Abstract
Decline in sperm numbers—indicating deteriorating fertility potential especially in Western countries, or a matter of participant bias, insufficient design of studies, and less appropriate laboratory techniques? Here is an attempt to highlight possible alternative explanations for the observed decline in sperm numbers in the last 40 years. A better understanding of factors determining the content of spermatozoa in the ejaculate and robust and reliable laboratory techniques is essential for the proper design and critical evaluation of investigations of global temporal trends in sperm numbers.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Annoying Facts
Reports on imminent or ongoing catastrophes tend to rapidly attain general attention. Without diminishing the gravity of signs of severe changes in the global male reproductive potential, it is essential that the scientific community continuously consider and evaluate the underlying evidence and reasonable alternative explanations. It is also essential to keep in mind that statistical methods do not prove the existence of true differences or relations. The statistical tests only estimate the probability that random variability has caused the observed data: if the probability for random distribution of results to cause the found results is less than 5% it is usually considered statistically significant. Still, in 5 out of 100 random instances results are outside the “reference range.” Furthermore, a reference limit based only on the distribution of results from healthy volunteers (or men with proven fertility) does not represent a limit between normal fertility and subfertility. The distribution of results from fertile men does not disclose anything about the distribution of results from subfertile men.
This chapter is dedicated to elucidating both factors that could constitute alternative explanations to a real decline in human sperm production and factors that could be possible contributors to a true general decline in human sperm production.
Among factors and facts to be considered when evaluating a possible decline in sperm counts is also the meaning of “semen quality,” sperm production, sperm transport, ejaculation, and laboratory techniques. Without taking sufficient attention to such aspects, the risk for mistaken conclusions increases—both for false premonitions and false dismissals of true threats to male reproductive potential.
What Is Semen Quality?
A very common simplification used in publications investigating male factors and semen analysis results in relation to fertility is the ambiguous term “semen quality.” The meaning of “quality” is a distinguishing characteristic. However, sperm concentration or total sperm count cannot be said to distinguishing factor for a man’s fertility potential in the entire range of results. It is true that men with few spermatozoa are less probable to contribute to a “spontaneous” pregnancy (Björndahl 2011), but no exact limit exists. For sperm numbers above, for example, 40 million, there is no direct relation between fertility and sperm number. For instance, 150 million sperm in the ejaculate does not mean a doubled fertility capacity compared to an ejaculate containing 75 million spermatozoa. The WHO semen manual (Cooper et al. 2010) is often misunderstood concerning the distributions of results and assumed reference limits: the distributions of results from fertile volunteers (in male contraceptive studies) only show those distributions, not limits that distinguish between subfertile men from fertile men.
Another misconception is the overuse of sperm concentration as a measure of quality, ignoring that this measure depends on two mainly independent functions: on one hand the production and transport of spermatozoa to the urethra and on the other the secretory function of primarily the seminal vesicles and the prostate. Variation in these functions can be considerable and if sperm production is the primary interest, the total number of spermatozoa is a better measure than the concentration.
Sperm Production
The starting point of spermatogenesis is related to the onset of puberty leading to gonadotrophins release from the pituitary causing high intratesticular androgen levels and the development of immature testicular “strings” into testicular tubuli seminiferi containing germ cells in different stages of development and “nursing” Sertoli cells. Once started, the process with high mitotic activity of spermatogonia and subsequent sperm production appears to continue without major changes even into high age provided the intratesticular androgen levels remain high. After several generations of mitotic divisions, some spermatogonia enter spermatogenesis by entering the meiotic division, where finally four gametes are formed from each immature germ cell. The quantity of sperm production is largely dependent on the number of stem cells and the number of generations of mitotic divisions—two factors that can be assumed to be genetically controlled. Based on the estimation of a magnitude of 50–100 million spermatozoa formed each 24 h, several hundreds of germ cell mitoses would occur every second. This would explain the wide range of sperm numbers that is considered as a sign of normal fertility potential.
From this point of view, possible factors threatening male reproductive potential would be interaction with the genetic control of spermatogenesis as well as normal pituitary and testicular endocrine functions (including use of anabolic steroids or dietary supplements with similar additions as well as hormone receptor mutations).
Sperm production being continuous requires a functional storage function which is believed to be one main function of the epididymides. The reservoir capacity is however, compared to other animals, limited. A maximum of 2 days of testicular sperm production is estimated to be stored in the cauda epididymis (Bedford 1994). Studies aiming at establishing baseline sperm production indicate that a maximum abstinence time ideally should be 42–54 h (at a maximum up to 60 h), corresponding to 1.75–2.25 days (or a maximum of 2.5 days), if the man has ejaculated every 42–60 h the week preceding ejaculate collection (Amann and Chapman 2009). Ignoring this could lead to difficulties establishing both the true baseline for sperm production and therefore create difficulties determining the effects of interventions like hormonal contraception (Behre et al. 2016).
Ejaculation
The process of sending spermatozoa to the gametes of another individual (i.e., oocytes) is a quite intricate and complicated function. Compared to the situation in water-living animals, mammals like humans have adapted to internal fertilization and fetal development. The process of ejaculations is effectuated by autonomous nerve signals stimulating secretomotor activity in accessory sex glands and contractions of smooth muscle cells in caudal epididymides, vasa deferentia, prostatic land acini, and seminal vesicles. Due to differences in the relation between lumen diameter, the thickness of smooth muscle tissue (wall thickness), and viscosity of fluids, the normal sequence of ejaculation is that spermatozoa are mixed with prostatic fluid and expelled before the emptying of the seminal vesicles (Fig. 1) (Björndahl and Kvist 2003). In the natural situation, this is to the advantage of the spermatozoa, since the seminal vesicular fluid, in general, has a mainly negative impact on sperm function (Lindholmer 1973) and sperm DNA protection by chromatin stability (Björndahl and Kvist 1990). The quality of the ejaculation is apparently dependent on the duration and quality of sexual arousal (Pound et al. 2002).
Schematic representation of the relative contribution of spermatozoa (green), prostatic fluid (zinc, blue), and seminal vesicular fluid (fructose; red) in an ejaculate collected with the six ejaculate fractions separate (split-ejaculate technique) (Björndahl and Kvist 2003)
The expulsion of the ejaculate is largely dependent on contractions of the striated muscle tissue surrounding the corpora cavernosa and thereby increasing the pressure in the urethra. The rhythmical contractions of the striated muscle tissue are also dependent on a proper erectile function to increase the urethra pressure and thereby execute an efficient ejaculation.
Any pharmaceutical treatment able to interact with autonomous nerve activity, smooth muscle, or striated muscle contraction is therefore likely to interfere with normal ejaculation. Psychoactive drugs like serotonin uptake blockers are suspected to be able to interact with normal ejaculation, as is antihypertensive drugs like α1-adrenoreceptor blockers (Doxazosine) that cannot only increase the risk for retrograde ejaculation but also interfere with sperm transport and emptying of accessory sex glands leading to azoospermic ejaculation or even “dry ejaculation” (orgasm without antegrade ejaculation, often mistaken for retrograde ejaculation if the presence of sperm in postorgasmic urine has not been investigated). Peripheral nerve damage, not uncommon in poorly controlled diabetes mellitus, is also a possible cause for interference with normal ejaculation.
Laboratory Techniques
It is a strength of the recent study indicating a temporal decline in sperm numbers that attempts have been done to eliminate publications based on substandard laboratory techniques by only including studies based on haemocytometer measurements of sperm numbers (Levine et al. 2017). By doing this, a number of common sources of errors are minimized. Studies using low volume chambers have a higher risk for using subsampling with aliquots with poor representativity of the entire ejaculate. Furthermore, counting still motile sperm in undiluted ejaculates also increases the risk for technical errors in assessing the sperm number, and counting less than 400 spermatozoa increases the influence of random errors to result in errors in the results (Fig. 2). To reduce the influence of random errors it is essential to compare replicate counting of at least 200 spermatozoa (Björndahl et al. 2016).
With regards to the assessment of sperm motility, the assessment of progressive motility, a controlled and constant temperature is important. Room temperature is not a defined temperature and within the usual range of temperatures expected to be that temperature, the velocity of motile spermatozoa will vary. The recommendation is therefore to have a controlled temperature close to 37 °C.
Discussion and Conclusions
The ideal studies for global assessment of a possible decline in sperm numbers would be studies where investigated samples have been collected after 3 days of daily ejaculations to exhaust the epididymal sperm storages. Without doing that the obtained sperm numbers are not directly related to sperm production but a combination of the number or stored spermatozoa and the daily production. The time for sexual abstinence cannot replace systematic exhaustion of epididymal sperm storages, and mathematical adjustment of sperm counts based on sexual abstinence before sample collection cannot do that either. Furthermore, the mathematical adjustment is based on an average with uncertainty, and the adjusted value then is burdened with both the basic uncertainty augmented with the added uncertainty due to the variation in the adjustment.
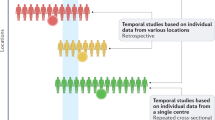
Furthermore, what does it mean that the average number of sperm has decreased among investigated men? Since all included studies are based on investigations of men recruited under different circumstances from different populations, there is a possibility that variation in recruitment or population may be a contributing factor to the observed differences. There could also be a shift in participants’ frequency of ejaculations before the investigated ejaculate, irrespective of the “days of abstinence” before the collection of the investigated ejaculate.
Few studies have followed the same men. There are indications that men without fertility problems may retain sperm production and functions better than men with fertility problems (Björndahl 2013). Following the same cohort of men could indicate age-related decline while examining birth-cohorts of men would be better to unravel temporal changes based on year of birth.
Although studies going back to early 1990s have indicated a possible ongoing decline in sperm production (Carlsen et al. 1992) contemporaneous studies of population fertility (based on Time-To-Pregnancy) have indicated that at that time a decrease in sperm number had not yet had an impact on the population fertility (Akre et al. 1999; Scheike et al. 2008).
From a global perspective, a decline was apparent in Western countries. In other parts of the world, a similar pattern of decline could not be shown. An important aspect of the latter is, of course, a lack of published investigations over time to the same extent as in Western countries. However, it still raises questions if a decline is mainly a problem in Western countries—or if it is a matter of changes of ethnical/genetical contributions to investigated populations in these countries. There is a general lack of knowledge if there are genetic differences (e.g., testicular size and thereby capacity for spermatogenesis) in different parts of the world, or if other factors like nutrition and exposure to gonadotoxic substances and pharmaceuticals may contribute both to differences and decline.
In conclusion, a general decline has been found in ejaculates examined in Western countries. If this is due to a true decline leading to a decline in fertility potential remains to be proven. To do that, more focused studies are needed, with a design to clearly define recruitment and status of participants, minimize bias by variability in sperm storage, and laboratory techniques.
References
Akre O, Cnattingius S, Bergstrom R, Kvist U, Trichopoulos D, Ekbom A (1999) Human fertility does not decline: evidence from Sweden. Fertil Steril 71(6):1066–1069
Amann RP, Chapman PL (2009) Total sperm per ejaculate of men: obtaining a meaningful value or a mean value with appropriate precision. J Androl 30(6):642–649
Bedford JM (1994) The status and the state of the human epididymis. Human Reprod 9(11):2187–2199
Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, McLachlan RI et al (2016) Efficacy and safety of an injectable combination hormonal contraceptive for men. J Clin Endocrinol Metab 101(12):4779–4788
Björndahl L (2011) What is normal semen quality? On the use and abuse of reference limits for the interpretation of semen analysis results. Hum Fertil (Camb) 14(3):179–186
Björndahl L (2013) Semen characteristics and aging: technical considerations regarding variability. In: Carrell D (ed) Paternal influences on human reproductive success. Cambridge University Press, Cambridge, pp 183–190
Björndahl L, Kvist U (1990) Influence of seminal vesicular fluid on the zinc content of human sperm chromatin. Int J Androl 13(3):232–237
Björndahl L, Kvist U (2003) Sequence of ejaculation affects the spermatozoon as a carrier and its message. Reprod Biomed Online 7(4):440–448
Björndahl L, Barratt CL, Mortimer D, Jouannet P (2016) ‘How to count sperm properly’: checklist for acceptability of studies based on human semen analysis. Hum Reprod 31(2):227–232
Carlsen E, Giwercman A, Keiding N, Skakkebaek NE (1992) Evidence for decreasing quality of semen during past 50 years. BMJ 305(6854):609–613
Cooper TG, Aitken J, Auger J, Baker HWG, Barratt CLR, Behre HM et al (eds) (2010) WHO laboratory manual for the examination and processing of human semen, 5th edn. World Health Organization, Geneva. 286 p
Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I et al (2017) Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 23(6):646–659
Lindholmer C (1973) Survival of human spermatozoa in different fractions of split ejaculate. Fertil Steril 24(7):521–526
Pound N, Javed MH, Ruberto C, Shaikh MA, Del Valle AP (2002) Duration of sexual arousal predicts semen parameters for masturbatory ejaculates. Physiol Behav 76(4–5):685–689
Scheike TH, Rylander L, Carstensen L, Keiding N, Jensen TK, Stromberg U et al (2008) Time trends in human fecundability in Sweden. Epidemiology 19(2):191–196
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this paper
Cite this paper
Björndahl, L. (2021). Is Decreasing Sperm Concentrations a Sign of a General Decay in Fertility Potential?. In: Björndahl, L., Flanagan, J., Holmberg, R., Kvist, U. (eds) XIIIth International Symposium on Spermatology. Springer, Cham. https://doi.org/10.1007/978-3-030-66292-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-66292-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66291-2
Online ISBN: 978-3-030-66292-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)