Abstract
Similar to other B-cell malignancies, the prognosis of post-transplant lymphoproliferative disorders (PTLD) subsequent to solid organ transplantation follows comparable principles. Despite remaining one of the most reliable prognostic systems, the International Prognostic Index does not fully hit the mark in regard to the complexity of PTLD and new proposed prognostic factors. Age, performance status, disease stage, and elevated LDH continue to be apropos prognostic factors in PTLD. The heterogeneous nature of PTLD and isolated single-center analyses make validation of updated risk factors difficult to ascertain. Biologic markers will undoubtedly become a backbone of prognostication once validated models become available. While wrought with limitations, when viewed as a conglomerate, certain patterns help provide clinical insight and guidance for practitioners treating PTLD. It is evident additional research in this serious complication of solid organ transplantation will be paramount to identifying future markers to help clinicians better prognosticate PTLD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Historical Background
Prognosis of post-transplant lymphoproliferative disorders (PTLD) follows the principles of other B-cell lymphomas. The prognosis of Hodgkin lymphoma patients has been established using the Ann Arbor staging system (Table 9.1) [1]. Originating in 1971, the Ann Arbor system classifies patients into risk categories based on anatomic stage. The Ann Arbor system was further validated in 1977 for non-Hodgkin lymphoma (NHL) and is currently the primary prognostic tool for both Hodgkin lymphoma and NHL [2]. However, anatomic staging alone is inadequate for estimating prognosis in NHL due to the hematogenous spread of disease characteristic of these lymphomas, a nonspecific clinical presentation, advanced Ann Arbor stage often present upon initial diagnosis, and outcomes better correlated with histopathology. Given the aforementioned challenges with prognostication of lymphomas, in 1993, the International Prognostic Index (IPI) was published. The IPI was the culmination of international level data to help develop a better prognostic-factor model for NHL [3]. NHL continues to follow IPI, or a modification of IPI, for assessment of prognosis (Tables 9.2 and 9.3).
Prognostic Factors for PTLD
Identification of prognostic factors for PTLD is complicated and tortuous due to the rapid changes in the understanding and management of this heterogeneous group of disorders. Similar to the treatment paradigm shift in NHL, the introduction of the anti-CD20 monoclonal antibody, rituximab, has drastically changed the treatment approach of PTLD. Thus, the assembly for prognostic outcomes in patient with PTLD has shifted.
In the current era, there continues to be modest, often conflicting, and outdated or non-generalizable data regarding prognostic factors for PTLD. The large majority of the data include retrospective literature from the pre-rituximab era, heavily weighted in renal transplant patients. Despite this heterogeneity and lack of robust consistency in the literature, we attempt to assemble a clinically meaningful overview of the information published to date (Table 9.4).
Of all prognostic factors, performance status has the most supporting evidence in the body of PTLD literature [7, 9,10,11,12, 15]. As ECOG PS increases, prognosis worsens, with poorer survival appearing to be most pronounced in PS greater than 1. PS may not be the most intuitive prognostic factor for PTLD, as compared to NHL, because PS is, in part, a marker of tolerance to chemotherapy regimens, whereas PTLD treatment in the current era involves reduced immunosuppression and rituximab, which are well-tolerated therapies. PTLD itself may resonate the toll the disease has on patients and is therefore a marker of tumor biology and behavior. PS is likely a reflection of a patient’s overall stamina and capacity to endure the disease process itself.
Patient age at diagnosis of PTLD is the next prognostic factor with the most supporting studies [8, 12, 24]. The International Prognostic Index defines age greater than 60 as the benchmark definition; in contrast, PTLD prognosis worsens in a relatively linear fashion as age advances beyond 55–60 [12]. Advanced age in patients with NHL is a poor prognostic factor due to comorbidities and is associated with a reduced capacity to tolerate chemotherapy. Patients with PTLD of advanced age, similar to PS, may be a surrogate marker for overall health and stamina to fight or endure PTLD. It is also postulated that a younger patient with PTLD may have different disease biology than PTLD in patients with advanced age. EBV status is by and large less likely to be negative in younger patients. Younger patients also have different exposures and different immune systems as compared to older patients. Finally, the biology of EBV-driven PTLD in younger patients, particularly children, arising from primary infection, is likely different from older patients who develop PTLD through viral re-activation [25, 26].
The presence of multifocal disease is an additional well-supported poor prognostic factor in PTLD [7,8,9, 12, 13, 24]. This is akin to the IPI in NHL, where both extranodal disease and advanced Ann Arbor stage confer poorer prognosis. In contrast to NHL, PTLD often present with allograft involvement. Even though allograft involvement qualifies as organ involvement by strict definition, it does not appear to confer as much risk of a poorer outcome as involvement of a non-allograft organ, and clearly is not as strong a risk factor alone as the presence of multifocal or multiorgan involvement. In renal transplant, isolated allograft involvement alone can be managed with surgical resection and therefore may improve prognosis [12].
Another poor prognostic factor is CD-20-negative status, mainly because this debars the use of rituximab therapy [12]. The lack of CD-20 suggests a divergent underlying cell of origin that leads to a different disease behavior, whether it be immature B cells who have yet to acquire CD-20 or a mature plasmacytic B cell that has shed CD-20. Rituximab will likely continue to be the predominant agent used in CD-20-positive PTLD, and therefore, it will be difficult to parse out the prognostic significance of CD-20 status outside the context of the effect of rituximab.
The introduction of rituximab in the current era and its relationship to prognostic factors are weighed in an article published in 2005 by Ghobrial and colleagues [10]. The study retrospectively evaluated 30 consecutive patients at a single center diagnosed with PTLD between 1999 and 2002. Rituximab was administered to 15 patients who were CD-20 positive and EBV positive and did not respond to frontline treatment with reduction in immunosuppression. There were 15 patients who did not meet the aforementioned criteria and had received alternative therapy, including observation, surgery, radiation, chemotherapy, or a combination of these therapies. There were differences observed in a number of characteristics between the rituximab group and the other treatment group. The average age was younger in the rituximab group (average age 37 vs. 50) and they developed disease sooner after transplant (average 8 months vs. >5 years out). Multivariate analysis for all 30 patients identified important prognostic factors. Overall survival in patients with CD-20-positive PTLD, low IPI (P = 0.004), and rituximab therapy (P = 0.03) was significant on multivariate analysis.
Prognostic factors for response to rituximab have been evaluated in two prospective clinical trials. Choquet and colleagues conducted a phase 2 multicenter trial of 46 solid organ transplant recipients with B-cell PTLD. The only factor predictor of response at 80 days in patients receiving rituximab was a normal LDH level (odds ratio = 6.9; P = 0.007) [27]. Lack of CNS disease is hypothesized to be a positive predictive marker for response to rituximab; however, clinical trials exclude patients with CNS disease and thus have not been evaluated prospectively. Furthermore, Oertel and colleagues conducted a prospective multicenter trial of 17 PTLD patients administered with rituximab therapy. The two factors predictive of response were EBV positivity (P < 0.0001) and a shorter time from transplantation to diagnosis (P = 0.036) [28]. These predictive markers to rituximab are logical given elevated LDH and EBV-negative PTLD are known poor prognostic features in PTLD. Response to rituximab can also be used as a prognostic feature to predict which patients will require subsequent therapy with chemotherapy. Trappe and colleagues suggest that PTLD patients treated sequentially with 4 infusions of rituximab followed by chemotherapy is preferable to rituximab monotherapy plus chemotherapy at disease progression [21, 23, 29]. If a patient is noted to respond well to rituximab, then chemotherapy is not necessary, and consolidation of a CR with four applications of rituximab with four additional courses of rituximab is even superior to chemotherapy consolidation.
The presence of CNS involvement signifies poorer outcomes [30]. One study, published by Trofe and colleagues, reviewed the Israel Penn registry specifically identifying cases of PTLD with CNS involvement [13]. Out of 910 cases, 15% had CNS involvement. Patients with CNS disease had a 3-year survival of 9.4% compared to those without CNS disease which was 49.4%. Isolated CNS disease conferred a 3-year overall survival of 29%. Patients with both CNS and non-CNS involvement of PTLD had a 3-year overall survival of 0. Another multicenter study of 80 solid organ transplant recipients removed treatment on Cox regression multivariate analysis and identified CNS disease, in addition to hypoalbuminemia, and bone marrow involvement as the most significant prognostic markers [19]. Hypoalbuminemia was later found to be non-significant in a subsequent single-center analysis [22].
The majority of PTLD cases are of B-cell origin; however, there are many case reports and small case series of T-cell PTLD. Classic PTLD arise from suppressed T-cell activity leading to EBV, which reside in B cells, ultimately inducing B-cell proliferation and transformation. In contrast, T-cell PTLD, in most cases, are not EBV positive and follow a distinct biologic mechanism, conceivably through altered T-cell proliferation related to T-cell-suppressive therapies. The onset of T-cell PTLD usually presents later and more commonly is associated with a primary extranodal site [31]. There remains a lack of large analyses of this rare subgroup; however, based on case reports and clinical experience, T-cell PTLD have been observed to have a poorer prognosis compared to classic B-cell PTLD [31, 32].
The type of organ transplanted noticeably has an impact on the incidence of PTLD. From highest to lowest, the incidence of PTLD occurs in the following order among solid organ transplants: intestinal, lung, heart, liver, and kidney. The intensity and duration of immunosuppression required for organ transplantation is related to incidence, as well as the mass of lymphoid tissue associated with a particular organ, which is why intestinal transplantation has the highest incidence of PTLD. Kidney and liver recipients who have PTLD appear to have better outcomes as compared to lung and heart recipients, which is related to the ease and safety of immunosuppression reduction [20, 33]. Kidney and liver rejection is reasonably easy to monitor with laboratory observation, and both organs are relatively tolerant of rejection allowing for more aggressive reduction in immunosuppression. In contrast, cardiac and lung transplant rejection is more likely to manifest as sudden death or rapid and frequently irreversible decompensation. The risks in rejection in these organs temper the extent to which immunosuppression is reduced. The opportunity of initiating a patient on dialysis makes kidney rejection manageable compared to other organ decompensation from rejection. In addition to the difficulty of immune suppression reduction, heart and lung transplant recipients treated for PTLD have a more aggressive disease course. A lack of response to rituximab monotherapy in the heart and lung transplant population predicts a lack of response to CHOP and early relapse as compared to liver or kidney transplant patients refractory to rituximab monotherapy [21, 23].
It was once thought that EBV-positive PTLD carried a more favorable prognosis compared to EBV-negative PTLD. Several biologic inferences with similar favorable prognosis supported this assumption. EBV-driven PTLD are primarily B cell in origin, conferring favorable prognosis compared to T-cell PTLD. EBV-positive PTLD are more likely to be CD-20 positive, which can be treated with rituximab, and therefore more favorable. Additionally, EBV-positive PTLD biology is more often polymorphic, which is observed to have more favorable outcomes compared to monomorphic disease. Finally, children have better outcomes compared to adults and are more likely to have EBV-positive disease. Despite these strong correlative observations, studies discussed elsewhere in this chapter have not found EBV status to be a reliable prognostic factor [7,8,9, 12, 13, 16]. By the same token, a 2006 study by Kremers and colleagues investigated 35 adult liver transplant patients and the prognostic significance of EBV status. Their study found the outcomes among 22 EBV-positive patients and 13 EBV-negative patients were nearly identical at both 1- and 5-year follow-up [34]. Ultimately, the data does not support EBV as a marker useful for prognostication. While EBV positivity may not be a useful prognosticator, patients who are EBV-naïve receiving an EBV-infected organ seem to be at the highest risk for PTLD development [18].
Another potential prognostic factor without robust evidence is the presence of monomorphic disease. Monomorphic disease suggests a clonal process resisted the natural checks on cell growth and survival. PTLD with monomorphic disease resemble traditional NHL in immunocompetent patients. A multi-institutional retrospective analysis of 56 pediatric heart transplant patients with PTLD identified 35 polymorphic cases and 19 monomorphic cases. Early onset PTLD were observed to be more commonly polymorphic. Nonetheless, there was no survival difference identified between these two histologic categories [26]. Curiously, one study of 107 PTLD patients identified monomorphic disease was not a significant prognostic factor by univariate analysis; however, upon multivariable analysis, it was identified as a poor prognostic factor in the author’s proposed PTLD prognostic index [35] (Table 9.5).
There are many other prognostic factors hypothesized to confer a negative prognostic value in PTLD; however, there remains scant or contradictory evidence supporting them. One of these factors is early vs. late onset of PTLD after transplantation. Some clinicians posit early onset PTLD indicate worse outcomes under the assumption early disease designates aggressive disease. In 2005, a large retrospective analysis of the Israel Penn registry between 1968 and 2004 was conducted, identifying 402 kidney transplant patients diagnosed with PTLD [12]. Survival was identified to be poorer in those patients diagnosed within 6 months of transplant (64%) compared to diagnosis beyond 6 months (54%, P = 0.04), as well as those diagnosed within 1 year compared to after 1 year from transplantation (60% vs. 55%, p < 0.04). A dissimilar report was published in 2001, reporting a single institution retrospective study of 30 lung transplant patients diagnosed with PTLD observed no survival difference between patients diagnosed before or after 1 year from transplant [37]. Another negative retrospective single-center analysis of 107 solid organ transplant patients found early onset PTLD, defined as within 1 year of transplant, commonly were EBV-positive, were CD-20-positive, and involved grafted organs. In spite of these biological markers, there were no differences observed in survival [35]. Given the conglomerate of literature, it is fair to presume both early and late PTLD can either behave as indolent in nature or behave aggressively, often leading to rapid decline.
Similar to adults, PTLD in children are a heterogeneous group of diseases. PTLD in children often have better outcomes compared to adults. The timing of PTLD in children is generally earlier after transplantation, is commonly EBV positive, and associated with primary EBV infection. There remains little data on prognostic factors in children with PTLD, although there remain some analyses [38].
A retrospective analysis of 55 pediatric patients with PTLD after solid organ transplant, by Maecker and colleagues, suggested prognostic factors in children largely mirror those in adults [15]. The authors identified stage IV disease, bone marrow involvement, CNS disease, and poor response to initial therapy were significantly associated with poor outcomes. EBV negativity and early onset of PTLD after transplantation were not significantly associated with poor outcomes. These findings are consistent with those identified in adult patients with PTLD. Additionally, as similar to adult NHL patients, children with c-myc translocation had worse outcomes. The presence of c-myc translocation may poorly predict event-free survival; therefore, as more robust data presents itself, it is not uncommon to recommend a cytogenetic analysis of patients. While monomorphic disease was not associated with prognostic value in this study, another single-center retrospective analysis of 32 patients showed conflicting results [39].
A recent publication studied the efficacy of low-dose chemotherapy in 36 children diagnosed with PTLD after failing first-line therapy. Patients in this study responded well to chemotherapy; however, patients who presented with fulminant disseminated disease (N = 4) did poorly [40].
As previously mentioned, one retrospective, multi-institutional analysis of 56 pediatric heart transplant patients with PTLD observed no survival difference between monomorphic and polymorphic diseases [26].
Compared to adult patients, there is less data on prognostic factors for PTLD in children, leading clinicians to extrapolate based on adult literature. The retrospective questionnaire study of centers who participate in the NAPRTS database investigated the pediatric kidney transplant population. There were 92 survey questionnaires evaluated from 35 different centers. Pediatric patients with PTLD within 1 year post-transplant were associated with better survival outcomes compared to PTLD after 1 year of transplantation (p = 0.032). The presence of EBV, CD20 positivity, and clonality were not found to be negative prognostic factors; however, several confounders in this study leave room for further investigation of these factors [41]. Additional research in the pediatric population is required to shed additional light on prognostic factors given the pitfalls of extrapolating data from adults, especially given the biologic and phenotypic differences recognized in each population.
Prognostic Indices
There have been several authors who attempted to develop a PTLD-specific prognostic index to replace the International Prognostic Index, which was developed for aggressive NHL who are otherwise immunocompetent. Unfortunately, these indices are restricted by the nature of their small sample sizes and generalizability of the patient populations being studied. However, clinicians still may find these other indices useful as long as they match their patient population where applicable. We summarize several of these articles in Table 9.5 to provide a comparison.
In 2001, Leblond and colleagues published a retrospective analysis from two institutions examining 61 patients diagnosed with PTLD between 1980 and 1999. There were 34 patients who had kidney transplants, 19 cardiac transplants, and all other patients had a lung or liver transplant [7]. The authors acknowledged two of the risk factors identified, PS and number of involved sites, could define a risk index by sorting patients into three groups: low-risk patients (PS <2 and <2 sites) whose median survival time had not yet been reached at well over 100 months of follow-up, intermediate-risk patients (PS ≥2 or ≥2 sites involved) with a median survival time of 34 months, and high-risk patients (PS ≥2 and ≥2 sites involved) with a median survival time of 1 month. The authors concluded this PTLD-specific index had a slight advantage when predicting survival as compared to the International Prognostic Index.
Another study in 2001, by Tsai and colleagues, examined prognostic factors in 42 patients who were treated with reduction in immunosuppression as initial therapy for PTLD [8]. Multivariable analysis identified that an elevated lactate dehydrogenase (LDH) ratio, organ dysfunction, and multiorgan involvement by PTLD were independent prognostic factors for lack of response to reduction of immunosuppression. Of the 18 patients lacking these poor prognostic factors, 89% responded to reduction in immunosuppression as opposed to three of five (60%) in patients with one risk factor and zero out of seven patients who had two to three risk factors. The notable overlap of the prognostic factors discovered in this study with the IPI suggests that PTLD behave much like lymphomas in immunocompetent hosts, despite a stark difference in management.
In 2005, Ghobrial and colleagues published a prognostic study including 107 patients diagnosed with PTLD at the Mayo Clinic between 1970 and 2003 [35]. The median survival for the entire cohort was 31.5 months (95% CI, 10.7–72.5 months). The median follow-up of living patients was 51.8 months (range, 5.6–202.6 months). An easy-to-use multivariable model for survival was created, which included poor performance status (3–4), monomorphic disease, and graft organ involvement. Patients who were identified with two or three of these factors had a 5.31 relative risk of death during follow-up compared with patients with zero or one factor present. As previously mentioned, monomorphic disease was useful in the multivariable model; however, it was not a prognostic factor in univariate analysis. When compared to the IPI, the authors concluded their three-variable model was superior (P = 0.006).
A 2007 study by Choquet and colleagues investigated the long-term efficacy of single-agent rituximab in PTLD patients [24]. Predictors of survival in patients treated with rituximab were age at diagnosis, performance status, LDH, and time from transplantation. The authors developed a PTLD-specific prognostic index in the setting of rituximab treatment, using LDH, age > 60, PS >1, and time from transplant as risk factors. Patients with no risk factors had an 88% 2-year survival. Patients with one risk factor had a 50% 2-year survival and no patients with two risk factors survived to 2 years. Compared to the IPI, the author’s PTLD prognostic index appeared to predict survival better.
A 2008 paper published by Oton and colleagues studied 84 solid organ transplant patients at a single center diagnosed with PTLD. The authors identified patients who had overexpression of BCL-2 (>50% staining), ECOG >2, and elevated white blood cell count had a median survival of 10 days. Patients with none of the aforementioned risk factors had a median survival of 1414 days [17]. Strong expression of the proto-oncogene, BCL-2, seemed to correlate with inferior outcomes, as similar to NHL. BCL-2 overexpression is proposed to provide prognostic significance and requires confirmatory studies to validate.
In 2008, Hourigan and colleagues published correspondence describing their retrospective study of 42 patients with PTLD after renal transplant [16]. The authors identified elevated LDH, PS >1, and presence of B symptoms were significantly associated with decreased survival. An analysis of this index was compared to the IPI, the Leblond (2001) PTLD prognostic index, the Choquet (2007) index, and the Ghobrial (2005) index. The authors found all indices, except the Ghobrial index, could separate patients into clinically meaningful survival groups (Fig. 9.1). It is important to note that the correspondence described an analysis of only renal transplant patients, while the other indices, including the Ghobrial index, identified a more diverse group of transplant recipients.
The French registry investigation by Caillard and colleagues in 2013 identified age > 55 years, serum creatinine >133 μmol/L (1.5 mg/dL), elevated LDH, disseminated lymphoma, brain localization, invasion of serous membranes, monomorphic PTLD, and T-cell PTLD as independent poor prognostic indicators of survival. The investigators incorporated age, serum creatinine, LDH, PTLD localization, and histology as a five-point prognostic score. PTLD mortality was low in patients with a score of 0 (3.3%; 95% CI, 0.4–9.5%), intermediate in a score of 1 (18.7%; 95% CI, 29–42.6%), high in a score of 2 or 3 (35.8%; 95% CI, 29–42.6%), and very high in patients with a score of 4 or 5 (60%; 95% CI, 40.5–76.1%). Patients with a score of 0 have a 5-year survival rate of 92%, whereas patients with a score of 4 to 5 have a 5-year survival rate of 35% [36]. This study was missing performance status, so it was unable to assess IPI and thus unable to compare this prognostic system against the IPI to see whether it is a suitable alternative. The scale was able to correctly prognosticate survival in this patient population and requires independent validation.
In 2015, Trappe and colleagues analyzed a cohort of 70 patients treated in the international multicenter phase II PTLD-1 trial in order to identify the risk factors for PTLD. The analysis confirmed the prognostic role of IPI, Ghobrial score, and PTLD prognostic index in relation to the overall survival. A high IPI and high PTLD prognostic index were associated with higher treatment-related mortality, primarily driven by age and ECOG performance status. It was identified that thoracic organ transplantation and response to rituximab were prognostic indicators for both time to progression and overall survival. IPI was broken down into low (<3) and high (≥3) [21].
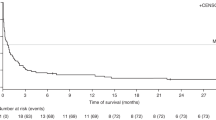
With low sample size as a limitation, Trappe and colleagues followed up their survey in 2016 with a prospective, international, multicenter phase II trial. Response to weekly doses of rituximab (375 mg/m2 IV) for 4 weeks was a significant predictor of time to progression and overall survival (P < 0.001). IPI <3 or ≥3 was confirmed in this trial to be a significant prognostic factor for overall survival. An IPI ≥3 conferred a negative prognostic value for overall survival (p = 0.001; 95% HR 2.540; CI, 1.461–4.418) and time to progression (p = 0.001; HR 3.338; 95% CI, 1.624–6.862). Overall survival and time to progression were further broken down according to IPI <3 or ≥3 (Fig. 9.2). Response to rituximab conferred a positive prognostic value for overall survival (p < 0.001; HR 0.320; 95% CI, 0.180–0.571) and time to progression (p < 0.001; HR 0.256; 95% CI, 0.119–0.549) [23]. Patients who did not respond to rituximab tended to have more aggressive disease when treated with CHOP and were more likely to have refractory disease and early treatment relapse [23].
A 2017 single institution study by Bishnoi and colleagues identified older age at PTLD diagnosis, recipient EBV status, bone marrow involvement, and initial best response were statistically significant prognostic factors (p < 0.05) [19]. Female gender was found to have a statistically significant better prognosis compared to males [Female gender HR 0.553 (p: 0.0427, CI 0.311–0.981)]. Similar to other studies, monomorphic PTLD performed poorly; however, it was not significant. EBV was not supported for predicting survival, yet it is still important for predicting pathogenesis. Interestingly, rituximab as upfront therapy did not impact overall survival and had a poor hazard ratio [0.589 (95% CI: 0.289–1.201)]. This single institution study confirmed previous risk factors associated with predicting PTLD.
As research will illuminate further dichotomy, PTLD will undoubtedly separate into various histologic, molecular, and clinical diagnoses, as similar to NHL. The perpetual forward momentum of our understanding of these disease processes, in addition to the foreseeable arrival of new treatment options, will make prognosis an ever-evolving issue for clinicians and patients. Biologic markers, such as BCL-2 and c-myc, will be more closely scrutinized to help refine diagnosis, prognosis, and management of PTLD. When considering the various prognostic factors identified in the heterogeneous body of PTLD literature, age, PS, sites of disease/extranodal disease, and LDH remain consistent. The IPI has helped predict response in the prior era of chemotherapy-based treatment, and newer PTLD indices now account for rituximab-based therapies and reduction of immunosuppression. In the face of evolving therapies in PTLD with newer options in the clinical setting, the prognostic factors have by and large remained the same. Regardless of the therapy used, in the end, the biology of PTLD has endured to follow similar principles as lymphoma.
Take-Home Pearls
-
The identification of prognostic factors for PTLD is complicated by the rapid changes in the understanding and management of what is a heterogeneous group of disorders.
-
Several factors such as poor PS, multifocal disease, tumor CD-20 negativity, CNS involvement, lack of response to rituximab monotherapy, and advanced age are consistently observed as poor prognostic features in PTLD.
-
Prognostic factors in PTLD continue to vary over time, especially due to the wide timeline of investigation spanning different diagnostic techniques, molecular testing, and available treatment options.
-
In the future, biologic markers such as c-myc and BCL-2 will be investigated to provide additional insight on diagnosis, prognosis, and management of PTLD.
References
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin’s disease staging classification. Cancer Res. 1971;31:1860–1.
Rosenberg SA. Validity of the Ann Arbor staging classification for the non-Hodgkin’s lymphomas. Cancer Treat Rep. 1977;61:1023–7.
Shipp MA, Harrington DP, Anderson JR, Armitage JO, Bonadonna G, Brittinger G, et al. A predictive model for aggressive non-Hodgkin’s lymphoma. The international non-Hodgkin’s lymphoma prognostic factors project. N Engl J Med. 1993;329(14):987–94.
AJCC: Hodgkin and non-Hodgkin lymphomas. In: Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.p. 607–11.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Maniar T, Tsai D. Prognostic Factors for PTLD. Post-Transplant Lymphoproliferative Disorders [Internet]. Springer Berlin Heidelberg; 2010;105–16. Available from: http://dx.doi.org/10.1007/978-3-642-01653-0_8.
Leblond V, Dhedin N, Brunee MF, Choquet S, Hermine O, Porcher R, et al. Identification of prognostic factors in 61 patients with post-transplantation lymphoproliferative disorders. J Clin Oncol. 2001;19(3):772–8.
Tsai DE, Hardy CL, Tomasezewski JE, Kotloff RM, Oltoff KM, Somer BG, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71(8):1076–88.
Muti G, Cantoni S, Oreste P, Klersy C, Gini G, Rossi V, et al. Post-transplant lymphoproliferative disorders: improved outcome after clinic-pathologically tailored treatment. Haematologica. 2002;87(1):67–77.
Ghobrial IM, Habermann TM, Ristow KM, Ansell SM, Macon W, Geyer SM, et al. Prognostic factors in patients with post-transplant lymphoproliferative disorders (PTLD) in the rituximab era. Leuk Lymphoma. 2005;46:191–6.
Bakker NA, van Imhoff GW, Verschuuren EAM, van Son WJ, Homan van der Heide JJ, Veeger NJGM, et al. Early onset post-transplant lymphoproliferative disease is associated with allograft localization. Clin Transpl. 2005;19(3):327–34.
Ghobrial IM, Haberman TM, Macon WR, Ristow KM, Larson TS, Walker RC, et al. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005;79(2):244–7.
Trofe JT, Buell JF, Beebe TM, Hanaway MJ, First MR, Alloway RR, et al. Analysis of factors that influence survival with post-transplant lymphoproliferative disorder in renal transplant recipients: the Israel Penn international transplant tumor registry experience. Am J Transplant. 2005;5(4):775–80.
Caillard S, Lelong C, Pessione F, Moulin B. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French registry. Am J Transplant. 2006;6:2735–42.
Maecker B, Jack T, Zimmerman M, Abdul-Khalig H, Burdelski M, Fuchs A, et al. CNS or bone marrow involvement as risk factors for poor survival in post-transplantation lymphoproliferative disorders in children after solid organ transplantation. J Clin Oncol. 2007;31:4902–8.
Hourigan MJ, Doecke J, Mollee PN, Gill DS, Norris D, Johnson DW, et al. A new prognosticator for post-transplant lymphoproliferative disorders after renal transplantation. Br J Haematol. 2008;141(6):904–7.
Oton AB, Wang H, Leleu X, Melhem MF, George D, Lacasce A, et al. Clinical and pathological prognostic markers for survival in adult patients with post-transplant lymphoproliferative disorders in solid transplant. Leuk Lymphoma. 2008;49:1738–44.
Knight JS, Tsodikov A, Cibrik DM, Ross CW, Kaminski MS, Blayney DW. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354–62.
Evens AM, David KA, Helenaowski I, Nelson B, Kaufman D, Kircher SM, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28(6):1038–46.
Yoon SO, Cho YE, Suh C, Kim KM, Han DJ, Lee SG, et al. Post-transplant lymphoproliferative disorders: clinicopathological analysis of 43 cases in a single center, 1990–2009. Clin Transpl. 2012;26:67–73.
Trappe RU, Choquet S, Dierickx D, Mollee P, Zaucha JM, Dreyling MH, et al. International prognostic index, type of transplant and response to rituximab are key parameters to tailor treatment in adults with CD20-positive B cell PTLD: clues from the PTLD-1 trial. Am J Transplant. 2015;15:1091–100.
Bishnoi R, Bajwa R, Franke AJ, Skelton WP, Wang Y, Patel N, et al. Post-transplant lymphoproliferative disorder (PTLD): single institutional experience of 141 patients. Exp Hematol Oncol. 2017;6:1–14.
Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, et al. Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful stratification into rituximab or R-CHOP consolidation in an international, prospective, multicenter phase II trial. J Clin Oncol. 2017;35:536–43.
Choquet S, Oertel S, Leblond V, Riess H, Varoqueaux N, Dörken B, et al. Rituximab in the management of post-transplant lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol. 2007;86(8):599–607.
Lucas KG, Burton RL, Zimmerman SE, Wang J, Cornetta KG, Robertson KA, et al. Semiquantitative Epstein-Barr virus polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood. 1998;92(10):3977–8.
Webber SA, Naftel DC, Fricker FJ, Olesnevich P, Blume ED, Addonizio L, et al. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet. 2006;367:233–9.
Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa AM, Vandenberghe P, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006;107:3503–7.
Oertel SH, Vershuuren E, Reinke P, Zeidler K, Papp-Váry M, Babel N, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). Am J Transplant. 2005;5:2901–6.
Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13:196–206.
Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P, First MR, et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc. 2005;37(2):954–5.
Haldas J, Wang W, Lazarchick J. Post-transplant lymphoproliferative disorders: T-cell lymphoma following cardiac transplant. Leuk Lymphoma. 2002;43(2):447–50.
Lundell R, Elenitoba-Johnson KSJ, Lim MS. T-cell posttransplant lymphoproliferative disorder occurring in a pediatric solid-organ transplant patient. Am J Surg Pathol. 2004;28(7):967–73.
Benkerrou M, Jais J-P, Leblond V, Durandy A, Sutton L, Bordigoni P, et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: prognostic factors and long-term outcome. Blood. 1998;92(9):3137–47.
Kremers WK, Devarbhavi HC, Wiesner RH, Krom RAF, Macon WR, Habermann TM. Post-transplant lymphoproliferative disorders following liver transplantation: incidence, risk factors and survival. Am J Transplant. 2006;6:1017–24.
Ghobrial IM, Habermann TM, Maurer MJ, Geyer SM, Ristow KM, Larson TS, et al. Prognostic analysis for survival in adult solid organ transplant recipients with post-transplantation lymphoproliferative disorders. J Clin Oncol. 2005;23:7574–82.
Caillard S, Porcher R, Provot F, Dantal J, Choquet S, Durrbach A, et al. Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol. 2013;31:1302–9.
Paranjothi S, Yusen RD, Kraus MD, Lynch JP, Patterson GA, Trulock EP. Lymphoproliferative disease after lung transplantation: comparison of presentation and outcome of early and late cases. J Heart Lung Transplant. 2001;20:1054–63.
Gross TG, Bucuvalas JC, Park JR, Greiner TC, Hinrich SH, Kaufman SS, et al. Low-dose chemotherapy for Epstein-Barr virus-positive post-transplantation lymphoproliferative disease in children after solid organ transplantation. J Clin Oncol. 2005;23:6481–8.
Hayashi RJ, Kraus MD, Patel AL, Canter C, Cohen AH, Hmiel P, et al. Posttransplant lymphoproliferative disease in children: correlation of histology to clinical behavior. J Pediatr Hematol Oncol. 2001;23:14–8.
Fohrer C, Caillard S, Koumarianou A, Ellero B, Woehl-Jaeglé M-L, Meyer C, et al. Long-term survival in post-transplant lymphoproliferative disorders with a dose-adjusted ACVBP regimen. Brit J Haem. 2006;134:602–12.
Dharnidharka VR, Martz KL, Stablein DM, Benfield MR. Improved survival with recent post-transplant lymphoproliferative disorder (PTLD) in children with kidney transplants. Am J Transplant. 2011;11:751–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tsai, D.E., Hughes, M.E. (2021). Prognostic Factors of PTLD after SOT. In: Dharnidharka, V.R., Green, M., Webber, S.A., Trappe, R.U. (eds) Post-Transplant Lymphoproliferative Disorders. Springer, Cham. https://doi.org/10.1007/978-3-030-65403-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-65403-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65402-3
Online ISBN: 978-3-030-65403-0
eBook Packages: MedicineMedicine (R0)