Abstract
Introduction
Human cytomegalovirus (HCMV) is a member of Betaherpesvirus family and is the leading infectious cause of neurosensory hearing loss, vision loss and neurocognitive disability among congenitally infected children. HCMV is a ubiquitous central nervous system (CNS) pathogen that causes significant morbidity and mortality in individuals that are immune compromised. Even more, HCMV is an important opportunistic pathogen that can cause life threatening disease in HIV patients and transplant recipients. HCMV has broad tropism for multiple cell types including cells of the vascular barrier systems. My aim is to explore HCMV infectivity in human vascular pericyte populations with implications for its role in human vascular diseases.
Methods
Human primary low passaged pericyte populations were examined for HCMV infectivity along with controls by means of phase microscopy, immunofluorescence, immunohistochemistry, electron microscopy, real-time reverse-transcription polymerase chain reaction (RT-PCR), and quantitative RT-PCR (qRT-PCR), the proinflammatory cytokines assay, and HCMV-GFP recombinant virus.
Results
I have discovered that human vascular pericytes from multiple barrier systems that includes the blood brain barrier (BBB ), inner blood retinal barrier (IRRB), glomerular barrier, and pericytes of vascular systems of the placenta, and adipose tissue are fully permissive for HCMV lytic replication. I also observed consistent induction of proinflammatory and angiogenic cytokines after HCMV infection in all of these pericyte populations. Even more, I have examined murine retinal pericyte and found that they are also permissive for mouse cytomegalovirus (MCMV) lytic replication.
Conclusions
The mechanisms associated with HCMV infection and the increased risks for the development of vascular diseases requires further investigation. Studies have shown that people exposed to HCMV infection had higher risk for vascular disease. There are antivirals that can effectively inhibit HCMV replication thus therapeutic modalities designed to specifically protect pericytes populations from HCMV infection could impact vascular disease outcomes especially among the elderly and immune compromise patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pericytes
- HCMV
- Cytomegalovirus
- Infection
- Inflammation
- Brain
- Placenta
- Kidney
- Ocular disease
- Adipose
- Liver
- Stellate cells
- Neurovascular unit
- Latency
- Neurodegenerative disease
- Virus replication
4.1 Introduction
Cytomegalovirus is the most common congenital virus infection in the world. HCMV congenital infections in newborn infants ranges from 0.5% to 0.7% in developed countries and from 1 to 2% in developing countries (Kenneson and Cannon 2007). Approximately 13% of newborns with congenital HCMV are symptomatic at birth and have clinical presentations that includes microcephaly, intrauterine growth restriction, seizures, brain calcifications, hepatosplenomegaly, thrombocytopenia and chorioretinitis (Dollard et al. 2007). Congenital human cytomegalovirus (HCMV) infections can result in abnormalities including intrauterine growth restriction (IUGR), vision loss, neurocognitive disability, motor deficits, seizures, and hearing loss (Nigro and Adler 2011; Stagno et al. 1986). Forty percent of mothers with primary HCMV infection during gestation transmit the infection to their babies (Boppana et al. 1992, 2005). Even more, 58% of transplacental transmission of HCMV occur in women who are seropositive with non-primary maternal infections (Kylat et al. 2006; Boppana et al. 2005). Annually, about 1 out of 200 babies in the US are born with congenital HCMV (Pass and Anderson 2014; Pass et al. 2006; Enders et al. 2011; Wang et al. 2011). Preventing congenital HCMV is a public health priority (Rawlinson et al. 2017). HCMV not only causes life-threatening disease in immunocompromised individuals (Lichtner et al. 2015; Beam et al. 2014), but also HCMV-associated pathologies that lead to long-term health risks represents an important health disparity in underserved communities. Higher infection rates are observed among non-Hispanic Blacks and Mexican Americans than among non-Hispanic Whites (Colugnati et al. 2007). Higher frequencies of new HCMV infections were found among non-Hispanic Blacks and Mexican Americans compared to Whites, and primary infections among adolescent girls 12–19 years of age were 50× more likely in seronegative non-Hispanic Blacks and 15× more likely in seronegative Mexican Americans than in non-Hispanic Whites (Colugnati et al. 2007). Higher rates of primary maternal infections among ethnic minorities resulted in increased rates of congenital disease, perinatal morbidity and higher health care expense.
The human blood-brain barrier (BBB) consists of brain microvascular endothelial cells, brain vascular pericytes, and astrocytes; together they are known as the neurovascular unit (NVU). Pericytes of the BBB play an essential role in a range of microvascular functions, including angiogenesis, vascular remodeling, regression, and stabilization, as well as generation and maintenance of the BBB (Bergers and Song 2005; Armulik et al. 2010, 2011; Hamilton et al. 2010). My studies have revealed that primary human pericytes from vascular compartments of the brain , retina, glomerulus, placenta, and adipose tissue are all permissive for HCMV lytic replication. Even more, I have observed that the pericyte component in vascular compartments of the brain , retina, glomerulus (mesangial cells/pericyte of the glomerulus) and placental vascular pericytes are the most permissive cell type for HCMV infection when compared to other related cells types (Alcendor et al. 2012; Wilkerson et al. 2015; Popik et al. 2019; Aronoff et al. 2017). I also find consistent upregulation of proinflammatory cytokines in these pericyte populations post HCMV infection when compared control cells (Alcendor et al. 2012; Wilkerson et al. 2015; Popik et al. 2019; Aronoff et al. 2017). This permissiveness for HCMV infection occurs across species as demonstrated when I used murine retinal pericytes and mouse cytomegalovirus (MCMV). The connection between HCMV and vascular disease has been well documented. The molecular mechanisms that governs HCMV universal tropism for human pericytes populations had not been appreciated. Therefore the impact of HCMV infection on pericyte populations and its role in the development and progression of vascular disease requires further investigation. Insights obtained from these studies could provide new information for developing novel therapeutic approaches for stroke and other disorders with a neurovascular vascular component, including Alzheimer’s disease and Parkinson Disease.
4.2 Cytomegalovirus Biology
It was Hugo Ribbert who in 1881 discovered the first evidence of “cytomegalia” as inclusions in human kidney and parathyroid gland cells (Ribbert 1904). Many years later it was collaborations between Smith, Rowe, and Weller in 1956 and 1957 that the virus was first isolated and referred as cytomegalovirus (Smith 1956; Rowe et al. 1956; Weller et al. 1957). Human cytomegalovirus or human herpesvirus type-5 (HHV-5) is a member of the human Herpesvirus family in the order Herpesvirales, in the family Herpesviridae, and in the subfamily Betaherpesvirinae (Brito and Pinney 2020). HCMV , has a lipid bilayer enveloped and a protective viral tegument protein layer with an icosahedral capsid (Fig. 4.1a) and an electron dense viral core (Fig. 4.1b) (Yu et al. 2017). HCMV has a double-linear stranded DNA genome of about 235 kb (Martí-Carreras and Maes 2019).
The seroprevalence of HCMV world-wide is between 45% and 100% in adult populations (Cannon et al. 2010). HCMV can cause primary infections in immunocompetent individuals in the form of a mononucleosis-like syndrome (Alberola et al. 2000). Most often these infections are asymptomatic but the virus can establish life-long latency and can reactivate in immunosuppressed individuals (Heald-Sargent et al. 2020). Even more immunocompetent individuals will often shed virus intermittently throughout the life time without clinical disease (Vancíková and Dvorák 2001). The primary target cells of HCMV are monocytes, lymphocytes, and epithelial cells, and the virus establishes latency as circularized episomes inside the nuclei of bone marrow progenitor cells that are CD33+ and CD34+ as well as peripheral blood mononuclear cells (Schottstedt et al. 2010; Collins-McMillen et al. 2018; Sinclair and Sissons 2006). However, HCMV has been shown to infect fibroblasts; epithelial, endothelial, stromal cells, and smooth muscle cells (Haspot et al. 2012); and adipocytes (Bouwman et al. 2008). Our recent studies suggest that vascular pericytes are primary target cells for HCMV and likely serve as amplification reservoirs for HCMV replication in multiple vascular bed. HCMV is a ubiquitous opportunistic pathogen and most notably HCMV can cause severe life-threatening disease in the HIV patient that goes undiagnosed or who is untreated for their HIV disease, the transplant patient under iatrogenic immunuosuppression, and a child of an infected mother that acquires a primary infection early in pregnancy (Boeckh and Geballe 2011). Viral latency and reactivation from latency along genetic strain variation that occurs among clinical strains of HCMV represents a major obstacle for the development of an effective anti-HCMV vaccine (Chen et al. 2019).
HCMV has been implicated in the development of atherosclerosis leading to coronary heart disease, malignant gliomas, systemic autoimmune diseases, type 1 diabetes, type 2 diabetes, Alzheimer’s disease, rheumatoid arthritis, systemic lupus erythematosus, and contributing to frailty via chronic inflammation (Blankenberg et al. 2001; Farias et al. 2019; Pawlik et al. 2003; Pak et al. 1988; Chen et al. 2012; Nell et al. 2013; Rothe et al. 2016; Berman and Belmont 2017; Wang et al. 2010). The role of HCMV in these chronic diseases have not been fully determined and need further study (Janahi et al. 2018).
4.3 Pericytes
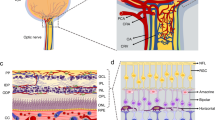
Pericytes, were first describes by Eberth and Rouget and were also known as Roget cells or mural cells in the 1870s. However the name “pericyte ” was introduced by Zimmermann in 1923 (Eberth 1871; Rouget 1873; Zimmermann 1923; Attwell et al. 2016). Pericytes were described as cells that were abluminal to endothelial cells that were later shown to have contractile properties due to their cytoplasmic extensions that wrap around the endothelial cells lining the capillaries (Fig. 4.2a-1, a-2) and venules throughout the body (Sims 1986; Bergers and Song 2005; Armulik et al. 2010, 2011; Hamilton et al. 2010; Rustenhoven et al. 2017). Primary pericytes can be cultivated to confluency and stained to confirm biomarker characterization (Fig. 4.2b-1, b-2, b-3). There are no unique antigenic biomarker for pericytes however they can be identified by their expression of the platelet-derived growth factor receptor-beta (PDGFRb) and of the neural/glial antigen 2 (NG2 proteoglycan) a co-receptor for PDGF (Fig. 4.2b-4) (Smyth et al. 2018; Stallcup 2018). The highest density of vascular pericytes in humans have been observed in the brain and retina (Sims 1986). There are functional differences among pericytes depending on their origin, morphology and their organ-derived vascular bed. Pericytes and microvascular endothelial cells share a common membrane (Birbrair 2018; Abbott et al. 2010) (Fig. 4.3a). The human blood-brain barrier (BBB) consists of brain microvascular endothelial cells, brain vascular pericytes, and astrocytes; together they are known as the neurovascular unit (NVU) (Brown et al. 2019) (Fig. 4.3b). Perciytes are pluripotent cells that are an essential component of the blood-brain-barrier and play major role in the BBB development, stability, angiogenesis, immunoregulation, function, and maintenance of the BBB (Fig. 4.3c). The physical contact and paracrine signaling between pericytes and endothelial cells are essential for new blood vessel formation, maturation, and maintenance. During cellular invasion after traumatic brain injury pericytes are among the first cell to invade the primary lesion in models of brain and spinal injury (Göritz et al. 2011; Birbrair et al. 2018; Dias et al. 2018; Hesp et al. 2018) in support of vascular remodeling.
Brain vascular pericytes. (a) Pericytes as they appear on rat brain capillaries (2a-1) Rat brain capillaries undergoing constriction by vascular pericytes (2a-2). (b) Primary human brain pericytes subconfluent (2b-1); Primary human pericytes moderately confluent (2b-2); Confluent primary human brain pericytes (2b-3); Primary human brain pericytes stained with a monoclonal antibody for NG-2 proteoglycan. Total magnification is 200×
Pericytes and the neurovascular unit (NVU). Brain capillaries featuring pericytes. (a) Cross-section of a brain capillary illustrating the capillary basement membrane, as well as the endothelial cells and brain pericytes abluminal to endothelial cells sharing a contiguous basement membrane. (b) Neurovascular unit (NVU) of the blood-brain barrier (BBB) featuring capillary endothelial cells with tight junction, pericytes and astrocytes. (c) The NVU and other brain parenchymal cells, including neurons and microglia. Important functions of pericytes are also shown listed 1–5
Recent studies have revealed that pericyte deficiency in the CNS can cause a breakdown of the BBB and lead to other degenerative changes in the brain . Most recently, pericytes dysfunction has been observed in Alzheimer’s disease (AD) pathology (Salmina et al. 2019; Yamazaki and Kanekiyo 2017; Liesz 2019; Cai et al. 2018). Studies reveal that Amyloid-β (Aβ) induces neurovascular dysfunction that leads to functional changes in the brain microvascular resulting in the impairment of capillaries to respond to neuronal activity (Nortley et al. 2019). Studies by Nortley et al., suggest that brain vascular pericytes link Aβ to vascular dysfunction in AD (Nortley et al. 2019). Using human brain slices and in a mouse model they reported that oxidative stress caused by toxicity of Aβ causes constriction of capillaries via the generation of reactive species (ROS) by the induction of NOX4 (reduced nicotinamide adenine dinucleotide phosphate oxidase 4). ROS then triggers the release of endothelin-1, which interacts with ETA receptors on pericytes to cause pericyte contraction and subsequent capillary constriction (Nortley et al. 2019). It is clear that AD is a multifactorial disease and the implication HCMV in AD pathogenesis is controversial. Infection due to reactivation of latent neurotropic viruses such as HCMV , Human herpesvirus 1 (HHV-1), Human herpesvirus 2 (HHV-2), and Varicella zoster virus, have been linked to AD neuropathology for decades but whether viral infections could be considered causative in AD or a factor that promotes the progression of AD continues to be investigated (Sochocka et al. 2017). There have been several recent reports that link HCMV as an infectious cause of AD . Laurain et al., reported virological and immunological characteristic of HCMV infection that were associated with AD (Lurain et al. 2013). They demonstrated HCMV serum antibody levels that were associated with the development of neurofibrillary tangles in AD patients (Lurain et al. 2013). In this same study, authors showed amyloid-β induction in human foreskin fibroblasts (HFF cells) infected clinical strains of HCMV . Even more they demonstrated that there was no induction of amyloid-β after herpes simplex virus type 1 (HSV-1) infection of HFF cells and no association of HSV-1 serum antibody titers and AD (Lurain et al. 2013). Studies by Barnes et al., using solid-phase enzyme-linked immunosorbent assay to detect type-specific immunoglobulin G antibody responses to HCMV and herpes simplex virus type 1 (HSV-1) in 849 archived serum samples showed that HCMV infection is associated with an increased risk of AD and a faster rate of cognitive decline in older diverse populations (Barnes et al. 2015). Other studies have suggested that HCMV and HSV-1 interactions play a role in AD (Lövheim et al. 2018). In a study by Loveheim et al., using plasma samples from 360 AD cases examined both HCMV and HSV IgG and IgM antibodies by ELISA assay and found interaction between HCMV and HSV1 to be associated significantly with AD development (Lövheim et al. 2018). However, the role of HCMV , HSV-1/2, and other neurotropic viruses in AD pathobiology is controversial and the mechanisms to support these associations as causative or supportive of progressive disease will require further investigations. The permissiveness of brain pericytes for HCMV infection and the role pericyte loss and dysfunction in AD associated vascular pathology could be linked and deserves further study. As pericyte death reliably indicates very mild cognitive impairments (Nation et al. 2019), it is likely that pericyte death is not only a symptom of AD but may also be a potential cause of AD . Hypoxia influences amyloid precursor protein (APP) mechanisms that lead to an increased production of b- and g-secretase and therefore increased Aβ production (Zlokovic 2011). This suggests that pericyte loss may be an early event in the progression of AD , as pericyte loss reduces cerebral blood flow, thus creating potential hypoxic areas throughout the brain .
4.4 Cytomegalovirus and Vascular Disease
Several serological and molecular-biological studies have shown an association between HCMV infection of endothelial cells and in the development of atherosclerosis (AS) (Gkrania-Klotsas et al. 2012; Muhlestein et al. 2000; Simanek et al. 2011; Spyridopoulos et al. 2016). A meta-analysis of data retrieved from electronic databases that involved 30 studies, 3328 cases and 2090 controls showed that HCMV infection is significantly associated with an increased risk for atherosclerosis. A meta-analysis conducted by Lv et al., involving 68 studies, from 24 countries (12,027 cases and 15,386 controls) suggests that HCMV infection is associated with an increased risk for vascular diseases (Lv et al. 2017). The study found that people exposed to HCMV infection had higher risk for vascular diseases (OR 1.70 [95% CI 1.43–2.03]) (Lv et al. 2017). HCMV infection has been shown to be a risk factor for the development of renal vascular disease. Most recently, Lee et al., have shown the high burden of HCMV results in poor vascular health in renal transplant patients compared to healthy controls (Lee et al. 2019). After Examining carotid intima-media thickness, FMD, eGFR and plasma levels of HCMV antibodies and the expression of ICAM-1, VCAM-1, P-selectin, sIFNαR2, sTNFR1, sCD14 and CRP they predicted that high viral burden was associated with poor endothelial health and vascular damage in renal transplant patients when compared to controls (Lee et al. 2019). In addition, a study by Huang et al., involving 200 patients diagnosed with stroke and 200 controls found a correlation between HCMV and stroke (Huang et al. 2012). HCMV seropositivity was higher in the stroke patients than in controls (55.0% vs. 23.5%; P < 0.0001). Huang et al., also observed that the presence of HCMV DNA increased the risk of stroke (Huang et al. 2012). The mechanisms associated with HCMV infection and the increased risks for the development of vascular diseases requires further investigation. Even more HCMV infection upregulates the endothelin receptor type B protein (ETBR) expression and mRNA expression in both endothelial cells and smooth muscle cells (Yaiw et al. 2015). ETBR, a G protein-coupled receptor that mediates the vascular effects of ET-1 a potent vasoconstrictor (Yaiw et al. 2015). HCMV may have a role in cardiovascular disease via upregulation of ETBR. ET-1 is also known to cause capillary constriction via ETA receptor interactions on pericytes.
4.4.1 Cytomegalovirus Infection of Pericytes in the Neurovascular Unit
The blood-brain barrier (BBB) interfaces the peripheral circulation and the central nervous system (CNS) allowing nutrients into the CNS and preventing blood-borne pathogens from harming the brain . This barrier is an elaborate network of tight junctions (TJ) between capillary endothelial cells that lack fenestrae and have a reduced capacity for pinocytosis (Ballabh et al. 2004; Engelhardt 2003). The TJ of the capillary endothelium is supported by astrocytic endfeet and pericytes. Cerebral vascular pericytes (CNS pericytes) have been shown to enhance TJ barrier function, stimulate expression of TJ proteins and reduce the paracellular permeability of the capillary endothelium (Garberg et al. 2005; Ramsauer et al. 2002; Shepro and Morel 1993; Tsukita et al. 2001). Pericytes are adult multipotent, contractile and migratory stem cells (Balabanov and Dore-Duffy 1998; Dore-Duffy 2008) that surround capillaries and actively communicate with other cells of the neurovasculature, including endothelial cells, astrocytes and neurons. Completely surrounded by a basal lamina, they also contribute to the deposition of the basal lamina during vascular development and angiogenesis (Dore-Duffy and Cleary 2011). Although a critical cellular component for the development and function of the BBB , the role of pericytes in HCMV infection and dissemination has largely been ignored (Lai and Kuo 2005). Rather, to date, astrocytes and brain microvascular endothelial cells (BMVEC) cells have been implicated as cell types that support HCMV dissemination at the blood-brain barrier level (Cheeran et al. 2009). Recent studies have revealed that pericyte deficiency in the CNS can cause a breakdown of the BBB and lead to other degenerative changes in the brain (Cai et al. 2017). Ribbert (1904) demonstrated murine CMV (MCMV) infection of pericytes in brown and white adipose tissue of young adult infected mice. These mice later developed viral-induced inflammatory lesions in peripancreatic and salivary gland adipose tissues. However it was Alcendor et al., that was the first demonstrate HCMV infection of primary human brain pericytes (Alcendor et al. 2012). Using a clinical isolate of HCMV (SBCMV), microscopy of infected pericytes showed virion production and typical cytomegalic cytopathology (Fig. 4.4a1, a-2, a-3). This finding was confirmed by the expression of major immediate early and late virion proteins and by the presence of HCMV mRNA (Fig. 4.4a4, a5) and finally by electron microscopy (Fig. 4.4a-6). Brain pericytes were fully permissive for CMV lytic replication after 96 h in culture compared to human astrocytes or human brain microvascular endothelial cells (BMVEC) (Fig. 4.4b-1, b-2, b-3, b-4, b-5, b-6). However, temporal transcriptional expression of pp65 virion protein after SBCMV infection was lower than that seen with the HCMV Towne laboratory strain (Alcendor et al. 2012). Using RT-PCR and dual-labeled immunofluorescence, proinflammatory cytokines CXCL8/IL-8, CXCL11/ITAC, and CCL5/Rantes were upregulated in SBCMV-infected cells, as were tumor necrosis factor-alpha (TNF-alpha), interleukin-1 beta (IL-1beta), and interleukin-6 (IL-6) (Alcendor et al. 2012). Pericytes exposed to SBCMV elicited higher levels of IL-6 compared to both mock-infected as well as heat-killed virus controls (Alcendor et al. 2012). A 6.6-fold induction of IL-6 and no induction TNF-alpha was observed in SBCMV-infected cell supernatants at 24 h post infection (Alcendor et al. 2012). Using archival brain tissue from a patient coinfected with HCMV and HIV, we also found evidence of HCMV infection of pericytes using dual-label immunohistochemistry, as monitored by NG2 proteoglycan staining (Alcendor et al. 2012). These studies suggests that HCMV lytic infection of primary human brain pericytes contribute to both virus dissemination in the CNS as well as neuroinflammation. A hypothetical model of HCMV infection of the NVU shows viral replication preference for brain pericytes when compared astrocytes and BMVEC (Fig. 4.5).
(a) Primary brain vascular pericytes. Phase contrast images of: (1) an uninfected subconfluent monolayer of primary brain vascular pericytes, (2) a confluent monolayer of brain vascular pericytes, and (3) pericytes 72 h after infection with SBCMV. Immunofluorescence staining of SBCMV-infected pericytes for (4) HCMV MIE protein and (5) pp65 late protein. (6) TEM of SBCMV-infected pericytes showing HCMV virions in the cytoplasm (see arrow). With the exception of the TEM, images were taken on a Nikon TE2000S microscope (200× magnification). HCMV human cytomegalovirus, MIE major immediate early protein, SBCMV primary HCMV isolate from a patient, TEM transmission electron microscopy. (b) Time course analysis of human cytomegalovius-GFP (HCMV-GFP) infection of BBB (blood-brain barrier) cells. For comparison purposes, (a) the top panel includes phase contrast images of human mock and infected brain microvascular endothelial cells, brain vascular pericytes and astrocytes. The bottom panel shows phase contrast images of infected brain microvascular endothelial cells, brain pericytes and astrocytes with a fluorescent overlay showing HCMV-GFP-positive cells. Total magnification is 200×
4.4.2 Cytomegalovirus Infection of Pericytes in Retinal Vascular Unit
The inner blood-retinal barrier (IBRB) consists of retinal microvascular endothelial cells covered with tightly associated pericytes and Müller cells; together, they make up the retinal vascular unit (RVU) (Wilkerson et al. 2015). My study showed that normal primary human retinal pericytes expressed the biomarker neuron-glial antigen 2 and that retinal pericytes are highly permissive for HCMV infection as demonstrated by cytomegalic cytopathology and expressed of the major immediate protein (MIE) and the late phosphorylated envelop protein 65 (Fig. 4.6a-1, a-2, a-3, a-4, a-5) and shown to support lytic replication of a recombinant GFP virus (Fig. 4.6a-6) (Wilkerson et al. 2015). qRT-PCR analysis showed full lytic replication of HCMV in retinal pericytes. Pericytes were the most permissive cell type for HCMV replication when compared to retinal endothelial cells and Mϋller cells (Fig. 4.6b1, b-2, b-3, b-4, b-5, b-6) Pericytes exposed to SBCMV expressed higher levels of vascular endothelial cell growth factor mRNA compared to controls (Wilkerson et al. 2015). Luminex analysis of supernatants from SBCMV-infected retinal pericytes had increased levels of macrophage inflammatory protein-1α, beta-2 microglobulin (B2-m), matrix metalloproteinase-3 and -9 (MMP3/9), and lower levels of IL-6 and IL-8 compared to controls. At 24 h post infection , pericytes expressed higher levels of IL-8, TIMP-1 (tissue inhibitor of metalloproteinase-1), and RANTES (regulated upon activation normal T cell-expressed and presumably secreted) but lower levels of MMP9 (Wilkerson et al. 2015). Time course analysis showed that both brain and retinal pericytes were more permissive for HCMV infection than other cellular components of the BBB (blood-brain barrier) and IBRB. Using a Tricell culture model of the IBRB (retinal endothelial, pericytes, Müller cells), retinal pericytes were most permissive for SBCMV infection (Wilkerson et al. 2015). SBCMV infection of this IBRB Tricell mixture for 96 h that resulted in increased levels of IL-6 , MMP9, and stem cell factor with a concomitant decrease in granulocyte-macrophage colony-stimulating factor and TNF-alpha (Wilkerson et al. 2015). In retinal pericytes, HCMV induces proinflammatory and angiogenic cytokines. In the IBRB, pericytes likely serve as an amplification reservoir which contributes to retinal inflammation and angiogenesis. Even more, I examined the permissiveness of normal murine retinal pericytes expressing alpha actin smooth muscle (Fig. 4.7a–c) for mouse cytomegalovirus (MCMV) infection . At 72 h post infection I observed pericytes being fully permissive for MCMV lytic replication as demonstrated by expression of the late MCMV M55 late protein (Fig. 4.7d) and lytic replication of a recombinant MCMV expressing green fluorescent protein (GFP) (Fig. 4.7e).
Human cytomegalovirus (HCMV) infectivity of primary human retinal pericytes. (a) Phase contrast and fluorescent images of retinal pericytes (1) uninfected subconfluent monolayer of retinal pericytes, (2) confluent monolayer of retinal pericytes and (3) pericytes 96 h after infection with the clinical strain SBCMV. Immunofluorescence staining of SBCMV-infected retinal pericytes for (4) major immediate protein (MIE) protein and (5) pp65 late stage protein. (6) Phase fluorescent overlay image of human retinal pericytes infected with a recombinant HCMV virus expressing GFP. All images were taken on a Nikon TE2000S microscope (200× magnification); (b) Time course analysis of human cytomegalovirus-GFP (HCMV-GFP) infection of IBRB (inner blood-retinal barrier) cells. (a) Top panel: phase contrast images of human mock and infected retinal microvascular endothelial cells, retinal pericytes and Müller cells. Bottom panel: phase contrast images of infected retinal microvascular endothelial cells, retinal pericytes and Müller cells with a fluorescent overlay showing HCMV-GFP-positive cells. Magnification 200×
MCMV infection of mouse retinal pericytes. Cultivated mouse pericytes infected with mouse cytomegalovirus. (a) Pericytes subconfluent; (b) Pericytes confluent; (c) Pericytes staining positive for alpha actin smooth muscle (green FITC/blue DAPI); (d) Phase image of pericytes infected with MCMV; (e) Pericytes staining positive for the MCMV M55 late protein; (f) Pericytes infected with a GFP-positive MCMV (virus infected appear green)
4.4.3 Cytomegalovirus Infection of Mesangial Cells: Pericytes of the Glomerular Vascular Unit
Mesangial cells are considered specialized pericytes of the renal glomerulus (Yamanaka 1988; Diaz-Flores et al. 2009; Lindahl et al. 1998; Smith et al. 2012). The glomerular vascular unit (GVU) consists of glomerular endothelial cells, podocytes, and mesangial cells (Popik et al. 2019). The molecular crosstalk between mesangial cells, podocytes, and glomerular endothelial cells is essential for glomerular filtration. Mesangial cells form a supporting framework that maintains the structural integrity of the glomerular tuft that includes the glomerular capillaries (Wilkerson et al. 2015). Mesangial cells affect glomerular hemodynamics by altering glomerular vascular resistance (Wilkerson et al. 2015). To date, mesangial cells, and their contribution to HCMV infection in the glomerulus is poorly understood. I showed that mesangial cells and glomerular endothelial cells but not podocytes were permissive for both lab adapted and clinical strains of HCMV (Fig. 4.8a) (Popik et al. 2019). A hypothetical model is shown to support this finding (Fig. 4.8b). Luminex analysis revealed dysregulation proinflammatory cytokines expressed by mesangial cells exposed to the SBCMV clinical strain (Popik et al. 2019). HCMV upregulation of angiogenic and proinflammatory cytokines could contribute to glomerular inflammation (Popik et al. 2019). Briefly, mesangial cells exposed to SBCMV for 96 h revealed an increase in the expression of B2-m (beta-2 microglobulin) ferritin, complement C3, IL-6 , IL-7, IL-8, RANTES, VEGF, and MMP-3 compared to mock infected controls (Popik et al. 2019). Taken together upregulation of these factor are known to greatly impact the development of kidney disease. However, because these data represent in vitro studies in primary cells infected at low multiplicities of infection this will require further investigation. Although I was able to demonstrate HCMV infection in pericytes in renal tissue from a transplant patient supporting in vivo for HCMV infection of pericytes in renal tissue (Popik et al. 2019).
Infection with the clinical strain (SBCMV) of GVU (Glomerular Vascular Unit) cells 96 h after infection. (a) A graph showing the number of infected SBCMV positive podocytes (open bars), glomerular endothelial cells (gray bars), and mesangial (black bars) per 4 × 106 total cells 96 h after infection. (b) Right panel: Immunohistochemical stained images of SBCMV infected podocytes, glomerular endothelial cells, and mesangial cells showing SBCMV positive cells (stained brown). All images were taken on a Nikon TE2000S microscope mounted with a charge-coupled device (CCD) camera at 200× magnification. (c) Hypothetical model of HCMV dissemination in the glomerulus of the human kidney showing HCMV infection of mesangial cells and glomerular endothelial cells but not podocytes
4.4.4 Cytomegalovirus Infection of Pericytes in at the Placental Vascular Barrier
Placental pericytes are essential for endothelial cell proliferation as well as for placental microvasculature stability and integrity but have largely been ignored in placenta biology (Price et al. 1990). Pericytes are also critical for placental vascular development and angiogenesis. A blood-placental barrier model consisting of trophoblasts and placental pericytes has been established. HCMV placental pathogenesis models that include placental pericytes have not been reported. The signaling mechanisms between placental pericytes, cytotrophoblast, and villous fibroblasts are largely unknown (Alcendor et al. 2012).
I was among the first (to my knowledge) to report the infectivity of human placental pericytes for HCMV , their potential role in viral dissemination in placental tissue, and the implications for HCMV-associated congenital disease (Aronoff et al. 2017). I first tracked HCMV in placental tissue obtained from a child with HCMV inclusion disease by dual labeled immunohistochemistry and showed overtime that the virus infects cytotrophoblasts and spreads into villous during dissemination (Fig. 4.9a). My findings show that placental pericytes strongly support HCMV replication, inducing proinflammatory and angiogenic cytokines that could contribute to viral dissemination, placenta inflammation, and dysregulation of placental angiogenesis. I showed that primary human placenta pericytes were more permissive for HCMV infectivity than either primary human cytotrophoblasts or villous fibroblasts (Fig. 4.9b, c). I also demonstrate the HCMV induction of giant cell formation only occurs infected pericytes (Fig. 4.9c see insert). Using a mixture of placental pericytes, cytotrophoblasts and villous fibroblasts, I find dysregulation of cytokines with a unique profile when compared with pericytes alone at early and late times post infection (Aronoff et al. 2017). I hypothesize that human placental pericytes are the most permissive cell type in the placenta for HCMV infection and serve as amplification reservoirs for HCMV dissemination in placental tissue that is governed by virus host-cell receptor entry.
HCMV dissemination in the placenta that includes placental pericytes. (a) HCMV entry into placenta tissue demonstrated by dual labeled IHC (trophoblasts, brown color, HCMV red color); (b) HCMV replication kinetics examined using a GFP recombinant virus in human placental fibroblasts, cytotrophoblasts and placental pericytes. Infections were performed in chamber slides in triplicate and the average number GFP positive cells are plotted in the graph; (c) HCMV-GFP positives cells after infection (insert for pericytes shows evidence of giant cell formation)
4.4.5 Cytomegalovirus Infection of Pericytes in Adipose Vasculature
The relevance of HCMV infection of pericytes in adipose vasculature is not apparent however I observed both full lytic replication in human adipose pericytes (obtained from Paula Dore Duffy from Wayne State University) data unpublished. To my knowledge there is only one report of mouse cytomegalovirus (MCMV) infection of mouse pericytes in salivary gland adipose vasculature tissue (Price et al. 1990). After MCMV infection of young adult mice they observed MCMV replication in adipocytes, fibroblasts, endothelial cells and pericytes in both brown and white adipose tissues from salivary glands as demonstrated by immunoperoxidase staining and electron microscopy (Price et al. 1990). However I shown human adipose tissue pericytes are fully permissive for HCMV lytic replication as demonstrated by infecting adipose pericytes with Toledo HCMV (lab strain) when compared to the mock infected control (Fig. 4.10). This is the first unpublished report of HCMV infection of human adipose vascular pericytes. This finding supports the notion that all human vascular pericytes are permissive for HCMV . Even more, it suggest that adipose tissue could serve as a reservoir for HCMV replication and dissemination which is a novel concept that will require further investigation.
4.5 Discussion and Conclusions
These studies described published evidence from my laboratory of HCMV lytic replication in human pericyte populations of the brain , retinal, placental, glomerular (mesangial cells), as well as unpublished evidence of HCMV infection and lytic replication in pericytes from human adipose tissue (Fig. 4.11). These studies relied mainly on in vitro data obtained from experiments using primary cells at low passage and infection at low multiplicity. Pericyte population were largely found to be the most permissive cell type for HCMV infection within neurovascular unit, the retinal vascular unit, placenta vascular barrier, and the glomerular vascular unit. In these vascular barrier systems, HCMV infection resulted in the induction of proinflammatory and angiogenic cytokines that likely contributes to vascular inflammation. Hence, vascular pericytes may represent a global reservoir for HCMV that can mobilized during immunosuppression. In addition, results from these studies support the notion that all pericytes populations, regardless of tissue origin, are permissive for HCMV infection , and that loss of HCMV immune surveillance or intermittent viral shedding over time could contribute to pericyte loss or dysfunction, microvascular instability, and subclinical progressive vascular disease. Pericytes have also been implication cerebral vascular diseases like AD However the role of HCMV , HSV-1/2, in AD remains highly controversial and requires further study.
Human vascular pericytes currently known to be permissive for HCMV lytic replication. Human vascular pericytes shown to be permissive for HCMV infection. (a) Brain pericyte; (b) Retinal pericytes; (c) Renal mesangial cells (kidney pericytes); (d) Placental pericytes; (e) Adipose pericytes (phase image). All cell types with the exception of adipose pericytes were stained with an antibody to the HCMV pp65 protein late protein. All images were taken at 200× magnification
References
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 1:13–25
Alberola J, Tamarit A, Igual R, Navarro D (2000) Early neutralizing and glycoprotein B (gB)-specific antibody responses to human cytomegalovirus (HCMV) in immunocompetent individuals with distinct clinical presentations of primary HCMV infection. J Clin Virol 2:113–122
Alcendor DJ, Charest AM, Zhu WQ, Vigil HE, Knobel SM (2012) Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflamm 9:95
Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K et al (2010) Pericytes regulate the blood-brain barrier. Nature 468:557–561
Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215
Aronoff DM, Correa H, Rogers LM, Arav-Boger R, Alcendor DJ (2017) A Placental pericytes and cytomegalovirus infectivity: implications for HCMV placental pathology and congenital disease. Am J Reprod Immunol 2017:78
Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T (2016) What is a pericyte? J Cereb Blood Flow Metab 2:451–455
Balabanov R, Dore-Duffy P (1998) Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res 6:637–644
Ballabh P, Braun A, Nedergaard M (2004) The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 1:1–13
Barnes LL, Capuano AW, Aiello AE, Turner AD, Yolken RH, Torrey EF, Bennett DA (2015) Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis 2:230–237
Beam E, Dioverti V, Razonable RR (2014) Emerging cytomegalovirus management strategies after solid organ transplantation: challenges and opportunities. Curr Infect Dis Rep 9:419
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology 7:452–464
Berman N, Belmont HM (2017) Disseminated cytomegalovirus infection complicating active treatment of systemic lupus erythematosus: an emerging problem. Lupus 4:431–434
Birbrair A (2018) Pericyte biology: development, homeostasis, and disease. Adv Exp Med Biol 1109:1–3
Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM et al (2018) Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther 5:122
Blankenberg S, Rupprecht HJ, Bickel C, Espinola-Klein C, Rippin G, Hafner G, Ossendorf M, Steinhagen K, Meyer J (2001) Cytomegalovirus infection with interleukin-6 response predicts cardiac mortality in patients with coronary artery disease. Circulation 24:2915–2921
Boeckh M, Geballe AP (2011) Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Investig 121:1673–1680
Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA (1992) Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J 11:93–99
Boppana SB, Fowler KB, Pass RF, Rivera LB, Bradford RD, Lakeman FD, Britt WJ (2005) Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J Pediatr 146:817–823
Bouwman JJ, Visseren FL, Bouter KP, Diepersloot RJ (2008) Infection-induced inflammatory response of adipocytes in vitro. Int J Obes 32:892–901
Brito AF, Pinney JW (2020) The evolution of protein domain repertoires: shedding light on the origins of the Herpesviridae family. Virus Evol 6(1):veaa001. https://doi.org/10.1093/ve/veaa001
Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA (2019) Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci 13:282
Cai W, Liu H, Zhao J, Chen LY, Chen J, Lu Z, Hu X (2017) Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res 2:107–121
Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q (2018) Role of blood-brain barrier in Alzheimer’s disease. J Alzheimers Dis 4:1223–1234
Cannon MJ, Schmid DS, Hyde TB (2010) Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20:202–213
Cheeran MC, Lokensgard JR, Schleiss MR (2009) Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 1:99–126
Chen S, de Craen AJ, Raz Y, Derhovanessian E, Vossen AC, Westendorp RG, Pawelec G, Maier AB (2012) Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old. Results from the Leiden 85-plus Study. Immun Ageing 1:18
Chen SJ, Wang SC, Chen YC (2019) Antiviral agents as therapeutic strategies against cytomegalovirus infections. Viruses 12(1):23
Collins-McMillen D, Buehler J, Peppenelli M, Goodrum F (2018) Molecular determinants and the regulation of human cytomegalovirus latency and reactivation. Viruses 10(8):20. pii: E444
Colugnati FA, Staras SA, Dollard SC, Cannon MJ (2007) Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis 7:71
Dias DO, Kim H, Holl D, Werne Solnestam B, Lundeberg J, Carlén M et al (2018) Reducingpericyte-derivedscarringpromotesrecoveryafterspinal cordinjury. Cell 173:153.e22–165.e22
Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Díaz-Flores JL (2009) Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24:909–969
Dollard SC, Grosse SD, Ross DS (2007) New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 5:355–363
Dore-Duffy P (2008) Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 16:1581–1593
Dore-Duffy P, Cleary K (2011) Morphology and properties of pericytes. Methods Mol Biol 686:49–68
Eberth CJ (1871) Handbuch der Lehre von der Gewegen des Menschen und der Tiere, vol 1. Engelmann, Leipzig
Enders G, Daiminger A, Bäder U, Exler S, Enders M (2011) Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol 52:244–256
Engelhardt B (2003) Development of the blood-brain barrier. Cell Tissue Res 1:119–129
Farias KPRA, Moreli ML, Floriano VG, da Costa VG (2019) Evidence based on a meta-analysis of human cytomegalovirus infection in glioma. Arch Virol 164(5):1249–1257
Garberg P, Ball M, Borg N, Cecchelli R, Fenart L, Hurst RD, Lindmark T, Mabondzo A, Nilsson JE, Raub TJ, Stanimirovic D, Terasaki T, Oberg JO (2005) In vitro models for the blood–brain barrier. Toxicol In Vitro 19:299–334
Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ (2012) Higher immunoglobulin G antibody levels against cytomegalovirus are associated with incident ischemic heart disease in the population-based EPIC-Norfolk cohort. J Infect Dis 2012(206):1897–1903
Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J (2011) A pericyte origin of spinal cord scar tissue. Science 333:238–242
Hamilton NB, Attwell D, Hall CN (2010) Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenerg 2:1–14
Haspot F et al (2012) Human cytomegalovirus entry into dendritic cells occurs via a macropinocytosis-like pathway in a pH independent and cholesterol-dependent manner. PLoS One 7:e34795
Heald-Sargent TA, Forte E, Liu X, Thorp EB, Abecassis MM, Zhang ZJ, Hummel MA (2020) New insights into the molecular mechanisms and immune control of cytomegalovirus reactivation. Transplantation 104:e118–e124. https://doi.org/10.1097/TP.0000000000003138
Hesp ZC, Yoseph RY, Suzuki R, Jukkola P, Wilson C, Nishiyama A et al (2018) Proliferating NG2-cell-dependent angiogenesis and scar formation alter axon growth and functional recovery after spinal cord injury in mice. J Neurosci 38:1366–1382
Huang ZR, Yu LP, Yang XC, Zhang F, Chen YR, Feng F, Qian XS, Cai J (2012) Human cytomegalovirus linked to stroke in a Chinese population. CNS Neurosci Ther 6:457–460
Janahi EMA, Das S, Bhattacharya SN, Haque S, Akhter N, Jawed A, Wahid M, Mandal RK, Lohani M, Areeshi MY, Ramachandran VG, Almalki S, Dar SA (2018) Cytomegalovirus aggravates the autoimmune phenomenon in systemic autoimmune diseases. Microb Pathog 120:132–139
Kenneson A, Cannon MJ (2007) Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 4:253–276
Kylat RI, Kelly EN, Ford-Jones EL (2006) Clinical findings and adverse outcomes in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. Eur J Pediatrics 165:773–778
Lai CH, Kuo KH (2005) The critical component to establish in vitro BBB model: Pericyte. Brain Res Brain Res Rev 2:258–265
Lee S, Brook E, Affandi J, Howson P, Tanudjaja SA, Dhaliwal S, Irish A, Price P (2019) A high burden of cytomegalovirus marks poor vascular health in transplant recipients more clearly than in the general population. Clin Transl Immunol 2:e1043
Lichtner M, Cicconi P, Vita S, Cozzi-Lepri A, Galli M, Lo Caputo S, Saracino A, De Luca A, Moioli M, Maggiolo F, Marchetti G, Vullo V, d’Arminio Monforte A, ICONA Foundation Study (2015) Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis 2:178–186
Liesz A (2019) The vascular side of Alzheimer’s disease. Science 6450:223–224
Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C (1998) Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125:3313–3322
Lövheim H, Olsson J, Weidung B, Johansson A, Eriksson S, Hallmans G, Elgh F (2018) Interaction between cytomegalovirus and herpes simplex virus type 1 associated with the risk of Alzheimer’s disease development. J Alzheimers Dis 3:939–945
Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, Bennett DA, Schneider JA (2013) Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 4:564–572
Lv YL, Han FF, Gong LL, Liu H, Ma J, Yu WY, Wan ZR, Jia YJ, Zhang W, Shi M, Liu LH (2017) Human cytomegalovirus infection and vascular disease risk: a meta-analysis. Virus Res 227:124–134
Martí-Carreras J, Maes P (2019) Human cytomegalovirus genomics and transcriptomics through the lens of next-generation sequencing: revision and future challenges. Virus Genes 2:138–164
Muhlestein JB, Horne BD, Carlquist JF, Madsen TE, Bair TL, Pearson RR, Anderson JL (2000) Cytomegalovirus seropositivity and C-reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation 102:1917–1923
Nation DA, Ho JK, Dutt S, Han SD, MHC L (2019) Alzheimer’s disease neuroimaging initiative neuropsychological decline improves prediction of dementia beyond Alzheime’s disease biomarker and mild cognitive impairment diagnoses. J Alzheimers Dis 4:1171–1182
Nell S, Lurain BA, Hanson JM, Leurgans SE, Landay AL, Bennett DA, Schneider JA (2013) Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 4:564–572
Nigro G, Adler SP (2011) Cytomegalovirus infections during pregnancy. Curr Opin Obstet Gynecol 23:123–128
Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, Kyrargyri V, Pfeiffer T, Khennouf L, Madry C, Gong H, Richard-Loendt A, Huang W, Saito T, Saido TC, Brandner S, Sethi H, Attwell D (2019) Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 19(6450):365
Pak CY, Eun HM, McArthur RG, Yoon JW (1988) Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet 8601:1–4
Pass RF, Anderson B (2014) Mother-to-child transmission of cytomegalovirus and prevention of congenital infection. J Pediatr Infect Dis Soc Suppl 1:S2–S6
Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S (2006) Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol 35:216–220
Pawlik A, Ostanek L, Brzosko I, Brzosko M, Masiuk M, Machalinski B, Gawronska-Szklarz B (2003) The expansion of CD4+CD28- T cells in patients with rheumatoid arthritis. Arthritis Res Ther 4:R210–R213
Popik W, Correa H, Khatua A, Aronoff DM, Alcendor DJ (2019) Mesangial cells, specialized renal pericytes and cytomegalovirus infectivity: implications for HCMV pathology in the glomerular vascular unit and post-transplant renal disease. J Transl Sci 2019:5
Price P, Eddy KS, Papadimitriou JM, Robertson TA, Shellam GR (1990) Cytomegalovirus infection of adipose tissues induces steatitis in adult mice. Int J Exp Pathol 4:557–571
Ramsauer M, Krause D, Dermietzel R (2002) Angiogenesis of the blood–brain barrier in vitro and the function of cerebral pericytes. FASEB J 16:1274–1276
Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, Daly K, Doutré S, Gibson L, Giles ML, Greenlee J, Hamilton ST, Harrison GJ, Hui L, Jones CA, Palasanthiran P, Schleiss MR, Shand AW, van Zuylen WJ (2017) Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 6:e177–e188
Ribbert H (1904) Ueber protozoenartige Zellen in der Niere eines syphilitischen Neugeborenen und in der Parotis von Kindern. Zbl All Pathol 15:945–948
Rothe K, Quandt D, Schubert K, Rossol M, Klingner M, Jasinski-Bergner S, Scholz R, Seliger B, Pierer M, Baerwald C, Wagner U (2016) Latent cytomegalovirus infection in rheumatoid arthritis and increased frequencies of cytolytic LIR-1+CD8+ T cells. Arthritis Rheumatol 2:337–346
Rouget C (1873) Me’moire sur le de’veloppement, la structure et les propriete’s physiologiques des capillaires sanguins et lymphatiques. Arch Physiol Norm Path 5:603–663
Rowe WP, Hartley JW, Waterman S, Turner HC, Huebner RJ (1956) Cytopathogenic agents resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol 92:418–424
Rustenhoven J, Jansson D, Smyth LC, Dragunow M (2017) Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci 3:291–304
Salmina AB, Komleva YK, Lopatina OL, Birbrair A (2019) Pericytes in Alzheimer’s disease: novel clues to cerebral amyloid angiopathy pathogenesis. Adv Exp Med Biol 1147:147–166
Schottstedt V, Blümel J, Burger R et al (2010) Human cytomegalovirus (HCMV)-revised. Transfus Med Hemother 2010(37):365–375
Shepro D, Morel NM (1993) Pericyte physiology. FASEB J 7:1031–1038
Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE (2011) Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 6:e16103
Sims DE (1986) The pericyte—a review. Tissue Cell 18:153–174
Sinclair J, Sissons P (2006) Latency and reactivation of human cytomegalovirus. J Gen Virol 87(Pt 7):1763–1779
Smith MG (1956) Propagation in tissue cultures of a cytopathogenic virus from human salivary gland virus (SGV) disease. Proc Soc Exp Biol Med 92:424–430
Smith SW, Chand S, Savage CO (2012) Biology of the renal pericyte. Nephrol Dial Transplant 27:2149–2155
Smyth LCD, Rustenhoven J, Scotter EL, Schweder P, Faull RLM, Park TIH, Dragunow M (2018) Markers for human brain pericytes and smooth muscle cells. J Chem Neuroanat 92:48–60
Sochocka M, Zwolińska K, Leszek J (2017) The infectious etiology of Alzheimer’s disease. Curr Neuropharmacol 7:996–1009
Spyridopoulos I et al (2016) CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results fromthe Newcastle 85+ study. Aging Cell 15:389–392
Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford C (1986) Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 14:1904–1808
Stallcup WB (2018) The NG2 proteoglycan in pericyte biology. Adv Exp Med Biol 1109:5–19
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Vancíková Z, Dvorák P (2001) Cytomegalovirus infection in immunocompetent and immunocompromised individuals--a review. Curr Drug Targets Immune Endocr Metabol Disord 2:179–187
Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP (2010) Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol 10:1144–1152
Wang C, Zhang X, Bialek S, Cannon MJ (2011) Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 2:e11–e13
Weller TH, MacAuley JC, Craig JM, Wirth P (1957) Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease. Exp Biol Med 94:4–12
Wilkerson I, Laban J, Mitchell J, Sheibani N, Alcendor DJ (2015) Retinal pericytes and cytomegalovirus infectivity: Implications for both CMV induced retinopathy and congenital ocular disease. J Neuroinflamm 12:2
Yaiw KC, Mohammad AA, Costa H, Taher C, Badrnya S, Assinger A, Wilhelmi V, Ananthaseshan S, Estekizadeh A, Davoudi B, Ovchinnikova O, Shlyakhto E, Rafnsson A, Khan Z, Butler L, Rahbar A, Pernow J, Söderberg-Nauclér C (2015) Human cytomegalovirus up-regulates endothelin receptor type B: implication for vasculopathies? Open Forum Infect Dis 4:ofv155
Yamanaka N (1988) Development of the glomerular mesangium. Pediatr Nephrol 2:85–91
Yamazaki Y, Kanekiyo T (2017) Blood-brain barrier dysfunction and the pathogenesis of Alzheimer’s disease. Int J Mol Sci 18(9):E1965. pii: E1965
Yu X, Jih J, Jiang J, Zhou ZH (2017) Atomic structure of the human cytomegalovirus capsid with its securing tegument layer of pp150. Science 356:6345. pii: eaam6892
Zimmermann KW (1923) Der feinere Bau der Blutkapillaren. Z Anat Entwicklungsgesch 68:29–109
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723–738
Acknowledgements
The author would like to thank Waldemar Popik and Atanu Khatua for past collaborations.
Dedication
Special dedication to Dr. James E.K. Hildreth MD, PhD.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Funding: D.J.A. was supported by the Meharry Zika Startup Grant and a Research Centers in Minority Institutions (RCMI) program grant (U54MD007586-01).
Disclosure of Interests: All authors declare they have no conflict of interest.
Ethical approval: Studies involving humans.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Approval was granted by the Institutional Review Boards (IRB) of Meharry Medical College, Johns Hopkins University Medical Center, and Vanderbilt University Medical Center. This includes all de-identified specimens from patients on slides.
Ethical approval: Studies involving animals.
This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent for participation and publication was obtained from all individual participants included in the study.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alcendor, D.J. (2021). Effects of Cytomegalovirus on Pericytes. In: Birbrair, A. (eds) Biology of Pericytes – Recent Advances. Stem Cell Biology and Regenerative Medicine, vol 68. Humana, Cham. https://doi.org/10.1007/978-3-030-62129-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-62129-2_4
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-62128-5
Online ISBN: 978-3-030-62129-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)