Abstract
Brainstem gliomas represent a heterogeneous group of tumors that most commonly occur in the pediatric population (Reddy and Wellons 3rd, Cancer J 9:107–112, 2003). The clinical behavior of brainstem gliomas is commonly related to the location within the brainstem with tumors of the medulla and midbrain being lower grade and tumors within the pons being aggressive and often rapidly fatal (Youland et al., J Pediatr Hematol Oncol 35:197–2052, 2013). Diffuse intrinsic pontine gliomas are associated with the H3.3K27M mutation (Gajjar et al., J Clin Oncol 33:2986–2998, 2015). Radiation therapy has been shown to demonstrate some efficacy in the treatment modality for brainstem gliomas (Merchant et al., J Clin Oncol 27:3598–3604, 2009). Treatment volumes should be designed to encompass imaging abnormality on both contrast-enhanced and FLAIR MRI sequences. Stereotactic techniques are useful given the often small and eloquent target.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 General Principles of Simulation and Target Delineation (Tables 16.1 and 16.2)
-
Information on simulation
-
Recommended imaging
-
Preoperative as well as postoperative imaging should be obtained.
-
Imaging sequences should include at least T1, T1 with contrast, T2, and FLAIR.
-
CT planning scan section thickness should ideally be ≤5 mm, although ≤2–3 mm remains ideal.
-
-
Patient positioning
-
Supine positioning and immobilization with a short aquaplast mask are recommended. The use of photon vs. proton radiotherapy may dictate head position or the need for other special setup devices like table-associated range shifter, etc.
-
-
-
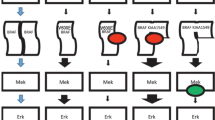
Recommendations for target delineation (Figs. 16.1 and 16.2)
-
Imaging sequences and special circumstances
-
Low-grade intrinsic gliomas tend to hypointense on T1-weighted and hyperintense on T2-weighted sequences, varying in degree following gadolinium infusion depending on independent tumor characteristics. Diffuse intrinsic pontine gliomas are expansile tumors that are homogeneously hypointense lesion on T1 and hyperintense on T2. MR T2 and T2 FLAIR are most likely to assist with defining the extent of disease and postoperative tumor bed (if applicable).
-
-
On-treatment imaging
-
Daily cone-beam CT or other stereotactic technique is recommended given the small tumor volumes and commonly pediatric patients that may require sedation and airway protection during treatment.
-
-
16.2 Dose Prescriptions
-
A dose of 50.4 Gy in 1.8 Gy fractions is generally recommended for low-grade intrinsic brainstem glioma; however, doses ranging from 45 to 50.4 Gy are acceptable depending on the extent of brainstem involvement and ability to meet the dose constraints of the organs at risk.
-
A dose of 54.0 Gy in 1.8 Gy fractions is generally recommended for diffuse pontine intrinsic glioma.
16.3 Treatment Planning Techniques (Fig. 16.3 and Table 16.3)
-
Modality
-
4–6 MV photons are typically utilized for treatment. Protons may also be considered.
-
-
Treatment technique
-
Three-dimensional conformal radiotherapy (3D-CRT), intensity-modulated radiotherapy (IMRT), or volumetric modulated arc therapy (VMAT) may be used with the goal of sparing the brain, brainstem, temporal lobes, hippocampi, cochlea, and pituitary/hypothalamic complex if possible.
-
Treatment planning is aimed so that at least 100% of the PTV is covered by 95% of the prescribed dose. Depending on tumor complexity and proximity to organs at risk (brainstem, cochlea, temporal lobes, spinal cord, etc.), achieving 95% coverage of the PTV by 95% of the prescribed dose is acceptable. Furthermore, no more than 10% of the PTV should receive greater than 110% of the prescription dose as determined by the dose volume histogram (DVH).
-
A goal is to achieve uniform dose distributions (utilize wedges, compensators, or any additional techniques).
-
If using proton beam therapy (PBT):
-
In patients with focal tumors or low-grade intrinsic brainstem gliomas, consider proton beam therapy, as these children are more likely to achieve a therapeutic benefit by reducing the risk of late toxicities of therapy from reducing radiation dose to normal tissue toxicity while maintaining target volume coverage.
-
Because lateral and range expansions may vary for each beam, a single PTV is no longer adequate and should not be used to determine the distal range for the individual proton beams. Instead of prescribing a uniform dose to a PTV, the treatment plan should be created to encompass the CTV in the setting of expected uncertainties. Thus, the distal target margin is based on the distal aspect of the CTV, range uncertainty, setup margin (SM), and internal margin (IM). The IM compensates for all tissue size and shape variation within the CTV. The SM accounts for daily dosimetric and setup uncertainties related to patient positioning, software, and equipment.
-
-
16.4 Side Effects
-
Acute side effects to evaluate during weekly on-treatment visits
-
Hair loss, fatigue, radiation dermatitis, headaches, nausea, aural fullness, risk of transient edema causing neurological symptoms or resulting in obstructive hydrocephalus.
-
-
Late side effects and complications
-
Temporary or permanent hair loss, injury to the cochlea, causing partial or full hearing loss in one or both ears; hypopituitarism leading to endocrine abnormalities and infertility; neurocognitive decline impacting memory, IQ, and behavior; risk of injury to cranial nerves in the region, which could result in swallowing dysfunction, requiring feeding tube or tracheotomy; injury to the circle of Willis and surrounding vessels, increasing the risk of vasculopathy and stroke; damage to the brainstem and normal brain tissue, which could result in permanent sensory deficit, paralysis, or death; risk of developing secondary malignancies.
-
-
Clinical pearls for addressing those side effects
-
If patients are on steroids at the time of radiotherapy, utilization of gastric prophylaxis (e.g., ranitidine) and evaluation for oral thrush are recommended.
-
Premedicate patients with ondansetron 1 h prior to radiotherapy for prevention of nausea. May need to add a combination of dexamethasone, prochlorperazine, and lorazepam if nausea breaks through prophylaxis.
-
References
ACNS0822 protocol. https://clinicaltrials.gov/ct2/show/NCT01236560
Huguenin M, Trivin C, Zerah M et al (2003) Adult height after cranial irradiation for optic pathway tumors: relationship with neurofibromatosis. J Pediatr 142:699–703
Indelicato DJ, Flampouri S, Rotondo RL et al (2014) Incidence and dosimetric parameters of pediatric brainstem toxicity. Acta Oncol 53:1298–1304
Further Reading
Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG (2015) Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol 33(27):2986–2998
Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA (2009) Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol 27(22):3598–3604
Reddy AT, Wellons JC 3rd (2003) Pediatric high-grade gliomas. Cancer J 9(2):107–112
Youland RS, Khwaja SS, Schomas DA, Keating GF, Wetjen NM, Laack NN (2013) Prognostic factors and survival patterns in pediatric low-grade gliomas over 4 decades. J Pediatr Hematol Oncol 35(3):197–205
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vern Gross, T.Z., Chan, M.D., Lucas, J.T. (2021). Brainstem Glioma. In: Halasz, L.M., Lo, S.S., Chang, E.L., Sahgal, A. (eds) Intracranial and Spinal Radiotherapy . Practical Guides in Radiation Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-64508-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-64508-3_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64507-6
Online ISBN: 978-3-030-64508-3
eBook Packages: MedicineMedicine (R0)