Abstract
The hypothalamus is a key regulatory brain region that coordinates and modulates various vital processes, including cardiovascular and respiratory functions, metabolism and mood, among others. This complex function is achieved by the concerted action of neurons, astrocytes, and microglia within this nucleus. However, various pathophysiological conditions, particularly those involving a neuroinflammatory process, disturb the normal function and interaction of these various cellular components of the nucleus. Here we provide a broad overview about the origin, development, and function of microglia and astrocytes and discuss their respective roles in the regulation of hypothalamic activity. We further provide insight into how cardiovascular diseases such as heart failure or hypertension affect neuronal and glial activity in the hypothalamus and impose detrimental consequences to the well-being of affected individuals. Finally, we discuss a recently developed technique that allows the three-dimensional reconstruction and analysis of glial cells, thereby providing unprecedented details about glial morphology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction: Neuron–Glia Interactions in the Brain
The idea that glial cells (i.e., astrocytes, oligodendrocytes, and microglia) are merely supportive elements within the brain, which provide stability and nutrients to neurons, has been overthrown many years ago. Research in the last two decades has not only revealed many more vital functions of glia, but highlighted various neuron–glia interactions that are crucial for proper signal communication, integration, and maintenance within and across different brain networks. In fact, the intricate interaction between neurons and astrocytes has been deemed so important that scientists coined the term “tripartite synapse,” which refers to the close interaction between the pre-synapse, post-synapse, and astrocytes.
1.1 Astrocytic Diversity
Astrocytes display astonishing variability across different species, brain regions, and even within the same functional units/networks in the brain. In different brain regions and during different times of development, astrocytes display distinct functional properties. The size of astrocytes can even increase with brain size and cognitive abilities. Interestingly, human astrocytes are up to threefold larger and tenfold more ramified than rodent astrocytes (Allen and Eroglu 2017). In addition, recent genetic studies have revealed significant differences in gene expression between rodent and human astrocytes, which could explain why certain unique features of the latter are cell-autonomous. While mouse cortical astrocytes contact approximately 100,000 synapses, their human counterparts have 2,000,000 synaptic contacts.
1.2 Astrocytes as Regulators Between Pre- and Post-synapse
Astrocytic processes ensheath synapses and this supportive formation is critical for normal synapse function, brain homeostasis, and neuronal health (Araque et al. 1999). Astrocytes mop up various neurotransmitters from the extracellular space, and the high expression of glutamate transporters in astrocytic processes prevents excessive extrasynaptic accumulation of glutamate, thereby providing protection against excitotoxicity. Furthermore, astrocytes control ionic balance at the synapse through various channels, such as potassium channels, to maintain a healthy/balanced ionic milieu, which is a prerequisite for proper synaptic transmission. While it is certain that astrocytes are equipped with metabotropic and ionotropic receptors, their precise relationship with astrocytic Ca2+ transients and subsequent gliotransmission remains controversial (Fiacco and McCarthy 2018; Savtchouk and Volterra 2018). However, it seems that astrocytic Ca2+ transients occur in a delayed/slower fashion than in neurons and recent findings highlighted the presence of Ca2+ microdomains in astrocytes (Agarwal et al. 2017).
Astrocyte–synapse interaction is critical for normal CNS development, and the main periods of synaptogenesis and axonal formation take place during the second and third postnatal week, after the differentiation of astrocytes is already completed. Early experiments in neuronal cultures showed that the addition of astrocytes increased both the number and strength of synapses and highlighted the important role of neuronal–glial interaction in synapse development. Over the years, these findings could be replicated and turned out to be true for many different species across the animal kingdom, including C. elegans, Drosophila, Xenopus, rodents, and humans. Moreover, astrocytes are involved in the formation of many synapse types, including glutamatergic, GABAergic, glycinergic, and cholinergic synapses.
Astrocytes secrete the so-called Thrombospondins, such as TSP1/2 and a protein named SPARCL1/Hevin, which are signals that control glutamatergic synapse formation. A number of astrocyte-derived signals have been identified, such as Gpc4/6 and TNF-α, which regulate AMPA receptor localization to post-synaptic terminals and increase AMPAR levels at existing synapses. Astrocytes also play an important role in the control of synapse numbers: In the developing brain, they directly phagocytose excess synapses via the astrocytic phagocytic receptors Mertk and Megf10 (Chung et al. 2013). Recent studies have corroborated the hypothesis that astrocyte-secreted signals regulate synaptogenic pathways within neurons (Baldwin and Eroglu 2017; Singh et al. 2016), adding another layer of complexity to the intricate neuron–glia interaction. However, mRNA profiling of isolated astrocytes from different brain regions in rodents and humans suggested that not all astrocytes have the same synaptogenic potential (Zhang et al. 2016). For detailed reviews about astrocyte–synapse interactions in the healthy and diseased brain, as well as neuron–glia interactions in the formation of the blood–brain barrier, we refer the reader to the following literature: (Allen and Eroglu 2017; Eroglu and Barres 2010).
Despite the fact that astrocytes have emerged as important communicative elements in neuron–glia communication during the last decade, another key function that they are involved with is the formation of the blood–brain barrier (BBB). The BBB outlines cerebral microvessels and is composed of endothelial cells, astrocytes, neurons, and pericytes (Zhao et al. 2015). The complex interactions between endothelial cells, extracellular matrix, basal lamina, pericytes, and astrocytes comprise a neurovascular unit, which regulates central nervous system (CNS) development and synaptic activity, and can even influence the permeability of the BBB. Within the neurovascular unit, astrocytes are bidirectional communication partners that receive signals from neighboring neurons and respond with the release of neuroactive substances.
2 Transfer of Power in the Glial Kingdom: Microglial Cells
While the importance of astrocytes for synapse development and signal integration within a functional brain cannot be questioned, another glial cell type that has been overlooked and underestimated for many years is microglia (see also Chap. 1). Microglia hide in plain sight and are the secret heroes of the brain immune system, despite being much smaller (hence the name, 5–10 μm diameter) than other glia in the brain. When Pio del Rio Hortega, a student of the famous Santiago Ramon y Cajal, first described microglia around 1920, he could not have foreseen how crucial these small glial cells actually are for normal brain function. During the last decade, microglia have emerged as key players in brain development, synapse formation and pruning, as well as in a plethora of vital immune-related functions. Microglia are specialized immune cells that take up residence in brain parenchyma and are among the first responding cells during injury, cell death, or entry of unwanted intruders that might access the brain due to a compromised blood–brain barrier. Microglia represent a major component in the immune response and participate in the neuroinflammatory response largely via the release of various pro-inflammatory cytokines. Research throughout the last decades has provided compelling evidence that microglia are more than just passive bystanders, and refuted the long-held dogma that microglia represent circulating CNS macrophages that enter the brain to replenish the local pool of immune cells. An overview of the respective roles of astrocytes and microglia in the developing and healthy adult brain can be found on Box 5.1.
2.1 Origin of Microglia: A Journey from the Yolk Sac to the Developing Brain
For a long time, it was believed that microglia represent a distinct pool of tissue-specific macrophages that populate the brain parenchyma and gain access to the brain through the BBB. However, recent research showed that microglia develop from c-KitloCD41lo progenitors that originate around embryonic day 7.25 (E7.25) in the yolk sac and find their way into the developing brain (Ginhoux et al. 2010). Several studies in zebrafish, mice, and chickens show that microglia are established during early embryogenesis, well before other glial cells such as astrocytes and oligodendrocytes arise. Microglia represent a self-sustainable pool of resident brain immune cells, which are not dependent on circulating macrophages (Ajami et al. 2007, 2011). Microglia in the embryonic, early postnatal, and adult CNS significantly differ in their expression, highlighting that adult brain microglia and microglia during early brain development are in fact functionally distinct entities. Once they reach the developing brain, microglia take over a variety of important functions and actively participate in shaping emerging brain circuits.
2.2 Role of Microglia in the Developing Brain: Neuronal Support and Synaptic Pruning
Microglia in the developing brain lack the characteristic ramified extensions of adult microglia (Lawson et al. 1990), which scan the environment for cues of sick neurons and tissue damage in inflammation. On the contrary, microglia in the developing brain are considerably more proliferative than in the adult and are actively involved in phagocytosis and tissue remodeling. By engulfing neighboring cells in the developing CNS and clearing apoptotic neuronal debris, microglia help to shape neuronal networks (Peri and Nusslein-Volhard 2008). In addition, microglia stimulate neurogenesis and support the survival, proliferation, and maturation of neuronal progenitor cells in the developing brain. During the early postnatal period, crosstalk of microglia and oligodendrocyte precursor cells has been described, suggesting far-reaching and long-lasting consequences of microglial activity in this period. The microglial chemokine receptor CX3XR1 has been highlighted as a key component of the microglia-mediated survival cascade, and microglia lacking CX3XR1 do not produce sufficient amounts of the neurotrophic insulin-growth factor (IGF)1 and subsequently fail to promote postnatal survival. Microglia actively participate in synaptic pruning via CX3XR1, and CX3XR1 knockout mice lacking the receptor in microglia display a significantly higher number of post-synaptic puncta, which is indicative of impaired synaptic pruning. Synapses can be actively tagged for engulfment by microglia via complement protein (C3), allowing a directed C3 receptor-mediated phagocytosis (Stevens et al. 2007). On the other hand, synapses can be protected from excess pruning via CD47, which represents a “don’t eat me” signal that stops microglia from engulfing the respective synapses (Lehrman et al. 2018). The reader is referred to Chap. 1 for a detailed description of the role of microglia in the development of the hypothalamus.
2.3 Microglia in the Adult Brain: Homeostasis and Immune Response
In the healthy adult brain, microglia extend their tiny processes throughout the extracellular space and constantly scan the environment for various cues. Although the cell bodies of microglia remain in place, their processes are anything but static (Nimmerjahn et al. 2005). Indeed, several studies suggest that microglia can encompass areas more than tenfold the size of their cell bodies and constantly interact with neighboring cells, although the precise underlying receptors, ion channels, and neurotransmitters for these interactions are under debate. Microglia display a remarkable heterogeneity and drastic differences in brain region-specific density, morphology, and genetics.
Upon acute injury within the CNS, microglial processes converge toward the site of injury and, after hours to days, they retract their processes and transform into their activated, ameboid form, which is responsible for mediating the appropriate immune response (Nimmerjahn et al. 2005). The signal-directed movement of microglia to the site of injury, known as chemotaxis, depends on purinergic P2Y12 receptors on microglia that bind to ATP or ADP released by various types of neural cells. In very rare cases, activated microglia can respond to noxious stimuli by transforming into a hyper-ramified phenotype, although the precise underlying mechanisms are currently unclear. It is important to know that at any given point, ramified, primed, reactive, or ameboid microglial phenotypes co-exist, even in the healthy brain, making it clear that a binary classification of active/inactive microglia is an oversimplification. Reactive microglia secrete various pro-inflammatory cytokines, such as IL-1, TNF-α, IL-18, IL-6, or IL-23, which in turn act on neural cells and promote further neuroinflammation (Prinz et al. 2019). In addition to cytokine release, microglia release reactive oxygen species (ROS) and nitrogen species, which are toxic to both neurons and oligodendrocytes, thereby actively promoting neuroinflammation-induced apoptosis. It was recently discovered that the combined secretion of TNF-α, IL-1α, and C1q by activated microglia turns astrocytes into a neurotoxic (A1) astrocyte phenotype. Astrocytes can release orosomucoid-2 to inhibit microglial activation, block the chemokine receptor type 5 of the microglial membrane, downregulate the inflammatory response, or further promote neuroinflammation and neurodegeneration. This bidirectional interaction allows an efficient and tailored response to various potentially harmful threats that the brain might be exposed to. Another recent study highlighted the “immune memory” of microglia, resulting in smaller volumes of experimental stroke and maintenance of IL-10 expression (Wendeln et al. 2018). For a comprehensive literature about microglial origin, microglial function during disease, and microglia–astrocyte interaction during neuroinflammation, we refer the reader to these reviews: (Liddelow and Barres 2017; Prinz et al. 2019; Prinz and Priller 2014). The respective functional and pathological roles of activated microglia and astrocytes are summarized in Box 5.1.
Box 5.1: Overview of Astrocyte and Microglia Functions in the Developing, Mature, and Diseased Brain
Cell type | Stage | Function | Reference |
|---|---|---|---|
Microglia | Development | Phagocytosis, tissue remodeling | Peri and Nusslein-Volhard (2008) |
Microglia | Development | Stimulation of neurogenesis, proliferation and support of cellular survival | Sierra et al. (2010) |
Microglia | Adult brain | Synaptic pruning: engulfment of synapses and regulation of post-synaptic puncta | Stevens et al. (2007) |
Microglia | Adult brain | Constant scanning of CNS for unwanted intruders and signs of injury | Prinz et al. (2019) |
Reactive microglia | Brain reacting to acute injury/immune response | Altered synaptic density, neurotoxic effect, neuroinflammation, cytokine release, uncontrolled and excessive synaptic pruning, peripheral macrophages gain access to CNS | Ajami et al. (2011); Ajami et al. (2007); Liddelow and Barres (2017); Liddelow et al. (2017) |
Astrocytes | Development | Synaptogenesis, axonal formation, regulation of synaptic strength | Zhang et al. (2016) |
Astrocytes | Development | Phagocytosis of excess synapses | Chung et al. (2013) |
Astrocytes | Development/Adult brain | Formation of the blood–brain barrier | Zhao et al. (2015) |
Astrocytes | Development/Adult brain | Control of glutamatergic, GABAergic, cholinergic, and glycinergic synapse formation | Ullian et al. (2001) |
Astrocytes | Adult brain | Regulation of the tripartite synapse, control of extracellular glutamate/GABA balance | Araque et al. (1999) |
Astrocytes | Adult brain | Active part in neuron–glia communication, gliotransmission | |
Reactive astrocyte | Brain reacting to acute injury/immune response | Compromised BBB, altered levels of neurotransmitters, compromised access of circulating signals to the brain, astrogliosis, retraction of astrocytic processes, brain swelling, neuronal death |
3 The Supraoptic and Paraventricular Nuclei of the Hypothalamus: Role in Homeostasis and Emotional Regulation
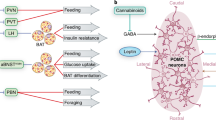
The supraoptic nucleus (SON) of the hypothalamus is situated at the base of the brain, just slightly above the optic tract, while the paraventricular nucleus (PVN) of the hypothalamus is located on both sides of the third ventricle (Fig. 5.1). Magnocellular neurosecretory cells (MNCs) synthesizing OT and VP are present in both the SON and PVN and release these neuropeptides into the systemic circulation via axonal projections to the posterior pituitary. The estimated total number of MNCs amounts to 100,000 in humans and 10,000 in rats. The SON plays an important role in regulating plasma osmolality, labor, and lactation, and has recently been proposed to be involved in the regulation of context-dependent fear memories (Hasan et al. 2019). While the SON comprises exclusively MNCs, the PVN harbors, in addition to MNCs, parvocellular neurons that project to the median eminence and various hindbrain regions (Althammer and Grinevich 2017). The PVN is a key structure for the regulation of sympathetic outflow and cardiovascular control, playing thus a major role in physiological homeostasis and neuroendocrine control (Stern 2015). These actions are mediated via direct innervation of sympathetic-related brainstem and spinal cord neurons. OT and VP neurons in both the SON and PVN have been implicated in emotional regulation and affect a plethora of different behaviors including anxiety, aggression, fear, depression, social behavior, and pair bonding. For comprehensive reviews on the physiological regulation MNCs and emotional control via OT/VP neurons, we refer the readers to the following literature: (Brown et al. 2013)
Oxytocin and vasopressin neurons in the rodent hypothalamus. Confocal images show rat vasopressin (green, from vasopressin-GFP transgenic rat) and oxytocin (magenta, immunofluorescence with oxytocin antibody) neurons located in the supraoptic and paraventricular nucleus. The scheme depicts the topographical location of the respective nuclei in the rat brain
3.1 Somato-dendritic Release of Oxytocin and Vasopressin
Both types of MNCs in the SON and PVN have the ability to release their respective neuropeptides from both dendrites and somata via a process called somato-dendritic release (Ludwig and Leng 2006). Somato-dendritic release of OT and VP mediates multiple and unique functions, which are distinct from those mediated by axonal, systemic release of these neuropeptides. For example, somato-dendritic release of OT and VP serves as an autocrine signal by which magnocellular neurosecretory neurons autoregulate their firing activity and systemic hormone release. Moreover, dendritically-released VP can diffuse in the extracellular space to act as a diffusible, interpopulation signaling molecule, coordinating the activity of sympathetic and neurosecretory PVN neurons (Son et al. 2013), playing thus a critical role in the generation of multimodal homeostatic responses by the PVN; (see Ludwig and Stern (2015) for a review on this subject).
3.2 Role of Neuro–glial Interaction in Regulating the Physiological Activity in the SON and PVN
Various forms of complex neuro–glial interactions have been shown to take place in the MNC system, primarily involving bidirectional communication between astrocytes and neurons. Astrocytes express an abundance of G protein-coupled receptors for various neurotransmitters, including glutamate, GABA, and various neuropeptides as well. Thus, astrocytes can readily sense neuronally-derived signals. For example, noradrenaline has been shown to stimulate α1-adrenoceptors on PVN astrocytes resulting in the release of ATP from the stimulated astrocytes. Astrocytes in the SON and PVN also express high levels of endothelin-B receptors, and have been shown to contribute to endothelin-mediated regulation of MNC firing activity in a nitric oxide-dependent manner (Filosa et al. 2012). Finally, dendritically-released VP from MNCs can evoke Ca2+ activity in surrounding astrocytes, a mechanism recently proposed to contribute to ghrelin-mediated modulation of VP activity in a nutritional-dependent manner.
Another key mechanism by which astrocytes influence MNC firing activity is via regulation of the concentration and time course of neurotransmitters in synaptic and perisynaptic areas. This is mediated by the activity of selective and powerful neurotransmitter transporters, particularly for the amino acid transmitters glutamate and GABA (see Chap. 2). Over the past 10 years, our laboratory has focused on a particular aspect of this phenomenon, namely the ability of astrocyte amino acid transporters to restrict the ability of extracellular neurotransmitters to access and activate extrasynaptic, particularly extrasynaptic glutamate NMDA receptors (eNMDARs). Differently from classical synaptic receptors, eNMDARs display a low degree of desensitization and a low affinity for glutamate, mediating a persistent, “tonic” excitatory current that is thought to globally influence neuronal excitability and the overall gain within a network of neurons. In this sense, we showed that activation of eNMDARs by ambient, extracellular glutamate levels results in a persistent inward current, which tonically stimulates SON neuronal activity (Fleming et al. 2011). Moreover, the strength of this tonic excitatory drive is directly regulated by astrocyte GLT1 activity. Thus, pharmacological block of GLT1 activity or glial retraction during dehydration results in an enhanced activation and contribution of eNMDARs to SON firing activity. We proposed this phenomenon to contribute to the homeostatic increase in neurosecretory firing activity during dehydration.
Importantly, we recently demonstrated that the neuropeptide Angiotensin II (AngII) can directly inhibit the activity of astrocyte GLT1 transporters, resulting in the buildup of extracellular glutamate and activation of SON and PVN neurons (Stern et al. 2016). Finally, we also demonstrated that changes in the expression and/or fraction of GLT1 in the SON and PVN can contribute to exacerbated hypothalamic neuronal activity in prevalent cardiovascular diseases, including heart failure and hypertension (Stern et al. 2016).
In summary, a significant amount of progress has been made regarding the role of astrocytes in the regulation of the magnocellular system, particularly under physiological conditions. Conversely, and compared also to other major CNS regions such as the cortex and hippocampus, much less is known about the contribution of microglial cells in shaping the normal activity of hypothalamic neurons and circuits, an area that clearly deserves to be further investigated. Figure 5.2 shows isolated microglia and astrocytes in high magnification as well as an overview of astrocytes, microglia, and OT neurons in the SON and PVN.
Microglia and astrocytes in the rat hypothalamus. Top confocal images show individual microglia (red, anti-IBA1 immunofluorescence) and astrocytes (green, anti-glutamine synthetase immunofluorescence, and magenta, anti-GFAP immunofluorescence) under high magnification. Bottom panels show the distribution of microglia (red) and astrocytes (magenta) in the oxytocin-expressing (green, anti-oxytocin immunofluorescence) supraoptic (left panel) and paraventricular (right panel) nuclei
3.3 Neuroinflammation in the SON and PVN in Disease Conditions
Neurohumoral activation, a process that involves exacerbated sympathoexcitatory activity, along with elevated circulating levels of neurohormones, including VP, AngII, and endothelins, among others, is a common pathophysiological phenomenon in highly prevalent cardiometabolic diseases, including hypertension, heart failure, diabetes, and obesity. Importantly, a direct correlation between the degree of neurohumoral activation and morbidity and mortality in these diseases is well-established. Thus, elucidating the precise underlying mechanisms mediating neurohumoral activation is of high clinical relevance.
A growing body of evidence supports a critical role for the SON and PVN in the onset and maintenance of neurohumoral activation in these diseases, particularly in hypertension and heart failure. Importantly, neuroinflammation within these hypothalamic nuclei has been identified as a key underlying pathophysiological mechanism. Thus, several studies have found elevated cytokine levels, astrogliosis, microglia activation and infiltration by peripheral immune cells, as well as disruption of the BBB. In extreme cases, a compromised BBB can lead to entry of peripheral macrophages, which further exacerbates neuroinflammation in the brain (Ajami et al. 2007, 2011). Still, the precise mechanisms and cascade of signaling events contributing to neuroinflammation in cardiometabolic diseases remain to be determined.
3.4 Role of the Renin–Angiotensin System (RAS) in Mediating Reactive Astrocytes and Microglia Cell Activation in the SON and PVN
A common denominator in several of these cardiometabolic diseases is activation of the renin–angiotensin system (RAS) (Diaz et al. 2019). Compelling evidence supports that overactivation of both the peripheral and brain RAS in hypertensive and heart failure conditions contributes to increased vascular tone, Na+ retention, volume expansion, and sympathohumoral activation. Indeed, some of the most efficient pharmacological tools available for the treatment of these diseases target either AngII receptors or angiotensin converting enzymes.
Importantly, AngII is also recognized as a potent pro-inflammatory molecule, and a growing body of evidence points to AngII as a candidate mediating hypothalamic neuroinflammation in cardiometabolic disease. AngII receptors in the brain have been assumed to be exclusively expressed in neurons. While still a controversial topic (de Kloet et al. 2015), we recently demonstrated that AngII AT1 receptors are also expressed both in astrocytes and microglial cells of the PVN. Indeed, we reported that AT1 receptor activation in astrocytes inhibits GLT1 transporter activity, resulting in the buildup of extracellular glutamate and activation of eNMDARs in PVN neurons. We proposed this to be one of the key mechanisms by which AngII mediates increased neuronal activity and neurohumoral outflow from the PVN (Stern et al. 2016).
Whether microglial cells can also be directly targeted by the RAS is also a controversial issue, with conflicting results described in the literature. In a recent paper, we demonstrated that AT1 receptor mRNA is present in PVN microglia, and that AT1 receptor stimulation results in microglia activation and oxidative stress (Biancardi et al. 2016). Interestingly, these AngII effects on microglia required Toll-like receptor 4-mediated signaling, supporting a functional interaction between the RAS and innate immune signaling in mediating RAS-dependent neuroinflammation in the PVN. Moreover, we also showed that AngII-mediated signaling contributes to microglia activation and PVN neuroinflammation during hypertension (Biancardi and Stern 2013).
3.5 Compromised PVN Blood–Brain Barrier Integrity as Part of the Neuroinflammatory Response During Hypertension
Finally, as stated above, glial cells play critical roles in maintaining the integrity of BBB. A compromised BBB permeability has been described in several neurological disorders that have a strong neuroinflammatory component, such as stroke, traumatic brain injury, hypertension, and dementia, among. Moreover, RAS blockade has been shown to have neuroprotective effects when used in these conditions. Thus, we aimed to investigate whether AngII pro-inflammatory effects in the PVN could also lead to BBB disruption in hypertensive conditions. To this end, we developed a relatively simple imaging approach, based on the intravascular infusion of two fluorescent dyes of different colors (red and green) and molecular sizes (70 kDa and 10 kDa, respectively), to quantitatively assess the level of disruption of BBB permeability, in this case, in a rat model of hypertension. The principle of this approach is that under control conditions with an intact BBB function, both dyes circulate intravascularly, without extravasating into the tissue parenchyma. During pathological conditions involving increased BBB permeability, the small-sized dye (green) will be able to extravasate into the parenchyma, whereas the large-sized dye (red) would still remain intravascularly. Thus, using simple imaging algorithms, it is possible to isolate and quantitatively measure pixels containing small green dye located extravascularly (see Fig. 5.3 and (Biancardi et al. 2014; Biancardi and Stern 2016) for detailed information about this procedure). Using this approach, we found that elevated circulating levels of AngII during hypertension resulted in the disruption of the BBB integrity, allowing access of circulating AngII to the PVN. Interestingly, the leaked AngII was found to be predominantly bound to microglial cells (Biancardi et al. 2014; Biancardi and Stern 2016).
Imaging approach to quantify changes in blood–brain-barrier permeability. (a1) Sample of labeling of the PVN microvasculature following the simultaneous intracarotid injection of the small size dextran-FITC 10 kDa (green) and the large size dextran-rhodamine 70 kDa (RHO70, red). (a2) The first step in this process consists in detecting individual pixels that contain both signals (green and red, shown in white color). These pixels showing colocalization are isolated and a separate image is generated (a3). Concurrently, a binary image containing only the FITC 10 green signal (small dye) is obtained (a4). Finally, to isolate and quantify the extravasated FITC 10 signal, image A3 is digitally subtracted from image A4, resulting in a new image containing only the extravascular FITC 10 signal (a5), which is then used for densitometry analysis. (b) Representative images showing increased extravasated small-size dextran-FITC 10 kDa (green dye) but not large size dextran-rhodamine 70 kDa (RHO70) (red dye) in the PVN of a hypertensive (b2), compared to a normotensive (b1) rat. A sample of FITC 10 dye located in the PVN parenchyma (empty arrows) around a nearby vessel (filled arrow) is shown in b3. Scale bars = 50 μm. 3V: third ventricle. Panels a and b modified from Biancardi et al., Hypertension 2014 and Biancardi and Stern, J Physiol 2016, respectively)
Finally, it is important to mention that a RAS-mediated neuroinflammatory response is not a phenomenon restricted to the PVN or hypothalamus, given that a contribution of the RAS to neuroinflammation in other brain regions and pathologies such as Parkinson’s disease (Rodriguez-Perez et al. 2018) has also been demonstrated recently.
4 Experimental Approach to Monitor Microglia Activation During the Neuroinflammatory Response
One of the main challenges in studying microglial morphology and function is choosing a reliable technique that reports structural and functional changes in an unbiased manner. While conventional measurements of cytokine mRNA or protein levels, as well as immunohistochemical staining for specific microglial markers, have been around for quite a while, they provide only indirect evidence of microglial activation. During neuroinflammation, microglia undergo a morphological transition from a highly ramified to a deramified state, retracting their fine processes and experiencing an overall reduction in cell volume (Nimmerjahn et al. 2005). Although it seems evident that a comprehensive knowledge of microglial deramification is of paramount importance, very little is known about the precise series of events that ultimately lead to this phenomenon, highlighting the need for tools that allow a detailed morphometric analysis of microglial remodeling. While classical markers such as increased microglial density, increased expression of ionized calcium-binding adapter molecule 1 (IBA1), and various cytokines are widely used to assess neuroinflammation, they fall short of addressing detailed microglial morphological changes during this pathological process. This is critical because diverse microglial morphometric features are not only associated with diverse microglial functions, but more importantly, they also have been associated recently with different stages in the spatio-temporal progression of the neuroinflammation process (Prinz et al. 2019). In this sense, classical two-dimensional maximum projection analysis is insufficient to provide detailed information about microglial features such as changes in cellular or somatic volume. In fact, it becomes clear that detailed three-dimensional analysis or microglia cell morphology is of paramount importance, especially considering microglial heterogeneity and brain region-specific differences in size, density, and activation stages.
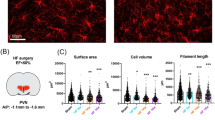
Our lab recently developed a comprehensive glial profiler on the basis of the IMARIS (Bitplane, Oxford Instruments) software, which allows rapid, unbiased, and flexible three-dimensional reconstruction of glial surface and filaments (Althammer et al. 2020). An overview of the individual steps of the three-dimensional reconstruction can be found on Fig. 5.4. The uploaded videos highlight the three-dimensional rotation and the features of the glial profiler. With this approach, we quantified and analyzed microglia and astrocytes in the PVN in rats with heart failure. We found that already 8 weeks after the surgical ligation of the coronary artery, microglia transitioned into a high-activity state and displayed somatic swelling and retraction of their processes. These two microglial alterations were highly correlated, indicating that somatic swelling and deramification are processes that occur in parallel within the same microglial cells. In addition, we found convincing evidence for A1 astrocytes 14 weeks after the surgery through genetic quantification of A1 markers via qPCR and assessment of astrocyte morphology using the 3D glial profiler.
Three-dimensional reconstruction of microglia via IMARIS. Raw z-stacks of fluorescently labeled microglia are used for the three-dimensional reconstruction. Surface and filaments are reconstructed in a two-step process and the final reconstruction can be used to calculate various glial parameters such as surface, cell volume, branches, and complexity. During injury, disease, and neuroinflammation, microglial cells retract their processes and become less ramified, which is usually referred to as microglial activation. This term can be slightly misleading, given that microglia are highly active cells even in the basal state. The precise description of the three-dimensional reconstruction of glial cells can be found in Althammer et al. (2020)
Recent technical advances have made it possible to monitor microglial activity, calcium transients, and morphometric changes in vitro and in vivo. While our recently developed glial profiler allows to monitor microglial population shifts and respective changes in microglial morphology, these tools are very useful to study acute effects of various compounds on microglial activity and function in individual, non-fixed cells. Further improvement of these techniques will be necessary to specifically manipulate microglial function in vivo and in vitro, with methods similar to those that have been used to activate/inhibit both neurons and microglia under various conditions.
5 Perspectives
The respective roles of astrocytes and microglia in healthy and diseased brains have been extensively studied (Eroglu and Barres 2010; Liddelow and Barres 2017). However, several questions regarding the transition from healthy to diseased states remain for both cell types and require further studies. For instance, it remains unclear how the still heavily debated concept of gliotransmission changes during various disease conditions. How do neurodegenerative diseases, chronic neuroinflammation, and CNS injury affect neuron–glia communication, and ultimately information processing and function in the brain? How exactly does disturbed astrocyte function and signaling translate into impaired network function, which can be observed in various cognitive disorders and neurodegenerative diseases? The recent discovery of A1 astrocytes that become neurotoxic upon induction by activated microglia represented a milestone in neuroinflammation research (Liddelow et al. 2017). However, the precise role and identity of the neurotoxin is currently still unknown (Liddelow and Barres 2017; Liddelow et al. 2017). While most of the work in the field of neuroinflammation has been focused on cognition-related brain areas, including the cortex and the hippocampus, important findings regarding the contribution of astrocytes and microglial cells to altered hypothalamic network functions in disease states has recently started to emerge. These studies provide compelling evidence for a critical contribution of altered astrocyte and microglia function to exacerbated neuronal activity as well as autonomic and neuroendocrine outputs (e.g., neurohumoral activation) in prevalent cardiovascular diseases, particularly heart failure. Moreover, a growing body of evidence supports astrogliosis in the arcuate nucleus as a key mechanism underlying compromised energy-related signaling in the hypothalamus during obesity. Finally, recent clinical studies provide compelling evidence supporting a high degree of comorbidity between cardiometabolic diseases and cognitive impairment and mood disorders (Hammond et al. 2018), with neuroinflammation standing as a potential common underlying mechanism. Thus, changes in hypothalamic neuro–glial communication in disease states could have important pathological impacts beyond autonomic and neuroendocrine regulation. Recent advances in microglia and astrocyte research have made it possible to label and manipulate glial cells in a cell type-specific manner to provide detailed insights into their origin, genetic variability, and functions during health and disease. Understanding the precise molecular architecture and functional roles of glia will help to develop tailored approaches to treat patients suffering from a myriad of cognitive, neurodegenerative, and even developmental diseases that involve one or more types of glial cells.
6 Key Literature
-
Allen and Eroglu (2017) Comprehensive review about the interaction between astrocytes with neurons and synapses.
-
Althammer et al. (2020) Development of a three-dimensional morphometric glial profiler used to highlight drastic changes to microglial and astrocytic morphology during heart failure.
-
Araque et al. (1999) Review of the seminal finding that astrocytes are an active participant in the tripartite synapse.
-
Brown et al. (2013) The most detailed and comphrehensive review about magnocellular neurons, their function, their in- and outputs and modulation of various vital physiological processes.
-
Eroglu and Barres (2010) The role of glia in synaptogenesis, synapse modulation and synapse elimination.
-
Liddelow and Barres (2017) The role of reactive astrocytes during neuroinflammation.
-
Ludwig and Leng (2006) The mechanism of somato-dendritic release of neuropeptides and its consequences on local networks and physiology.
-
Prinz et al. (2019) Comprehensive overview of microglial concepts, microglial development and microglial function.
-
Stern (2015) Review about the relationship of somato-dendritic release and neuroendocrine integration in the hypothalamus and its link to pathophysiological conditions.
-
Zhao et al. (2015) Fantastic review on the blood-brain barrier and its role in the healthy and diseased brain.
References
Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, Bergles DE (2017) Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93:587–605 e587
Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10:1538–1543
Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM (2011) Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14:1142–1149
Allen NJ, Eroglu C (2017) Cell biology of astrocyte-synapse interactions. Neuron 96:697–708
Althammer F, Grinevich V (2017) Diversity of oxytocin neurons: beyond magno- and parvocellular cell types? J Neuroendocrinol.
Althammer F, Ferreira-Neto HC, Rubaharan M, Roy RK, Patel AA, Cox DN, Stern J (2020) Three-dimensional morphometric analysis reveals time-dependent structural changes in microglia and astrocytes in the central amygdala and hypothalamic paraventricular nucleus of heart failure rats. J Neuroinflamm. https://doi.org/10.1186/s12974-020-01892-4
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–215
Baldwin KT, Eroglu C (2017) Molecular mechanisms of astrocyte-induced synaptogenesis. Curr Opin Neurobiol 45:113–120
Biancardi VC, Stern J (2013) Angiotensin II contributes to microglial cell activation in the PVN of hypertensive rats. FASEB J 27(1 suppl):699.618
Biancardi VC, Stern JE (2016) Compromised blood-brain barrier permeability: novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. J Physiol 594:1591–1600
Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE (2014) Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 63:572–579
Biancardi VC, Stranahan AM, Krause EG, de Kloet AD, Stern JE (2016) Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol 310:H404–H415
Brown CH, Bains JS, Ludwig M, Stern JE (2013) Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol 25:678–710
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504:394–400
de Kloet AD, Liu M, Rodriguez V, Krause EG, Sumners C (2015) Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am J Physiol Regul Integr Comp Physiol 309:R444–R458
Diaz HS, Toledo C, Andrade DC, Marcus NJ, Rio RD (2019) Neuroinflammation in heart failure: new insights for an old disease. J Physiol. https://doi.org/10.1113/JP278864
Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468:223–231
Fiacco TA, McCarthy KD (2018) Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J Neurosci 38:3–13
Filosa JA, Naskar K, Perfume G, Iddings JA, Biancardi VC, Vatta MS, Stern JE (2012) Endothelin-mediated calcium responses in supraoptic nucleus astrocytes influence magnocellular neurosecretory firing activity. J Neuroendocrinol 24:378–392
Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE (2011) State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol 589:3929–3941
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845
Hammond CA, Blades NJ, Chaudhry SI, Dodson JA, Longstreth WT Jr, Heckbert SR, Psaty BM, Arnold AM, Dublin S, Sitlani CM et al (2018) Long-term cognitive decline after newly diagnosed heart failure: longitudinal analysis in the CHS (Cardiovascular Health Study). Circ Heart Fail 11:e004476
Hasan MT, Althammer F, Silva da Gouveia M, Goyon S, Eliava M, Lefevre A, Kerspern D, Schimmer J, Raftogianni A, Wahis J et al (2019) A fear memory engram and its plasticity in the hypothalamic oxytocin system. Neuron 103:133–146 e138
Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39:151–170
Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, Walker AJ, Heller MD, Umemori H, Chen C et al (2018) CD47 protects synapses from excess microglia-mediated pruning during development. Neuron 100:120–134 e126
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487
Ludwig M, Leng G (2006) Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7:126–136
Ludwig M, Stern J (2015) Multiple signalling modalities mediated by dendritic exocytosis of oxytocin and vasopressin. Philos Trans R Soc Lond B Biol Sci 370
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Peri F, Nusslein-Volhard C (2008) Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133:916–927
Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15:300–312
Prinz M, Jung S, Priller J (2019) Microglia biology: one century of evolving concepts. Cell 179:292–311
Rodriguez-Perez AI, Sucunza D, Pedrosa MA, Garrido-Gil P, Kulisevsky J, Lanciego JL, Labandeira-Garcia JL (2018) Angiotensin type 1 receptor antagonists protect against alpha-synuclein-induced neuroinflammation and dopaminergic neuron death. Neurotherapeutics 15:1063–1081
Savtchouk I, Volterra A (2018) Gliotransmission: beyond black-and-white. J Neurosci 38:14–25
Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7:483–495
Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ, Kim IH, Manhaes AC, Rodrigues WS Jr, Pamukcu A et al (2016) Astrocytes assemble thalamocortical synapses by bridging NRX1alpha and NL1 via Hevin. Cell 164:183–196
Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE (2013) Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron 78:1036–1049
Stern JE (2015) Neuroendocrine-autonomic integration in the paraventricular nucleus: novel roles for dendritically released neuropeptides. J Neuroendocrinol 27:487–497
Stern JE, Son S, Biancardi VC, Zheng H, Sharma N, Patel KP (2016) Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 68:1483–1493
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B et al (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178
Ullian EM, Sapperstein SK, Christopherson KS, Barres BA (2001) Control of synapse number by glia. Science 291:657–661
Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, Wagner J, Hasler LM, Wild K, Skodras A et al (2018) Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556:332–338
Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163:1064–1078
Acknowledgements
The authors thank Myurajan Rubaharan for providing the IMARIS videos. We also thank financial support provided by NIH R01HL090948 and R01NS094640 (JES), DFG postdoctoral fellowship AL 2466/1-1 (FA), as well as funding from the Center for Neuroinflammation and Cardiometabolic Diseases.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Labeling of three-dimensional microglia in different colors allows for the individual assessment of their morphometric properties based on IBA1 immunofluorescence (MP4 5987 kb)
High magnification visualization of reconstructed microglia (green) and their original IBA1 immunofluorescence (magenta) (MP4 7001 kb)
Reconstruction of microglial filaments (green, using IMARIS based on IBA1 immunofluorescence) used to analyze process length, number of branches and spines. The blue seed points placed in the center of the microglial soma serve as the origin for the IMARIS-based calculations of the filament reconstructions (MP4 10043 kb)
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Althammer, F., Stern, J.E. (2021). The Multifaceted Roles of Hypothalamic Astrocytes and Microglial Cells in Neuroendocrine and Autonomic Regulation in Health and Disease. In: Tasker, J.G., Bains, J.S., Chowen, J.A. (eds) Glial-Neuronal Signaling in Neuroendocrine Systems. Masterclass in Neuroendocrinology, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-62383-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-62383-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62382-1
Online ISBN: 978-3-030-62383-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)