Abstract
Lately, misusing and counterfeiting pharmaceuticals in the drug supply chain have grown evidently worldwide, especially in the developing world. A pharmaceutical product is counterfeit when having a fake identity (ID) or origin. A flawed drug supply chain is among the major causes of counterfeiting pharmaceuticals either for the complexity of tracing pharmaceuticals, the lack of transparency, or exchanging limited or irrelevant data between all the entities during the transactions. The counterfeit drugs adversely influence both patients’ health and the economic status of the actual producers. Transforming the existing drug supply chain by applying monitoring techniques between the manufacturer and the consumer would address such issues. The blockchain technology applies a digital signature where each block has a unique crypto ID, ensuring solid management over possession. The Internet of Things (IoT)-integrated blockchain is a competently distributed ledger (DLT), which preserves an entirely changeless register of the data on transactions avoiding faking and accessible by all parties for more transparency. This chapter explores counterfeiting pharmaceuticals and the innovative managing of the drug supply chain securely via the IoT-integrated blockchain framework. Adopting this framework would enhance managing pharmaceuticals in the drug supply chain, hence delivering better healthcare service.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- IoT
- Blockchain
- IoT-integrated blockchain
- Supply chain
- Drug supply chain
- Counterfeit drugs
- Counterfeit pharmaceuticals
- Pharmaceuticals
- Pharmaceutical industry
- Medicine
7.1 Introduction
Counterfeiting pharmaceuticals in the drug supply chain is a critical public health issue worldwide. Pharmaceutical products are vital elements in healthcare to prevent and treat diseases, hence saving lives. Lately, counterfeiting pharmaceuticals has grown evidently leading to poor healthcare delivery and more deaths yearly [2]. The World Health Organization (WHO) describes counterfeit pharmaceuticals as falsified or substandard drug products. Falsified drug products have illegal or/and intentionally misrepresented identity, contents, or origin, while substandard drug products are allowed products, which could not fit the quality specification standards [23].
The Organization for Economic Cooperation and Development (OECD) considers trading counterfeit pharmaceuticals among the highly profitable markets for illicitly traded goods, valued yearly by billions of dollars. Professionals forecast that the rate of trading counterfeit pharmaceuticals and hence its revenues would double the legal ones. Meanwhile, drug companies are afflicting the results of falling income worth hundreds of billions of dollars with the added prices of adopting the costly safeguarding drug supply chain technologies, and hence, lower resources are there for research and development of novel drugs [1, 3].
Universally, counterfeit pharmaceuticals get in the market via the drug supply chain, which grows more challenging for the globalization of manufacturing processes where the pharmaceutical products are usually distributed in other countries than those who produce active substituents. Missing transparency in the drug supply chain cycle lets illicit players interfere with corruption or replace pharmaceuticals across the drug supply chain, compromising medications prior to reaching the patients. In the United States, most medications are produced with active substituents from either China or India. China, followed by India, is the major country for manufacturing counterfeit pharmaceuticals where millions of counterfeit antibiotic tablets were confiscated in 2009 [10]. A famous incidence is the made-in-China impure heparin that was distributed in 11 countries and caused the death of 81 individuals in the United States in 2008 [15].
Securing the drug supply chain should involve monitoring pharmaceuticals from the laboratory to the patient. The Falsified Medicines Directive (FMD), a track and trace project adopted by the European Union, encodes all pharmaceuticals via an automated drug supply chain in the pharmaceutical companies. Internet of Things (IoT) techniques like the blockchain could trace and remember items in the drug supply chain minimizing both interruptions like thieving and counterfeiting pharmaceuticals. Adopting the IoT-integrated blockchain to trace pharmaceuticals across the drug supply chain from the active constituents’ vendors to the producer to the patient would enhance managing pharmaceuticals. This chapter examines the drug supply chain and its limitations. It then explores the IoT-integrated blockchain framework in the pharmaceutical industry [15].
7.2 The Drug Supply Chain and Counterfeiting Pharmaceuticals
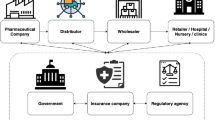
A drug supply chain is linking between an agency and the different vendors for properly manufacturing and distributing products to end users. In the pharmaceutical industry, managing the drug supply chain could enable the agencies to well use properties and investments for both making profits and meeting the end users’ needs. Smoothly managing the drug supply chain could genuinely affect and prosper the entire approach for a business involving less complicated processes such as selecting vendors, storage, and distribution and precise statistics. In the drug supply chain, counterfeit pharmaceuticals are a significant problem endangering patients and costing the pharmaceutical industry vast sums of money. Counterfeit pharmaceuticals are not marketed under the original trade name and violate the patents of pharmaceutical companies [22, 23].
There are several gates where counterfeit pharmaceuticals can be introduced into the drug supply chain. Gate-1 is where the vendors might provide impure, expired, poor-quality, or different raw materials for manufacturing pharmaceuticals, hence compromising the quality of the manufactured products and the safety of the consumers. Gate-2 is where most of the counterfeit pharmaceuticals or active constituents are introduced into the drug supply chain via the manufacturer. Counterfeiting a pharmaceutical product could involve using improper constituents, dose, or labeling and even marketing a placebo rather than the authentic drug. A counterfeit pharmaceutical products’ manufacturers develop products that resemble the authentic ones but with poor quality.
Gate-3 is where the manufacturers of the counterfeit pharmaceuticals target marketing their products, hence selling them directly to the distributor via fake networks. Usually, this process could range from simply shipping fake pharmaceuticals to applying very sophisticated trailing techniques for breaching current legal networks with poor security [20]. Gate-4 is where the pharmacies are the core of the whole issue. It might happen to receive a counterfeit pharmaceutical while buying a certain brand medication from a local or even a big pharmacy. Lately, more individuals are interested in online purchasing of medications for less money. Typically, such medications come with diverse labels from various places and a promise of the exact drug while it might not. In places with poor laws, online pharmacies could market counterfeit or fake pharmaceuticals to gain more for lower costs.
7.3 Blockchain Technology
In this digital era, applying technology, such as blockchain, to the drug industry could enhance all procedures enabling better services. Blockchain technology is a sort of distributed ledger (DLT), which keeps a changeless record of all data on transactions and devoid manipulations. Unlike conventional storage techniques, DLT applies nodes and separate computers to record, share, and synchronize the data of a transaction. Afterward, blockchain cluster data in blocks linked together in a chain. This framework could be used in the distributed setting of the drug supply chain where vendors, healthcare facilities, pharmacies, and patients produce separate but yet to be coordinated data.
Exposing the data on transactions in the markets might lead to leakage and manipulations; hence data should be housed securely in repositories. Blockchain ensures a confidential environment and protects anonymous public data via decentralization applying encoding asymmetrically and algorithms for hashing [18]. The algorithms of hashing map differently lengthened input data into similarly constant length output hashes uniquely representing their blocks, thus preventing any backward mapping targeting retrieving the original message’s data and allowing approvals from all entities.
Modifications in the block’s source data yield an output of diverse hash, hence interrupting the chain. Following the hashing procedure, every block would have a unique digital signature, and the chain is established based on the prior block signature hence an anonymous signature for the upcoming block guaranteeing all transactions executed via the proper entity. Such a hashing algorithm would prevent manipulating the blockchain, smoothly locating a hashed record, and creating random threads eliminating housed data redundancy in the database.
Added to the hashing encryption, another secure technique for authentication and confidentiality is called asymmetric encryption or private key cryptography where every blockchain party has a public and private key. The transactions’ encoding keys differ from the decoding ones where the public key is accessed by all parties, while the private one is only accessed by the party generating it for encoding contents to be then decoded by the other party through its private key. Figure 7.1 shows that one entity uses the public key to send a specific content, which gets encrypted and then decrypted by the other entity’s private key to read it.
In the blockchain, decentralization, a transparent medium, enables exchanging and recording the data; therefore, entities looking up records in such a credible distributed system could find solid and transparent data on transactions [11]. Hence, securing explicitly open and trustworthy repositories that are needed for the drug supply chain in the pharmaceutical business worldwide where the required data is smoothly accessed and tracked by all involved entities is shown in Fig. 7.2 [17].
The transparency offers readily available data on the source of elements and aspects of the procedure, with different levels of accessibility, to all entities [7]. Hence, the social accountability of all entities for their activities affecting the behavior of consumers and allowing competing parties to keep their credibility. Yearly, the drug supply chain is challenged progressively by counterfeit pharmaceuticals, deficiency, or cancellation of drug products that adversely affect patients and healthcare systems; therefore regulating entities are moving ahead to protect the drug supply chain [5]. Adopting blockchain has evidently optimized transparency for achieving operational objectives regarding the source of raw materials and reframed end services or products hence, the more supply chain transparency, producing changeless, persistent, and distributed records to enable monitoring [7].
7.4 The Applications of Integrating IoT with the Blockchain
The IoT has an influencing role in the logistics department of the pharmaceutical industry and presented many opportunities. IoT integration in the blockchain has improved the logistic operations in a big way [12]. The IoT brings ease for supply chain operations and reduces the risks that can cause a long-lasting disaster. Some applications of the IoT in the drug supply chain are the following:
7.4.1 Overcoming the Shortage of Medicines
The employment of the IoT from the digital technologies can ensure a synchronized supply of medicine resulting in patients getting the medicines on time. The IoT can help manage the best inventory based on company-defined business rules so that it can make planned decisions about manufacturing and assist in sending related products to the market [12].
7.4.2 Information Transmission Network in the Drug Supply Chain
Due to improper drug handling and counterfeiting issues, manufacturers and governments are facing the challenge of being consistent with regulations and ensuring the safety of customers’ lives. IoT devices and solutions can be used to record drug information throughout the supply chain [12].
7.4.3 Enhance Supply Chain Security
Safety of the supply chain is improved with the help of the IoT in a process of facilitating two-way communication between information seekers and devices, tracking inventory about the location and current status of packaging. Supply chain security can be safeguarded by RFID format labels, two-dimensional barcodes, and smart labels for packaging. The IoT can easily track the movement of drug inventory at each checkpoint [25]. It saves stakeholders in the supply chain from losing millions of dollars and at the same time ensures that while delivering real products to customers, it can also stop counterfeiters.
7.4.4 Freight Tracking
According to the report of Freight Watch International Supply Chain Intelligence Center, pharmaceutical companies suffered heavy load losses due to theft of goods. On the other hand, due to the use of intelligent freight tracking and data collection methods, the average loss value of the US pharmaceutical industry has dropped by about 55% from 2010 to 2012. This clearly shows the importance of tracking the goods just in case [8].
7.4.5 Temperature Control
In addition to managing temperature peaks during transportation, the quality of medicines should also be ensured. The temperature of the drugs being transported can be tracked to ensure that they remain within the specified stability range. If the drug storage conditions deviate from the specified temperature range, an environmental sensor will be embedded, and a programmed sensor will be used to sound an alarm. In addition to reducing drug waste and ensuring effectiveness, these are the key solutions that guarantee acquiescence with international and regional laws and regulations [19].
7.5 The Proposed IoT-Integrated Blockchain Framework
Today, the growing counterfeit pharmaceuticals have turned problematic since black marketplaces are supplying likewise medications with no monitoring for quality. For monitoring all undesirable acts in the drug supply chain until arriving the consumer, an IoT-integrated blockchain framework should be adopted for tracking purity, delivery, fraud, and making data about contaminated and counterfeit pharmaceuticals available for verifying the source and originality of the provided medications [5].
Following exporting a medication, all the chemical and technical data are warehoused on the producer system; meanwhile, the IoT links between the received medications all along until reaching the consumer. Yet, the unavoidable and undesirable acts of fraud happen but also detectable. Figure 7.3 shows a radio-frequency identification (RFID)-based drug supply chain that monitors the system for securing the chain and ensuring the delivery of quality healthcare services. The blockchain-based IoT framework would secure the drug supply chain against the yearly ever encountered network breaches worldwide that threaten data about both companies and patients [14] where it benefits from the vital blockchain feature in securing transactions that, upon verification, are not liable for falsification.
Figure 7.4 shows connected parties via a decentralized network where all are contacting the verified and approved medications’ transactions. When a party executes an activity, all the other parties get alerted and could assess verification via an authorized algorithm. Once a new block is verified, it would be added to the chain, hence minimizing sharing files where all transactions and verifications are substitute evidence of conventional paperwork.
Counterfeit pharmaceuticals could imply wasting money on products that might contain allergenic impurities, hence threatening health and causing an economic load. Discovering counterfeit products depends on validating data by all bodies at the drug supply chain. Adopting blockchain coupled with integration with IoT devices would facilitate management via tracking, reporting ownership, and intelligent contract rewarding, which are promising outputs eliminating fake data and counterfeit products.
7.6 The Case of Modum.io
The IoT technology is being used in many sectors including the transmission of real-time information and the monitoring of assets throughout the chain [24]. Also, other technologies integrated with the IoT (such as blockchain) can advance the security and reliability of these resources. There are many start-ups in which the IoT is integrated into blockchain technology for supply chain networks. Among several start-ups, Modum.io is a start-up that uses IoT sensor device blockchain technology to accentuate data invariability and make “temperature records” accessible for the public. Modum results in a decrease in operational costs in the pharmaceutical industry. This section attempts to explain how these technology combinations work better. We will also discuss the case of Modum, which mainly works in this field.
Modum is a use case that illustrates the blockchain and the IoT in the same application, which was established in cooperation with the University of Zurich (UZH). These companies seek to establish a drug sharing network integrated with blockchain technology. The company’s goal is to integrate the concept of the IoT to monitor changes in the status of drugs [9]. The technology checks whether certain transport standards are met to ensure that the quality is maintained until the goods reach their destination. Modum.io AG works closely with the UZH to jointly develop sensor equipment and its related software program. The planning of this project began in 2015, leading to the development phase in 2016, and was established in July 2016.
Modum.io monitors transportation of medicinal products and gathers all the necessary data by integrating IoT sensors with blockchain technology [13]. The integrity of data is thus guaranteed by the use of this technology, thus making it impossible to change the records. After delivery of the supply, a smart contract is initiated that guarantees compliance with the temperature. The data can be verified by any party once it enters the blockchain system and thus remains unchanged. The data/results are reported to the recipient and distributor and can be accessed publicly. It is foreseeable that customers can also check the temperature in the future. However, the serial number of each pharmaceutical package must be executed first.
The architecture of Modum.io AG is designed as front-end, back-end, and IoT-integrated sensor devices. The temperature records that are registered/stored in the front-end are verified by the Ethereum network of blockchain. Smart contracts are regulated by the use of Ethereum Virtual Machine, and data can be verified through smart contracts [16]. To meet the compliance with temperature data, for every new shipment, a smart contract is issued that maintains this responsibility. To store the raw temperature data and credentials of the user, an interactive database is used. Linkage of blockchain networks and the front-end users is established via a server. This linkage can modify or create a smart contract and the database stores the data. The new shipments can be registered through mobile devices, and the temperature data is sent to the server in a trackable manner. A thermal device (sensor), compatible with Bluetooth technology, is configured to send data to the mobile device at a fixed polling interval. A Bluetooth-integrated thermal device (sensor) sends the data at defined intervals to the mobile device.
SensorTag4’s IoT sensor provides the temperature data and can be placed at a critical location for consignment. The sensor has the functions of identification and can communicate a particular temperature at specific points. In addition, it requires a low energy Bluetooth connection, which is nowadays available in most of the mobile devices. Modum.io AG has established a prototype, and the first pilot project is accomplished with a pharmaceutical distributor. From July to August, they conducted a pilot project to deliver medical products from a trader to a medical retailer every week. 55 batches in total were sent. 29 batches were selected by the retailer to 1 site, 21 batches to another setting, and 05 batches of goods were sent within [4].
7.7 Discussions
The presence of counterfeit drugs in the global supply chain is causing a public health crisis. Pharmaceutical drugs are important components of the healthcare setup, bear crucial importance in diagnosing and curing diseases, and are a valuable asset in saving lives. However, counterfeit drugs are increasing over the years, resulting in deaths. According to estimates, there are millions of deaths because of counterfeit drugs ending the efficacy of healthcare. Complex and insufficient drug supply chain leads to the worst scenario of counterfeit drugs According to experts, it is estimated that the counterfeit pharmaceutical drug trade is growing double as compared to legitimate pharmaceuticals. However, it is still difficult to estimate the economic loss due to counterfeit drugs around the world.
Many traditional technologies have been suggested for the tracking and tracing of medicines, such as the barcode scanning system, RFID, mobile technology, and others. Still, counterfeit drug debacles happen on a global scale. The best way of preventing counterfeit drugs in the supply chain is the use of blockchain technology. It safeguards an absolute chain of transaction ledger, tracking each step of the drug supply chain.
Many serious issues are encountered by the pharmaceutical supply chain because of drug shortage and counterfeit drugs. Law enforcement and related agencies are concerned with the increased number of cases every year resulting in a burden on the public health system. To cover all the issues in the drug supply chain, an IoT-integrated blockchain system should be implemented to trace and keep track of transportation, contamination, and theft. Besides, the IoT-integrated system will provide information regarding falsified and contaminated drugs to authenticate the source and validity of the supplied medicines.
Nowadays, there are high regulations in the distribution of medicinal products for human use. The transfer of these products from the manufacturer to those who will use requires the utmost importance and involves various mediators. Now the supply chains are responsible to report any deviations such as temperature to the distributor and also to the recipient of the medicinal product, and the temperature of every parcel should also be monitored at all times. Thus, pharmaceutical companies are enforced to order special services from the logistics department. Blockchain technology provides a solution to the decentralized process in which data of the medicinal products can be stored and accessed during the logistic process by both parties ensured through a smart contract.
7.8 Insights for the Future
With the blockchain traceability and transparency features, the hash of the transferred pharmaceutical product, the physical asset, could validate the drug supply chain at all phases [21]. In the temperature-monitored drug supply chain, the so-called cold chain, several pharmaceuticals are highly affected by temperature and need extreme care during transport. The IoT-integrated blockchain could be applied in managing the cold chain where a sensor for heat could be included in each pharmaceutical batch for ongoing monitoring of the temperature. If the temperature highly differs from the limit, the pharmaceutical batch should be terminated in the blockchain, where it could no longer be marketed to anybody in the drug supply chain and should be wasted [6].
7.9 Conclusions
This chapter explored the IoT-integrated blockchain technique for managing the drug supply chain where it emphasized implementing the blockchain to minimize counterfeit pharmaceuticals in the drug supply chain. First, it described key entities in the drug supply chain, then highlighted the issues in the pharmaceutical industry, and identified the likely breaches in the drug supply chain facilitating leakage of counterfeit pharmaceuticals. However, regarding the richness in research covering the problem of the counterfeit pharmaceuticals in the drug supply chain, the technological aspects were yet inadequately covered. Next, it proposed the technical solution involving blockchain, RFID, and the IoT to enhance monitoring the pharmaceutical products traveling across the drug supply chain. Consequently, there are several advantages of adopting the IoT-integrated blockchain, which could address the issue of counterfeit pharmaceuticals in the drug supply chain along with other domains in the pharmaceutical industry including integrity and controlling misusing and fraud.
References
K. Acri, They Cost Us Billions and They Can Kill: Counterfeit Drugs Are Invading Canada | Financial Post (2 March 2018). https://business.financialpost.com/opinion/they-cost-us-billions-and-they-can-kill-counterfeit-drugs-are-invading-canada
V. Ahmadi, S. Benjelloun, M. El Kik, T. Sharma, H. Chi, W. Zhou, Drug governance: IoT-based blockchain implementation in the pharmaceutical supply chain, in 2020 Sixth International Conference on Mobile and Secure Services (MobiSecServ), (IEEE, Piscataway, 2020), pp. 1–8. https://doi.org/10.1109/MobiSecServ48690.2020.9042950
E.A. Blackstone, J.P. Fuhr, S. Pociask, The health and economic effects of counterfeit drugs. Am. Health Drug Benefits 7(4), 216–224 (2014) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4105729/
T. Bocek, B.B. Rodrigues, T. Strasser, B. Stiller, Blockchains everywhere – A use-case of blockchains in the pharma supply-chain, in 2017 IFIP/IEEE Symposium on Integrated Network and Service Management (IM), vol. 2017, (IEEE, Piscataway), pp. 772–777. https://doi.org/10.23919/INM.2017.7987376
J.B. Choi, J. Rogers, E.C. Jones, The impact of a shared pharmaceutical supply chain model on counterfeit drugs, diverted drugs, and drug shortages, in 2015 Portland International Conference on Management of Engineering and Technology (PICMET), (IEEE, Piscataway, 2015), pp. 1879–1889. https://doi.org/10.1109/PICMET.2015.7273165
F. Tian, A supply chain traceability system for food safety based on HACCP, blockchain internet of things, in 2017 International Conference on Service Systems Service Management, (IEEE, Piscataway, 2017), pp. 1–6. https://doi.org/10.1109/ICSSSM.2017.7996119
K. Francisco, D. Swanson, The supply chain has no clothes: Technology adoption of blockchain for supply chain transparency. Logistics 2(1), 2 (2018). https://doi.org/10.3390/logistics2010002
A. Jablokow, How Will IoT Revolutionize Pharmaceutical Manufacturing? IoT For All (17 April 2020). https://www.iotforall.com/drugs-continuous-manufacturing-and-iot/
N. Kshetri, 1 Blockchain’s roles in meeting key supply chain management objectives. Int. J. Inf. Manag. 39, 80–89 (2018). https://doi.org/10.1016/j.ijinfomgt.2017.12.005
H. Lock, B. Team, Fight the Fakes: How to Beat the $200bn Medicine Counterfeiters. The Mail & Guardian (10 June 2019). https://mg.co.za/article/2019-06-10-00-fake-medicine-makers-blockchain-artificial-intelligence/
Y. Lu, The blockchain: State-of-the-art and research challenges. J. Ind. Inf. Integr. 15, 80–90 (2019). https://doi.org/10.1016/j.jii.2019.04.002
S. Mishra, A. Dash, B.K. Mishra, Chapter 9—An insight of Internet of Things applications in pharmaceutical domain, in Emergence of Pharmaceutical Industry Growth with Industrial IoT Approach, ed. by V. E. Balas, V. K. Solanki, R. Kumar, (Academic, London, 2020), pp. 245–273. https://doi.org/10.1016/B978-0-12-819593-2.00009-1
S. Norton, CIO Explainer: What Is Blockchain? WSJ (2 February 2016). https://blogs.wsj.com/cio/2016/02/02/cio-explainer-what-is-blockchain/
K.V.O. Rabah, Challenges & opportunities for blockchain powered healthcare systems: A review. Mara. Res. J. Med. Health Sci. 1(1), 45–52 (2017)
V. Rees, The impact of counterfeit drugs in south and south-east Asia. Eur. Pharm. Rev. (2019). https://www.europeanpharmaceuticalreview.com/article/92194/the-impact-of-counterfeit-drugs-in-south-and-south-east-asia/
M. Sahu, 10 Best Tools for Ethereum Development Every Blockchain Developer Should Know About. UpGrad Blog (24 March 2020). https://www.upgrad.com/blog/tools-for-ethereum-development/
C.G. Schmidt, S.M. Wagner, Blockchain and supply chain relations: A transaction cost theory perspective. J. Purch. Supply Manag. 25(4), 100552 (2019). https://doi.org/10.1016/j.pursup.2019.100552
T. Scott, A. Post, J. Quick, S. Rafiqi, Evaluating feasibility of blockchain application for DSCSA compliance. SMU Data Sci. Rev. 1(2), 4 (2018) https://scholar.smu.edu/datasciencereview/vol1/iss2/4
U. Sharma, Transforming Pharma Logistics with IOT. Express Pharma (1 November 2018). https://www.expresspharma.in/pharma-logistics/transforming-pharma-logistics-with-iot/
M. Snyder, Keeping Counterfeit Medicines Out of the Supply Chain (Pharmaceutical Processing World, 28 January 2016). https://www.pharmaceuticalprocessingworld.com/keeping-counterfeit-medicines-out-of-the-supply-chain/
S. Tendulkar, A. Rodrigues, K. Patel, H. Dalvi, System to fight counterfeit drugs, in Advanced Computing Technologies and Applications, ed. by H. Vasudevan, A. Michalas, N. Shekokar, M. Narvekar, (Springer, Singapore, 2020), pp. 465–470. https://doi.org/10.1007/978-981-15-3242-9_43
M. Tremblay, Medicines counterfeiting is a complex problem: A review of key challenges across the supply chain. Curr. Drug Saf. 8(1), 43–55 (2013). https://doi.org/10.2174/1574886311308010007
WHO, WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products (World Health Organization, 2017). http://www.who.int/medicines/regulation/ssffc/publications/gsms-report-sf/en/
B. Yan, G. Huang, Supply chain information transmission based on RFID and internet of things. 2009 ISECS Int. Colloq. Comput. Commun. Control Manage. 4, 166–169 (2009). https://doi.org/10.1109/CCCM.2009.5267755
W. Zhou, E.J. Yoon, S. Piramuthu, Simultaneous multi-level RFID tag ownership & transfer in health care environments. Decis. Support. Syst. 54(1), 98–108 (2012). https://doi.org/10.1016/j.dss.2012.04.006
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rayan, R.A., Zubair, M.A.M. (2021). IoT-Integrated Blockchain in the Drug Supply Chain. In: Choudhury, T., Khanna, A., Toe, T.T., Khurana, M., Gia Nhu, N. (eds) Blockchain Applications in IoT Ecosystem. EAI/Springer Innovations in Communication and Computing. Springer, Cham. https://doi.org/10.1007/978-3-030-65691-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-65691-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65690-4
Online ISBN: 978-3-030-65691-1
eBook Packages: EngineeringEngineering (R0)