Abstract
Targeted therapy and checkpoint inhibitors have been approved by US Food and Drug Administration (FDA) and European Medical Agency (EMA) for advanced and adjuvant treatment of melanoma. The first checkpoint inhibitor to be approved by FDA and EMA for treatment of melanoma was ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody, based on proven benefit in terms of overall survival and recurrence-free survival in advanced and early disease, respectively. Programmed death-1 (PD-1) checkpoint inhibitor nivolumab was approved in advanced disease in 2014 and for the adjuvant treatment of stage IIIB–IIIC/stage IV melanoma in 2017 on the basis of an improvement in recurrence-free survival versus the active comparator ipilimumab. In this chapter, we review the activity, efficacy, and toxicity of nivolumab and summarize new potential therapeutic strategies, which could potentially expand its spectrum of activity in melanoma.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The first approval of Nivolumab for the treatment of advanced melanoma occurred in December 2014 on the basis of results of either phase I CA209-003 or phase III CA209-037 trials [1, 2]. Thus, the Food and Drug Administration (FDA) approved Opdivo® for pretreated locally advanced or metastatic melanoma patients. From the early indication of Ipilimumab® and Vemurafenib® in 2011, Dabrafenib and Trametinib in 2013, and Pembrolizumab in 2014, Opdivo was the seventh innovative drug approved for the treatment of metastatic melanoma. In 2015, FDA approved the combination of Nivolumab and Ipilimumab in BRAF V600 wild-type unresectable or metastatic melanoma and in the early 2016 for advanced melanoma, independently of the BRAF mutational status. Finally, in December 2017 and July 2018, the FDA and the European Medicine Agency (EMA) extended the Nivolumab indication to the adjuvant setting in melanoma patients with stage III as well as resected with “No Evidence of Disease” (NED).
Based on the results shown by these agents, great efforts in discovering new immune checkpoint inhibitors (ICIs) to ameliorate the overall clinical results for the treatment of melanoma are ongoing in several prospective clinical trials.
Of note, in the wake of enthusiasm generated by the anti-PD1 combination with anti-CTLA4, the association of Nivolumab with other ICIs has been explored in clinical trials, and both LAG3 inhibitors and anti-NTKR agents showed promising results. In particular, the combination with anti-CTLA4 MoAbs produced relevant results in terms of duration and depth of response, although a high incidence of serious adverse events (AEs) has been reported. In this chapter, we review the activity, efficacy, and toxicity of nivolumab in metastatic and adjuvant setting and summarize new potential therapeutic strategies, which could potentially expand its spectrum of activity in melanoma.
Nivolumab in Advanced Disease
The CA209-003 was the first phase I trial testing the tolerability and activity of Nivolumab in advanced solid cancer, followed by phase II Checkmate 172 and 037 and phase III Checkmate 037 and 066, which explored the efficacy of Nivolumab in advanced melanoma [1,2,3,4,5].
Checkmate 003 , a phase I open-label, multicenter, multidose, dose-escalation trial, investigated the safety, clinical activity, pharmacodynamic, and immunologic effect of nivolumab (MDX-1106) in patients with advanced refractory solid tumors [1]. Patients with metastatic melanoma, advanced colorectal cancer (CRC), castrate-resistant prostate cancer, non-small-cell lung cancer (NSCLC), or renal cell carcinoma (RCC) received a single intravenous infusion (i.v.) of MDX-1106 in a dose-escalating program at 0.1–10 mg/kg every 2 weeks up to 96 weeks or until progressive disease, unacceptable toxic effects as well as withdrawal of consent. The primary endpoint of the study was safety and tolerability. An emendation of the study allowed the collection of data concerning the overall survival (OS). The last 5 years’ update [1] included a cohort of 270 patients bearing advanced melanoma (n. 107, 39,6%), NSCLC (n. 129, 47,8%), or RCC (n. 34, 12,6%). In melanoma cohort, 57% and 6.5% of patients experienced any grade or grade 3–4 AEs, respectively. The majority of AEs involved the skin (40.2%), the gastroenteric tract (18.7%), the endocrine system (13.1%), and the liver (8.4%). An objective overall response (ORR) according to the RECISTs criteria was achieved in 31.8% of patients, while a stable (SD) and a progressive disease was reported in 21.5% (23/107) in 38.3% (41/107) of patients, respectively. The 5-year OS was 34.2%, with a median OS of 17.3 months. Poor prognostic factors included liver and bone metastases, whereas good performance status (PS) and development of treatment-related AEs positively impacted on OS. At the median follow-up of 30 months, the progression-free survival (PFS) was 26% in patients receiving Nivolumab at 3 mg/kg. It is noteworthy that many responders (n = 21) who discontinued the drug for any reason, apart from the progression, maintained a response longer than 16 weeks.
The efficacy of Nivolumab in melanoma patients who progressed after Ipilimumab and/or a BRAF inhibitor was tested in Checkmate 037, a phase III randomized trial comparing Nivolumab vs. chemotherapy [2]. Four hundred and five patients were randomly assigned 2:1 to receive Nivolumab (3 mg/kg i.v. every 2 weeks) or investigator’s choice chemotherapy (Dacarbazine, DTIC or Carboplatin and Paclitaxel) and were stratified on the basis of PD-L1 expression, BRAF status, and best response to Ipilimumab. Treatment beyond progression was only allowed in the Nivolumab arm. Primary endpoints were the ORR and OS. The study did not meet its co-primary survival endpoint, Nivolumab showed higher and durable response without any benefit in terms of survival compared to chemotherapy [2]. Median OS was of 15.7 months in the Nivolumab arm and 14.4 months in patients receiving chemotherapy (HR: 0.95; CI: 0.73–1.24), the median PFS was 3.1 months vs. 3.7 months (HR:1.0; CI: 0.78 to 1.436). The ORR was 27% and 10% with nivolumab and chemotherapy, respectively; the median duration of response favored the nivolumab arm (32 months vs 13 months). In addition, a lower rate of grade 3–4 AEs (14% in Nivolumab vs. 34% in the chemotherapy arm) was demonstrated. The ECOG PS, brain metastases, and high LDH levels were associated with poor survival. The reported AEs in the Nivolumab arm involved skin (38%), GI (18%), liver (11%), and endocrine glands (7.8%). The reasons for failing to meet the primary endpoint can be only hypothesized: (1) the high percentage of patients with brain metastases or elevated LDH levels; (2) the crossover to anti-PD1 or anti-PDL1 antibody for patients progressing upon chemotherapy; (3) the higher proportion of patients dropped out after assignment to the chemotherapy arm and before starting chemotherapy.
The efficacy of Nivolumab vs Dacarbazine in the first-line treatment of BRAF wt advanced melanoma patients was investigated in Checkmate 066 trial [3]. This was a randomized phase III, double-blind clinical study that enrolled 418 BRAF wt melanoma patients to receive 1:1 Nivolumab (3 mg/kg every 2 weeks) or Dacarbazine (1000 mg/mq every 3 weeks). Patients with clinical benefit from Nivolumab or without significant AEs could be treated beyond progression. Crossover to Nivolumab was allowed for patients who progressed during Dacarbazine, in an open-label extension phase of the trial. The primary endpoint was the OS while the secondary endpoints were PFS and ORR. The median OS with Nivolumab vs Dacarbazine was 37.5 months vs. 11.2 months, whereas the 3-year OS rate was of 51.2% vs. 21.6%. Median PFS was 5.1 and 2.2 months, respectively, whereas the 3-year PFS rate was 32.2% and 2.9% for Nivolumab and Dacarbazine groups, respectively. The benefit of Nivolumab in terms of mOS was reached regardless of the PD-L1 expression. The secondary endpoint of the study was ORR. Complete and partial responses, respectively, were reported for 19.0% (40 of 210) and 23.8% (50 of 210) of patients in the nivolumab group compared with 1.4% (3 of 208) and 13.0% (27 of 208) of patients in the dacarbazine group.
The benefit was obtained even for patients with brain metastases or elevated LDH. Indeed, a clinical response was observed up to 160 weeks after starting treatment, regardless of therapy discontinuation. In addition, the long-term survival at 3 years was seen independently of the radiological response. Post hoc analysis of OS in patients who discontinued treatment due to disease progression showed that mOS from randomization was 21.5 months in patients treated with Ipilimumab following Nivolumab, 35.4 and 17.4 months for those treated with Nivolumab or Ipilimumab after Dacarbazine, respectively. Among 68 patients who discontinued Nivolumab and received Ipilimumab, 10.3% obtained an OR. Grade 3–4 AEs occurred in 15% of patients treated with Nivolumab and 17.6% of patients in the Dacarbazine arm, whereas no deaths due to AEs were registered.
Based on results of phase III CheckMate 037, the efficacy of Nivolumab has been investigated in Checkmate 172, which enrolled a challenging subgroup of patients excluded from clinical trials: Briefly, CheckMate 172 was a phase II single-arm study that explored the use of Nivolumab in previously treated, unresectable, stage III or IV melanoma (regardless of BRAF mutation status) patients who progressed upon Ipilimumab. Furthermore, patients with different melanoma subtypes, brain metastases, autoimmune diseases, ECOG 2 and previous grade 3–4 immune-related AEs (irAEs) were included [4, 5]. Patients received 3 mg/kg of Nivolumab every 2 weeks up to 2 years. Primary endpoint of the study was the safety; secondary endpoints included (1) incidence of all grade ≥3 not conventional AEs, (2) median time to onset and resolution of grade 3/4 select AEs, and (3) OS. Exploratory endpoints were safety, tolerability, and OS. Among challenging subgroups, patients who experienced an Ipilimumab-related grade 3/4 irAE and those with autoimmune disease showed the longest median OS (21.5 months and 18. months, respectively) without difference with overall population (median OS, 21.4 months). Patients with brain metastases and ECOG PS 2 had a lower median OS (11.6 months and 2.4 months, respectively). The 18-month OS rate was 53.8% in the general population and 42.3%, 18.8%, 59.3%, and 58.2% in patients with brain metastases, ECOG 2, Ipilimumab-related grade 3/4 irAE, and autoimmune disease, respectively. These data confirmed the worse outcome of patients with a poor ECOG PS, although the majority of them harbored additional adverse clinical prognostic factors including brain metastases (27.3%), rare histotypes such as mucosal melanoma (13.6%) and high LDH levels (78.8%).
The 18-month median OS was 25.3 months in non-acral cutaneous melanoma, 25.8 months in acral cutaneous melanoma, 12.6 months in uveal melanoma and 11.5 months in mucosal melanoma patients, while the OS rate was 57.5%, 59%, 34.8%, and 31.5%, respectively, thus confirming the known worse prognosis of mucosal and uveal melanomas. Similar to Checkmate 003, the most common toxicities were cutaneous (26.4% any grade, 1.2% grade 3 or 4), endocrine (16.9% any grade, 1.8% grade 3 or 4), gastrointestinal (13.5% any grade, 1.4% grade 3 or 4), hepatic (8.2% any grade, 2.8% grade 3 or 4), pulmonary (2.2% any grade, 0.5% grade 3 or 4), and renal (1.7% any grade, 0.1% grade 3 or 4). Patients with ECOG 2 and autoimmune disease experienced toxicities at gastrointestinal tract and endocrine system. Interestingly, this analysis produced relevant insights for the management of patients who experienced immune-related AEs following the treatment with ipilimumab, but also of those with a concomitant autoimmune disease. The first subgroup had a lower incidence of grade 3/4 AEs as compared to the general population (11.9% versus 18.2%), the latter showed limited grade 3–4 AEs and, interestingly, the OS that resulted similar to the overall population, thus suggesting that these patients can be safely treated with anti-PD1.
Although subgroup analyses of CheckMate 066 and CheckMate 172 provided a proof of principle of Nivolumab activity as monotherapy in patients with brain metastasis, the most robust data on the activity as a single agent or in combination derive from the phase 2 CheckMate 204 study and the anti-PD-1 Brain Collaboration (ABC) Trial, a phase II trial of Nivolumab Plus Ipilimumab or Nivolumab alone in patients with melanoma brain metastases [6, 7] .
In the ABC study, 60 were asymptomatic, and, of these, 35 received a combination of nivolumab and ipilimumab (cohort A) and 25 received nivolumab monotherapy (cohort B). Sixteen patients who had failed local therapy or were neurologically symptomatic and/or had leptomeningeal disease (LMD) received nivolumab monotherapy (cohort C). Intracranial responses were achieved in 51%, 20%, and 6%, and 12-month OS was 63%, 60%, and 31% in cohorts A, B, and C, respectively. The intracranial OR in cohort A was 59% for treatment-naive patients and 25% in patients previously treated with BRAF inhibitors. However, ABC was not designed/powered to be comparative between treatment arms [6].
The safety and efficacy of nivolumab and ipilimumab was also evaluated in CheckMate 204. In the most recent update, 119 patients had been treated: 101 patients with asymptomatic MBMs and 18 patients with either symptomatic MBMs or who were receiving up to 4 mg of oral dexamethasone. The intracranial RR was 54% in patients with asymptomatic MBMs, including CRs in 29%. The 6-month intracranial PFS was 63% and the median PFS was not reached. In patients who were either symptomatic or requiring steroids at the time of treatment initiation, the RR was 22%, although only 1/11 patients (9%) receiving steroids experienced a response [7, 8].
Efficacy of Ipilimumab Plus Nivolumab Regimen
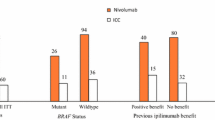
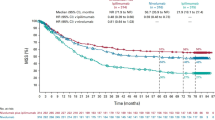
CheckMate 067 is one of the most relevant trials evaluating the combination of ICIs. The trial was designed to compare the combo regimen vs Ipilimumab but was underpowered to compare the combo regimen with Nivolumab. Patients were randomized to receive one of the following strategies: (1) Nivolumab plus Ipilimumab for four doses followed by Nivolumab alone, (2) Nivolumab alone or (3) Ipilimumab alone [9]. The median OS at 60 months was longer than 60 months (median not reached), 36.9 months, and 19.9 months, in the Nivolumab/Ipilimumab, Nivolumab alone, and Ipilimumab alone cohorts, respectively. With regard to the landmark analysis, the 5-year OS was 52%, 44%, and 26%, in the Nivolumab/Ipilimumab, Nivolumab alone and in the Ipilimumab cohorts, respectively. The ORR was 58% in the Nivolumab/Ipilimumab group, 45% in the Nivolumab group, and 19% in the Ipilimumab group with 22%, 19%, and 6% of CR, respectively. This advantage was observed in all subgroups, independently of BRAF mutation status, PD-L1 expression as well as extension of disease. In addition, patients who discontinued treatment early for an AE had a survival benefit almost similar to the overall population [10]
Ongoing Promising Clinical Trials
Results of CA224-020, a phase 1/2 study evaluating Relatlimab (LAG3 inhibitor) with and without Nivolumab for the treatment of solid tumors were presented at ESMO 2017 [11]. The lymphocyte activation gene 3 (LAG3) is an additional target of ICI that negatively regulates the activity of effector T cells. Based on the dual inhibition of the LAG3 and PD1 activity exerted by Relatlimab and Nivolumab, a signal of potential clinical benefit in previously treated metastatic or unresectable melanoma patients resistant to an anti-PD(L)1 MoAb has been demonstrated. The ORR was 11.5%, the disease Control Rate (DCR) 49%, with a safety profile similar to Nivolumab alone, while the response rate was higher in patients with LAG-3 expression ≥1%. Based on these results, a randomized, double-blind, phase II/III study of Relatlimab in combination with Nivolumab versus Nivolumab alone in previously untreated metastatic or unresectable (CA224-047) melanoma is actually ongoing
Bempegaldesleukin (NKTR-214) is a CD122-preferential IL-2 pathway agonist able to increase tumor-infiltrating lymphocytes, T-cell clonality, and PD-1 expression. Safety and tolerability of NKTR-214 was proven in PIVOT-02 phase 1/2 trial. After over 18 months of follow-up, in untreated advanced melanoma, NKTR-214 plus Nivolumab showed clinical activity, with an ORR of 53%, and a remarkable 34% of CR, independently of PD-L1 expression [12].
The PIVOT IO 001, a phase III, randomized, open-label study of NKTR-214 plus Nivolumab vs Nivolumab monotherapy in patients with untreated advanced melanoma is still ongoing (NCT03635983).
Nivolumab in Adjuvant Setting
Adjuvant therapy is actually considered the best option for stage III melanoma patients in order to reduce the risk of recurrence. Recently, ipilimumab at 10 mg/kg showed a significant improvement in terms of RFS and OS in stage III melanoma compared to placebo but with a high incidence of severe irAEs [13]. Moreover, new options have emerged and the results from the CheckMate 238 and the IMMUNED trials have been reported [14, 15].
The CheckMate 238 trial investigated the efficacy of Nivolumab vs Ipilimumab in stage IIIB/C and radically resected stage IV melanoma patients. Patients were randomly assigned 1:1 to receive Nivolumab 3 mg/kg every 2 weeks or Ipilimumab 10 mg/kg every 4 weeks for 4 doses and every 12 weeks thereafter for 1 year or less or until disease recurrence or unacceptable toxicity. The primary endpoint was recurrence-free survival (RFS), and exploratory endpoints included distant metastasis-free survival (DMFS) and OS. Furthermore, exploratory analyses included predictive biomarkers of outcome. At a median follow-up of 36 months, the Nivolumab arm had better RFS compared with the Ipilimumab arm (hazard ratio [HR] = 0.68; 95% confidence interval [CI] = 0.56–0.82, p < 0.0001). The 3-year RFS rate was 58% vs 45% with Nivolumab and Ipilimumab, respectively. Distant metastasis-free survival was improved in the Nivolumab arm as well (HR = 0.78; 95% CI = 0.62–0.99). Surrogate analyses suggested that a high tumor mutational burden, the interferon-gamma gene expression signature, as well as a high infiltration of CD8+ T cells and low density of myeloid-derived suppressor cell correlate with improved RFS in both arms. The majority of first-occurrence treatment-related adverse events (TRAEs) with adjuvant Nivolumab occurred early during treatment (0–3 months) while rarely (<2.5%) after the last dose. Almost all TRAEs were solved within 6 months. No association was observed between early TRAEs and RFS [16].
With regard to the radically resected stage IV disease, the IMMUNED study has been recently reported [15]. The IMMUNED was a phase II randomized clinical trial evaluating Nivolumab/Ipilimumab vs single-agent Nivolumab vs placebo, in patients with radically resected or irradiated stage IV disease and considered to be at high risk for recurrence. This was the first prospective randomized placebo-controlled clinical study in patients with stage IV melanoma without evidence of disease. Overall, 167 patients with high-risk stage IV disease were randomly assigned to Nivolumab at 3 mg/kg (with maintenance Nivolumab), Nivolumab at 1 mg/kg plus Ipilimumab at 3 mg/kg (with maintenance Nivolumab) or placebo. Notwithstanding the limited median time of treatment with the combination therapy (6.4 weeks), it yielded a 2-year recurrence-free survival rate of 70%, which compared favorably with Nivolumab (42%) or placebo (14%). Relapses occurred in 81% of the placebo arm, 56% of the Nivolumab arm, and 27% of the combination arm. Distant relapses were reported in 44%, 39%, and 14%, respectively. Nivolumab alone was better tolerated than the combo regimen, with lower incidence of grade 3 or 4 AEs and less toxicity leading to treatment discontinuation. The combination of ICIs has been further evaluated in the phase III CheckMate-915 study. This trial included 1943 patients with IIIB/C and radically resected stage IV melanoma patients.
Participants were treated with 240 mg of intravenous Nivolumab every 2 weeks and 1 mg/kg Ipilimumab every 6 weeks or 480 mg of Nivolumab every 4 weeks for 12 months. Results are not still available, preliminary data suggest modest RFS benefit with the combination in patients with PD-L1 levels lower than 1% [17].
Nivolumab in the Neoadjuvant Setting
The outcome of patients with palpable node, locally advanced stage III melanoma still remains poor. The neoadjuvant approach is well suited for melanoma: prototype tumor for drug development, there is accessible tissue, and provides rapid results. Nevertheless, some caveat should be considered including patients not responding might deteriorate losing the opportunity of curative surgery, irAEs might hamper surgery, neoadjuvant therapies require more patient management, timing scans, day clinic appointments, and surgery planning. Nivolumab alone or in combination with ipilimumab has been investigated in four major clinical trials [18,19,20,21]. Amaria et al. reported results from a randomized phase 2 study of neoadjuvant nivolumab versus combined ipilimumab with nivolumab in 23 patients with high-risk resectable melanoma. Treatment with combined ipilimumab and nivolumab yielded high response rates (RECIST ORR 73%, pCR 45%) but substantial toxicity (73% grade 3 trAEs), whereas treatment with nivolumab monotherapy yielded modest responses (ORR 25%, pCR 25%) and low toxicity (8% grade 3 trAEs). Immune correlates of response were identified, demonstrating higher lymphoid infiltrates in responders to both therapies and a more clonal and diverse T-cell infiltrate in responders to nivolumab monotherapy. Blank et al. reported the results of the OpACIN trial, a randomized phase Ib trial [22]. A total of 20 patients with palpable stage III melanoma were randomized to four cycles of adjuvant or neoadjuvant Ipilimumab 3 mg/kg plus Nivolumab 1 mg/kg treatment (two cycles before and after surgery). In the neoadjuvant arm, all patients underwent complete lymph node dissection after at least one course of neoadjuvant therapy. One patient in the adjuvant arm discontinued therapy due to disease progression. The other patients discontinued the therapy due to development of grade 3/4 AEs. Nine of ten patients in the neoadjuvant arm were evaluated for a pathologic response, and seven of them achieved a response: three patients obtained a pCR, three patients achieved “near” pCR, defined as ≤10% of viable tumor cells; one patient experienced a partial pathologic response (pPR), defined as ≤50% viable tumor cells. Two patients without PR relapsed. At a median follow-up of 21.6 months in this group, none of the seven patients with a PR relapsed. In terms of AEs, 90% patients stopped therapy due to grade 3/4 AEs. In relation to the severe toxicity of the standard Ipilimumab plus Nivolumab dosing schedule, the OpACIN-neo-trial was designed to identify a less toxic dosing schedule of Ipilimumab plus Nivolumab. Patients had resectable stage III melanoma only involving lymph nodes, and measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Patients were randomly assigned (1:1:1) to one of three neoadjuvant dosing schedules: group A, two cycles of Ipilimumab 3 mg/kg plus Nivolumab 1 mg/kg once every 3 weeks intravenously; group B, two cycles of Ipilimumab 1 mg/kg plus Nivolumab 3 mg/kg once every 3 weeks intravenously; or group C, two cycles of Ipilimumab 3 mg/kg once every 3 weeks directly followed by two cycles of Nivolumab 3 mg/kg once every 2 weeks intravenously. The primary endpoints were the proportion of patients with grade 3–4 irAEs within the first 12 weeks and the proportion of patients achieving a radiological objective response and PR at 6 weeks. Patients were enrolled and randomly assigned to one of the three groups: 30 patients in group A, 30 in group B, and 26 in group C. During the first 12 weeks, grade 3–4 irAEs were observed in 12 (40%) of 30 patients in group A, six (20%) of 30 in group B, and 13 (50%) of 26 in group C. The difference in grade 3–4 toxicity between groups B and A was 20% (95% CI −46 to 6; p = 0·158) and between groups C and A was 10% (−20 to 40; p = 0·591). The most common grade 3–4 AE was the increase in liver enzymes in group A and colitis in group C. A patient in group A died 9.5 months after starting the treatment because of late-onset immune-related encephalitis. Nineteen (63%) of 30 patients in group A, 17 (57%) of 30 in group B, and nine (35%) of 26 in group C achieved a radiological objective response, while PR occurred in 24 (80%) patients in group A, 23 (77%) in group B, and 17 (65%) in group C. With regard to immune biomarkers, subgroup analyses revealed IFN-gamma signature and TMB correlated with response while PDL-1 expression did not. Moreover, low bacterial alpha diversity was associated with severe irAEs and poor anti-melanoma responses. In addition, similarly to the Opacin trial, the neoadjuvant immunotherapy led to a greater expansion of tumor-resident T-cell clones in the peripheral blood as compared with adjuvant treatment [23]. Immune signature analysis demonstrated that patients with a high IFN/T-cell/BATF3 signature had a better clinical outcome [24,25,26] as well as high/intermediate IFN signatures identified patients showing long-term responses.
Based on these evidences, OpACIN-neo identified a tolerable neoadjuvant dosing schedule (group B: two cycles of Ipilimumab 1 mg/kg plus Nivolumab 3 mg/kg) that gains a PR in a high proportion of patients. However, the optimal duration of neoadjuvant therapy is not yet standardized (Identification of the Optimal Combination Dosing Schedule of Neoadjuvant Ipilimumab Plus Nivolumab in macroscopic stage III melanoma [20]. Very recently, the PRADO trial has been designed in an attempt to reduce the extension of surgery and tailor the adjuvant strategy according to the response to the neoadjuvant approach [21].
Several neoadjuvant studies in melanoma are ongoing or planned to be started in the near future. A randomized phase II trial will investigate the activity of Nivolumab with or without Ipilimumab or Relatlimab (anti-LAG-3 monoclonal antibody) before surgery in patients with resectable stage IIIB–IV melanoma (NCT02519322). Immunotherapy with Nivolumab, Ipilimumab or Relatlimab may restrain the ability of the immune system to counterattack tumor cell growing and spreading. Upfront treatment with Nivolumab alone or in combination with Ipilimumab or Relatlimab might reduce the tumor size and limit the removal of normal nearby tissue. Moreover, a pilot phase I trial study is exploring VX15/2503 (Pepinemab) with or without Ipilimumab and/or Nivolumab for treatment of inoperable stage IIIB–D melanoma patients (NCT03769155). Another ongoing study is a phase II neoadjuvant trial of Nivolumab in combination with HF10 oncolytic viral therapy in resectable stage IIIB, IIIC, and IVM1a melanoma (Neo-NivoHF10) (NCT03259425).
References
Topalian SL, Hodi FS, Brahmer JR, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5:1411–20.
Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36:383–90.
Ascierto P, Long GV, Robert C, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019;5(2):187–94.
Schadendorf D, Ascierto PA, Haanen J, et al. Safety and efficacy of nivolumab in challenging subgroups with advanced melanoma who progressed on or after ipilimumab treatment: a single-arm, open-label, phase II study (CheckMate 172). Eur J Cancer. 2019;121:144–53.
Nathan P, Ascierto PA, Haanen J, et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: a single-arm, open-label, phase II study (CheckMate 172). Eur J Cancer. 2019;119:168–78.
Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–81.
Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and Ipilimumab in melanoma metastatic to the brain. NEJM. 2018;379(8):722–30.
Tawbi HA, Forsyth PA, Hodi S, et al. Efficacy and safety of the combination of nivolumab (NIVO) plus ipilimumab (IPI) in patients with symptomatic melanoma brain metastases (CheckMate204). J Clin Oncol. 2019;37(15_suppl):9501.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–46.
Schadendorf D, Wolchok JD, Hodi S, et al. Efficacy and safety outcome in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35(34):3807–14.
Ascierto P, Bono P, Bhatia S, et al. Efficacy of BMS-986016, a monoclonal antibody that targets LAG-3, in combination with Nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (MEL PRIOR IO) in all-comer and biomarker-enriched populations. Ann Oncol. 2017;28:605–49.
Adi D, Tannir NM, al BSE. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov. 2020;10:1–16.
Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. NEJM. 2016;375:1845–55.
Weber J, del Vecchio M, Mandala M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III/IV melanoma: 3-year efficacy and biomarker results from the phase 3 CheckMate 238 trial. Ann Oncol. 2019;30(S5):v533–63.
Zimmer L, Livingstone E, Hasse JC, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;395(10236):1558–68.
Mandalà M, Larkin J, Ascierto PA, et al. An analysis of nivolumab-mediated adverse events and association with clinical efficacy in resected stage III or IV melanoma (CheckMate 238). J Clin Oncol. 2019;37(15_suppl):9584.
Bristol-Myers Squibb Announces Update on CheckMate -915 for Opdivo (Nivolumab) Plus Yervoy (Ipilimumab) Versus Opdivo Alone in Patients with Resected High-Risk Melanoma and PD-L1 <1% [press release] 2019. Princeton, New Jersey, November 20.
Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–54.
Rozeman EA, Fanchi L, van Akkooi ACJ, et al. (Neo-)Adjuvant Ipilimumab + Nivolumab (Ipi + Nivo) in palpable stage 3 melanoma - updated relapse free survival (RFS) data from the Opacin trial and first biomarker analyses. Ann Oncol. 2017;28:428–48.
Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–60.
Blank CU, Pennington T, Versluis JM, et al. First safety and efficacy results of PRADO: a phase II study of personalized response-driven surgery and adjuvant therapy after neoadjuvant ipilimumab (IPI) and nivolumab (NIVO) in resectable stage III melanoma. J Clin Oncol. 2020;38(15_suppl):10002.
Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–61.
Batten M, Shanahan ER, Silva IP et al (2019) Low intestinal microbial diversity is associated with severe immune-related adverse events and lack of response to neoadjuvant combination antiPD1, antiCTLA4 immunotherapy [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019; Atlanta (GA), Mar 29–Apr 3 AACR, Cancer Res 2019;79:abstract 2822.
Spranger S, Dai D, Horton B, et al. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31(5):711–23.
Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5.
Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blokade. J Clin Investig. 2017;127(8):2930–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Stucci, L.S., Todisco, A., Mandalà, M., Tucci, M. (2021). Nivolumab in Melanoma: An Overview of Medical Literature and Future Perspectives. In: Rutkowski, P., Mandalà, M. (eds) New Therapies in Advanced Cutaneous Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-030-64009-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-64009-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64008-8
Online ISBN: 978-3-030-64009-5
eBook Packages: MedicineMedicine (R0)