Abstract
A noteworthy advancement through nanotechnological intervention has been noticed in every sphere of life, including pharmaceutical industry and consumer products. Despite its tremendous benefits, the indiscriminate utilization of nanomaterials in marketed products and their ensuing release into the ecosystems spur serious concern and have potential adverse environmental impacts. However, very little is known on environmental toxicity and risk modeling for nanomaterial emissions to the environment and little or no data exist on reliable quantitative measurements of nanomaterials at actual release concentrations.
In this context, the present work aims to compile and present recent advances, potential hazards and risks to the environment as well as regulatory background of engineered nanomaterials. As many issues regarding the bioavailability, uptake, and the life cycle assessment remain to be explored, we herein highlight and discuss the progress and updates on research of toxicity of engineered nanomaterials used, highlighting the pressing need within the field of econanotoxicity. In addition, grey areas, challenges, and tentative directions for the way forward are suggested.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
With the advent of nanotechnology which is of widespread significance, exponential developments have been observed in science and industries like pharmaceuticals, cosmetics, foods, textile, electronics, etc. (Guzmán et al. 2006). Nanoparticles (NPs) or nanomaterials (NMs) are defined as natural or man-made substances that exist in singly or as aggregated/agglomerated form within the range of 1–100 nm (number size distribution in at least one of the dimensions with 50% or more of the particles), along with a volume-specific surface area of at least 60 m2 cm−3 (EU Commission 2011; Loureiro et al. 2018). More often, nanoparticles are found naturally but their extensive commercial use have put forth the synthetic production of these particles for various tailor-made applications with unique optical, electronic, chemical, biological, and mechanical properties and are termed as engineered nanoparticles (ENPs). Globally, numerous ENP-based products are available for healthcare, energy, and environmental applications (Goswami et al. 2017). Since 2000, the global market value of ENPs has increased from US$ 125 million to US$ 7.3–12.7 billion in between 2008–2016. It is slated to reach approximately between US$ 11.8–16.8 billion by 2022–2025 (Lai et al. 2017; He et al. 2018).
This escalating production and applications of these ENPs results in their exposure in the environmental media and interacts with various trophic levels of the ecosystems. Presently, around 63–91% of ENPs are disposed in landfills while the remaining are being released into atmosphere (0.1–1.5%), soils (8–28%), and water bodies (0.4–7%) (Keller et al. 2013). Thus, despite multifaceted benefits for commercial purpose, their presence may cause hazardous biological effects in the nature. The unique properties of these nanoparticles leading to detrimental effect in environment mainly comprises of (i) high specific surface area, (ii) sufficient reactive sites on the surface, and (iii) their easy mobility (Wiesner et al. 2006). In this direction, researchers have reported the interactions of nanoparticles with living organisms and little, if any, information is available on the fate and behavior of these nanoparticles within the environment and on human health (Handy et al. 2008). Thus, to narrow the scope of this review, the present chapter aims to emphasize the widespread contamination of the environment due to nanoparticles manufacturing and waste disposal, and highlights the importance of econanotoxicity of engineered nanomaterials to the waste management community.
11.2 Naturally Occurring and Engineered Nanoparticles
With increased anthropogenic activities along with the technological advancements, nanoparticles generate enormous waste materials contaminating the biosphere and pose serious ecological risks. However, nanoparticles still existed and leached into the environment even before the formal emergence of the field of nanotechnology . Naturally occurring nanoparticles are ubiquitous in nature. Several geological processes are known to produce natural nanoparticles such as in the form of combustion by-product, automobile exhaust, aerosols, and volcanoes (Bystrzejewska-Piotrowska et al. 2009). Further, in biological processes, biomolecules like protein, nucleic acids, ATP, membranes, cells, organelles, etc. are directly released into the environment from the organisms, leading to the formation of nanoparticles as a result of degradation of biological matters (Bhatt and Tripathi 2011). However, many of these natural and incidental nanomaterials also have certain distinctive characteristics that cannot be denied from an environmental chemistry perspective (Bernhardt et al. 2010).
Unlike the naturally occurring nanoparticles that are formed heterogeneously and disseminated in the environment, ENPs are mostly homogeneous in terms of size, shape, and structure. The two approaches for the production of ENPs are top-down and bottom-up fabrication method (Bhatt and Tripathi 2011). In the first method, lithographic techniques cut large materials into sizes less than 30 nm. Alternatively, macromaterial are ground in a ball mill for producing NPs having size less than 30 nm (Borm et al. 2006). In contrast, bottom-up synthesis process is a more suitable method to convert extremely small molecules or atoms to nanometer level (Christian et al. 2008). The diameter-tuning of nanoparticles is especially imperative and is regulated with media in which they are synthesized. While temperature and reaction time are important within the realms of wet-phase synthesis protocol, precursor concentration, as well as reaction temperature, controls the diameter of ENPs in gas phase. Moreover, dispersing additives are used to stop aggregation of the synthesized nanoparticles during mechanical milling; they comprise a film or coat throughout the NPs to prevent aggregation (Borm et al. 2006). However, the unique qualities of the ENPs result in new chemical reactions, thereby making the prediction of its environmental impact and fate more difficult which in turn calls for significant multidisciplinary advances to know about their impacts (Wiesner et al. 2006; Handy et al. 2008).
11.3 Different Classes of Engineered Nanoparticles
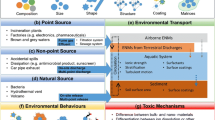
As discussed previously, the NPs relevant in the environment can be categorized into natural and engineered nanoparticles . The ENPs are further categorized into various classes, including (i) carbonaceous nanomaterials (fullerene compounds, nanotubes, nanowires, etc.), (ii) metal oxides [bismuth trioxide (Bi2O3), chromium dioxide (CrO2), cerium dioxide (CeO2), molybdenum trioxide (MoO3), titanium dioxide (TiO2), zinc oxide (ZnO)], and binary oxides, (iii) semiconductor materials [quantum dots (QDs)], (iv) zero-valent metals [ferric (Fe3+) or ferrous, dissolution of the metal salt and its reduction to the zero-valent state, etc.], and (v) nanopolymers (dendrimers, liposomes, etc.). Figure 11.1 gives an overview of the nanoparticles and their distribution in the environment.
Of carbonaceous nanomaterials, the first class of fullerenes (C60-atom hollow sphere) originated in 1985. They are naturally non-ionogenic but gain charge under selective conditions that possess a negative zeta potential and shows optical, elastic, mechanical, and thermal properties (Brant et al. 2005). Further, in 1991, the carbon nanotubes (CNTs), which are the cylindrical fullerene derivative, were synthesized. Sheets of carbon atoms are linked covalently to form one-dimensional hollow cylindrical shape (Smart et al. 2006). CNTs are of two distinct types, namely, single-walled nanotubes (SWNTs) and multiwalled nanotubes (MWNTs). The structure of SWCNTs can be visualized as single-layered graphene sheets that are wrapped up into seamless cylinder. In MWCNTs, two or more concentric layers of graphene sheets with different length and sizes are found (Cao 2004). CNTs and fullerenes find their application in various sectors like medical, plastics, catalysts, fuel electrodes, electrochemical capacitors, wastewater purification system, sensing appliance, etc. (Klaine et al. 2008).
Another type of ENPs comprises of metal-containing materials like metal oxides and binary oxides. The two common methods of their preparation are precipitation with stabilization and flame pyrolysis (Christian et al. 2008). In metal oxides, crystalline TiO2 is an excellent band-gap semiconductor that has a large energy gap of 3.2 eV (Bellardita et al. 2007; Klaine et al. 2008; Lihitkar et al. 2007; Reijnders 2008). Another example of the same class is ZnO which finds application in cosmetics due to a band-gap energy of 3.36 eV, and high dielectric constant (Singh et al. 2007; Christian et al. 2008).
Quanta dots (QDs) semiconductors with nanocrystalline diameter (2–10 nm) possesses unique magnetic and catalytic properties and constitute the third class of ENPs (Schmid 2004). Examples include core type, core-shell type, or alloyed QDs like chalcogenides of metals (Murray et al. 2001; Logothetidis 2006). They are widely used in experimental medicines, attached to surface ligands or introduced into live organisms for intracellular in vivo analysis, biomedical imaging, targeted therapeutics, etc. (Alivisatos et al. 2005; Roszek et al. 2005; Logothetidis 2006; Klaine et al. 2008).
Nanoscale zero-valent metals that are generally prepared by the reduction of metal salts are also widely used. One such example is the synthesis of zero-valent iron by reducing the ferric (Fe3+) or ferrous (Fe2+) salts with a sodium borohydride (NaBH4). Also, gold and silver NPs are synthesized chemically through metal or metallic salt dissolution in a suitable solvent to reduce them to the zero-valent state (Li et al. 2006). Further, these NPs exhibit unique optical properties called as surface plasmon resonance (SPR) (Noguez 2007).
The last class of ENPs is dendrimers, defined as a complex, highly branched polymers of 1–10 nm diameter. They are asymmetrical and are transformed into globular forms with increase in branching (Caminati et al. 1990). During synthesis of dendrimers, in a process of emulsion polymerization, ammonium per sulfate is used for initiating free radical polymerization. For example, an aqueous emulsion of monomer like styrene or methyl acrylate is prepared using water and sodium dodecyl sulfate or a sulfonate as a surfactant (Shim et al. 2004). Their diverse applications range from biomedicine to surface modification. Some uses of common ENPs are enlisted in Table 11.1.
11.4 Engineered Nanomaterials in Pharmaceuticals: Biological and Environmental Interactions
The introduction and use of nanotechnology in the pharmaceutical industry exhibited remarkable potential and remarkable efforts are in progress worldwide to fulfill the promise of the nanorevolution. Previously, nanotherapies were mainly used as vaccine or cancer therapy, whereas recent trends toward engineering nanomaterials as personalized medicine for the prevention of diseases by employing nanotherapeutics with other advanced nanotechnologies, such as nanobots and nanodevices (Goswami et al. 2017).
The intervention dates back to 1930 when first nanoscale iron colloidal preparation was administered in human. It has been reported that currently there are 43 approved drug formulations commonly referred as nanomedicines, and approximately 789 clinical trials are ongoing pertaining to 25 devices and 122 therapeutics (Weissig et al. 2014; Weissig and Guzman-Villanueva 2015). Milled nanocrystals and liposomes were the first-generation products that used nanomaterials to enhance bioavailability or drug exposure at action sites, respectively for poorly water-soluble drugs. TRICOR® is an example that contains active ingredient (Fenofibrate) crystals milled into the nanosize range (Tyner et al. 2015). The first approved new drug application (NDA) was Gris-PEG (griseofulvin ultramicro size, <1000 nm) that targeted treatment of fungal infections. Further, the first US FDA-approved nanotechnology-enabled product was Doxil® nanodrug (stealth liposomes encapsulating about 10,000 doxorubicin molecules) that came into being in 1995 for treating AIDS-related Kaposi’s sarcoma. Numerous unique features such as (i) increased biodistribution, (ii) enhanced targeting, and (iii) potential of stimuli-sensitive microenvironments payload release facilitated the development of nanotherapeutics of huge antibody–drug conjugates, small-molecule platforms, polymeric nanoparticles , albumin nanoparticles , metal-based nanoformulations, etc. In vaccine therapy, virosomes (e.g., InflexalV® and Epaxal®), consisting of unilamellar phospholipid membrane nanovesicles integrating virus-derived glycoproteins (100–150 nm) are considered an efficient delivery system. In viral gene therapy, the European Medicines Agency (EMA) in 2012 approved the first product for lipoprotein lipase deficiency that used adeno-associated virus (AAV), allowing stable gene transfer and enduring transgene expression. Various other products, such as aprepitant, fenofibrate, megasterol acetate, and rapamycin are being marketed using the NanoCrystal® or the DissoCube® technology.
The ever-increasing usage of engineered nanomaterials in pharmaceuticals has provoked scientific community to question their possible negative effect on ecology and animal health. Moreover, the unique properties of engineered nanomaterials make them highly reactive (chemically and biologically), able to interact with the neighboring matters including biological organisms as well as the environmental components that results in toxicity as a result of biological and environmental interactions.
11.5 Physicochemical Properties of Engineered Nanomaterials and Their Toxicity
The indispensable application of the ENPs in different sectors including pharmaceuticals results in their dissemination into the environment. In fact, the very similar properties that direct toward the scientific and technical benefits of nanotechnology also result in exclusive biological effects. Thus, it is imperative to execute physicochemical characterization of engineered nanoparticles like size, shape, structure, surface charge, composition, crystallinity, aggregation, concentration, etc. These properties play significant role in the interaction of the ENPs with the cells thereby leading to toxicity (Fig. 11.2). Hence, the toxicity of the nanomaterials with respect to some of the important physicochemical properties are enlisted here.
11.5.1 Effect of Particle Size
The toxicity of nanomaterials is dependent on its size which in turn is dependent on its capability to move into the biological systems and their modification of structures, thereby interfering with critical biological functions (Lovrić et al. 2005; Aggarwal et al. 2009). Li et al. (2015) suggested that the size of nanoparticles plays a critical role in cellular uptake, efficient processing of particle in the endocytic pathway as well as physiological response of cells to nanoparticles (Li et al. 2015). Various researchers have highlighted the fact that one of the key mechanisms leading to in vivo toxicity of the ENPs is generating oxidative responses due to the formation of free radicals where size has a pivotal role to play. The generated free radicals affect the biological systems mainly through DNA damage, lipid peroxidation, and inflammatory responses. Particles with size below 1 μm enter into cells whereas when the particles are >1 μm, the nanoparticles will react with cells through the formation of certain proteins on their surface. Park et al. (2011) compared the various toxicity effects of variable sized silver (Ag) nanoparticles (Park et al. 2011). They inferred that for all toxicity endpoints, 20 nm Ag nanoparticles were more toxic than the larger counterparts. However, when compared with Ag ions, Ag particles with size above 20 nm were found to be less toxic than Ag ions. On the contrary to the assumption that small-sized nanoparticles enter the cells more easily causing damages, Yin et al. (2005) witnessed the in vitro effects of particle size on the cytotoxicity of nickel ferrite in Neuro-2A cell line and concluded that the cytotoxicity was independent of the particle size (Yin et al. 2005). Thus, it can be inferred that the mechanism of nanoparticle-mediated toxicity is complicated and size cannot be regarded as the only influential parameter.
11.5.2 Effect of Shape and Structure
Apart from size, toxicity is also dependent on the shape and structure and has been reported for myriads of nanoparticles . Difference in shapes and structure of nanomaterials like planes, spheres, fibers, tubes, polyhydra, etc. often results in alterations in their toxicity . In in vivo, membrane-wrapping processes during endocytosis or phagocytosis are influenced by these ENPs. Endocytosis of spherical nanoparticles is reported to be faster and comparatively less toxic when compared to that of rod or fiber-shaped nanoparticles . Nonspherical nanomaterials more likely flow through capillaries causing other biological consequences (Gatoo et al. 2014). Zhang et al. (2010) conducted a compared toxicity of graphene and carbon nanotubes and found an induction of concentration and shape-dependent cytotoxic effects (Zhang et al. 2010). Moreover, even at low concentrations, graphene induced a stronger metabolic activity emphasizing the effect of shape on cellular toxicity .
11.5.3 Effect of Surface Charge
Surface charge plays a crucial role in toxicity of ENPs as they interact with the biological systems. Surface charge primarily regulates (i) selective adsorption of nanoparticles , (ii) colloidal behavior, (iii) plasma protein binding, (iv) blood–brain barrier integrity, and (v) transmembrane permeability. Mostly, positively charged NPs show enhanced opsonization as well as induce hemolysis and platelet aggregation in comparison to negatively charged and neutral nanoparticles (Goodman et al. 2004). For example, positively charged Si nanoparticles (Si–NP–NH2) are more cytotoxic in comparison to neutral and negatively charged ones (Bhattacharjee et al. 2010).
11.5.4 Effect of Composition and Crystalline Structure
Toxicity is also influenced by the composition and crystalline structure of nanoparticles . It has been observed that soluble forms of silver and copper nanoparticles triggered toxicity in various tested organisms like zebrafish, daphnids, and algal species, whereas TiO2 of the same dimensions did not cause any toxicity . Thus, compositions of NPs are integral in determining the toxicities (Griffitt et al. 2008). Regarding crystal structure, it has been observed that rutile TiO2 nanoparticles induce lipid peroxidation, oxidative DNA damage, and micronuclei formation in the absence of light when compared to the anatase nanoparticles having similar size and chemical composition (Gurr et al. 2005).
11.5.5 Effect of Aggregation and Concentration
Aggregation and concentration can be regarded as the final aspects regarding the toxicity of nanomaterials. Among others, the aggregation of ENPs is mainly dependent on the size, surface charge, and composition. Thus, carbon nanotubes induce cytotoxic effects due to accumulation of aggregates for long span of time (Yang et al. 2008). Further, the pulmonary interstitial fibrosis is enhanced by agglomerated carbon nanotubes than well-dispersed carbon nanotubes (Wick et al. 2007). Regarding the effect of concentration, generally, increase in the nanoparticles concentration leads to decrease in toxicity at higher concentration (Gatoo et al. 2014). Santos et al. (2010) reported that the nontoxic threshold concentration for thermally hydrocarbonized and carbonized porous silicon particles was toxic at 2 mg mL−1, whereas for thermally oxidized porous silicon particles, it was 4 mg mL−1 (Santos et al. 2010).
11.6 Ecological Accumulation of Engineered Nanoparticles
There are predominantly three aspects that need to be taken care of while evaluating the impact of engineered nanomaterials in the environmental matrix: (i) their mobility (movement along with transfer) from one place to another or from one recipient to another (for example, from soil to drinking water or food plants), (ii) the possible ecotoxicity to living organisms in aqueous environment, sediments and soils that they likely come into contact, and (iii) to what extent engineered nanomaterials are altered once they are exposed in the environment along with the mechanism behind it. Organisms undergo several routes of exposure to pollutants leading to their uptake. Some of the relevant routes and endpoints are bioavailability, bioconcentration, bioaccumulation, and biomagnification. Table 11.2 enlists some existing and representative biological accumulation studies of synthesized engineered nanomaterials using most commonly used organisms and ecologically relevant contact conditions.
11.6.1 Bioavailability
According to Peijnenburg (2015), bioavailability is the chemical fractions which is accessible or made accessible for uptake causing positive or negative effects in organisms (Peijnenburg et al. 2015). In addition, Ortega-Calvo et al. (2015) defined bioavailability as the component that includes the dissolved fractions of a chemical in soil, whereas bioaccessibility comprises the fraction which may be bioavailable in the long term (Ortega-Calvo et al. 2015). Gaiser et al. (2012) viewed bioavailability in terms of nutritional efficiency, that is, the portion that is taken up, incorporated and utilized for storage and metabolism (Gaiser et al. 2012). The bioactive fraction, in totality, is related to the targeted organelle or particle and the interactions between particles, and thus, to the physiological and biochemical reactions generated and generally termed as biomarkers. It has been observed that engineered nanomaterials in marine ecosystems have a tendency to aggregate more as compared to aqueous freshwater because of surface charge screening in seawater due to high salts, thus, lessening the bioavailability of nanomaterials. Although the bioavailability is decreased, the cited works indicated that engineered nanomaterials are still bioavailable to organisms in marine systems (Table 11.2).
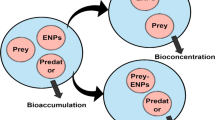
11.6.2 Bioconcentration
Bioconcentration is the procedure through which toxicants are passively absorbed by the living organisms from the environmental matrix exclusively through respiratory and/or dermal surfaces. For quantitatively measuring this process, bioconcentration factor (BCF) is conventionally calculated which is expressed as the ratio of the particle concentration in an organism to that in exposure medium (usually water or medium). BCF, expressed in terms of L kg−1, are usually expressed as chemical mass per L and chemical mass per kg biomass, respectively. BCF is measured at its steady state and is a net effect of uptake and elimination processes, taking care of metabolic transformation, fecal egestion, gill elimination, and growth dilution. The approximation of BCF can invite a few ambiguities as literature reports are either merely abstractive or it is difficult converting to BCF used for assessment. For example, the mean log BCF values for daphnids in case of many ENPs are quite broad, and vary from 3.16 to 5.64. On the other hand, the engineered nanomaterials mean log BCF values in fish varies from 1.27–2.87, which are 1–2-folds lesser than those of daphnids (Hou et al. 2013). However, bioconcentration can only be determined in controlled environmental settings.
11.6.3 Bioaccumulation
Bioaccumulation occurs if exposure takes place through contaminated food along with ambient sources and bioaccumulation endpoint (bioaccumulation factor, BAF) is defined as the ratio of the concentration of the substance or chemical in an organism (specific genus) (chemical mass per kg biomass) to the exposure concentration in water (chemical mass per L) (Hou et al. 2013). However, in case of exposure to soil or a benthic environment, the bioaccumulation endpoint is typically characterized by the ratio of chemical concentration in an organism to that in the sediment and termed as biota-sediment accumulation factor (BSAF). As per the USEPA Toxic Substances Control Act (TSCA), bioaccumulative substances have log BCF values in the range 3–3.7 and those with log BCF values ≥3.7 are considered very bioaccumulative substances. It has been shown that the bioaccumulation potential of nanoparticles to fish through oral route or food exposure is relatively low. In case of earthworms, several reports have revealed that the bioaccumulation potential of metal oxide or metallic nanoparticles .
11.6.4 Biomagnification
Biomagnification is the accumulation of a toxicant or chemical or pollutant by an organism due to water and food intake and it results in a concentration higher than that would have achieved from water contact alone and thus higher than expected from equilibrium (Hou et al. 2013). The biomagnification end point, the biomagnification factor (BMF), is the extent to which the concentration increases from one trophic level to next higher level. More precisely, BMFs are expressed as the ratio of the fugacity of a chemical entity in the predator to that in the prey, rather than as an expression of concentrations as discussed just above. In general, a BMF >1 signifies that biomagnification exists in a given food web. The comparatively greater bioaccumulation and partial depuration of engineered nanomaterials in lower trophic level organisms like daphnid results into the chance for trophic transfer and biomagnification through the food chain. Werlin et al. (2011) reported that CdSe QD titer in ciliated protozoa (Tetrahymena thermophila) is ~5 times higher than that in the bacteria (Pseudomonas aeruginosa), demonstrating that biomagnification occurs (Werlin et al. 2011). In contrast, due to the lack of QD internalization into bacterial cells, Holbrook et al. (2008) failed to observe trophic transfer from bacteria (Escherichia coli) to ciliates (Tetrahymena thermophila)-rotifers (Brachionus calyciflorus) (Holbrook et al. 2008). The difference would imply that uptake of QDs by bacteria is dependent on microbial isolates and/or QD exterior functionalization. In the absence of bacteria, QDs could be uptaken by ciliates and trophic transferred to the predator, rotifers. However, the body burden in rotifers is less than that in ciliates (BMF = 0.29–0.62), implying no biomagnification. Trophic transfer has also been observed in many high trophic level aquatic food webs, including QDs and Ag NPs transfer from algae to daphnia, QDs or nTiO2 transfer from daphnia to fish, clamworm to juvenile turbot.
11.7 Toxicity and Environmental Impact of Nanoparticles
Eventually, most of the ENPs are considered to be xenobiotic in nature and their potential release and fate pattern remain poorly understood (Mraz 2005; Oberdörster et al. 2005). Toxic NPs generate reactive oxygen species (ROS) that causes damages to membrane stabilization, protein damage and oxidation, nucleic acids degradation, release of harmful and toxic components, etc. (Klaine et al. 2008). Since ENPs are extensively used in biological applications, variable doses of ENPs should be administered in vivo to evaluate the ecotoxicological aspects of ENPs (Kunzmann et al. 2011).
Figure 11.3 explains the mechanisms of toxicity exerted by ENPs in living organizations. Once ENPs enter the living organism through endocytic pathways via motor proteins and cytoskeletal structures, they get transferred to the endolysosomal network within vesicles. Thereafter, the ENPs traverse the cytoplasm gain to access the nucleus causing cytotoxicity in the host organism (Shang et al. 2014). Moreover, ENPs may interact with membrane-bound cellular receptors like growth factor receptors and integrins to induce proliferation, differentiation, and migration.
In microbes, ENPs are accumulated either in the cells or adhere near the cell wall as electron dense structures (Feng et al. 2000). Accumulation of inorganic nanoparticles mostly occurs in the cytoplasm. This leads to the damage of bacterial membrane and create the access of NPs easy leading to the modification in cell by intracellular potassium leakage (Navarro et al. 2008). It must also be noted that surface coatings comprising of simple or complex organic moieties can act as carbon source for bacteria.
Further, plants also regularly encounter nanoparticles in environmental matrix. While atmospheric nanoparticles are shown to be adhered to leaves and other aerial parts of plants, roots encounter in proximity with aquatic or soil matrix nanoparticles . Thus, the entry points of ENPs in plant tissues are either through the underground roots or the aerial parts (e.g., cuticles, trichomes, stomata, stigma, and hydathodes), together with wounds and root joints (Fig. 11.4A, B). ENPs must navigate a sequence of biotic and abiotic barriers for uptake and translocation (Fig. 11.4C). The ENPs are internalization into the cells from the cell wall and occur through endocytosis (Corredor et al. 2009). Successive symplastic transfer afterward is dependent upon the size control limits of the plasmodesmata (Šamaj et al. 2004). Many literature reports suggest that ENPs have been found both in the apoplast and symplast; however, it remains to be established which route is more dominant.
Over the past decade, many experiments have been performed on the short-term acute toxic effects of ENPs. However, elucidation on chronic endpoints is relatively new area of research and it received impetus only after the first forum convened in Stockholm in 2007 (Kostarelos et al. 2007). Reports from the US EPA has revealed that titanium dioxide nanoparticles used in cosmetics have the potential to create brain damage in mice (Long et al. 2006). Nanosized titanium dioxide generates reactive oxygen species in brain microglia and affects neurons in vitro (Long et al. 2006). Most metal oxide nanoparticles show genotoxic and cytotoxic properties on fish cells (Handy et al. 2008; Vevers and Jha 2008). In the presence of magnetic nanoparticles of <10 nm dimension, neuronal cells enter a latent state and stop to react to chemical signals (Johnson 2007). Fullerenes are also reported to kill liver, skin and brain cells in vitro (Lewinski et al. 2008). Those nanoparticles which have been degraded in the cellular milieu could build up intracellularly, leading to either gene modification(s) or destruction of organelle integrity. Carbon-, metal-, and semiconductor-based nanoparticles , at high doses, exert cellular toxicity effects in a dose- and time-dependent manner. In case of reproductive system, literature cited works suggest that nanoparticles accumulate in the testes by traversing the blood–testes barrier and exert damage on sperm cells (McAuliffe and Perry 2007).
11.8 Risk Assessment of Engineered Nanoparticles
Repeated release as well as contact of ENPs with many elements of environment and trophic levels and likely hazards call for the strategy development or set patterns to test the probable risks of engineered nanomaterials. The toxicity of engineered nanomaterials is dependent upon their basic physicochemical properties and added functional chemico-biological features. Thus, these basic properties need to be evaluated while investing their probable ecological toxicity that becomes difficult because (i) their actual concentration in environment is much less than the measurable limits for most experimental tests and (ii) in addition to intentional ENPs, environment also consists of naturally produced NPs (Lead and Wilkinson 2006). Therefore, development/updating of currently available system to attain an improved screening potential and high selective recognition are the prerequisite. The first step toward this is pre-fractionation, that is, reduction of the mixture of particles in the real samples using stirring, centrifugation, or filtration. Size fractionation can be achieved through membranes (ultrafiltration, nanofiltration, and dialysis) as well as chromatography (Hassellöv et al. 2008). After that the size of ENPs can be examined through several instrumentations, light scattering is a frequently used method. Post analysis and characterization of ENPs, both the short- and long-term effects of ENPs on living organisms are tested. Establishment of a dose–response relationship by subjecting the organism to varying concentrations of NPs is a common pattern in almost all nanotoxicity-related studies (Navarro et al. 2008). The environmental hazards associated with chemical substances are assessed through standard ecotoxicity tests that focuses on the target/nontarget test species, endpoints protocols and measurement. The standard endpoints that are calculated [for example, lethal concentrations (LC), effective concentrations (EC) or no observed effect concentrations (NOECs)] are usually for higher organisms. In case of microbes and algae, the endpoint is population growth because of their fast growth (Crane and Scott 2012).
11.9 Nanowaste: Guidelines/Regulatory Measures
While the exponential growth of nanotechnology offers many benefits, they also contribute in generating wastes. Many of the nanomaterials-based manufacturing and products are discharged into the environment as a result of their disposal in waste streams (Moore 2007; Powell et al. 2008). Currently, industrial data on handling of discarded nanomaterials and their end-of-life scenarios remain elusive. At present, there is no centralized policy explicitly to tackle the ecotoxicity and safety inference of nanotechnology . There are no national or global safety guidelines or regulatory measures on manufacturing and characterization for nanomaterials at workplace. At the moment, regulatory government bodies in the USA (i.e., EPA, FDA, NIOSH) and in European Union (i.e., OECD, ECHA) have drafted strong technical guidelines and legislations to control the potential risks of ENPs. Considering the lack of the current risk assessment model and regulatory frameworks, the Woodrow Wilson International Center Project on Emerging Nanotechnologies (PEN) have highlighted the end-of-life directive of nanotechnologies (Breggin and Pendergrass 2007). However, much like usual chemical substances, research, and development with ENPs must be accomplished with great safety and responsibility. All federal, state, and local requirements must be dealt with while handling, transporting, storing, using or disposing chemicals, including nanomaterials.
The Occupational Safety and Health Administration (OSHA) stress on employers to sustain a secure and healthy working environment, “free from recognized hazards likely to cause death or serious physical harm”. As per OSHA guidelines, training and orientation programs on material safety data sheets and labeling and signage must be performed to educate the laboratory personnel so as to make them aware of the risks associated with workplace hazards. The transportation, treatment, disposal, and cleanup of hazardous waste come under the purview of The Resource Conservation and Recovery Act of 1976 (RCRA). Nanomaterials that have potential to be treated as a “hazardous waste” in RCRA are subject to this rule. Nanomaterials that are “chemical substances” under the Toxic Substances Control Act (TSCA) and which are not on the TSCA Inventory must be reported to the US Environmental Protection Agency (EPA). The usual practice is that a chemical substance that is not on the TSCA Inventory of Chemical Substances must be manufactured or imported with a prior “Premanufacture Notice” submitted to the EPA.
In case of all commercially available new pesticide products, the US EPA approval is necessary as per the ‘Federal Insecticide, Fungicide, and Rodenticide Act’ before registration along with subsequent evaluation, product composition and characterization, proper labeling mentioning proposed use of the material and data of extensive health and safety testing need to be submitted to US EPA. Furthermore, the US Food and Drug Administration also presently regulate an extensive array of nanotechnology or nanomaterials-enabled products (e.g., a nanomaterial for biomedical use).
11.10 Concluding Remarks, Challenges, and Perspectives
As the potentials and possibility of nanomaterials is well established, green and sustainable growth of nanotechnology is particularly imperative keeping environmental concern in mind. Environmental legislations must be promoted to develop ENPs with innovative parameters such as minimal mobility in environmental media and little or no toxicological effects for humans and ecology. The amount of introduction of nanoparticle in the ecological media is mounting speedily due to the numerous alternative green methods available today in both academia and industry. To date, enumeration of analytical environmental concentrations (hazard and exposure) of many popular nanoparticles is still not available. However, release and monitoring of ENPs/ENMs are required to be computed based on risk assessment and life cycle design concept.
Furthermore, to comprehend the long-lasting effect of ENPs/ENMs on the human health and ecology, extensive ecotoxicological data regarding their bioaccumulation and trophic transfer are required.
While it is widely accepted that many cited works have been carried out over the past decade, it goes without saying that the potential negative effects of engineered nanoparticles have been neglected. Here, we review the major observations emanating from recent works.
-
There is dearth of evidence on the transformation of engineered nanoparticles .
-
(a)
For instance, how transformations take place or expected at various conditions such as types of electrolytes used and their concentrations; pH of the preparing solution; nanoparticles ’ particle size and effect of coating, if any; interactions with the environmental media and different physicochemical conditions, etc.? What are the transformation pathways of the nanoparticles ? How does bioactivity and biotransformation or modification affected by media composition and trophic interaction?
-
(b)
How many potential stable species of transformed/aged nanoparticles exist in natural media and how do they interact with biota?
-
(a)
-
In-depth evidence from in vivo studies is required to truly reflect on fate, behavior, and transport of the engineered nanoparticles as the in vitro ecological studies do not necessarily mirror the factual effects of engineered nanoparticles in natural environmental media.
-
Although a plethora of literature data are available on greener synthesis of nanoparticles , there is lack of approach toward cost-effective quality by design products, a thorough appreciative understanding and production of safer by design (ecosafe) products.
-
Long-term experiments along with life cycle analysis to squarely reflect the release and exposure conditions at all ecosystem level are crucial to minimize the possible ecotoxicity of nanoparticles in different species.
-
The effects of forms (single or clustered, pristine or transformed), aging, transformation (both chemical and biological), and elemental compositional analysis and speciation on the inventory analysis warrant immediate attention.
-
The information about how the large-scale productions of nanoparticles affect the long-term impact in an ever changing environment is essential.
In a nutshell, addressing these research gaps and agglomeration on a common research platform is required to extend a rational framework for safeguarding the ecology that will result in a greener and safer earth around.
Abbreviations
- AAV:
-
Adeno-associated virus
- Ag:
-
Silver
- BAF:
-
Bioaccumulation factor
- BCF:
-
Bioconcentration factor
- Bi2O3:
-
Bismuth trioxide
- BMF:
-
Biomagnification factor
- CdSe:
-
Cadmium selenide
- CeO2:
-
Cerium dioxide
- CNTs:
-
Carbon nanotubes
- CrO2:
-
Chromium dioxide
- EC:
-
Effective concentrations
- Eg:
-
Energy gap
- EMA:
-
European Medicines Agency
- ENPs:
-
Engineered nanoparticles
- EPA:
-
Environmental Protection Agency
- ET:
-
Evapotranspiration
- Fe2+:
-
Ferrous
- Fe3+:
-
Ferric
- InP:
-
Indium phosphide
- LAI:
-
Leaf area indexes
- LC:
-
Lethal concentrations
- MoO3:
-
Molybdenum trioxide
- MWNTs:
-
Multiwalled nanotubes
- NDA:
-
New drug application
- NMs:
-
Nanomaterials
- NOECs:
-
No observed effect concentrations
- NPs:
-
Nanoparticles
- OSHA:
-
Occupational Safety and Health Administration
- PEI:
-
Polyethyleneimine
- PEN:
-
Project on Emerging Nanotechnologies
- PVP:
-
Polyvinylpyrrolidone
- QDs:
-
Quantum dots
- RCRA:
-
Resource Conservation and Recovery Act of 1976
- ROS :
-
Reactive oxygen species
- SELs:
-
Size exclusion limits
- SPR:
-
Surface plasmon resonance
- SWNTs:
-
Single-walled nanotubes
- TiO2:
-
Titanium dioxide
- TSCA:
-
Toxic Substances Control Act
- US FDA:
-
United States Food and Drug Administration
- ZnO:
-
Zinc oxide
- ZnS:
-
Zinc sulfide
- ZnSe:
-
Zinc selenide
References
Aggarwal P, Hall JB, McLeland CB et al (2009) Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev 61:428–437. https://doi.org/10.1016/j.addr.2009.03.009
Alivisatos AP, Gu W, Larabell C (2005) Quantum dots as cellular probes. Annu Rev Biomed Eng 7:55–76. https://doi.org/10.1146/annurev.bioeng.7.060804.100432
Bellardita M, Addamo M, Di Paola A, Palmisano L (2007) Photocatalytic behaviour of metal-loaded TiO2 aqueous dispersions and films. Chem Phys 339:94–103. https://doi.org/10.1016/j.chemphys.2007.06.003
Bernhardt ES, Colman BP, Hochella MF et al (2010) An ecological perspective on nanomaterial impacts in the environment. J Environ Qual 39:1954. https://doi.org/10.2134/jeq2009.0479
Bhatt I, Tripathi BN (2011) Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 82:308–317. https://doi.org/10.1016/j.chemosphere.2010.10.011
Bhattacharjee S, de Haan LHJ, Evers NM et al (2010) Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part Fibre Toxicol 7:25. https://doi.org/10.1186/1743-8977-7-25
Borm PJA, Robbins D, Haubold S et al (2006) The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol 3. https://doi.org/10.1186/1743-8977-3-11
Brant JA, Lecoanet H, Hotze M, Wiesner MR (2005) Surface charge acquisition and characteristics of fullerene aggregates (n-C60) in aqueous suspensions. Environ Sci Technol 39:6343–6351
Breggin LK, Pendergrass J (2007) Where does the nano go?: End-of-life regulation of nanotechnologies. Project on Emerging Nanotechnologies at the Woodrow Wilson International Center for Scholars, Washington, DC.
Bystrzejewska-Piotrowska G, Golimowski J, Urban PL (2009) Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag 29:2587–2595. https://doi.org/10.1016/j.wasman.2009.04.001
Caminati G, Turro NJ, Tomalia DA (1990) Photophysical investigation of starburst dendrimers and their interactions with anionic and cationic surfactants. J Am Chem Soc 112:8515–8522. https://doi.org/10.1021/ja00179a041
Cao G (2004) Nanostructures and nanomaterials – synthesis, properties and applications
Christian P, Von Der Kammer F, Baalousha M, Hofmann T (2008) Nanoparticles: Structure, properties, preparation and behaviour in environmental media. Ecotoxicology 17:326–343. https://doi.org/10.1007/s10646-008-0213-1
Corredor E, Testillano PS, Coronado MJ et al (2009) Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol 9:45. https://doi.org/10.1186/1471-2229-9-45
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211-212:112–125. https://doi.org/10.1016/j.jhazmat.2011.11.073
Croteau MN, Misra SK, Luoma SN, Valsami-Jones E (2011) Silver bioaccumulation dynamics in a freshwater invertebrate after aqueous and dietary exposures to nanosized and ionic Ag. Environ Sci Technol 45:6600–6607. https://doi.org/10.1021/es200880c
EU Commission (2011) Commission Recommendation of 18 October 2011 on the definition of nanomaterial. Off J Eur Commun: Legis 2011/696/EU
Feng QL, Wu J, Chen GQ et al (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668
Gaiser BK, Fernandes TF, Jepson MA et al (2012) Interspecies comparisons on the uptake and toxicity of silver and cerium dioxide nanoparticles. Environ Toxicol Chem 31:144–154. https://doi.org/10.1002/etc.703
Gatoo MA, Naseem S, Arfat MY et al (2014) Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. https://doi.org/10.1155/2014/498420
Geffroy B, Ladhar C, Cambier S, Treguer-Delapierre M, Brèthes D, Bourdineaud JP (2012) Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: the role of size, concentration and exposure time. Nanotoxicology 6:144–160. https://doi.org/10.3109/17435390.2011.562328
Ghafari P, St-Denis CH, Power ME, Jin X, Tsou V, Mandal HS, Bols NC, Tang XS (2008) Impact of carbon nanotubes on the ingestion and digestion of bacteria by ciliated protozoa. Nat Nanotechnol 3:347. https://doi.org/10.1038/nnano.2008.109
Goodman CM, McCusker CD, Yilmaz T, Rotello VM (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem 15:897–900. https://doi.org/10.1021/bc049951i
Goswami L, Kim KH, Deep A et al (2017) Engineered nano particles: nature, behavior, and effect on the environment. J Environ Manag 196:297–315. https://doi.org/10.1016/j.jenvman.2017.01.011
Griffitt RJ, Luo J, Gao J et al (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27:1972–1978. https://doi.org/10.1897/08-002.1
Gurr JR, Wang AS, Chen CH, Jan KY (2005) Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 213:66–73. https://doi.org/10.1016/j.tox.2005.05.007
Guzmán KAD, Taylor MR, Banfield JF (2006) Environmental risks of nanotechnology: national nanotechnology initiative funding, 2000–2004. Environ Sci Technol 40:1401–1407. https://doi.org/10.1021/es0515708
Handy RD, Henry TB, Scown TM et al (2008) Manufactured nanoparticles: their uptake and effects on fish – a mechanistic analysis. Ecotoxicology 17:396–409. https://doi.org/10.1007/s10646-008-0205-1
Hassellöv M, Readman JW, Ranville JF, Tiede K (2008) Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 17:344–361. https://doi.org/10.1007/s10646-008-0225-x
He X, Fu P, Aker WG, Hwang HM (2018) Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. J Environ Sci Heal Part C Environ Carcinog Ecotoxicol Rev 36:21–42. https://doi.org/10.1080/10590501.2017.1418793
Holbrook RD, Murphy KE, Morrow JB, Cole KD (2008) Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat Nanotechnol 3:352–355. https://doi.org/10.1038/nnano.2008.110
Hou WC, Westerhoff P, Posner JD (2013) Biological accumulation of engineered nanomaterials: a review of current knowledge. Environ Sci 15:103–122. https://doi.org/10.1039/C2EM30686G
Hu CW, Li M, Cui YB, Li DS, Chen J, Yang LY (2010) Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem 42:586–591. https://doi.org/10.1016/j.soilbio.2009.12.007
Johnson RC (2007) Studies warn of nanoparticle health effects. EE Times 4:13
Johnston BD, Scown TM, Moger J, Cumberland SA, Baalousha M, Linge K, van Aerle R, Jarvis K, Lead JR, Tyler CR (2010) Bioavailability of nanoscale metal oxides TiO2, CeO2, and ZnO to fish. Environ Sci Technol 44:1144–1151. https://doi.org/10.1021/es901971a
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1692. https://doi.org/10.1007/s11051-013-1692-4
Klaine SJ, Alvarez PJJ, Batley GE et al (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851. https://doi.org/10.1897/08-090.1
Kostarelos K, Lacerda L, Pastorin G et al (2007) Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotechnol 2:108–113. https://doi.org/10.1038/nnano.2006.209
Kunzmann A, Andersson B, Thurnherr T et al (2011) Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta 1810:361–373. https://doi.org/10.1016/j.bbagen.2010.04.007
Lai RWS, Yeung KWY, Yung MMN et al (2017) Regulation of engineered nanomaterials: current challenges, insights and future directions. Environ Sci Pollut Res:1–18. https://doi.org/10.1007/s11356-017-9489-0
Lead JR, Wilkinson KJ (2006) Aquatic colloids and nanoparticles: current knowledge and future trends. Environ Chem 3:159–171. https://doi.org/10.1071/EN06025
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4:26–49. https://doi.org/10.1002/smll.200700595
Li X, Elliott DW, Zhang W (2006) Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Crit Rev Solid State Mater Sci 31:111–122. https://doi.org/10.1080/10408430601057611
Li D, Fortner JD, Johnson DR, Chen C, Li Q, Alvarez PJ (2010) Bioaccumulation of 14C60 by the earthworm Eisenia fetida. Environ Sci Technol 44:9170–9175. https://doi.org/10.1021/es1024405
Li X, Liu W, Sun L et al (2015) Effects of physicochemical properties of nanomaterials on their toxicity. J Biomed Mater Res Part A 103:2499–2507. https://doi.org/10.1002/jbm.a.35384
Lihitkar NB, Abyaneh MK, Samuel V et al (2007) Titania nanoparticles synthesis in mesoporous molecular sieve MCM-41. J Colloid Interface Sci 314:310–316. https://doi.org/10.1016/j.jcis.2007.05.069
Logothetidis S (2006) Nanotechnology in medicine: the medicine of tomorrow and nanomedicine. Hippokratia 10:7–21
Long TC, Saleh N, Tilton RD et al (2006) Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol 40:4346–4352. https://doi.org/10.1021/es060589n
Loureiro S, Tourinho PS, Cornelis G et al (2018) Nanomaterials as soil pollutants. In: Soil pollution, pp 161–190. https://doi.org/10.1016/B978-0-12-849873-6.00007-8
Lovern SB, Owen H, Klaper R (2008) Electron microscopy of gold nanoparticle intake in the gut of Daphnia magna. Nanotoxicology 2:43–48. https://doi.org/10.1080/17435390801935960
Lovrić J, Bazzi HS, Cuie Y et al (2005) Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med (Berl) 83:377–385. https://doi.org/10.1007/s00109-004-0629-x
McAuliffe ME, Perry MJ (2007) Are nanoparticles potential male reproductive toxicants? A literature review. Nanotoxicology 1:204–210. https://doi.org/10.1080/17435390701675914
Misra SK, Dybowska A, Berhanu D, Croteau MN, Luoma SN, Boccaccini AR, Valsami-Jones E (2011) Isotopically modified nanoparticles for enhanced detection in bioaccumulation studies. Environ Sci Technol 46:1216–1222. https://doi.org/10.1021/es2039757
Moore J (2007) Nanowaste needs attention of EPA, industry and investors. Project on Emerging Nanotechnologies. EurekAlert
Mraz SJ (2005) Nanowaste: the big threat? Mach Des 77:46–53
Murray CB, Sun S, Gaschler W et al (2001) Colloidal synthesis of nanocrystals and nanocrystal superlattices. IBM J Res Dev 45:47–56. https://doi.org/10.1147/rd.451.0047
Navarro E, Baun A, Behra R et al (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386. https://doi.org/10.1007/s10646-008-0214-0
Noguez C (2007) Surface Plasmons on metal nanoparticles: the influence of shape and physical environment. J Phys Chem C 111:3806–3819. https://doi.org/10.1021/jp066539m
Novak S, Drobne D, Valant J, Pipan-Tkalec Ž, Pelicon P, Vavpetič P, Grlj N, Falnoga I, Mazej D, Remškar M (2012) Cell membrane integrity and internalization of ingested TiO2 nanoparticles by digestive gland cells of a terrestrial isopod. Environ Toxicol Chem 31:1083–1090. https://doi.org/10.1002/etc.1791
Oberdörster G, Maynard AA, Donaldson K et al (2005) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol 2:8. https://doi.org/10.1186/1743-8977-2-8
Ortega-Calvo JJ, Harmsen J, Parsons JR et al (2015) From bioavailability science to regulation of organic chemicals. Environ Sci Technol 49:10255–10264. https://doi.org/10.1021/acs.est.5b02412
Park MVDZ, Neigh AM, Vermeulen JP et al (2011) The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32:9810–9817. https://doi.org/10.1016/j.biomaterials.2011.08.085
Peijnenburg WJGM, Baalousha M, Chen J et al (2015) A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit Rev Environ Sci Technol 45:2084–2134. https://doi.org/10.1080/10643389.2015.1010430
Petersen EJ, Huang Q, Weber WJ Jr (2008) Bioaccumulation of radio-labeled carbon nanotubes by Eisenia foetida. Environ Sci Technol 42:3090–3095. https://doi.org/10.1021/es071366f
Pipan-Tkalec Ž, Drobne D, Jemec A, Romih T, Zidar P, Bele M (2010) Zinc bioaccumulation in a terrestrial invertebrate fed a diet treated with particulate ZnO or ZnCl2 solution. Toxicology 269:198–203. https://doi.org/10.1016/j.tox.2009.08.004
Powell MC, Griffin MPA, Tai S (2008) Bottom-up risk regulation? How nanotechnology risk knowledge gaps challenge federal and state environmental agencies. Environ Manag 42:426–443. https://doi.org/10.1007/s00267-008-9129-z
Reijnders L (2008) Hazard reduction for the application of titania nanoparticles in environmental technology. J Hazard Mater 152:440–445. https://doi.org/10.1016/j.jhazmat.2007.12.047
Roberts AP, Mount AS, Seda B, Souther J, Qiao R, Lin S, Ke PC, Rao AM, Klaine SJ (2007) In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna. Environ Sci Technol 41:3025–3029. https://doi.org/10.1021/es062572a
Roszek B, De Jong WH, Geertsma RE (2005) Nanotechnology in medical applications – state-of-the-art in materials and devices. RIVM Rep 265001001:1–123
Šamaj J, Baluška F, Voigt B (2004) Endocytosis, actin cytoskeleton, and signaling. Plant Physiol 135:1150–1161. https://doi.org/10.1104/pp.104.040683.1150
Santos HA, Riikonen J, Salonen J et al (2010) In vitro cytotoxicity of porous silicon microparticles: effect of the particle concentration, surface chemistry and size. Acta Biomater 6:2721–2731. https://doi.org/10.1016/j.actbio.2009.12.043
Schmid G (2004) Nanoparticles: from theory to application, pp 368–421
Shang L, Nienhaus K, Nienhaus GU (2014) Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnol 12:b26. https://doi.org/10.1186/1477-3155-12-5
Shim SE, Lee H, Choe S (2004) Synthesis of functionalized monodisperse poly(methyl methacrylate) nanoparticles by a RAFT agent carrying carboxyl end group. Macromolecules 37:5565–5571. https://doi.org/10.1021/ma049930j
Shoults-Wilson W, Reinsch BC, Tsyusko OV, Bertsch PM, Lowry GV, Unrine JM (2011) Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia foetida). Nanotoxicology 5:432–444. https://doi.org/10.3109/17435390.2010.537382
Singh S, Thiyagarajan P, Mohan Kant K et al (2007) Structure, microstructure and physical properties of ZnO based materials in various forms: bulk, thin film and nano. J Phys D Appl Phys 40:6312. https://doi.org/10.1088/0022-3727/40/20/S15
Smart SK, Cassady AI, Lu GQ, Martin DJ (2006) The biocompatibility of carbon nanotubes. Carbon N Y 44:1034–1047. https://doi.org/10.1016/j.carbon.2005.10.011
Sun H, Zhang X, Zhang Z, Chen Y, Crittenden JC (2009) Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite. Environ Pollut 157:1165–1170. https://doi.org/10.1016/j.envpol.2008.08.022
Tao X, Fortner JD, Zhang B, He Y, Chen Y, Hughes JB (2009) Effects of aqueous stable fullerene nanocrystals (nC60) on Daphnia magna: evaluation of sub-lethal reproductive responses and accumulation. Chemosphere 77:1482–1487. https://doi.org/10.1016/j.chemosphere.2009.10.027
Tervonen K, Waissi G, Petersen EJ, Akkanen J, Kukkonen JV (2010) Analysis of fullerene-C60 and kinetic measurements for its accumulation and depuration in Daphnia magna. Environ Toxicol Chem 29:1072–1078. https://doi.org/10.1002/etc.124
Tyner KM, Zou P, Yang X et al (2015) Product quality for nanomaterials: current US experience and perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7:640–654. https://doi.org/10.1002/wnan.1338
Unrine JM, Tsyusko OV, Hunyadi SE, Judy JD, Bertsch PM (2010) Effects of particle size on chemical speciation and bioavailability of copper to earthworms (Eisenia foetida) exposed to copper nanoparticles. J Environ Qual 39:1942–1953. https://doi.org/10.2134/jeq2009.0387
Vevers WF, Jha AN (2008) Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology 17:410–420. https://doi.org/10.1007/s10646-008-0226-9
Weissig V, Guzman-Villanueva D (2015) Nanopharmaceuticals (Part 2): products in the pipeline. Int J Nanomedicine 10:1245–1257. https://doi.org/10.2147/IJN.S65526
Weissig V, Pettinger TK, Murdock N (2014) Nanopharmaceuticals (Part 1): products on the market. Int J Nanomedicine 9:4357–4373. https://doi.org/10.2147/IJN.S46900
Werlin R, Priester JH, Mielke RE et al (2011) Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat Nanotechnol 6:65–71. https://doi.org/10.1038/nnano.2010.251
Wick P, Manser P, Limbach LK et al (2007) The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett 168:121–131. https://doi.org/10.1016/j.toxlet.2006.08.019
Wiesner MR, Lowry GV, Alvarez P et al (2006) Assessing the risks of manufactured nanomaterials. Environ Sci Technol 40:4336–4345
Yang ST, Wang X, Jia G et al (2008) Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol Lett 181:182–189. https://doi.org/10.1016/j.toxlet.2008.07.020
Yin H, Too HP, Chow GM (2005) The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials 26:5818–5826. https://doi.org/10.1016/j.biomaterials.2005.02.036
Zhang Y, Ali SF, Dervishi E et al (2010) Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived pc12 cells. ACS Nano 4:3181–3186. https://doi.org/10.1021/nn1007176
Zhao CM, Wang WX (2010) Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna. Environ Sci Technol 44:7699–7704. https://doi.org/10.1021/es101484s
Zhu X, Chang Y, Chen Y (2010) Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 78:209–215. https://doi.org/10.1016/j.chemosphere.2009.11.013
Acknowledgments
Debasree Kundu acknowledges Science and Engineering Research Board (SERB), New Delhi for providing National Postdoctoral Fellowship (File No. PDF/2015/000382). Mohd Faheem Khan is thankful to Indian Institute of Technology Guwahati for providing research fellowship to pursue doctoral studies.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive licence to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kundu, D., Khan, M.F., Gogoi, M., Patra, S. (2021). Environmental Impact and Econanotoxicity of Engineered Nanomaterials. In: Kumar, V., Guleria, P., Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Nanotoxicology and Nanoecotoxicology Vol. 1. Environmental Chemistry for a Sustainable World, vol 59. Springer, Cham. https://doi.org/10.1007/978-3-030-63241-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-63241-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63240-3
Online ISBN: 978-3-030-63241-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)