma PC (2014) Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh, India. Springerplus 3(1):134–140
Bond R (2010) Superficial veterinary mycoses. Clin Dermatol 28(2):226–236
Carlotti B (2002) Dermatophytosis due to Microsporum persicolor (13 cases) or Microsporum gypseum (20 cases) in dogs. Vet Dermatol. https://doi.org/10.1046/j.1365-3164.1999.00115.x
Cavaleiro C, Pinto E, Goncalves MJ, Salgueiro L (2006) Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J Appl Microbiol 100(6):1333–1338
Chollet A, Wespi B, Roosje P, Unger L, Venner M, Goepfert C (2015) An outbreak of Arthroderma vanbreuseghemii dermatophytosis at a veterinary school associated with an infected horse. Mycoses. https://doi.org/10.1111/myc.12301
Dalis JS, Kazeem HM, Kwaga JKP, Kwanashie CN (2019) Prevalence and distribution of dermatophytosis lesions on cattle in Plateau state, Nigeria. Veterinary World 12(9):1484–1490
de Hoog GS, Dukik K, Monad M, Packeu A, Stubbe D, Hendrickx M et al (2017) Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 182(1–2):5–31. https://doi.org/10.1007/s11046-016-0073-9
Elavarashi E, Kindo AJ, Kalyani J (2013) Optimization of PCR-RFLP directly from the skin and nails in cases of dermatophytosis, targeting the ITS and the 18S ribosomal DNA regions. J Clin Diagn Res 7(4):646
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL et al (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194
Frymus T, Gruffydd-Jones T, Pennisi MG, Addie D, Belak S, Boucraut-Baralon C et al (2013) Dermatophytosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg 15(7):598–604. https://doi.org/10.1177/1098612X13489222
Garcia-Solache MA, Casadevall A (2010) Global warming will bring new fungal diseases for mammals. MBio 1(1):e00061–e00010. https://doi.org/10.1128/mBio.00061-10
Hainer BL (2003) Dermatophyte infections. Am Fam Physician 67(1):101–110
Hayette MP, Sacheli R (2015) Dermatophytosis, trends in epidemiology and diagnostic approach. Curr Fungal Infect Rep 9(3):164–179
Ibrahim SY, El-Salam MMA (2015) Anti-dermatophyte efficacy and environmental safety of some essential oils commercial and in vitro extracted pure and combined against four keratinophilic pathogenic fungi. Environ Health Prev Med 20(4):279–286
Kakande T, Batunge Y, Eilu E, Shabohurira A, Abimana J, Akinola SA et al (2019) Prevalence of dermatophytosis and antifungal activity of ethanolic crude leaf extract of Tetradenia riparia against dermatophytes isolated from patients attending Kampala international university teaching hospital, Uganda. Dermatol Res Pract. https://doi.org/10.1155/2019/9328621
Kawasaki M (2011) Verification of a taxonomy of dermatophytes based on mating results and phylogenetic analyses. Med Mycol J 52(4):219–295
Kelly BP (2012) Superficial Fungal Infections. Pediatr Rev 33(4):22–37
Kontoyiannis DP (2017) Antifungal resistance: An emerging reality and a global challenge. J Infect Dis 216(3):S431–S435. https://doi.org/10.1093/infdis/jix179
Kraemer A, Mueller RS, Werckenthin C, Straubinger RK, Hein J (2012) Dermatophytes in pet Guinea pigs and rabbits. Vet Microbiol 157(1–2):208–213
Makimura K, Tamura Y, Mochizuki T, Hasegawa A, Tajiri Y, Hanazawa R, Uchida K, Saito H, Yamaguchi H (1999) Phylogenetic classification and species identification of dermatophyte strains based on DNA sequences of nuclear ribosomal internet transcribed spacer 1 regions. J Clin Microbiol 37(4):920–924
Maldonado YA (2011) Pneumocystis and other less common fungal infections. In: Infectious diseases of the fetus and newborn, 7th edn. https://doi.org/10.1016/B978-1-4160-6400-8.00034-1
Martinez-Rossi NM, Peres NTA, Rossi A (2016) Pathogenesis of dermatophytosis: sensing the host tissue. Mycopathologia 182(1–2):215–227. https://doi.org/10.1007/s11046-016-0057-9
Moretti A, Agnetti F, Mancianti F, Nardoni S, Righi C, Moretta I, Morganti G, Papini M (2013) Dermatophytosis in animals: epidemiological, clinical and zoonotic aspects. G Ital Dermatol Venereol 148:563–572
Moriello KA, Coyner K, Paterson S, Mignon B (2017) Diagnosis and treatment of dermatophytosis in dogs and cats. Vet Dermatol 28:266–e68. https://doi.org/10.1111/vde.12440
Pal M (2017) Dermatophytosis in an adult cattle due to Trichophyton verrucosum. Anim Husb Dairy Vet Sci 1. https://doi.org/10.15761/AHDVS.1000106
Pereira L, Dias N, Carvalho J, Fernandes S, Santos C, Lima N (2014) Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J Appl Microbiol 117(6):1601–1613
Pollock C (2003) Fungal diseases of laboratory rodents. Vet Clin North Am Exot Anim Pract 6(2):401–413
Rajagopalan M, Inamadar A, Mittal A, Miskeen AK, Srinivas CR, Sardana K et al (2018) Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol 18(1):1–11
Refai M, El-Yazid HA, El-Hariri M (2013) Monograph on dermatophytes – a guide for isolation and identification of dermatophytes, disease and treatment. https://www.academia.edu.eg
Roller JA, Westblom TU (1986) Microsporum nanum infection in hog farmers. J Am Acad Dermatol 15(5):935–939. https://doi.org/10.1016/s0190-9622(86)70252-1
Ronavari A, Igaz N, Gopisetty MK, Szerencses B, Kovacs D, Papp C et al (2018) Biosynthesized silver and gold nanoparticles are potent antimycotics against opportunistic pathogenic yeasts and dermatophytes. Int J Nanomedicine 13:695–703
Rudramurthy SM, Shaw D (2017) Overview and update on the laboratory diagnosis of dermatophytosis. Clin Dermatol Rev 1(3):3–11. https://doi.org/10.4103/CDR.CDR_35_17
Sahni K, Singh S, Dogra S (2018) Newer topical treatments in skin and nail dermatophyte infections. Indian Dermatol Online J 9(3):149–164
Seeliger HPR (1985) The discovery of Achorion schoenleinii. Mykosen 28:161–182
Sepahvand A, Eliasy H, Mohammadi M, Safarzadeh A, Azarbaijani K, Shahsavari S, Alizadeh M, Beyranvand F (2018) A review of the most effective medicinal plants for dermatophytosis in traditional medicine. Biomed Res Therapy 5(6):2378–2388
Seyedmousavi S, Bosco SMG, Hoog S, Ebel F, Elad D, Gomes RR et al (2018) Fungal infections in animals: a patchwork of different situations. Med Mycol 56(1):S165–S187. https://doi.org/10.1093/mmy/myx104
Shalaby MFM, El-din AN, El-Hamd MA (2016) Isolation, identification, and in vitro antifungal susceptibility testing of dermatophytes from clinical samples at Sohag University hospital of Egypt. Electron Physician 8(6):2557–2567
Sharma R, Rajak RC, Pandey AK, Graser Y (2006) Internal transcribed spacer (ITS) of rDNA of appendaged and non-appendaged strains of Microsporum gypseum reveals Microsporum appendiculatum as its synonym. Antonie Van Leeuwenhoek 89:197–202
Shivanna R, Inamadar AC (2017) Clinical failure of antifungal therapy of dermatophytosis: Reoccurrence, resistance, and remedy. Indian J Drugs Dermatol 3(1):1–3
Vanam HP, Mohanram K, Reddy KSR, Poojari SS, Anuradha PR, Kandi V (2018) First report of concomitant Tinea faciei and Pityriasis folliculorum: a dermatomicrobiological rarity. Cureus 10(7):e3017
Weitzman I, Summerbell RC (1995) The dermatophytes. Clin Microbiol Rev 8:240–259
White TC, Findley K, Dawson TL Jr, Scheynius A, Boekhout T, Cuomo CA, Xu J, Saunders CW (2014) Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med 4(8):a019802
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gautam, S.S., Navneet, Babu, N., Kumar, S. (2021). Current Perspective of Dermatophytosis in Animals. In: Gupta, A., Pratap Singh, N. (eds) Fungal Diseases in Animals. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-69507-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-69507-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69506-4
Online ISBN: 978-3-030-69507-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)