Abstract
Nail diseases are often challenging to dermatologists. Reflectance confocal microscopy (RCM) is a noninvasive diagnostic tool that achieves a nearly histological resolution. It was initially developed for the evaluation of skin neoplasms, but more recently, it has also been applied for different diseases such as psoriasis, atopic dermatitis, lichen planus, mycosis fungoides, discoid lupus erythematosus, cutaneous infections, and infestations.
Skin appendages, such as hair and nails, are particularly suited to be examined by RCM: all these body sites are sensitive areas where noninvasive imaging techniques are of high interest to spare biopsies and excisions.
In case in which biopsies are necessary, RCM can represent a very useful tool for the intraoperative examination of the nail bed and the nail matrix after reclination of the nail plate.
Ex vivo RCM can be performed intraoperatively immediately on the surgical tissue to have a quick examination before final histopathology.
Limitations are the higher cost than traditional optical microscope or dermoscopy, the need to have experienced specialized dermatologists for the acquisition and interpretation of the images, and the limited achievable depth.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Reflectance confocal microscopy (RCM) is a noninvasive high-resolution imaging technique that has been applied in dermatology for the diagnosis of melanocytic and non-melanocytic tumors and inflammatory and infectious diseases and, in the last years, also for the examination of skin appendages such as hairs and nails. It allows a noninvasive and in vivo examination of the nail plate, potentially reducing the number of biopsies that can irreversibly alter the structure of this sensitive area. RCM represents a valid tool for the diagnosis of onychomycosis and has shown better sensitivity and specificity than the microscopic examination with KOH preparation. Regarding melanonychia, it is unable to explore nail matrix owing to the limited penetration depth, but it can be used intraoperatively to visualize melanocytes in the nail matrix since the eponychium can be reclined during the surgical biopsy. Ex vivo fluorescence confocal microscopy (FCM) can also play a role in the preoperatory diagnosis of other nail tumors such as squamous cell carcinoma, onycholemmal carcinoma, onychomatricoma, onychopapilloma, glomus tumors, or neurinoma; it allows to perform a quick examination before final histopathology and to outline tumor margins. In the future, RCM could be used for a noninvasive diagnosis of inflammatory nail disease and to monitor the response to topical or systemic therapy. Limitations are the higher cost, the requirement to have experienced specialized dermatologists for the acquisition and interpretation of the images, and the limited achievable depth.
In memory of Bruno Fouilloux.
Confocal Microscopy and Nails
Confocal microscopy has been developed by Marvin Minsky in 1955 [1]. Since 1993, it has been applied in vivo in the field of dermatology [2]. It provides real-time high-resolution black and white horizontal sections of the entire epidermis and superficial dermal layer up to ~200 μm of depth. Unlike conventional microscopes, confocal microscopy enables to create virtual sections inside the skin and to identify also dynamic events such as blood flow [3]. Confocal microscopy can be used in the reflectance or the fluorescence mode. In reflectance confocal microscopy (RCM, 830 nm laser), different contrast depends on the refractive index of the inner structures: melanin and keratin are endogenous chromophores; therefore, stratum corneum, hair shaft, acrosyringium, pigmented keratinocytes, and melanocytes appear hyper-refractive [4]. Images appear in grayscale with dark hyporeflective structures (black and dark gray) and bright hyperreflective structures (white and light gray). In fluorescence confocal microscopy (FCM, laser wavelength of 488 nm and 658 nm), the nonfluorescent structures are dark (black and dark gray), and the fluorescent structures are white. Autogenous fluorescence of the skin and/or fluorescent agents can be exploited. In vivo confocal microscopy is mainly used in the reflectance mode, and fluorescence mode is practiced for research, whereas in ex vivo conditions, both reflectance and fluorescence modes are used, and reflectance and fluorescence can be also used simultaneously (fusion mode). In ex vivo conditions, several fluorescent agents have been tested, including acridine orange, fluorescein, patent blue, methylene blue, Nile blue, and toluidine blue, but only acridine orange is used in practice, as it provides strong contrast for cells by staining nuclear DNA. With acridine orange, the nuclei of the cells are fluorescently stained in white with an increase in contrast of keratinocytes, hair follicle epithelium, sebaceous and eccrine glands, fibroblasts, and tumor cells relative to the surrounding tissue.
Three devices dedicated to the skin are commercially available: two work in vivo (VivaScope 1500 and 3000, Caliber, New York, USA, distributed in Europe by the company MAVIG GmbH, Munich, Germany) and one ex vivo (VivaScope 2500, Caliber). The traditional wide probe camera VivaScope 1500 can work in both reflectance and fluorescence modes, provides images of 500 μm in diameter that can be stitched together in mosaic images of maximum 8 mm and should be fixed to the tissue with a special window, and needs mineral oil as interface. The convex surface of the nail and the concavity of the transition between the nail plate and the surrounding skin make difficult to place and hold the device during in vivo imaging. To overcome this technical problem, the handheld device (VivaScope 3000) can be used for the nail. VivaScope 3000 with ultrasound gel used as interface is better than VivaScope 1500 for nail examination because no extra window is needed, and the ultrasound gel allows adapting the tip of the device to the irregular nail surface to be observed. The second generation of VivaScope 3000 is more suitable than the newest version of VivaScope 3000 due to its smaller tip of 0.5 cm instead of 1.5 cm. The handheld camera acquires single images of 920 μm in diameter in reflectance mode, and no mosaics are obtainable. The acquisition of in vivo images is fast; it takes just a few minutes, especially with the handheld camera. Optical resolution is in the cellular scale: <2 μm in the horizontal plane and <5 μm in the vertical plane. The ex vivo device is used on freshly excised unfixed and not cut surgical specimen. Notably, it does not modify the tissue and does not prevent subsequent histopathological examination. Different from in vivo examination, ex vivo examination gives the opportunity to observe the sample from its lateral sides and its bottom according to how the specimen is mounted. The ex vivo microscope produces horizontal images of 750 × 750 μm of the different layers of the skin up to a thickness of 200 μm [5]. Single images are automatically stitched together into a reconstructed mosaic image to a maximum size of 20 × 20 mm [5]. The lateral and axial spatial resolutions are 1 μm and 3 μm, respectively.
RCM has been used in clinical routine for the diagnosis of several melanocytic and non-melanocytic tumors [6,7,8], and it has been demonstrated to be superior to dermoscopy for the diagnosis of melanoma [9]. More recently, it has also been applied for different diseases such as psoriasis, atopic dermatitis, lichen planus, mycosis fungoides, discoid lupus erythematosus [10,11,12,13,14,15], and cutaneous infections and infestations [16, 17] and for the examination of skin appendages, such as hair [18] and nails [19, 20].
Healthy Nail
Nails are particularly suitable to be examined with RCM owing to the nail plate transparency that allows a deeper penetration of the laser up to 400–500 μm [20]. In 1995, Kaufman et al. affirmed that the examination of nail apparatus with conventional light microscope, transillumination, and reflected light illumination was not useful because of the high reflectivity of the keratinized surface of the nail plate, and they first considered RCM as a new instrument for the study of nail unit in vivo [19]. Their microscope allowed to visualize not only changes seen in the capillary nail fold but also the nail plate and nail bed. Lower nail plate showed discrete cells, some with nuclear fragments, which were visible as bright spots; underlying epithelial ridge in nail bed revealed keratogenous zone and underlying rootlike epidermal ridges [19].
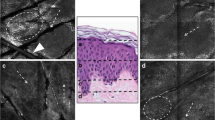
In 2011, Sattler et al. have described the morphology of nail unit under in vivo RCM [21]. Nail surface, nail plate, and nail bed in thin nails (<500 μm) could be examined with handheld RCM (Fig. 6.1). The difference of intensity of reflection allowed to distinguish three different layers:
-
A superficial layer with a brighter reflection
-
An intermediate layer with slightly poorer signal
-
A deeper brighter zone
Clinical (a) and in vivo reflectance confocal microscopy (RCM) aspect of a thumbnail plate (b–f). Three zones of different brightness can be differentiated going deeper into the nail plate: a bright surface (b), a dark central part (c), and a bright deepest part (d). The transition to the underlying nail bed is visible as wavelike structures, which are directed toward the fingertip (e). The transition between the proximal part of the nail plate and the skin (f) is characterized by normal honeycomb pattern of the epidermis (yellow asterisk) that continues into stellate figures corresponding to epidermal keratinocytes sectioned obliquely (red asterisk) and by a hyperreflective stripe corresponding to the cuticle (black asterisk)
Furthermore, transition zones have been described [20, 21]:
-
A transition area to the underlying nail bed that shows wavelike structures, which are directed toward the fingertip
-
A transition area between the proximal nail plate and the skin that is characterized by three adjacent layers:
-
(i)
Normal honeycombed pattern of the epidermis
-
(ii)
Stellate-shaped epidermal keratinocytes, i.e., keratinocytes sectioned obliquely
-
(iii)
Hyperreflective or moderate reflective band representing cuticle
-
(i)
Onychomycosis
Onychomycosis is a very common fungal infection of the nail caused by dermatophytes, yeasts, or molds [22]. It represents about 50% of ungual pathology [23]. Its diagnosis can be challenging because it is often difficult to distinguish from other nail disorders such as traumatic onycholysis, psoriasis, or lichen ruber planus [24]. Treatment often requires several months of systemic medication and can cause serious adverse reactions such as hepatitis or drug-induced lupus erythematosus [25]. Therefore, mycologic study is required before antifungal therapy is prescribed. Conventional diagnostic methods include KOH preparation for optical microscopy examination, culture, PCR, and histopathology with PAS staining [26].
KOH preparation is a cheap and easy to execute method that provides a result after about 30 minutes. However, it is invasive, and the sample collection must be correct in order to avoid false-negative results or false-positive results such as contaminant molds [27]. Dermatophyte culture allows the identification of the fungal species, but it takes a long time and can be contaminated. Recently, distinctive dermoscopic signs of distal subungual onychomycosis have been described such as jagged proximal edge with spikes of the onycholytic area and longitudinal striae [28, 29]. Utility of in vivo RCM for the diagnosis of onychomycosis has been known since 1994 when Pierard et al. considered RCM as “a door opened to the future,” able to help clinician in the identification of onychomycosis [30]. The first case of onychomycosis confirmed by in vivo RCM was reported by Hongcharu et al. in 2000 [31]. RCM revealed network of branched high-reflective hyphae just below the surface of the nail plate (Fig. 6.2) and was able to confirm the diagnosis with higher precision and faster results compared with KOH preparation. However, other authors asserted that this tool is too expensive to be used in clinical practice [27].
In 2012, Rothmund et al. considered 50 patients with suspected onychomycosis and compared sensitivity and specificity of six diagnostic methods including in vivo RCM [32]. RCM analysis was performed with VivaScope 1500 on the most suspicious area. Diagnosis of onychomycosis was made when hyper-refractive lengthy structures and bright aggregates (corresponding to spores) were observed . RCM had better sensitivity (79% vs. 74%) and specificity (81% vs. 76%) than KOH preparation. Fungal culture showed the lowest sensitivity and the worst negative predictive value. PCR showed the highest sensitivity, followed by RCM, PAS, and KOH preparation. RCM and KOH showed a high specificity, and RCM exhibited the best positive predictive value. RCM can also provide information about location and density of fungal cells [33]. Nevertheless, only PCR and culture allow identification of species. It is not clear if RCM is able to distinguish dermatophytes from molds or yeasts since no specific RCM studies have been conducted yet [33, 34].
Melanonychia
A big variety of conditions present longitudinal pigmented bands on the nail plate including melanocytic nevus, malignant melanoma, lentigo, ethnic melanonychia, pigmented onychomycosis, pigmented Bowen’s disease, drug-induced hyperpigmentation, and subungual hematoma [35]. The prevalence of melanonychia is approximately 1% [36]. Dermoscopy is a useful tool which can better identify the cases in which pathological examination is indicated [37]. With RCM, it is possible to visualize melanocytes of Hutchinson’s sign (Fig. 6.3), but melanocytes of the nail bed are difficult to be identified (Fig. 6.4), and nail matrix is impossible to be observed owing to the limited penetration depth. Debarbieux et al. tried to evaluate the feasibility of an intraoperative diagnosis by in vivo RCM [38, 39] since the eponychium could be reclined during the surgical biopsy to visualize nail matrix pigmentation. Four different patterns were identified: pattern 1: numerous atypical bright roundish cells at the dermoepithelial junction, usually associated with dendritic cells (pagetoid cells were occasionally identified in a few cases); pattern 2: numerous nests; pattern 3: very bright cobblestone pattern; and pattern 4: variably densely distributed dendritic basal cells, with neither nests, roundish cells, nor atypical cells in the dermis. Pattern 1 was the most relevant for melanoma diagnosis, pattern 2 usually indicated the presence of acral nevus, pattern 3 could be associated to melanoma, and pattern 4 could be found either in very early in situ melanomas or in lentigo with slight melanocytic hyperplasia [40]. Correlation with histopathology was good, and perioperative in vivo RCM allowed an extemporaneous diagnosis and one-step surgical treatment, thus reducing the pain induced by multiple invasive surgical procedures on the nail apparatus, as well as the postoperative disability period. Moreover, if in vivo RCM is unable to provide a sure diagnosis , ex vivo confocal microscopy can be used. Ex vivo confocal microscopy could be particularly useful for nail tumors in order to confirm intraoperatively the diagnosis of the biopsy specimen and to proceed to the final excision without waiting for the histological examination. Notably, it is more suitable than the optical examination of frozen sections of this site because nail tumors are often very small in size, and it is desirable that no material is wasted in producing sections that subsequently cannot be submitted to conventional histopathology [5]. Debarbieux et al. also used the in vivo device for the ex vivo examination of nail biopsies of pigmented subungual melanoma in a series of eight cases [38]. However, this procedure has limitations in that the specimen is not fixed, tends to move, and is difficult to orient, unlike when using the dedicated ex vivo device.
Other ungual tumors different from melanoma can present with melanonychia striata. Fernandes Massa et al. have described a case of pigmented squamous cell carcinoma (SCC) of the nail bed [41]. In vivo and ex vivo confocal microscopy of the distal matrix were performed intraoperatively: in vivo RCM showed small, bright nonnucleated cells corresponding to pigmented keratinocytes; a cobblestone pattern; and rare, small dendritic cells. The proximal nail bed revealed large, roundish structures with small, bright cells at their periphery within the papillary dermis, which were different from atypical melanocytes. These roundish structures could be better visualized with ex vivo RCM which showed small, bright nonnucleated cells with a structureless center [41].
RCM represents also a preliminary screening instrument for nail matrix nevus. Only two case reports have been described: both cases showed hyperreflective roundish cells in the deeper part of the nail plate that were uniform in size and shape and seemed to correspond to melanocytes on histopathology [42]. However, it should be noticed that these cells are difficult to differentiate from hyperreflective corneocytes that can be found in case of onycholysis and that no comparison with malignant melanocytes has been reported yet. Interestingly, in both nevi, no dendritic hyperreflective cells were observed. In our experience, we found that in nevi, when the nail bed was reachable, we could observe an alteration of the transition area from the nail plate to the underlying nail bed: wavelike structures were thickened and greyish and formed cord-like structures with some bulbous projections that remind cutaneous melanocytic nests under RCM (Fig. 6.4). It should be observed that in vivo RCM can be also used to examine skin lesion destroying the nail plate such as in case of malignant tumors (Fig. 6.3).
Nonpigmented Nail Tumors
Although most of the nonpigmented nail tumors are of epithelial origin, amelanotic melanoma can occur. The diagnosis of these tumors is often challenging and delayed because they can mimic other nail disorders such as onychomycoses, onychodystrophy, and verruca vulgaris [43,44,45].
SCC is the most common malignant tumor of the nail apparatus and usually presents in the form of Bowen’s disease and amelanotic melanoma [46]. Fingernails are selectively affected, especially the thumb [45]. Its RCM and FCM features are well described both in vivo and ex vivo in the skin but are less studied in the nail. Amelanotic subungual melanoma represents only a small fraction of all malignant melanomas [43], and it usually manifests as a vascular or ulcerating nodule [47]. RCM recognizes amelanotic melanocytes, but this aspect has not been deeply studied for the melanoma of the nail apparatus [20].
A pilot study conducted on ten patients with nonpigmented nail tumors (four in situ SCC, two invasive epithelial tumors, three benign epithelial tumors, and one nodular melanoma) has described for the first time the microscopic features examined with the ex vivo FCM [48]. Images were taken intraoperatively on the surgical specimens after immersion in acridine orange, a specific staining of the nucleus. Invasive SCC of the nail matrix and onycholemmal carcinoma showed well-demarcated epithelial nests composed of cells with nuclear pleomorphism (variable size and shape of the nucleus) invading the dermal area. Therefore, in these cases, it would be possible to perform wide excision of the tumors just after the observation of a biopsy specimen under ex vivo FCM shortening the management. In situ SCC displayed cytoarchitectural atypia: roundish cells in the upper layers of epithelium corresponding to dyskeratotic keratinocytes and “blurred cells,” i.e., large pseudo-acanthotic cells with blurry limits [48]. However, it was impossible to distinguish epithelial cells from inflammatory cells or atypical melanocytes using the fluorescence mode after acridine staining because acridine stains nuclei of cells of different nature.
Benign tumors were also observed with ex vivo FCM [48]. In particular, onychomatricoma exhibited epithelial proliferation with acanthosis and papillomatosis without atypia; moreover, small fusiform cells corresponding to fibroblasts were observed inside the papillae (Fig. 6.5) [48]. Onychomatricoma has also been examined with in vivo RCM (Fig. 6.6) [49, 50]. Sanchez et al. have described multiple parallel channel-like structures through the nail plate that correspond to the digitating fibroepithelial projections arising from the matrix [49]. These cavities appeared hyporeflective and were surrounded by a brighter matrix epithelium (Fig. 6.6a). Their hyporeflectance could be induced by their content in serum and blood which have low refraction of light on RCM [50]. If in vivo RCM is used perpendicularly to the distal ungual margin, hyporeflective roundish areas corresponding to the transversal sections of the tumor cavities can be observed (Fig. 6.6b) [50]. Onychopapilloma is a very circumscribed epithelial tumor of the nail bed, characterized by thin digitiform epithelial projections within the upper dermis that were visible under ex vivo FCM [48]. Not only nonpigmented epithelial tumors can be recognized under ex vivo FCM but also other amelanotic non-epithelial tumors such as glomus tumor and neurinoma [5].
Leukonychia
Leukonychia is a white discoloration of the nail; apparent and true variants have been described. Apparent leukonychia derives from pathological changes (e.g., edema) in the nail bed, whereas true leukonychia results from disorder of keratinization occurring in the nail matrix with a consequent involvement of the nail plate itself [51]. When an external process alters the nail plate’s growth, the discoloration of the nail plate is called pseudoleukonychia.
Main in vivo RCM features of leukonychia were first described by Sattler et al. in 2012 [21]; in vivo RCM revealed detachment of single hyperreflective corneocytes (Fig. 6.7). However, it is well known that white discoloration of the nail can be a sign of different diseases such as trauma and lichen planus, and there is no study in literature which has described the differences in the morphology or amount or depth of dyskeratotic corneocytes [20].
Inflammatory Disorders
Unlike inflammatory skin diseases , inflammatory nail pathologies have not been studied in detail with in vivo RCM microscopy. Only few cases of nail psoriasis have been described [20], and they showed large hyperreflective irregular areas on the surface and inside of the nail plate corresponding to hyperkeratosis (Fig. 6.8). The lateral margins of the nail displayed delamination of the nail plate [20].
Clinical (a) and in vivo reflectance confocal microscopy (RCM, b,c) aspect of nail psoriasis. In vivo RCM shows large hyperreflective irregular areas on the surface of the nail plate (red asterisk) (b) and inside the nail plate (yellow asterisk) (c), likely corresponding to areas of keratin densification. Normal nail plate is also visible (green asterisk)
Conclusion
RCM and FCM are noninvasive diagnostic techniques that can be used for a better evaluation of nail diseases. In vivo RCM represents a promising adjunctive diagnostic tool for the diagnosis of onychomycoses and for other nail plate diseases. The in vivo examination does not require any sample collection; the probe can be applied directly to the nail surface without any preparation, and it permits to quickly evaluate the entire nail plate. In some cases, in vivo RCM allows to avoid biopsies that cause pain and discomfort to the patient and can irreversibly alter the nail growth. In the future, RCM could also be used for noninvasive diagnosis of inflammatory nail disease and to monitor the response to topical or systemic therapy. In vivo RCM is often not able to explore the nail bed and cannot explore the nail matrix because the nail thickness restricts laser penetration. However, it can represent a very useful tool for the examination of the nail bed and the nail matrix if used intraoperatively after reclination of the nail plate and/or eponychium. Limitations are the higher cost than traditional optical microscope or dermoscopy, the need to have experienced specialized dermatologists for the acquisition and interpretation of the images, and the limited achievable depth.
In cases in which biopsies are necessary, ex vivo RCM and FCM can be performed intraoperatively immediately on the surgical tissue to have a quick examination before final histopathology. Ex vivo RCM is more suitable for melanocytic skin cancers, whereas ex vivo FCM is more suitable for non-melanocytic skin cancers.
References
Minsky M. Memoir on inventing the confocal scanning microscope. Scanning. 1988;10:128–38.
Piérard GE. In vivo confocal microscopy: a new paradigm in dermatology. Dermatology. 1993;186:4–5.
Cinotti E, et al. Quantification of capillary blood cell flow using reflectance confocal microscopy. Skin Res Technol. 2014;20:373–8.
Nwaneshiudu A, et al. Introduction to confocal microscopy. J Invest Dermatol. 2012;132:1–5.
Cinotti E, Perrot JL, Labeille B, Cambazard F, Rubegni P. Ex vivo confocal microscopy: an emerging technique in dermatology. Dermatol Pract Concept. 2018;8:109–19.
González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100:59–69.
Guida S, et al. Update on the use of confocal microscopy in melanoma and non-melanoma skin cancer. G Ital Dermatol Venereol. 2015;150:547–63.
Ulrich M, et al. Reflectance confocal microscopy for noninvasive monitoring of therapy and detection of subclinical actinic keratoses. Dermatol (Basel, Switzerland). 2010;220:15–24.
Dinnes J, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
Astner S, González S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182–91.
Agozzino M, et al. Reflectance confocal microscopy features of seborrheic dermatitis for plaque psoriasis differentiation. Dermatology. 2014;229:215–21.
Moscarella E, et al. Pilot study on reflectance confocal microscopy imaging of lichen planus: a real-time, non-invasive aid for clinical diagnosis. J Eur Acad Dermatol Venereol. 2012;26:1258–65.
Broggi G, Lacarrubba F, Verzì AE, Micali G, Caltabiano R. Confocal microscopy features of patch-stage mycosis fungoides and their correlation with horizontal histopathological sections. A case series. J Cutan Pathol. 2019;46:163–5.
Ardigo M, et al. Preliminary evaluation of in vivo reflectance confocal microscopy features of discoid lupus erythematosus. Br J Dermatol. 2007;156:1196–203.
Ardigo M, Cota C, Berardesca E, González S. Concordance between in vivo reflectance confocal microscopy and histology in the evaluation of plaque psoriasis. J Eur Acad Dermatol Venereol. 2009;23:660–7.
Cinotti E, Perrot JL, Labeille B, Cambazard F. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754–63.
Cinotti E, Labeille B, Cambazard F, Perrot JL. Reflectance confocal microscopy in infectious diseases. G Ital Dermatol Venereol. 2015;150:575–83.
Ardigo M, Agozzino M, Franceschini C, Lacarrubba F. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487–96.
Kaufman SC, Beuerman RW, Greer DL. Confocal microscopy: a new tool for the study of the nail unit. J Am Acad Dermatol. 1995;32:668–70.
Cinotti E, et al. Confocal microscopy for healthy and pathological nail. J Eur Acad Dermatol Venereol. 2014;28:853–8.
Sattler E, Kaestle R, Rothmund G, Welzel J. Confocal laser scanning microscopy, optical coherence tomography and transonychial water loss for in vivo investigation of nails. Br J Dermatol. 2012;166:740–6.
Leung AK, Lam JM, Leong KF, Hon KL, Barankin B, Leung AA, Wong AH. Onychomycosis: an updated review. Recent Patents Inflamm Allergy Drug Discov. 2019. https://doi.org/10.2174/1872213X13666191026090713.
Chabasse D, Pihet M. Mycological diagnosis of onychomycosis. J Mycol Med. 2014;24:269–78.
Allevato MAJ. Diseases mimicking onychomycosis. Clin Dermatol. 2010;28:164–77.
Michaelis TC, Sontheimer RD, Lowe GC. An update in drug-induced subacute cutaneous lupus erythematosus. Dermatol Online J. 2017;23.
Beifuss B, Borelli C, Korting HC. Mycological laboratory. Hautarzt Z Naturforsch C J Biosci. 2006;57:487–8, 490–2.
de Chauvin MF. New diagnostic techniques. J Eur Acad Dermatol Venereol. 2005;19:20–4.
Piraccini BM, Balestri R, Starace M, Rech G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509–13.
Alessandrini A, Starace M, Piraccini BM. Dermoscopy in the evaluation of nail disorders. Skin Appendage Disord. 2017;3:70–82.
Piérard GE, et al. Diagnostic microscopique des onychomycoses. In: Annales de dermatologie et de vénéréologie, vol. 121. Paris: Masson; 1994. p. 25–9.
Hongcharu W, Dwyer P, Gonzalez S, Anderson RR. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42:214–6.
Rothmund G, et al. Confocal laser scanning microscopy as a new valuable tool in the diagnosis of onychomycosis - comparison of six diagnostic methods. Mycoses. 2013;56:47–55.
Arrese J-E, Quatresooz P, Piérard-Franchimont C, Piérard G-E. Nail histomycology. Protean aspects of a human fungal bed. Ann Dermatol Venereol. 2003;130:1254–9.
Pharaon M, et al. Diagnosis and treatment monitoring of toenail onychomycosis by reflectance confocal microscopy: prospective cohort study in 58 patients. J Am Acad Dermatol. 2014;71:56–61.
Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49–54.
Duhard E, Calvet C, Mariotte N, Tichet J, Vaillant L. Prevalence of longitudinal melanonychia in the white population. Ann Dermatol Venereol. 1995;122:586–90.
Thomas L, Dalle S. Dermoscopy provides useful information for the management of melanonychia striata. Dermatol Ther. 2007;20:3–10.
Debarbieux S, et al. Perioperative confocal microscopy of the nail matrix in the management of in situ or minimally invasive subungual melanomas. Br J Dermatol. 2012;167:828–36.
Routine use of perioperative in vivo reflectance confocal microscopy of the nail matrix in melanonychia striata: a report of 30 cases. – PubMed – NCBI. https://www.ncbi.nlm.nih.gov/pubmed/27699771
Fattouh K, et al. Routine use of perioperative in vivo reflectance confocal microscopy of the nail matrix in melanonychia striata: a report of 30 cases. Br J Dermatol. 2017;177:570–3.
Fernandes Massa A, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198–9.
Zeng X, Qiu Y, Peng J, Xiang W. Reflectance confocal microscopy as a preliminary screening tool for nail matrix nevus: two cases report. Skin Res Technol. 2019;25:758–60.
Ishii L, Richmond NA, Carstens SJ, Vincek V. An amelanotic nail bed melanoma presenting as persistent onychodystrophy. Dermatol Online J. 2018;24.
Riahi RR, Cohen PR, Goldberg LH. Subungual amelanotic melanoma masquerading as onychomycosis. Cureus. 2018;10:e2307.
Dalle S, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871–4.
Park J-H, Lee D-Y, Kim N. Nail neoplasms. J Dermatol. 2017;44:279–87.
Harrington P, O’Kelly A, Trail IA, Freemont AJ. Amelanotic subungual melanoma mimicking pyogenic granuloma in the hand. J R Coll Surg Edinb. 2002;47:638–40.
Debarbieux S, et al. Intraoperative diagnosis of nonpigmented nail tumours with ex vivo fluorescence confocal microscopy: 10 cases. Br J Dermatol. 2015;172:1037–44.
Sanchez M, Hu S, Miteva M, Tosti A. Onychomatricoma has channel-like structures on in vivo reflectance confocal microscopy. J Eur Acad Dermatol Venereol. 2014;28:1560–2.
Cinotti E, et al. Imaging technique for the diagnosis of onychomatricoma. J Eur Acad Dermatol Venereol. 2018;32:1874–8.
Kim SW, Kim MS, Han TY, Lee JH, Son S-J. Idiopathic acquired true leukonychia totalis and partialis. Ann Dermatol. 2014;26:262–3.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fiorani, D., Perrot, J.L., Rubegni, P., Cinotti, E. (2021). In Vivo and Ex Vivo Confocal Microscopy for Nail Diseases. In: Baran, R.L. (eds) Advances in Nail Disease and Management. Updates in Clinical Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-030-59997-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-59997-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59996-6
Online ISBN: 978-3-030-59997-3
eBook Packages: MedicineMedicine (R0)