Abstract
Cardiovascular disease is a leading cause of death in many societies. Arterial stiffness is an initial sign of structural and functional changes in the arterial wall. Pulse wave velocity (PWV) is the gold standard for non-invasive evaluation of aortic stiffness and a modifiable cardiovascular risk factor. Curcumin is a major component of turmeric with known anti-inflammatory and anti-oxidative effects. Since arterial stiffness is affected by inflammation and oxidative stress, it may be improved by curcumin supplementation. The purpose of this clinical trial was to investigate the potential effects of curcumin on improving arterial stiffness in patients with metabolic syndrome. This placebo-controlled, double-blind, randomized clinical trial was conducted among metabolic syndrome patients. Sixty-six eligible individuals were randomly assigned to active intervention or control groups. The active intervention group received curcumin supplement at a dose of 500 mg daily for 12 weeks, whereas the control group received placebo capsule. Physical activity, daily dietary energy intake, anthropometric body composition, and biochemical hemodynamic and arterial stiffness parameters were evaluated at baseline and at the end of the study. Body weight decreased significantly in the curcumin group compared to placebo. Also, curcumin intervention improved PWV, which remained significant after adjustment for potential confounding factors (p = 0.011). The current clinical trial demonstrated that daily intake of 500 mg of curcumin for 12 weeks can lead to the improvement of arterial stiffness and weight management among subjects with metabolic syndrome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Arterial stiffness
- Vascular stiffness
- Vascular aging
- Arterial aging
- Pulse wave velocity
- Augmentation index
- Curcuminoid
- Curcumin
- Turmeric
- Metabolic syndrome
- Obesity
1.1 Introduction
Cardiovascular disease (CVD) is the most prevalent cause of death in the world [1]. Arterial stiffness, specifically aortic stiffness, is a primary sign of structural and functional changes in arterial walls and is a predictor of cardiovascular events [2, 3]. Arterial stiffness explains the reduced ability of an artery in dilation and constriction in response to pressure alterations [4]. Collagen and elastin are two important proteins in the arterial wall and any imbalance between them, such as caused by inflammation or increased luminal pressure, results in increased collagen, reduced elastin and subsequently enhanced stiffness of arterial wall [5, 6].
Various methods, both invasive and noninvasive, have been accepted to assess arterial resilience. Pulse wave velocity (PWV) and wave reflection are two noninvasive methodologies for vascular stiffness assessment [7, 8]. Augmentation pressure (AP) and augmentation index (AIX) are measures of pulse wave reflection and evaluated using the pulse wave analysis (PWA) technique [9]. Large elastic artery stiffness and systemic arterial stiffness are evaluated through aortic PWV and wave reflection, respectively [10]. Aortic PWV, as the ‘gold-standard’ measurement of arterial stiffness, has been determined by carotid-femoral PWV (cf-PWV) [2, 11]. Also, AIX can demonstrate the CVD risks independently of peripheral pressures, as shown in a recent meta-analysis [12].
Several situations can reduce vascular elasticity such as aging, central obesity, smoking, diabetes, hypertension, inflammation disease, metabolic syndrome and genetic factors [8, 13]. Metabolic syndrome is one of the major causes of CVD, and has been described as one of the main public health global challenges [14, 15]. According to the International Diabetes Federation (IDF) definition, metabolic syndrome can be diagnosed with central obesity and the existence of two or more other clinical features that include elevated blood pressure, increased levels of triglyceride and fasting plasma glucose, and reduced HDL-cholesterol concentrations [16, 17]. Lifestyle modifications, such as improved dietary habits, can have a favorable effect on vascular stiffness [18]. Turmeric is a source of an orange-yellow pigment polyphenolic compound called curcumin [1,7-bis-(4-hydroxy-3-methoxy-phenyl)-1,6-hepta diene-3,5-dione] [19]. Curcumin has been reported to have many beneficial effects on health [20,21,22,23,24,25,26,27,28]. It has been shown that curcumin can induce nitric oxide production and reduce oxidative stress and inflammation in animal and in vitro models of vascular-related disorders [29,30,31,32]. A recent preclinical study in young and older male mice showed that 4 weeks of curcumin supplementation resulted in improved endothelial function and arterial stiffness by enhancement of nitric oxide bioavailability and reduced oxidative stress [33].
The purpose of this study was to test the hypothesis that 12 weeks of curcumin supplementation would lead to improved arterial stiffness indices in metabolic syndrome patients.
1.2 Methods
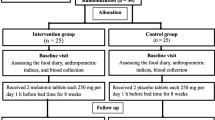
This randomized, double-blinded, placebo-controlled clinical trial with parallel design was conducted at the Persian cohort center of Imam Reza hospital, Mashhad, Iran. In this trial, 200 new cases of metabolic syndrome were assessed using inclusion and exclusion criteria. Of these cases, 66 individuals aged 30–60 years met the IDF criteria [17] and were incorporated into this 12 week study (Fig. 1.1). Exclusion criteria were the following: pregnancy; lactation; smoking; drug abuse; use of statins, contraceptive pills, analgesic, antidiabetic, antiplatelet, or anti-inflammatory drugs; consumption of antioxidants, multivitamins, multivitamin-mineral or herbal supplements 3 months before starting the study; and a history of diabetes, kidney failure, cancer , gallstones, calcium oxalate stones, autoimmune, biliary or obstructive diseases.
This investigation was approved by the Ethics Committee of Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran (serial no. IR.MUMS.MEDICAL.REC.1397.452), in accordance with the Declaration of Helsinki. In addition, this study was registered on Iranian Registry of Clinical Trials website (clinical trial registration no. IRCT20180619040151N2). At the beginning of the study, the nature, side effects, and advantages of the study were illustrated to volunteers and their written informed consent was obtained. All measurements were done at the Persian cohort center of Imam Reza hospital after 12 h fasting (water allowed) and > 24 h refrainment from physical activity.
1.2.1 Randomization Procedure
After conducting the screening and consent steps, the randomization procedure was performed using a stratified permuted block scheme, in which the stratification was based on age and gender. Subsequently, all participants were randomly allocated to either the curcumin or the placebo group.
1.2.2 Interventions
Curcumin [500 mg (95% total curcuminoids), provided by Karen Pharma and Food Supplement Company] or placebo capsules [500 mg of lactose, provided by the Faculty of Pharmacy, Mashhad University of Medical Sciences] were taken by the participants once per day with the midday meal. Every four weeks during the trial, in-person check-in visits were implemented to change the intervention capsules and evaluate participant compliance by survey and pill count. Additionally, tolerability and side effects of the interventions were assessed during these check-in visits.
1.2.3 Dietary and Physical Activity Assessment
Average daily dietary energy intake was evaluated by two-day dietary recall at baseline and at week 12. Dietary recall data were analyzed by Nutritionist IV software (N-Squared Computing, Salem, OR, USA). Also Physical activity was estimated by the long version of International Physical Activity Questionnaire (IPAQ) at baseline and week 12.
1.2.4 Anthropometric and Body Composition Assessment
Anthropometric and body composition measures were taken with subjects wearing light-weight clothing with no shoes on. Standing height was measured to the nearest 0.5 cm using a wall-mounted stadiometer. Waist, hip, wrist and neck circumferences were determined by a tension-gated tape at baseline and week 12. Waist circumference was measured to the nearest 0.5 cm at the midway of the distance between the lower rib margin and the iliac crest at the end of a gentle expiration and in the direction of the horizontal plane. Hip circumference was measured to the nearest 0.5 cm around the widest portion of the gluteal area in standing position [34]. Wrist circumference (WrC) was measured around the bony prominences of the radial and ulnar styloids [35]. Neck circumference (NC) was measured at the midpoint of the neck or just below the laryngeal prominence (‘Adam’s apple’) ‘in men with an obvious Adams apple, while the tape was placed vertically [34]. WrC and NC measures were taken to the nearest 0.1 cm. Also, weight and body composition parameters were determined via a bioelectrical impedance body composition analyzer (InBody 770, Biospace Co., Ltd. Seoul, South Korea). To decrease examiner-related errors, all the measurements executed by the same person.
1.2.5 Laboratory Parameters
Blood samples were collected from the antecubital vein after 12 h overnight fasting at baseline (0 weeks) and at the end of 12 weeks intervention . Serum concentrations of fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using the BT1500 chemistry analyser (Biotecnica Instruments S.p.A., Rome, Italy).
1.2.6 Blood Pressure and Arterial Stiffness Measurements
Brachial and aortic blood pressure, aortic pulse pressure (PP), mean arterial pressure (MAP,) heart rate (HR), AIX, AIX75, AP, and arterial age measurements were obtained after the participants had rested in a supine posture for at least 10 min in a calm, thermoneutral room. Measurements were obtained with the SphygmoCor XCEL System (Sphygmocor; AtCor Medical, Sydney, Australia) by a trained physician. After PWA, cf-PWV was measured for assessment of aortic stiffness using the SphygmoCor XCEL System.
1.2.7 Statistical Analyses
The sample size was statistically calculated to achieve a power of 90 according to change in AIX75 in the Sugawara [36] investigation. Statistical analysis was performed using SPSS 16. Assessment of data normality was performed using the One-Sample Kolmogorov-Smirnov test. In addition, histogram plots were evaluated visually, and it was observed that data distribution for normality was acceptable. Finally, linear regression was used to confirm the final results.
1.3 Results
A total of 66 metabolic syndrome patients were initially enrolled in the study but five placebo group participants (one smoking, three unwilling to continue, one adherence less than 80%) and one curcumin group participant (reflux side effect) did not complete the study (Fig. 1.1). The baseline characteristics of the final 60 study participants, who were randomly assigned into the two treatment arms, are shown in Table 1.1. None of the participant characteristics were significantly different between the two groups at baseline (all P > 0.5), except for waist to hip (WHR) ratio and A Body Shape Index (ABSI), which were both higher in the control group (P < 0.05). In the current study, energy intake and physical activity did not change in either the curcumin and placebo groups compared to baseline.
1.3.1 The Effect of Curcumin on and Anthropometric, Body Composition, and Serological Tests
The effects of curcumin supplementation on anthropometric, body composition and biochemical tests are presented in Table 1.1 and Table 1.2. After 12 weeks of intervention, a statistically significant reduction in mean body weight was observed in the curcumin compared to the placebo group but body composition and other anthropometric parameters showed no significant changes. A decreasing trend was observed in anthropometric and body composition parameters, including waist circumference, neck circumference, body mass index (BMI), and visceral fat area after curcumin treatment relative to placebo. In both groups, liver enzymea (ALT and AST) were decreased significantly but there was no significant difference between the groups.
1.3.2 The Effect of Curcumin on Arterial stiffness and Hemodynamic Parameters
Table 1.3 indicates that there was no difference between the groups in vascular stiffness and hemodynamic parameters at the baseline of the study. As shown in Table 1.4, a significant reduction in PWV was observed following 12 weeks of curcumin intervention compared to placebo. Also, it was shown that after adjusting for confounding factors including age, gender, change in physical activity and energy intake by regression, the curcumin treatment significantly reduced aortic PWV, relative to placebo (Table 1.5). Finally, brachial and aortic systolic blood pressure (SBP) was reduced but totally hemodynamic parameters were not significantly improved with curcumin consumption compared to placebo.
1.4 Discussion
The present investigation demonstrated that 12 weeks of regular ingestion of curcumin supplement ameliorated aortic stiffness in metabolic syndrome patients. We assessed PWV as a principal marker of large arteries stiffness and observed that curcumin supplementation significantly reduced PWV. Analysis of potential sex differences did not show any significant improvement in arterial stiffness parameters in women who received curcumin intervention. Initial evidence in a preclinical study performed by Fleenor et al. [33] demonstrated that dietary curcumin supplementation improves age-related large elastic artery stiffness by nitric oxide bioavailability restoration, oxidative stress reduction and normalization of collagen I and advanced glycation end products (AGES) deposition in the arterial wall.
This finding is consistent with research showing that arterial stiffness (PWV or carotid arterial compliance) significantly improves after several weeks to months of curcumin treatment [20, 37, 38]. However, two studies reported that curcumin ingestion does not affect PWV [36, 39]. A recent study conducted by Campbell et al. demonstrated that only subjects with a higher baseline value of aortic PWV (arterial stiffness) responded to curcumin supplementation. In the present investigation, the mean age of patients receiving curcumin was 44 years old and the mean baseline cf-PWV value was 7.6 m/s. However, the mean cf-PWV for healthy 40–49 year-old subjects was found to be 7.2 m/s [40]. This suggests that there may be differences in the measured mean baseline cf-PWV across different studies.
We found that the reflection wave indices (aortic AP, AIX, and AIX75) were not affected by the curcumin intervention. In agreement with this finding, Sugawara et al. [36] found that curcumin significantly decreased aortic AIX75 only when combined with exercise training. AIX is a complicated variable that shows the stiffness of smaller muscular arteries as well as microvascular density, number and location of terminal arterioles that give rise to reflected waves, the velocity of the pressure wave, and the pattern of left ventricular ejection. In addition, in contrast to PWV, AIX is influenced by gender and anthropometric measurements [41].
Today lifestyle modification is the main strategy for prevention of CVD, and weight management is a key factor in this objective. For this objective, curcumin has been reported to have beneficial effects on obesity management [42,43,44,45]. A preclinical study suggested that curcumin has antiobesity effects through downregulating the expression of peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer binding protein α, which are key transcription factors in adipogenesis and lipogenesis [46]. This results in suppression of adipocyte differentiation, fatty acid esterification, and adipokine-induced angiogenesis in adipose tissue, and induction of fatty acid oxidation and increased apoptosis of adipocytes. In humans, 10 weeks of curcumin supplementation significantly decreased mean body weight in overweight type 2 diabetes patients [42]. consistent with this finding, we observed that 12 weeks of curcumin ingestion significantly decreased body weight compared to the placebo group. Although not statistically significant, the curcumin intervention also tended to decrease WC, NC, BMI, and visceral fat area, while these parameters had an increasing trend in the placebo group.
Metabolic syndrome is a serious health condition of impaired glucose tolerance and, consequently, elevation of fasting plasma glucose is one of its criteria. Many animal studies have demonstrated that curcumin anti-inflammatory and antioxidant activities may be responsible, at least in part, for its anti-hyperglycemic effects [47,48,49]. Studies in humans have been inconsistent as some have confirmed the curcumin anti-hyperglycemic effect [42, 43, 50], and others have found no effect [51, 52]. In our study, FPG did not significantly change with curcumin supplementation compared to placebo. Also, we did not observe any significant changes in lipid profiles between the groups. Many preclinical studies have found that curcumin reduces serum cholesterol levels via upregulating the expression of hepatic LDL receptors, inhibition of LDL oxidation, enhancement of cholesterol excretion by increasing bile acid secretion, and suppressing the expression of genes involved in cholesterol biosynthesis [53, 54]. Furthermore, a recent animal study showed that curcumin reduced serum TG concentrations through inhibition of sterol regulatory element-binding protein 1 (SREBP-1c), liver X receptor alpha (LXR-α), and the target lipogenic enzymes fatty acid synthase and acetyl CoA carboxylase [55]. To our knowledge and according to literature review, curcumin should be consumed in higher doses or in a higher efficacy form (nano-formulation or in combination with an adjuvant) to influence FPG and lipid profiles.
Since the liver is the main organ for drug metabolism and elimination, it should be considered that hepatotoxic reactions may take place there in the present study. Such drug-induced hepatotoxicity manifestations are various, ranging from a mild elevation of liver enzymes to fatal hepatic failure [56]. During the present clinical trial, liver function was not affected by the interventions, as determined by the lack of effect on ALT and AST, which are commonly used as biomarkers of liver damage.
This study was limited by its short-term duration of follow-up that precluded the possibility of assessing hard cardiovascular endpoints. Furthermore, although the mean baseline cf-PWV values of participants were modestly elevated, not all participants had high PWV. The inability to present a mechanistic view for the beneficial effects of curcumin on vascular aging was another limitation of this study.
1.5 Conclusions
In the present study, we observed the favorable effects of 12 weeks of curcumin supplementation on arterial stiffness and weight control. We also demonstrated that curcumin intake for 12 weeks was well tolerated. Further trials are warranted to confirm the present findings in target populations with elevated arterial stiffness.
References
Castellano JM, Narula J, Castillo J, Fuster V (2014) Promoting cardiovascular health worldwide: strategies, challenges, and opportunities. Rev Esp Cardiol (Engl Ed) 67(9):724–730
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D et al (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27(21):2588–2605
Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT et al (2017) Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol 8:1058. https://doi.org/10.3389/fimmu.2017.01058
Cecelja M, Chowienczyk P (2012) Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis 1(4):1–10
Shirwany NA, Zou M-h (2010) Arterial stiffness: a brief review. Acta Pharmacol Sin 31(10):1267
Zieman SJ, Melenovsky V, Kass DA (2005) Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25(5):932–943
O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE (2002) Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 15(5):426–444
Oliver JJ, Webb DJ (2003) Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol 23(4):554–566
Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E (2013) High aortic augmentation index predicts mortality and cardiovascular events in men from a general population, but not in women. Eur J Prev Cardiol 20(6):1005–1012
Janner JH, Godtfredsen N, Ladelund S, Vestbo J, Prescott E (2012) The association between aortic augmentation index and cardiovascular risk factors in a large unselected population. J Hum Hypertens 26(8):476–484
Durmus I, Kazaz Z, Altun G, Cansu A (2014) Augmentation index and aortic pulse wave velocity in patients with abdominal aortic aneurysms. Int J Clin Exp Med 7(2):421–425
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 31(15):1865–1871
Albu A, Tache S, Mavritsakis N, Potoră C (2017) Physical exercise and arterial stiffness in elderly. Palestrica of the Third Millennium Civilization and Sport 18(2):100–104
Alberti KGM, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366(9491):1059–1062
de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N (2004) Prevalence of the metabolic syndrome in American adolescents: findings from the third National Health and Nutrition Examination Survey. Circulation 110(16):2494–2497
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C (2004) Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109(3):433–438
Saely CH, Koch L, Schmid F, Marte T, Aczel S, Langer P et al (2006) Adult treatment panel III 2001 but not international diabetes federation 2005 criteria of the metabolic syndrome predict clinical cardiovascular events in subjects who underwent coronary angiography. Diabetes Care 29(4):901–907
Fleenor BS (2013) Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis 4(2):76–83
Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK (2004) Turmeric and curcumin: biological actions and medicinal applications. Curr Sci 87(1):44–53
Campbell MS, Berrones AJ, Krishnakumar I, Charnigo RJ, Westgate PM, Fleenor BS (2017) Responsiveness to curcumin intervention is associated with reduced aortic stiffness in young, obese men with higher initial stiffness. J Funct Foods 29:154–160. https://doi.org/10.1016/j.jff.2016.12.013
Hassanzadeh S, Read MI, Bland AR, Majeed M, Jamialahmadi T, Sahebkar, A (2020) Curcumin: an inflammasome silencer. Pharmacol Res 159:104921. https://doi.org/10.1016/j.phrs.2020.104921
Iranshahi M, Sahebkar A, Hosseini ST, Takasaki M, Konoshima T, Tokuda H (2010) Cancer chemopreventive activity of diversin from Ferula diversivittata in vitro and in vivo. Phytomedicine 17(3–4):269–273
Soleimani V, Sahebkar A, Hosseinzadeh H (2018) Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother Res 32(6):985–995
Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A (2019) Immune modulation by curcumin: the role of interleukin-10. Crit Rev Food Sci Nutr 59(1):89–101
Ghandadi M, Sahebkar A (2017) Curcumin: An effective inhibitor of interleukin-6. Curr Pharm Des 23(6):921–931
Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía LE, Majeed M, Sahebkar A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res (Stuttg). 2018 Jul;68(7):403-409. doi: 10.1055/s-0044-101752.
Teymouri M, Pirro M, Johnston TP, Sahebkar A (2017) Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: a review of chemistry, cellular, molecular, and preclinical features. Biofactors 43(3):331–346
Momtazi AA, Derosa G, Maffioli P, Banach M, Sahebkar (2016) A role of micrornas in the therapeutic effects of curcumin in non-cancer diseases. Mol Diagn Ther 20(4):335–345
Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R et al (2003) Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371(3):887–895
Fang XD, Yang F, Zhu L, Shen YL, Wang LL, Chen YY (2009) Curcumin ameliorates high glucose-induced acute vascular endothelial dysfunction in rat thoracic aorta. Clin Exp Pharmacol Physiol 36(12):1177–1182
Jain SK, Rains J, Croad J, Larson B, Jones K (2009) Curcumin supplementation lowers TNF-α, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-α, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal 11(2):241–249
Lee H-S, Lee M-J, Kim H, Choi S-K, Kim J-E, Moon H-I et al (2010) Curcumin inhibits TNFα-induced lectin-like oxidised LDL receptor-1 (LOX-1) expression and suppresses the inflammatory response in human umbilical vein endothelial cells (HUVECs) by an antioxidant mechanism. J Enzyme Inhib Med Chem 25(5):720–729
Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M et al (2013) Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48(2):269–276
Aswathappa J, Garg S, Kutty K, Shankar V (2013) Neck circumference as an anthropometric measure of obesity in diabetics. N Am J Med Sci 5(1):28–31
Capizzi M, Leto G, Petrone A, Zampetti S, Papa RE, Osimani M et al (2011) Wrist circumference is a clinical marker of insulin resistance in overweight and obese children and adolescents. Circulation 123(16):1757–1762
Sugawara J, Akazawa N, Miyaki A, Choi Y, Tanabe Y, Imai T et al (2012) Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. Am J Hypertens 25(6):651–656
Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S (2014) Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem 25(2):144–150
Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R et al (2013) Effects of curcumin intake and aerobic exercise training on arterial compliance in postmenopausal women. Artery Res 7(1):67–72
Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR (2017) Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 9(1):187–205
Collaboration TRVfAS (2010) Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 31(19):2338–2350
McNulty M, Mahmud A, Feely J (2007) Advanced glycation end-products and arterial stiffness in hypertension. Am J Hypertens 20(3):242–247
Hodaie H, Adibian M, Sohrab G, Hedayati M (2017) The effects of curcumin supplementation on control glycemic and anthropometric indices in overweight patients with type 2 diabetes. Iranian J Endocrinol Metab 19(1):1–9
Rahimi HR, Mohammadpour AH, Dastani M, Jaafari MR, Abnous K, Mobarhan MG et al (2016) The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed 6(5):567–577
Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A et al (2016) Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytother Res 30(9):1540–1548
Akbari M, Lankarani KB, Tabrizi R, Ghayour-Mobarhan M, Peymani P, Ferns G et al (2019) The effects of curcumin on weight loss among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 10:649. https://doi.org/10.3389/fphar.2019.00649
Ejaz A, Wu D, Kwan P, Meydani M (2009) Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr 139(5):919–925
El-Moselhy MA, Taye A, Sharkawi SS, El-Sisi SF, Ahmed AF (2011) The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-α and free fatty acids. Food Chem Toxicol 49(5):1129–1140
He H-J, Wang G-Y, Gao Y, Ling W-H, Yu Z-W, Jin T-R (2012) Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes 3(5):94–104
Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Zachariah B (2016) Curcumin prevents inflammatory response, oxidative stress and insulin resistance in high fructose fed male Wistar rats: potential role of serine kinases. Chem Biol Interact 244:187–194
Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S (2012) Curcumin extract for prevention of type 2 diabetes. Diabetes Care 35(11):2121–2127
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A (2016) Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: a randomized controlled trial. J Cardiovasc Pharmacol 68(3):223–229
Saberi-Karimian M, Parizadeh SMR, Ghayour-Mobarhan M, Salahshooh MM, Dizaji BF, Safarian H et al (2018) Evaluation of the effects of curcumin in patients with metabolic syndrome. Comp Clin Pathol 27(3):555–563
Panahi Y, Ahmadi Y, Teymouri M, Johnston TP, Sahebkar A (2018) Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J Cell Physiol 233(1):141–152
Shehzad A, Ha T, Subhan F, Lee YS (2011) New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr 50(3):151–161
Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Sripradha R, Badhe B (2016) Curcumin inhibits hyperlipidemia and hepatic fat accumulation in high-fructose-fed male Wistar rats. Pharm Biol 54(12):2857–2863
Andrade RJ, Aithal GP, Björnsson ES, Kaplowitz N, Kullak-Ublick GA, Larrey D et al (2019) EASL clinical practice guidelines: drug-induced liver injury. J Hepatol 70(6):1222–1261
Conflict of Interest
None of the authors had declarations of interest to publish.
Funding
This research was financed by Research Council of the Mashhad University of Medical Sciences, Mashhad, Iran. The results reported in this paper have been derived from a postgraduate thesis (Thesis No: 1445) in Mashhad University of Medical Sciences, Mashhad, Iran.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alidadi, M. et al. (2021). The Effect of Curcumin Supplementation on Pulse Wave Velocity in Patients with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. In: Barreto, G.E., Sahebkar, A. (eds) Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health. Advances in Experimental Medicine and Biology, vol 1308. Springer, Cham. https://doi.org/10.1007/978-3-030-64872-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-64872-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64871-8
Online ISBN: 978-3-030-64872-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)